Introduction
Situs inversus is a rare congenital anomaly that
occurred in 1/8,000-1/25,000 people globally in 2001 (1). Situs inversus totalis (SIT) is an
uncommon congenital anomaly that often occurs concomitantly with
other disorders (1,2), including congenital heart disease,
ciliary dyskinesia, Kartagener's syndrome and polysplenia (3–5). Chronic
myeloid leukemia (CML) is a malignant clonal disorder of
pluripotent hematopoietic stem cells and accounts for 15% of all
adult leukemia cases in the United States in 2007 (6). The diagnosis of CML is performed on the
basis of detecting the breakpoint cluster region protein
(BCR)-Abelson murine leukemia viral oncogene homolog 1 (ABL) gene
or Philadelphia chromosome [t(9; 22)(q34; q11.2)] (7). The translocation is detected using
routine cytogenetics, fluorescence in situ hybridization
(FISH) and molecular reverse-transcription polymerase chain
reaction tests (7). The global CML
annual incidence rate was 1.6–2.0/100,000 people per year (8). The CML annual incidence rate in China
was 0.36/10 million people in 1992, which was decreased compared
with that in Western countries (9).
The first generation of tyrosine inhibitor imatinib as a first-line
treatment of patients with CML is associated with a 10-year
survival rate of 85–90% (10). To the
best of our knowledge, the literature reports only 1 case of CML in
a patient exhibiting SIT; the patient was a 69-year-old male
exhibiting chronic granulocytic leukemia and SIT, which had induced
a right-sided splenomegaly that was originally diagnosed as a
hepatomegaly (11). The present study
reported a case of CML with complete situs inversus in a
68-year-old female patient. A series of imaging studies revealed
total mirror reversal of the thoracic and abdominal organs. A bone
marrow smear supported the diagnosis of CML in the accelerated
phase. The co-occurrence of CML and SIT prompted the present study
to evaluate whether these conditions may be associated.
Case report
A 68-year-old female patient was admitted to the
Department of Hematology at The First Affiliated Hospital of
Lanzhou University (Lanzhou, China) on September 3, 2012. The
patient reported a 2-month history of general weakness and night
sweats and a 1-month history of experiencing abdominal distension
and tenderness in the right hypochondrium. A chest X-ray and
conventional electrocardiogram revealed a right-sided heart.
Abdominal ultrasound revealed SIT, a condition characterized by
total mirrored reversal of the thoracic and abdominal organs.
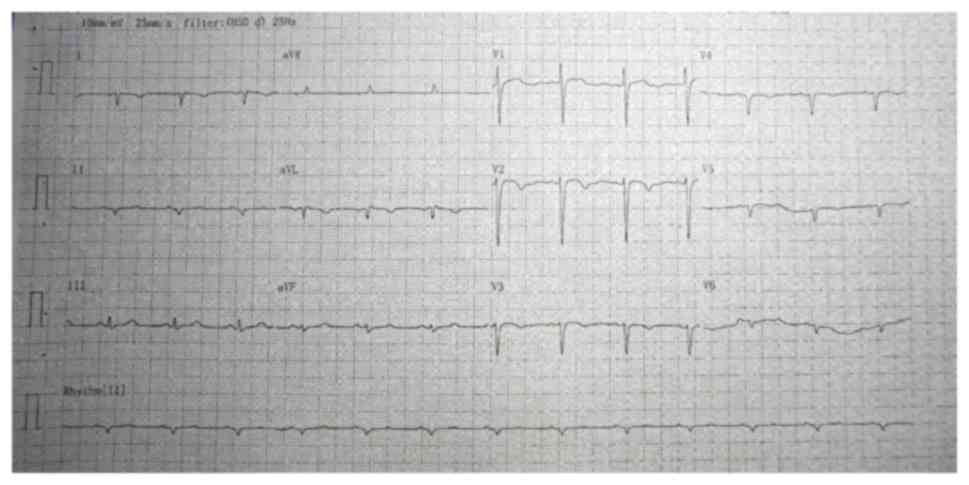
A conventional electrocardiogram revealed
dextrocardia (Fig. 1). A chest X-ray
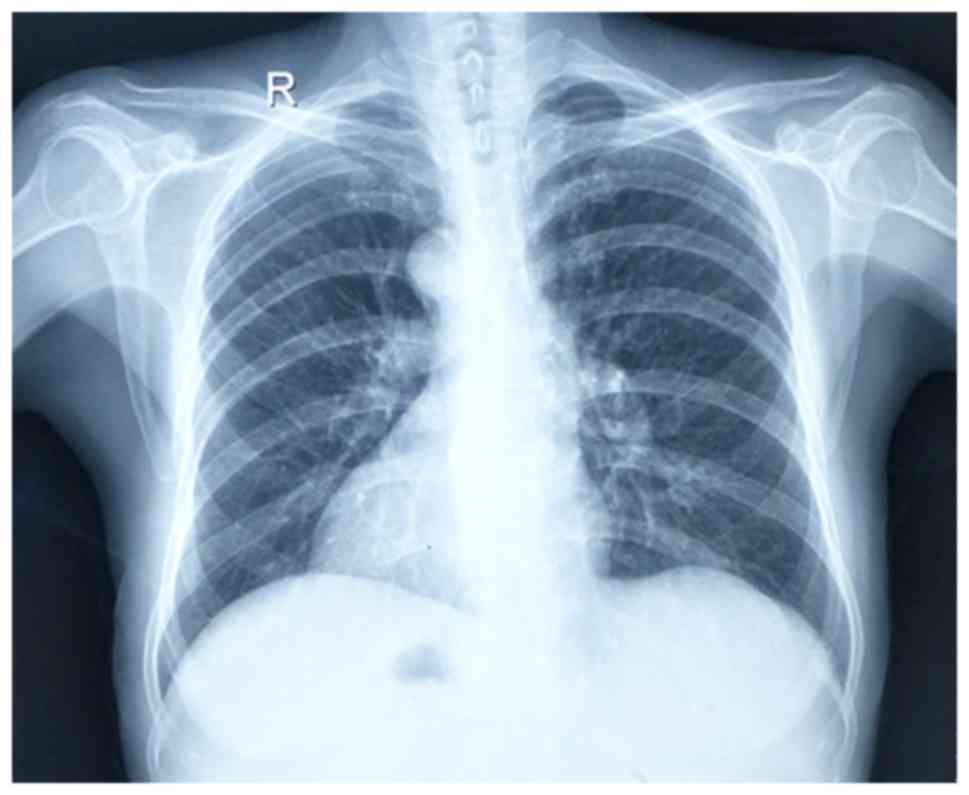
revealed heart and stomach gas on the right side (Fig. 2). An abdominal ultrasound examination
indicated SIT and spleen enlargement. A complete blood count test
obtained the following results: 12% original and naive cells
(normal range, 0%); hemoglobin, 109 g/l (normal range, 110–150
g/l); 1.897×1010 leukocytes/l (normal range,
0.400–1.000×1010 leukocytes/l); 1.176×1010
neutrophils/l (normal range, 0.200–0.700×1010
neutrophils/l); 2.85×109 lymphocytes/l (normal range,
1.50–4.00×109 lymphocytes/l); 1.52×109
monocytes/l (normal range, 0.00–0.45×109 monocytes/l);
3.8×108 eosinophils/l (normal range,
0.5–3.0×108 eosinophils/l); 2.47×109
basophils/l (normal range, 0.00–0.20×109 basophils/l)
and 3.86×1011 platelets/l (normal range,
1.00–3.00×1011 platelets/l). A bone marrow smear
demonstrated a myeloid:erythroid ratio of 3.68:1 (normal range,
2.00–4.00:1), with original granular cells accounting for 18.0% of
the total cells (normal range, 0.5–2.0%), and supported the
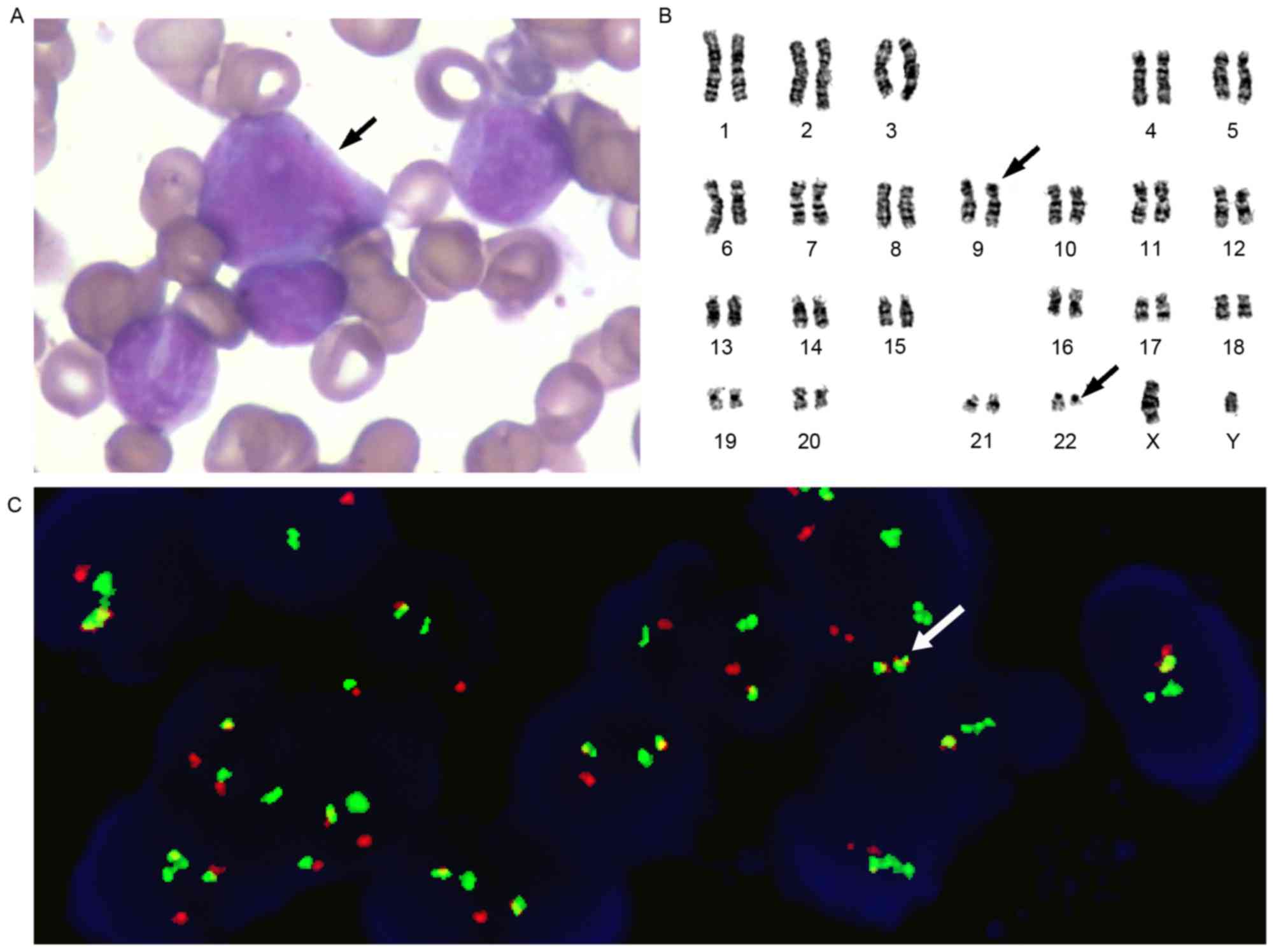
diagnosis of CML in the accelerated phase. Karyotype analysis
demonstrated 46, XX, t (9;22; q34;q11.2) in the 20 assessed cells
and was the sole abnormality observed. FISH was performed using the
gene locus-specific probe (GLP) BCR/GLP ABL1 to identify the
BCR-ABL1 fusion gene. The rate of BCR-ABL1 fusion was 84%, as
determined using FISH (Fig. 3). The
present study extracted the bone marrow samples from the patients
and sent the BCR-ABL1 fusion gene to undergo a quantitative
polymerase chain reaction. The method was determined using
fluorescence quantitative polymerase chain reaction. This method is
from the bone marrow mononuclear cells in the separation and
purification of RNA, TRIzol (TransStart Inc., Beijing, China)
reagent was used to extract total RNA. Subsequently, reverse
transcription of cDNA was performed using the reverse transcription
kit (Takara Bio Inc., Otsu, Japan), using RNA as a template. The
temperature protocol were as follows: 37°C for 15 min, 85°C for 5
sec and kept at 4°C until use. The fluorophore used for the
polymerase chain reaction was Dalian's Tap enzyme (Takara Bio
Inc.). The target gene primers were as follows: BCR-ABL, bcr:
5′-AGGGTGCACAGCCGCAACGGC-3′ and abl, 5′-GGCTTCACTCAGACCCTGAGG-3.
The reference gene was β-actin. The reference gene primers were as
follows: β-actin, forward: 5′-GGAGATTACTGCCCTGGCTCCTA-3′ and
reverse: 5′-GACTCATCGTACTCCTGCTTGCTG-3′. The thermocycling
conditions were as follows: 95°C for 30 sec for 1 cycle, 95°C for 5
sec and 60°C for 20 sec for 40 cycles. The results were expressed
as 2−ΔΔCq (12). The
quantity of BCR-ABL transcript was normalized to the ABL expression
level. The copy number of BCR-ABL fusion gene and the copy number
of ABL were calculated, and the result was expressed as the ratio
of BCR-ABL copy number to ABL copy number. The present patient's
BCR-ABL1 fusion gene quantitative detection revealed a BCR-ABL1
fusion gene-positive, a BCR-ABL1 copy number of
4.96×104, and a BCR-ABL1/ABL1 ratio of 5:18. Imatinib
mesylate (600 mg/day) was subsequently administered from September
12, 2012 until the patient ceased taking imatinib mesylate
following discharge from the hospital on September 16, 2012.
The patient last presented on November 20, 2013 with
dizziness, fatigue, and abdominal distention and pain. A bone
marrow smear demonstrated a myeloid:erythroid ratio of 57.33:1.00,
with original granular cells accounting for 21% of the total cells,
and supported the diagnosis of CML in the blast crisis phase; CML
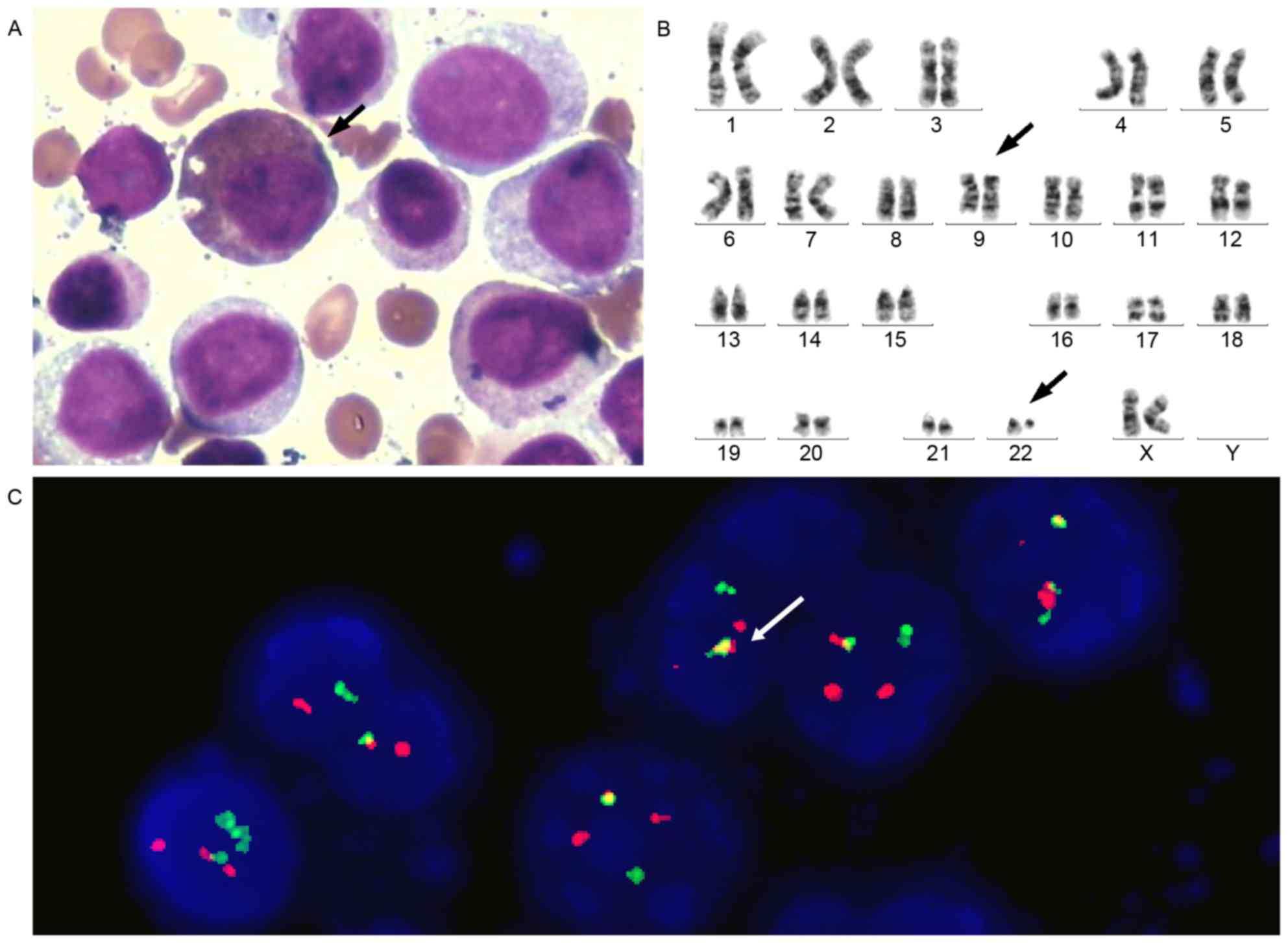
had progressed to acute myeloid leukemia (AML) M2a. Karyotype
analysis also revealed 46, XX, t(9;22;q34;q11.2) in the 20 assessed
cells. The positive rate of BCR-ABL1 fusion was 90%, as determined
by FISH (Fig. 4).
The initial white blood cell (WBC) count was
2.495×1010/l (normal range, 4–10×1010/l).
After 3 days of treatment with imatinib mesylate (600 mg/day) the
WBC count of the patient had increased to 5.083×1010/l,
at which point the imatinib mesylate treatment was replaced by the
continuous intravenous infusion of cytarabine (200 mg/m2
day). After 2 days of cytarabine treatment, the WBC count had
decreased to 1.7×1010/l and the previous relevant
symptoms were no longer present. The patient was discharged from
the hospital on November 28, 2013 and succumbed to AML 3 months
subsequently. The patient's family provided written informed
consent for the present study to be published.
Discussion
The anatomical arrangement of human organs may be
described using the following categories: Situs solitus; situs
inversus; and situs ambiguous. The normal position of the thoracic
and abdominal viscera is referred to as situs solitus. Situs
inversus is a rare congenital anomaly and is classified as either
SIT or partial situs inversus (13).
SIT is associated with an exchange in the position of the thoracic
and abdominal viscera between the left and right sides, resulting
in a mirror reversal of the thoracic and abdominal organs (14). Situs ambiguous is an uncommon
condition in which the cardiac and visceral organs are abnormally
distributed without mirror imaging from conventional anatomy
(15).
Situs inversus is not a premalignant condition.
However, rare synchronous and primary malignancies have been
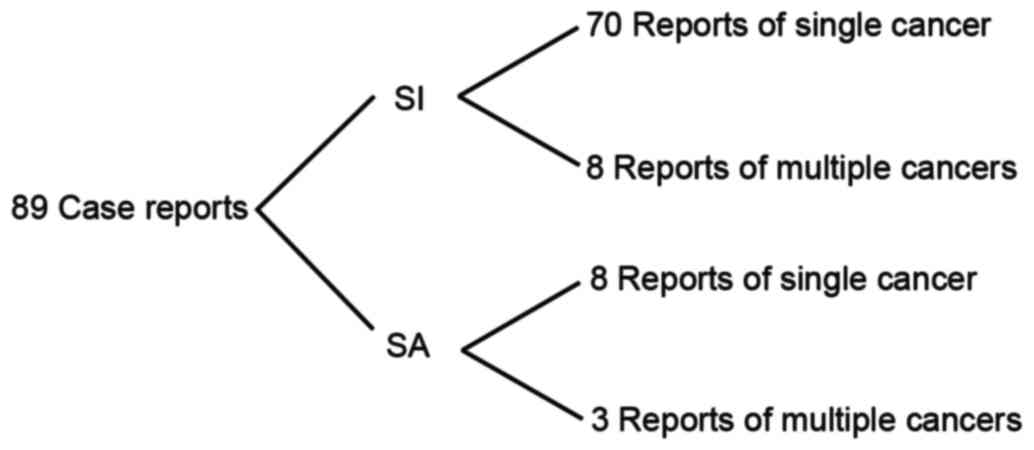
reported in the literature; the PubMed database includes 89 case
reports of patients with cancer exhibiting situs inversus or situs
ambiguous between 1952 and 2015 (Fig.
5). Among these were 78 and 11 cases of cancer in patients
exhibiting situs inversus and situs ambiguous, respectively, and
only 1 case of CML in a patient exhibiting SIT (11). Among these 89 case reports in the
Pubmed database, the four most common cancers reported in patients
with situs inversus included gastric cancer, colorectal
adenocarcinoma, lung cancer and renal cell carcinoma (Table I). Information on multiple primary
malignancies in patients with situs inversus or situs ambiguous
(Table II), and cases of lymphoma
and chronic granulocytic leukemia with situs inversus or situs
ambiguous was provided (Table
III).
 | Table I.Types of malignancy in patients with
SI or SA. |
Table I.
Types of malignancy in patients with
SI or SA.
| Type of cancer | No. of cases | SI/SA |
|---|
| Gastric cancer | 15 | 14/1 |
| Colorectal
adenocarcinoma | 11 | 11/0 |
| Lung cancer | 10 | 10/0 |
| Renal cell
carcinoma | 9 | 6/3 |
| Esophageal (squamous
or adenocarcinoma) | 7 | 7/0 |
| Bile duct cancer | 5 | 3/2 |
| Lymphoma | 5 | 5/0 |
| Hepatocellular
carcinoma | 4 | 4/0 |
| Pancreatic
adenocarcinoma | 4 | 3/1 |
| Endometrial
cancer | 2 | 2/0 |
| Chronic granulocytic
leukemia | 2a | 2/0 |
| Periampullary
carcinoma | 2 | 1/1 |
| Appendiceal mucinous
adenocarcinoma | 1 | 1/0 |
| Adrenal tumor | 1 | 1/0 |
| Bladder cancer | 1 | 1/0 |
 | Table II.Patients with multiple types of
primary malignancy and SI or SA. |
Table II.
Patients with multiple types of
primary malignancy and SI or SA.
| Type of cancer | SI/SA | (Refs.) |
|---|
| Combined
hepatocellular and cholangiocellular carcinoma | 1/0 | (24) |
| Synchronous colonic
tumors on the proximal transverse and sigmoid colon | 1/0 | (25) |
| Metachronous
rectosigmoid colon and gastric cancer | 1/0 | (25) |
| Lung cancer and
mediastinal tumor | 1/0 | (26) |
| Synchronous double
cancer originating from the stomach and rectum | 1/0 | (27) |
| Synchronous early
cancer of the esophagus and larynx | 1/0 | (28) |
| Laryngeal
neoplasms | 1/0 | (29) |
| Hepatocellular and
early signet ring cell gastric carcinoma | 1/0 | (30) |
| Double cancer of
the stomach and esophagus | 0/1 | (31) |
| Gastrointestinal
neoplasms, bladder and mammary cancer, and basal cell
carcinoma | 0/1 | (16) |
| Carcinoma of the
right breast, bladder cancer, endometrial adenocarcinoma and
non-Hodgkin lymphoma of the scalp | 0/1 | (32) |
 | Table III.Patients with lymphoma or leukemia
and SI or SA. |
Table III.
Patients with lymphoma or leukemia
and SI or SA.
| Type of cancer | Age | Sex | SI/SA | (Refs.) |
|---|
| Hairy cell
leukemia | 73 | Male | 1/0 | (33) |
| Mantle cell
lymphoma | 73 | Male | 1/0 | (34) |
| Malignant lymphoma
of the stomach | 51 | Female | 1/0 | (2) |
| Splenic
lymphomatous infiltration | 65 | Male | 1/0 | (35) |
| Ileocecal
lymphoma | 4 | Male | 1/0 | (36) |
| Chronic
granulocytic leukemia | 69 | Male | 1/0 | (11) |
| Chronic
granulocytic leukemiaa | 68 | Female | 1/0 |
|
Although the occurrence of malignancy in patients
with SIT may be coincidental, certain studies have suggested a
possible association between malignancy and SIT (16–18).
Previous studies have proposed that unidentified genes affecting
the left-right axis arrangement may be associated with cancer
susceptibility (19,20). Although this may potentially explain
the occurrence of cancer in the patient with SIT assessed in the
present study, no genes have been conclusively identified as
candidates for this dual role to the best of our knowledge
(16). The pathogenetic mechanisms
underlying SIT were elucidated by previous studies, and it was
shown that kinesin (KIF)3, an intracellular motor protein, has an
important role in establishing left-right asymmetry (17). Nodal flow is autonomously generated by
the rotation of cilia tilted toward the posterior of the cells of
the ventral node. KIF3 molecular motors assist in the development
of these cilia but are dysfunctional in SIT (21,22). It
has also been reported that the KIF3 complex is involved in neural
(N)-cadherin transport to the cell surface. The cell adhesion
molecules N-cadherin and β-catenin are not transported to the cell
surface in patients with SIT due to the absence of function in the
KIF3 complex (18). Consequently, the
excess β-catenins in the cytoplasm enter the nucleus and activate
genes associated with cell proliferation, thereby facilitating the
development and progression of cancer in patients with SIT
(17). Cell-cell adhesion assists in
coordinating cellular organization and collective cell migration,
and is critical for the directional looping of developing embryonic
organs (23). A previous study
assessed the influence of collective cell migration on the breaking
of left-right symmetry, and established a 2D microscale system that
may be useful for fetal drug screening to assist in identifying and
potentially inhibiting birth defects associated with alterations in
cell-cell adhesion (24).
Since the data in the literature indicated that the
incidence of situs inversus with overall malignancy or leukemia is
extremely low, the present study concluded that the occurrence of
CML with SIT in the patient assessed in the present study may be by
chance. However, the diagnosis of SIT may also be established by
future, larger studies, which could provide a means of predicting
the disease and methods to prevent its progression.
References
|
1
|
Aylsworth AS: Clinical aspects of defects
in the determination of laterality. Am J Med Genet. 101:345–355.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murakami S, Terakado M, Misumi M, Tsuji Y,
Okubo K, Hirayama R, Inoue K and Arai E: Situs inversus totalis
with malignant lymphoma of the stomach: Report of a case. Surg
Today. 33:533–536. 2003.PubMed/NCBI
|
|
3
|
Schmutzer KJ and Linde LM: Situs inversus
totalis associated with complex cardiovascular anomalies. Am Heart
J. 56:761–768. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katsuhara K, Kawamoto S, Wakabayashi T and
Belsky JL: Situs inversus totalis and Kartagener's syndrome in a
Japanese population. Chest. 61:56–61. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park SS, Min BW, Kim WB, Han HJ, Yong HS,
Kim SJ, Kim CS and Mok YJ: Double cancer of the stomach and
oesophagus with situs ambiguus with polysplenia: The importance of
preoperative evaluation. Dig Liver Dis. 37:799–802. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
National Comprehensive Cancer Network:
NCCN clinical practice guideline in oncology: Chronic myelogenous
leukemia. V.2.2008. NCCN Web Site. 8-28-2007. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#cllJuly
26–2017
|
|
7
|
Bennett JM, Dsouza KG, Patel M and O'Dwyer
K: ‘Preleukemic or smoldering’ chronic myelogenous leukemia
(CML):BCR-ABL1 positive: A brief case report. Leukemia Res Rep.
4:12–14. 2014. View Article : Google Scholar
|
|
8
|
Druker BJ and Lee SJ: Chapter 43: Chronic
leukemias: Section 1: Chronic myelogenous leukemia. DeVitaVincent
T..Lawrence, Theodore S..Rosenberg and Steven A: Devita, Hellman
& Rosenberg's Cancer: Principles and Practice of Oncology.
Wolters Kluwer - Lippincott Williams & Wilkins; Philadelphia,
PA, USA: 8th Edition. pp. 2268–2277. 2008
|
|
9
|
Yang CL and Zhang XB: An epidemiological
ivvestigation of leukemia and aplastic anemia. Investigation on
incidence of leukemia in China. J China Acad Med Sci. 14:12–18.
1992.
|
|
10
|
Kalmanti L, Saussele S, Lauseker M, Müller
MC, Dietz CT, Heinrich L, Hanfstein B, Proetel U, Fabarius A,
Krause SW, et al: Safety and efficacy of imatinib in CML over a
period of 10 years: Data from the randomized CML-study IV.
Leukemia. 29:1123–1132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kasili EG and Gaya Z: A case of chronic
granulocytic leukemia with a right sided splenomegaly: Case-report.
East Afr Med J. 65:140–143. 1988.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YW, Ryu H, Kim DS and Kim IY: Double
primary malignancies associated with colon cancer in patients with
situs inversus totalis: Two case reports. World J Surg Oncol.
9:1092011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Varano NR and Merklin RJ: Situs inversus:
Review of the literature, report of four cases and analysis of the
clinical implications. J Int Coll Surg. 33:131–148. 1960.PubMed/NCBI
|
|
15
|
RS CVK and SL R: Robbins Pathologic Basis
of Disease. (4th edition). Asia-Pac J Clin onco. 777:1989.
|
|
16
|
Galiatsatos P, Kasprzak L, Chong G, Jass
JR and Foulkes WD: Multiple primary malignancies in a patient with
situs ambiguus. Clin Genet. 69:528–531. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haruki T, Maeta Y, Nakamura S, Sawata T,
Shimizu T, Kishi K, Miyasaka S, Maeta H, Morimoto K and Taniguchi
I: Advanced cancer with situs inversus totalis associated with KIF3
complex deficiency: Report of two case. Surg Today. 40:162–166.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teng J, Rai T, Tanaka Y, Takei Y, Nakata
T, Hirasawa M, Kulkarni AB and Hirokawa N: The KIF3 motor
transports N-cadherin and organizes the developing neuroepithelium.
Nat Cell Biol. 7:474–482. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Worley KE, Shieh D and Wan LQ: Inhibition
of cell-cell adhesion impairs directional epithelial migration on
micropatterned surfaces. Integr Biol (Camb). 7:580–590. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bamford RN, Roessler E, Burdine RD,
Saplakoğlu U, dela Cruz J, Splitt M, Goodship JA, Towbin J, Bowers
P, Ferrero GB, et al: Loss-of-function mutations in the EGF-CFC
gene CFC1 are associated with human left-right laterality defects.
Nat Genet. 26:365–369. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peeters H, Debeer P, Bairoch A, Wilquet V,
Huysmans C, Parthoens E, Fryns JP, Gewillig M, Nakamura Y, Niikawa
N, et al: PA26 is a candidate gene for heterotaxia in humans:
Identification of a novel PA26-related gene family in human and
mouse. Hum Genet. 112:573–580. 2003.PubMed/NCBI
|
|
22
|
Hirokawa N: Determination of left-right
asymmetry: Role of cilia and KIF3 motor proteins. News Physiol Sci.
15:562000.PubMed/NCBI
|
|
23
|
Hirokawa N, Tanaka Y, Okada Y and Takeda
S: Nodal flow and the generation of left-right asymmetry. Cell.
125:33–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Teng J, Rai T, Tanaka Y, Takei Y, Nakata
T, Hirasawa M, Kulkarni AB and Hirokawa N: The KIF3 motor
transports N-cadherin and organizes the developing neuroepithelium.
Nat Cell Biol. 7:474–482. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Worley KE, Shieh D and Wan LQ: Inhibition
of cell-cell adhesion impairs directional epithelial migration on
micropatterned surfaces. Integr Biol (Camb). 7:580–590. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kamiike W, Itakura T, Tanaka H, Hatanaka
N, Nakamuro M, Miyata M and Izumi H: Hepatic segmentectomy on
primary liver cancer with situs inversus totalis. HPB Surg.
9:169–173. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Horie M, Arai H, Noguchi S, Suzuki M,
Sakamoto Y and Oka T: Kartagener syndrome with lung cancer and
mediastinal tumor. Nihon Kokyuki Gakkai Zasshi. 48:375–378.
2010.(In Japanese). PubMed/NCBI
|
|
28
|
Iwamura T, Shibata N, Haraguchi Y, Hisashi
Y, Nishikawa T, Yamada H, Hayashi T and Toyoda K: Synchronous
double cancer of the stomach and rectum with situs inversus totalis
and polysplenia syndrome. J Clin Gastroenterol. 33:148–153. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dulganov KP and Nelip VE: Synchronous
early cancer of the esophagus and larynx in a patient with complete
situs viscerum inversus. Vopr Onkol. 36:1125–1126. 1990.(In
Russian). PubMed/NCBI
|
|
30
|
Rosseau Guerra R, Amat Daumy O, De Armas
Haramboure L and Andial A: Case of multiple cancer and situs
inversus. Arch Cuba Cancerol. 11:61–65. 1952.(In Undetermined
Language). PubMed/NCBI
|
|
31
|
Kim YI, Tada I, Kuwabara A and Kobayashi
M: Double cancer of the liver and stomach with situs inversus
totalis-a case report. Jpn J Surg. 19:756–759. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vijayakumar V and Brandt T: Prolonged
survival with isolated levocardia and situs inversus. Clev Clin J
Med. 58:243–247. 1991. View Article : Google Scholar
|
|
33
|
Pathak P, Zilberman V, Avezbakiyev B and
Gotlieb V: Hairy cell leukemia in a patient with situs inversus
totalis: An extremely rare combination. Future Oncol. 9:753–756.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yun S, Vincelette ND, Phan T and Anwer F:
Spontaneous tumour lysis syndrome associated with contrast dye
iohexol use in mantle cell lymphoma. BMJ Case Rep. 2014:pii:
bcr2014204113. 2014. View Article : Google Scholar
|
|
35
|
Trautner M, Szyszko T, Gnanasegaran G and
Nunan T: Interesting image. Situs inversus totalis in newly
diagnosed lymphoma: Additional value of hybrid imaging. Clin Nucl
Med. 35:26–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Patel RV, Mehta MH, Bhoot NH, Gondalia JS
and Ghodadra JK: Ileocecal lymphoma presenting as an epigastric
mass. Indian Pediatr. 29:519–521. 1992.PubMed/NCBI
|