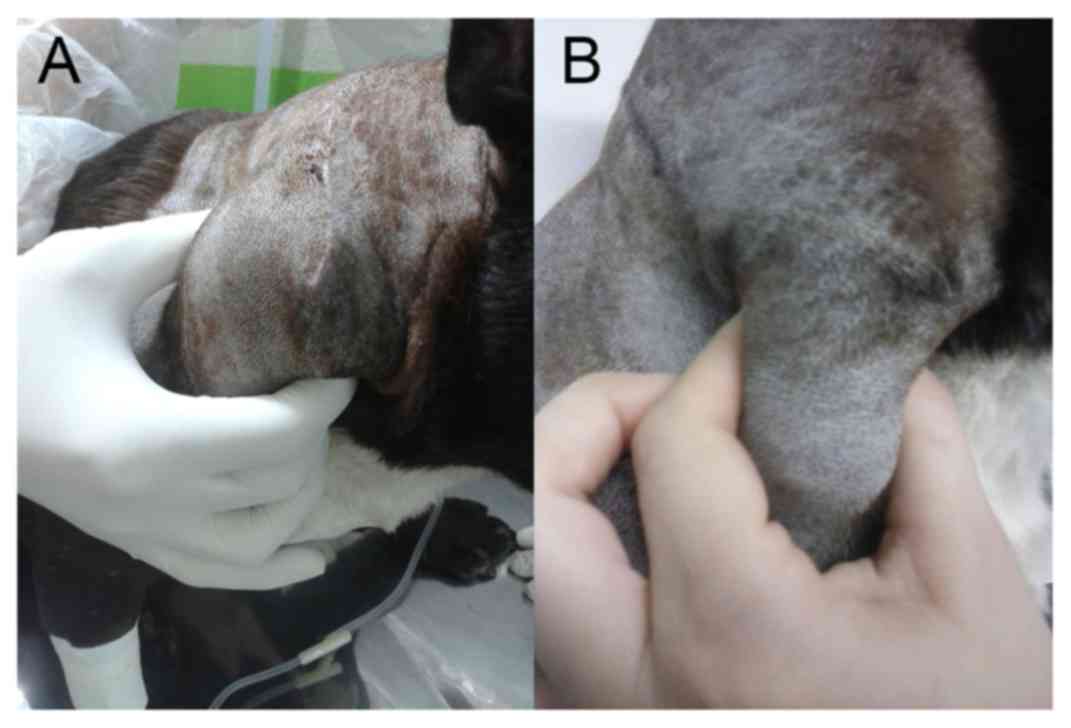
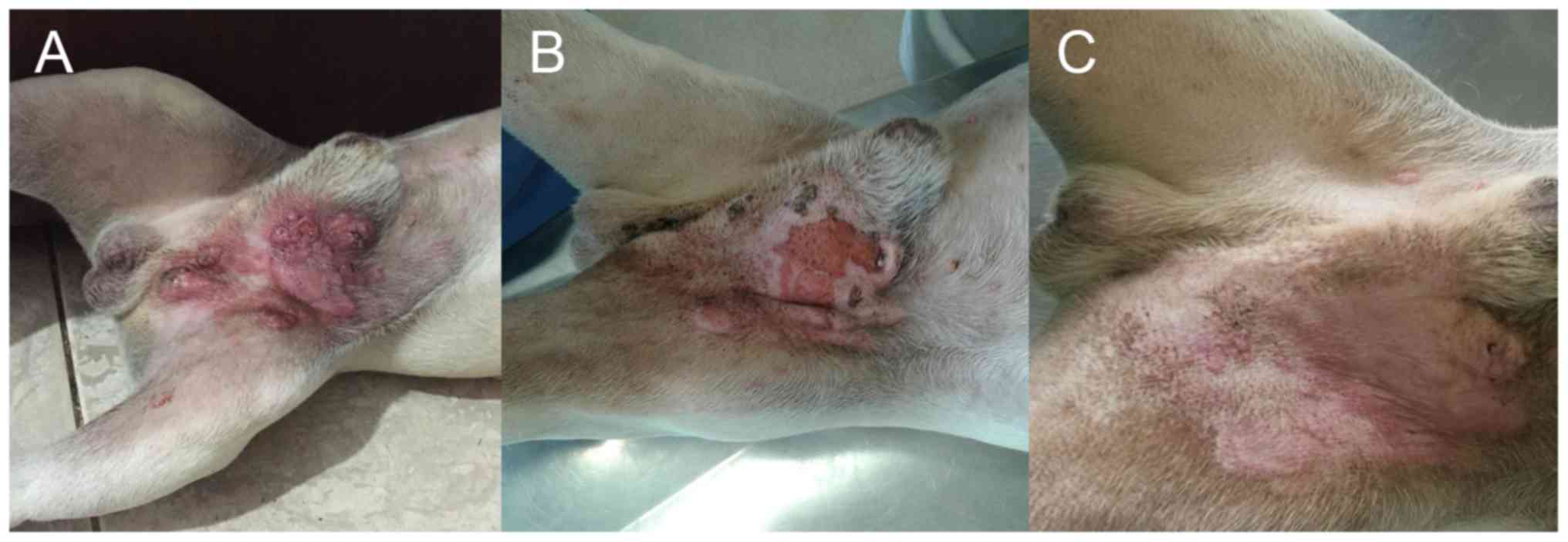
Introduction
Mast cell tumour (MCT) is the most common cutaneous
malignancy in dogs (1). In view of
the wide variation in its biological behaviour, many prognostic
factors have been proposed and evaluated in an attempt to improve
decision making in the management of this neoplasm (2–4). Among the
therapeutic approaches, surgery stands out as the optimal treatment
offering the highest rate of cure for most low to intermediate
grade MCTs (1,3,5). However,
for high grade or biologically aggressive tumours, surgical benefit
is limited and metastasis may occur in up to 90% of cases (2,3). Numerous
drugs, including glucocorticoids, chemotherapeutic agents and
tyrosine kinase inhibitors (TKIs) have been used for treatment of
non-resectable MCTs, but the prognosis for such tumours remains
guarded to poor (1,3,4). Tyrosine
kinases (TKs) are enzymes located on the cell surface, cytoplasm or
nucleus, that catalyze the transfer of phosphate groups from
adenosine triphosphate molecules (ATP), leading to cellular
signaling transmission. In cancer cells, several abnormalities may
be found in specific protein kinases, which allows transduction of
intracellular signals that ultimately will cause changes in gene
transcription, increase cell proliferation, invasion and survival
(6,7).
Genetic and epigenetic changes can result in alteration in
oncogenes or tumour suppressor genes expression leading to
constitutively activated TKs, or abnormal TKs interactions
(7–9).
Several molecular abnormalities have been identified
and characterized in Veterinary Medicine, particularly in canine
MCTs (10). Gain of-function
mutations involving the KIT receptor and its pathway are considered
relevant for the prognosis and treatment of MCT (11–14).
Dysregulation of several TKs have been found in
different human cancers. Monoclonal antibodies like trastuzumab and
cetuximab that respectively target HER-2 and EGFR TK receptors have
been approved for human breast cancer (7,15).
Imatinib mesylate is a small molecule TKI with a multi-target
action towards KITr, PDGFR and Bcr-Abl protein. Imatinib is a well
recognized and effective treatment for human gastro-intestinal
stromal tumours and chronic myeloid leukemia (16). In veterinary medicine, imatinib was
occasionally used in the treatment of canine MCT (17), and greatest effort was directed to the
development ofsimilar TKI for veterinary use (7,10).
Masitinib mesylate and toceranib phosphate, are TKIs
licensed for use in dogs with non-resectable Grade II or III MCTS
in Europe and the United States. They both act intracellularly in
the protein kinases KITR and PDGFR α/β, where masitinib also
operates in Lyn, Fyn and Lck (18),
and toceranib in VGFR and Flt-3 (19). The action against multiple therapeutic
targets, allows these molecules to interfere more effectively in
the different pathways responsible for cancer progression (7). However, despite the development of such
drugs and their increasing use in clinical practice, there is still
a lack of established factors that can predict the response to
treatment of canine MCTs toTKIs (4,10).
The objective of this study was to evaluate
measurable responses of canine MCT to TKIs, correlating this with
clinical, histopathological, imunohistochemical and genetic
prognostic factors.
Materials and methods
Subject selection and treatment
This study included subjects retrospectively
collected from the Queen's Veterinary School Hospital (QVSH) at the
University of Cambridge (Cambridge, UK) (n=10), and prospectively
enrolled from the Veterinary Hospital of the Universidade Federal
de Minas Gerais (UFMG, Belo Horizonte, MG, Brazil)(n=14). The dogs
were enrolled if presented with macroscopic cutaneous MCT and stage
II, III or IV disease, considered to be at high-risk of MCT related
death. For classification as a high-risk stage II, only a
cytological diagnosis of certain metastasis, was accepted (20).
Incisional biopsies of primary tumours were
performed and subjected to histological (Patnaik and Kiupel grading
systems), immunohistochemical (Ki-67 and KITr) and genetic
(c-kit oncogene) assessment. Clinical staging was performed
by physical examination, abdominal ultrasound, fine needle
aspiration and cytology of regional lymph nodes, satellite or
distant skin lesion and suspected visceral lesions. Lymph node
metastasis were identified, on fine needle aspirates (FNA) using
cytological criteria previous published (20).
Dogs were treated with masitinib, at a dosage
ranging from 8 to 12.5 mg/kg q 24 h or toceranib at a dosage of
2.5–2.7 mg/kg q 48 h. The concomitant use of prednisone or
prednisolone, at an initial dosage of 40 mg/m2, daily,
(7–10 days), followed by a dosage of 25 mg/m2, daily or
every other day was often used, along with gastric acid inhibitors
(omeprazole, ranitidine), for controlling paraneoplastic effects
related to degranulation of mast cells.
Follow up information was collected from the
subjects medical records or when necessary by telephone call
conversation with the referring veterinary surgeon or the owner.
Subjects which failed to comply with TKIs treatment or attendance
during the clinical follow-up were excluded from this study.
This study was approved by the Ethics Committee on
Animal Use (UFMG, protocol 384/2013) and Department's Ethics and
Welfare Committee (University of Cambridge, protocol CR 138).
Histological analysis
The surgical specimens of the primary tumours were
fixed in 10% formalin, cut in longitudinal sections for paraffin
embedding, and 4 µm sections were mounted in glass and stained with
hematoxylin-eosin and toluidine blue. Histopahological examination
was performed by FC and RH, and tumour grading was defined through
the systems proposed by Patnaik et al (21) and Kiupel et al (22).
Immunohistochemical analysis
Sections of 4 µm were cut from a representative
block for each case and collected on gelatin-coated slides. The
slides were deparaffinized and rehydrated in an alcohol series.
Antigenal retrieval was performed with an antigen retrieval
solution (Target Retrieval Solution Citrate pH 6, DakoCytomation,
Glostrup, Denmark) under pressurized heat (20–25 mmHg, 125°C/2
min). Endogenous peroxidase was blocked by immersion in 3% hydrogen
peroxide and protein blockage (Thermo Scientific UltraVision™
Protein Block; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Primary antibodies CD117 (policlonal, 1:800; DakoCytomation,
Glostrup, Denmark) and MIB-1 (monoclonal, 1:25; DakoCytomation)
were incubated at 4°C, for 16 h (overnight) for KITr and Ki-67
reactions, respectively. Secondary antibody (Advance HRP Link;
DakoCytomation) was incubated in the humidity chamber for 30 min
and the reaction was amplified by the polymer (Advance HRP Enzyme;
DakoCytomation). The reaction was revealed with the chromogen
3,3-diaminobenzidinetetrahydrochloride (Liquid DAB +
SubstratChromogen System; DakoCytomation) and stained with Harris
hematoxylin.
The immunolabelling pattern for KITr was evaluated,
by counting membrane, focal or difuse cytoplasmic immunoexpression
(KIT patterns I, II or III, respectively) in 100 mast cells at a
×40 magnification. Each MCT was assigned with the highest staining
pattern present in at least 10% of the neoplastic cell population
or present in large clusters of neoplastic cells within the tumour,
as described by Kiupel et al (23). Ki-67 value was determined as the
percentage of positive nuclei in at least 500 neoplastic cells in
3–5 high power fields (×40 magnification). Every nucleus with
evidence of immune labelling was considered positive for Ki-67.
This approach was described by Scase et al (24). Previously tested canine MCT samples
were used as positive control for KITr and Ki-67, and negative
controls were obtained by replacing the primary antibody by normal
serum.
Screening of mutations in the c-kit
oncogene
The polymerase chain reaction (PCR) for
amplification of the fragment of interest in the c-kit
oncogene, was performed by Progen, in Vetpat Laboratory (Campinas,
SP, Brazil), from the DNA extraction in paraffin embedded tumour,
by the proteinase K method. The primers used in the bleaching of
the reaction were designed with the help of the BLAST software
(Basic Local Alignment Search Tool®, NCBI) and
manufactured by Invitrogen (São Paulo, SP, Brazil), as c-kit
forward: 5′-ATCTGTCTCTCTTTTCTCCCCC-3′ (sense) and c-kit
reverse: 5′-TGGGGTTCCCTAAAGTCATTGT-3′ (antisense). The product
generated by these pair of primers had 225 bp in the absence of
mutations (native c-kit). Reactions were prepared and
planned in a GenPro thermocycler (BIOER Technology), with a
maintenance at 95°C for five min, then 30 cycles of 94°C for 45 sec
for denaturation of DNA strands, 63°C for 45 sec to pairing and
annealing of primers and 72°C for one minute to extension, to be
finally maintained at 72°C for ten min for molecular stabilization.
The amplified material was separated by electrophoresis at 100V,
with free amperage. Canine healthy skin samples and milique water
were used as positive and negative controls, respectively.
Assessment of response and
toxicity
Tumour response to TKI was based on measurements of
the primary tumour and all target lesions (including metastatic
lymph nodes) before and two-weeks after starting treatment, as
recommended by the Response Evaluation Criteria for Solid Tumours
(RECIST, v.1.0) (25). Complete
response (CR) was defined as a complete disappearance of the
mass(es), partial response (PR) was defined as at least 30%
reduction in size, stable disease between 20% reduction and 20%
increase in size, progressive disease was defined as an increase in
size of the mass of more than 20%. Overall response rate (ORR) was
calculated based on the total number of subjects that achieved
complete and partial response (CR+PR). The disease-free interval
(DFI), for subjects who achieved complete remission and overall
survival (OS) for all subjects were calculated from the start of
TKI administration. Cytology was used to confirm the diagnosis in
case of progressive disease and appearance of new lesions. Side
effects related to the use of TKI were recorded according to
Veterinary Cooperative Oncology Group-Common Terminology Criteria
for Adverse Events (VCOG-CTCAE v.1.1) (26).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism (v.6.01). A matrix correlation was built through Spearmann
test for searching association between prognostic factors and
overall survival. DFI and OS were estimated through Kaplan-Meier
curve and the log-rank test of Cox-Mantel was used to compare the
curves, according to prognostic factors. P<0.05 was considered
to indicate a statistically significant difference. Significant
correlations were considered strong when they occurred in over than
49% of the studied population (r>0.07), moderate, as occurred in
9–49% (0.3<r<0.7), and weak, when they occurred in less than
9% of the population (r<0.3).
Results
A total of 24 dogs were included in this study
(Table I). Fourteen cases were
enrolled prospectively, from the Veterinary Hospital, UFMG and 10
cases were retrospectively included, identified from medical
records of subjects treated at the QVSH, University of Cambridge.
Tyrosine kinase inhibitors were used as first line therapy in 11
dogs and as a rescue treament in 13 dogs. All except one subject
received concomitant prednisone (n=13, all from UFMG) or
prednisolone (n=10, from QVSH). Sixteen subjects had received
previous chemotherapeutic agents including: lomustine (n=9),
vinblastine (n=4), lomustine followed by chlorambucil (n=2),
lomustine followed by vinblastine (n=1). Toceranib was used instead
of masitinib in four subjects.
 | Table I.Clinical, histopahological,
immunohistochemical and genetic features of 24 dogs submitted to
treatment with tyrosine-kinase inhibitors for treatment of
measurable disease. |
Table I.
Clinical, histopahological,
immunohistochemical and genetic features of 24 dogs submitted to
treatment with tyrosine-kinase inhibitors for treatment of
measurable disease.
| N | Breed | Age (months) | Staging | Grade
(Patnaik/Kiupel) | Mitotic index | Ki-67 (%) | KITr | c-kit oncogene exon
11 mutational status | Previous
treatment | Clinical
response | Follow-up | Disease-free
interval | Overall
survival |
|---|
| 01a (M) | Sharpei | 72 | III | Grade 2/high
grade | 6 | 7.0 | KIT I | Native | Prednisone,
lomustine | CR | Euthanasia due to
disease progression | 40 | 133 |
| 02a (T) | French Bulldog | 48 | III | Grade 3/high
grade | 20 | 33.0 | KIT III | Native | Prednisone,
vimblastine | CR | Natural death due
to disease progression | 124 | 131 |
| 03a (T) | Crossbreed | 59 | III | Grade 3/high
grade | 60 | 28.7 | KIT II | Native | Prednisone,
lomustine | PD | Natural death due
to disease progression | – | 30 |
| 04 (M) | French Bulldog | 35 | III | Grade 3/high
grade | 5 | 19.0 | KIT III | Native | Prednisone,
lomustine | PR | Euthanasia due to
disease progression | – | 103 |
| 05a (M) | Schnauzer | 158 | III | Grade 2/low
grade | 3 | 26.0 | KIT I | Native | Prednisone | PD | Euthanasia due to
disease progression | – | 49 |
| 06 (M) | Crossbreed | 115 | III | Grade 2/low
grade | 2 | 13.0 | KIT I | Native | Prednisone,
lomustine | SD | Euthanasia due to
disease progression | – | 123 |
| 07a (T) | Cocker spaniel | 133 | III | Grade 3/high
grade | 2 | 22.0 | KIT III | Native | Prednisone,
lomustine | SD | Euthanasia due to
disease progression | – | 125 |
| 08a (M) | Pinshcer | 144 | III | Grade 2/high
grade | 4 | 13.0 | KIT I | ITD | Prednisone | CR | Natural death due
to disease progression | 140 | 164 |
| 09 (M) | Crossbreed | 123 | III | Grade 2/low
grade | 3 | 29.0 | KIT II | ITD | Prednisone,
lomustine | CR | Still alive but
with signs of disease progression | 240 | 280 |
| 10a (M) | Pinscher | 132 | III | Grade 3/high
grade | 4 | 13.0 | KIT II | Native | Prednisone | SD | Still alive | – | 208 |
| 11a (M) | Crossbreed | 162 | II | Grade
3/high-grade | 41 | 14.6 | KIT II | Native | Prednisone,
lomustine | PD | Euthanasia due to
disease progression | – | 47 |
| 12a (T) | Schnauzer | 156 | IV | Grade 2/high
grade | 33 | 34.0 | KIT I | Native | Prednisolone,
vimblastine | PD | Euthanasia due to
disease progression | – | 15 |
| 13 (M) | Schnauzer | 144 | III | Grade 2/low
grade | 1 | 28.0 | KIT I | ITD | Prednisone, | PD | Euthanasia due to
disease progression | – | 12 |
| 14a (M) | Pinscher | 120 | III | Grade 2/low
grade | 4 | 22.0 | KIT II | ITD | Prednisone,
lomustine, chlorambucil | PD | Euthanasia due to
disease progression | – | 42 |
| 15 (M) | Sharpei | 120 | III | Grade 3/high
grade | 44 | 46.0 | KIT II | Native | Prednisolone, | CR | Euthanasia due to
disease progression | 400 | 427 |
| 16a (M) | Jack Russel
Terrier | 140 | III | Grade 3/high
grade | 15 | 9.0 | KIT II | ITD | Prednisolone, | PR | Euthanasia due to
disease progression | – | 57 |
| 17a (M) | Labrador | 48 | II | Grade 2/low
grade | 2 | 6.3 | KIT II | Native | Prednisolone | SD | Euthanasia due to
disease progression | – | 161 |
| 18a (M) | Boxer | 60 | II | Grade 2/high
grade | 2 | 15.3 | KIT II | Native | Prednisolone | PR | Euthanasia due to
disease progression | – | 101 |
| 19a(M) | Labrador | 117 | II | Grade 2/low
grade | 2 | 6.0 | KIT II | Native | Prednisolone | PR | Euthanasia due to
disease progression | – | 66 |
| 20a (M) | Dogue de
Bordeaux | 29 | II | Grade 3/high
grade | 28 | 37.8 | KIT III | ITD | Prednisolone | CR | Natural death due
to disease progression | 114 | 203 |
| 21a (M) | Greyhound | 132 | II | Grade 3/high
grade | 4 | 9.3 | KIT II | Native | Prednisolone | CR | Euthanasia due to
disease progression | 52 | 160 |
| 22a (M) | Border Collie | 145 | III | Grade 2/low
grade | 5 | 12.4 | KIT I | Native | Prednisolone | PD | Euthanasia due to
disease progression | – | 47 |
| 23a (M) | Poodle | 36 | III | Grade 2/high
grade | 14 | 5.4 | KIT II | Native | Prednisolone,
vimblastine | PDPR | Euthanasia due to
disease progression | – | 23 |
| 24 (M) | Labrador | 105 | III | Grade 3/high
grade | 12 | 6.8 | KIT II | Native | – |
| Euthanasia due to
disease progression | – | 288 |
|
|
|
|
|
|
|
|
|
|
|
| | 86 | 141 |
An objective response was obtained in 12/24 subjects
(50%), seven of which had CR (29%) and five PR (21%) as shown in
Figs. 1 and 2, respectively. Stable (n=4; 17%) or
progressive disease (n=8; 33%) was observed in 12 subjects (50%).
One subject developed partial remission with masitinib, as a first
line therapy, resulting in the tumour becoming resectable. Surgery
was performed and the subject continued masitinib treatment with a
DFI of 86 days, and an OS of 288 days (144 days after surgery).
The overall survival time for all subjects in this
study was 113 days but DFI and OS for subjects who achieved CR was
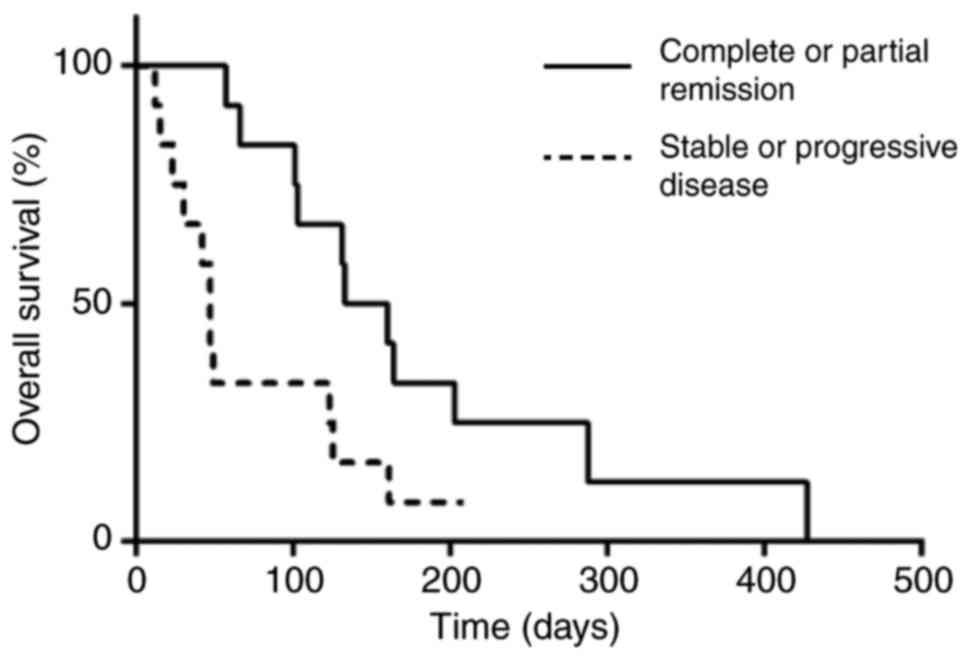
140 and 164 days. In a matrix correlation only the initial response
to TKIs was associated with OS (P=0,03; rS=0,578). As
shown in fig. 3, subjects who
achieved measurable responses during the first weeks of treatment
(n=12) reached the median at 146 days, while those who remained
with stable or progressive disease (n=12) reached the median at 47
days (P=0.02).
Eleven subjects were treated with TKIs as a first
line treatment, but 81.8% (9/11) of these, were treated only after
post surgical recurrence of the tumour. The ORR for tumours treated
with TKI as first line treatment was 54.5% (6/11). Thirteen dogs
received TKIs as a second line treatment and 69,2% (9/13) of these
had previous surgery as well. The ORR for tumours treated with TKI
as a second line treatment was 46,2% (6/13). The difference in ORR
between the two groups of subjects treated with TKIs as a first or
second line treatment was not statistically significant. There was
also no significant difference in DFI and OS for the same two
groups of subjects, however a tendency for significance in OS was
found between the first line treatment compared to the second line
treatment group (160 and 103 days, respectively; P=0,2).
Similarily, there was no difference in ORR between subjects treated
on the first presentation of MCT or after post surgical recurrence
of the tumour, however a tendency for significance in OS was found
between non-recurrent and recurrent MCTs (123 and 66 days,
respectively; P=0,09).
Clinical staging was also not statistically related
to prognosis, and subjects in stage II (n=6) and III (n=17),
reached a median OS of 130 and 123 days, respectively (P=0,8).
There was also no influence of histological grade, mitotic index
(1–60 mitotic figures in 10 high-power fields) and Ki-67 value
(5,4–46,0%) in OS of these subjects.
Abnormalities in KIT expression were identified in
17/24 (71%) MCTs, 12 with KIT II-pattern and four with a KIT
III-pattern, but there was also no correlation with OS.
Nevertheless, objective responses (CR+PR) were obtained in 28%
(2/7), 54% (7/13) and 75% (3/4) of subjects whose tumours presented
with KIT expression pattern I, II and III, respectively, although
the number was not appropriate for a contingency analysis.
Duplications in exon 11 of the c-kit gene were identified in
6/24 subjects (24%). Of these, measurable responses were observed
in 4/6 (67%). A similar rate of response was found for subjects
without any identified mutations, through the elected method (8/18,
44% of response to TKI). There was also no difference in OS,
according to the mutational status in the exon 11 of the
c-kit oncogene.
Positive correlations were found between mitotic
index and both grading systems (P=0,009; rS=0,523 for
Patnaik grading system; P=0,001; rS=0,617 for Kiupel
grading system), KITr pattern and Patnaik grading system
(P<0,00001; rS=0,676) and both grading systems
(P=0,0006; rS=0,650). Ki67 was not correlated with MI or
Patnaik and Kiupel grade.
Side effects were relatively common and are reported
in Table II. One dog developed
severe illness after 133 days of masitinib. The subject presented
with a grade 4 non-regenerative anaemia with concomitant
thrombocytopenia, grade 2 azotemia and grade 4 proteinuria
resulting in hypoalbuminemia/ascites (nephrotic syndrome). The dog
was treated with total blood transfusion and fluidtherapy and the
drug was suspended, but despite the subject's recovery, tumour
recurrence was noted 28 days later and the dog was euthanized.
 | Table II.Adverse side effects observed in 24
dogs with advanced staged mast cell tumours treated with tyrosine
kinase inhibitors (23 were also treated with glucocorticoids). |
Table II.
Adverse side effects observed in 24
dogs with advanced staged mast cell tumours treated with tyrosine
kinase inhibitors (23 were also treated with glucocorticoids).
| Adverse side
effect | Grade | Frequency (%) |
|---|
| Anaemia | Grade 2 | 1/24 (4.2) |
|
| Grade 4 | 1/24 (4.2) |
|
Thrombocytopenia | Grade 4 | 1/24 (4.2) |
| Neutropenia | Grade 1 | 16/24 (66.7) |
| ALP increase | Grade 1 | 12/24 (50) |
| ALT increase | Grade 1 | 1/24 (4.2) |
|
| Grade 2 | 2/24 (8.3) |
| Azotemia | Grade 2 | 1/24 (4.2) |
| Proteinuria
(increase urine protein/creatinine ratio) | Grade 1 | 1/24 (4.2) |
|
| Grade 3 | 1/24 (4.2) |
|
| Grade 4 | 1/24 (4.2) |
|
Hypoalbuminaemia | Grade 4 | 1/24 (4.2) |
Discussion
In this study, as previously reported by Smrkovski
et al (27), a 50% ORR was
observed in dogs with unresectable MCTs, treated with TKIs.
The OS of dogs in this study was lower in comparison
to reports of Smrkvoski et al (27) and Hahn et al (28). Three main hypotheses might explain
this difference: Firstly, our study included a high number of
subjects with post surgical recurrent MCTs (18/24) which showed
reduced survival rates compared to non recurrent MCT, althought
this was not statistical significant. A poor outcome is
historically reported for recurrent MCTs with related death rates
reaching 86–100% of cases as reported by Patnaik et al
(21); Secondly, TKIs were
administered after failure of chemotherapy in over half of these
subjects. As shown by Hahn et al (28), better responses were obtained when
mastinib mesylate was used as a first line treatment. However, in
our study, no differences were seen in OS in subjects treated as a
first or second line treatment with TKIs. A third and most likely
hypothesis is that the concomitant use of glucocorticoids might
have impaired a favourable and prolonged response to TKIs. The
mechanisms involved in tumour resistance to TKIs are still largely
unknown, but appear to involve abnormalities in genes responsible
for the synthesis of other areas of the targeted proteins, or
development of alternative cellular pathways (29). Another mechanism of resistance is the
overexpression of ABC transporters (30). Imatinib and dasitinib are TKIs similar
to masitinib in its mechanism of action and they are substrates of
the ABC transporters, such as P-glycoprotein (P-gp, ABCB1) and
breast cancer resistance protein (BCRP, ABCG2), both induced by the
administration of glucocorticoids (30). Masitinib is also a P-gp substrate and
P-gp overexpression can increase the resistance to masitinib
(31). However, multitarget TKIs
similar to toceranib, as sunitinib, have been found to inhibit the
ABC (32,33). The authors and collaborators found a
significant increase in survival in subjects treated with masitinib
alone compared to subjects treated with masitinib in combination
with prednisolone (data still not published). The efficacy of
mastinib could be reduced by the development of a rapid drug
resistance caused by the induction of P-gp, from previous or
concurrent prednisolone treatment, while toceranib could or could
not be affected. In our study only four subjects were treated with
toceranib and prednisolone, too few to allow any conclusion.
Further studies are needed to evaluate the benefit of adding
corticosteroids to masitinib or toceranib.
In this study, one subject received adjuvant therapy
with masitinib, once this TKI resulted in partial response of its
previous unresectable disease, making it resectable. This subject
reached an OS of 288 days from the beginning of neoadjuvant
treatment with masitinib, superior to the median obtained in this
study (113 days). This observation could suggest that TKIs
responses in the treatment of gross disease may also be useful in
the adjuvant scenario. In the presence of minimal residual disease,
a reduced development of tumour resistance and even a synergism
with other therapeutic approaches could be hypothesized. New
clinical trials are required to evaluate the response of canine MCT
to these drugs in the adjuvant setting.
In this case series, including dogs with advanced
staged disease, the initial response to TKIs was the most
significant prognostic factor, as previously reported by Smrkovski
et al (27) and Grant et
al (34). In contrast,
histological grade, mitotic index, Ki-67 value, KITr pattern and
even the mutational status in exon 11 of the c-kit oncogene
had no impact on OS for these subjects. Increased response rate was
found in subjects with II and III KITr staining patterns and in the
presence of ITD in the exon 11 of c-kit oncogene, however
due to the low number in each subcategory the statistical
significance could not be evaluated. The prognostic value of KITr
immunelabelling pattern has been evaluated in some studies and
although Kiupel et al (2004) showed that the KITr
immunelabelling pattern could be a prognostic factor for canine MCT
(23), this was not confirmed in more
recent studies (35,36). The relevance of c-kit
mutational status, as a predictor for TKI response was suggested in
older studies (17,19,28). We
found an increase response rate in samples arboring c-kit
mutations compared with samples with absent mutations, however the
number of cases was too small to draw any significant
conclusion.
The main limitations of this study were the
relatively low number of samples and the heterogenicity of the
subjects and type of treatment used, however this is often a common
problem in studies of canine MCTs. Genetic assessment of exon 11
was performed using PCR analysis rather than genetic sequencing, so
point mutations in the exon 11, could not be assessed. Primers
applied in this study were limited only to the exon 11, but whereas
there might be c-kit activating mutations in other loci,
like exons 2, 5, 6, 7, 8, 9 and 15, (14), these are not proven, at the current
state of our knowledge, to be of prognostic or predictive
significance.
Although the number of cases in this study was
small, there was no correlation between mitotic index and Ki-67
value, which differs from the study conducted by Berlato et
al (37). However a moderate
correlation was found between mitotic index and both grading
systems, Patnaik's grading system and KITr pattern. As expected and
previously demonstrated by Giantin et al (35), both grading systems were also
moderately correlated with each other.
Masitinib and toceranib are generally well tolerated
in dogs, although mild and self-limiting side effects may occur.
However, clinical-pathological abnormalities should always be
monitored, once severe side effects may occur, like non
regenerative anaemia and moderate to severe proteinuria, as seen in
our study and also by Miller et al (38).
In conclusion, TKIs can be effective in the
treatment of macroscopic advanced staged canine MCTs. Nevertheless,
there is lack of factors that could strongly predict the response
to treatment. Similar to other studies, we found that the initial
response to treatment is the only reliable prognostic factor for
those subjects regardless of theclinical stage, histological grade
and mitotic index. Nevertheless, history of recurrent MCTs and
previous chemotherapeutic agents may reduce response rate. As found
in our preliminary results, concomitant use of glucocorticoids may
impair the response to TKIs and possibly induce early TKI
resistance resulting in reduced OS. Differently to other similar
papers published before all samples were evaluated for Ki-67 value,
immunohistochemical pattern of KITr and even the mutational status
in exon 11 of the c-kit oncogene. Ki67, KITr immunostaining
and c-kit mutation did not give any further relevant
informations regarding prognosis and or in the prediction of
response to TKIs in the cohort of high-risk MCTs examined, although
expression of KIT II and III might result in higher response
rate.
Acknowledgements
This study was supported by the National Council for
Scientific and Technological Development (CNPq) and Coordination
for the Improvement of Higher Education Personnel (CAPES).
References
|
1
|
Dobson JM and Scase TJ: Advances in the
diagnosis and management of cutaneous mast cell tumours in dogs. J
Small Anim Pract. 48:424–431. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Welle MM, Bley CR, Howard J and Rüfenacht
S: Canine mast cell tumours: A review of the pathogenesis, clinical
features, pathology and treatment. Vet Dermatol. 19:321–339. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
London CA and Thamm DH: Mast cell
tumorsSmall Animal Clinical Oncology. Withrow SJ, Vail DM and Page
RL: 5th. WB Saunders Co; Philadelphia, PA: pp. 335–355. 2013,
View Article : Google Scholar
|
|
4
|
Warland J, Brioschi V, Owen L and Dobson
J: Canine mast cell tumours: Decision-making and treatment. In
Pract. 37:315–332. 2015. View Article : Google Scholar
|
|
5
|
Simpson AM, Ludwig LL, Newman SJ, Bergman
PJ, Hottinger HA and Patnaik AK: Evaluation of surgical margins
required for complete excision of cutaneous mast cell tumors in
dogs. J Am Vet Med Assoc. 224:236–240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amora A and Scholar EM: Role of tyrosine
kinase inhibitors in cancer therapy. J Pharmacol Exp Ther.
315:971–979. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
London CA: Tyrosine kinase inhibitors in
veterinary medicine. Top Companion Anim Med. 24:106–112. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weinstein IB and Joe A: Oncogene
addiction. Cancer Res. 68:3077–3080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silva RLA: Oncogenes e genes supressores
de tumorOncologia molecular. Ferreira CG and Rocha JCC: 2nd.
Atheneu, São Paulo, SP: pp. 43–59. 2010
|
|
10
|
Webster JD: Small molecule kinase
inhibitors in veterinary oncology. Vet J. 205:122–3. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zemke D, Yamini B and Yuzbasiyan-Gurkan V:
Mutations in the juxtamembrane domain of c-KIT are associated with
higher grade mast cell tumors in dogs. Vet Pathol. 39:529–535.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Webster JD, Yuzbasiyan-Gurkan V, Kaneene
JB, Miller R, Resau JH and Kiupel M: The role of c-KIT in
tumorigenesis: Evaluation in canine cutaneous mast cell tumors.
Neoplasia. 8:104–111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Avery AC: Molecular diagnostics of
hematologic malignancies in small animals. Vet Clin North Am Small
Anim Pract. 42:97–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takeuchi Y, Fujino Y, Watanabe M,
Takahashi M, Nagawa T, Takeuchi A, Bonkobara M, Kobayashi T, Ohno
K, Uchida K, et al: Validation of the prognostic value of
histopathological grading or c-kit mutation in canine cutaneous
mast cell tumours: A retrospective cohort study. Vet J.
196:492–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sleijfer S, Wiemar E and Verweij J: Drug
Insight: Gastrointestinal stromal tumors (GIST)-the solid tumor
model for cancer-specific treatment. Nat Clin Pract Oncol.
5:102–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Isotani M, Ishida N, Tominaga M, Tamura K,
Yagihara H, Ochi S, Kato R, Kobayashi T, Fujita M, Fujino Y, et al:
Effect of tyrosine kinase inhibition by imatinibmesylate on mast
cell tumors in dogs. J Vet Intern Med. 22:985–988. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
London CA, Malpas PB, Wood-Follis SL,
Boucher JF, Rusk AW, Rosenberg MP, Henry CJ, Mitchener KL, Klein
MK, Hintermeister JG, et al: Multi-center, placebo-controlled,
double-blind, randomized study of oral toceranib phosphate
(SU11654), a receptor tyrosine kinase inhibitor, for the treatment
of dogs with recurrent (either local or distant) mast cell tumor
following surgical excision. Clin Cancer Res. 15:3856–3865. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ogilvie GK, Hensel P, Kitchell BE,
Dubreuil P and Ahn A: Masitinib-a targeted therapy with
applications in veterinary oncology and inflammatory diseases. CAB
Rev. 6:1–11. 2011. View Article : Google Scholar
|
|
20
|
Krick EL, Billings AP, Shofer FS, Watanabe
S and Sorenmo KU: Cytological lymph node evaluation in dogs with
mast cell tumours: Association with grade and survival. Vet Comp
Oncol. 7:130–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patnaik AK, Ehler WJ and MacEwen EG:
Canine cutaneous mast cell tumor: Morphologic grading and survival
time in 83 dogs. Vet Pathol. 21:268–274. 1984. View Article : Google Scholar
|
|
22
|
Kiupel M, Webster JD, Bailey KL, Best S,
DeLay J, Detrisac CJ, Fitzgerald SD, Gamble D, Ginn PE, Goldschmidt
MH, et al: Proposal of a 2-tier histologic grading system for
canine cutaneous mast cell tumors to more accurately predict
biological behavior. Vet Pathol. 48:147–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kiupel M, Webster JD, Kaneene JB, Miller R
and Yuzbasiyan-Gurkan V: The use of KIT and tryptase expression
patterns as prognostic tools for canine cutaneous mast cell tumors.
Vet Pathol. 41:371–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scase TJ, Edwards D, Miller J, Henley W,
Smith K, Blunden A and Murphy S: Canine mast cell tumors:
Correlation of apoptosis and proliferation markers with prognosis.
J Vet Intern Med. 20:151–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nguyen SM, Thamm DH, Vail DM and London
CA: Response evaluation criteria for solid tumours in dogs (v1.0):
A veterinary cooperative oncology group (VCOG) consensus document.
Vet Comp Oncol. 13:176–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Veterinary Co-operative Oncology Group
(VCOG), . Veterinary co-operative oncology group-common terminology
criteria for adverse events (VCOG-CTCAE) following chemotherapy or
biological antineoplastic therapy in dogs and cats v1.0. Vet Comp
Oncol. 2:195–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smrkovski OA, Essick L, Rohrbach BW and
Legendre AM: Masitinibmesylate for metastatic and non-resectable
canine cutaneous mast cell tumours. Vet Comp Oncol. 13:314–321.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hahn KA, Ogilvie G, Rusk T, Devauchelle P,
Leblanc A, Legendre A, Powers B, Leventhal PS, Kinet JP, Palmerini
F, et al: Masitinib is safe and effective for the treatment of
canine mast cell tumors. J Vet Intern Med. 22:1301–1309. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Antonescu CR, Besmer P, Guo T, Arkun K,
Hom G, Koryotowski B, Leversha MA, Jeffrey PD, Desantis D, Singer
S, et al: Acquired resistance to imatinib in gastrointestinal
stromal tumor occurs through secondary gene mutation. Clin Cancer
Res. 11:4182–4190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dohse M, Scharenberg C, Shukla S, Robey
RW, Volkmann T, Deeken JF, Brendel C, Ambudkar SV, Neubauer A and
Bates SE: Comparison of ATP-binding cassette transporter
interactions with the tyrosine kinase inhibitors imatinib,
nilotinib, and dasatinib. Drug Metab Dispos. 38:1371–1380. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mealey KL and Fidel J: P-glycoprotein
mediated drug interactions in animals and humans with cancer. J Vet
Intern Med. 29:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shukla S, Robey RW, Bates SE and Ambudkar
SV: Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine
kinase inhibitor, blocks function of the ATP-binding cassette (ABC)
transporters P-glycoprotein (ABCB1) and ABCG2. Drug Metab Dispos.
37:359–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoffmann K, Franz C, Xiao Z, Mohr E, Serba
S, Büchler MW and Schemmer P: Sorafenib modulates the gene
expression of multi-drug resistance mediating ATP-binding cassette
proteins in experimental hepatocellular carcinoma. Anticancer Res.
30:4503–4508. 2010.PubMed/NCBI
|
|
34
|
Grant J, North S and Lanore D: Clinical
response of masitinib mesylate in the treatment of canine
macroscopic mast cell tumours. J Small Anim Pract. 57:283–290.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giantin M, Granato A, Baratto C, Marconato
L, Vascellari M, Morello EM, Vercelli A, Mutinelli F and Dacasto M:
Global gene expression analysis of canine cutaneous mast cell
tumor: Could molecular profiling be useful for subtype
classification and prognostication? PLoS One. 9:e954812014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Casagrande TA Costa, de Oliveira Barros
LM, Fukumasu H, Cogliati B, Chaible LM, Dagli ML and Matera JM: The
value of molecular expression of KIT and KIT ligand analysed using
real-time polymerase chain reaction and immunohistochemistry as a
prognostic indicator for canine cuteaneous mast cell tumours. Vet
Comp Oncol. 13:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Berlato D, Murphy S, Monti P, Stewart J,
Newton JR, Flindall A and Maglennon GA: Comparison of mitotic index
and Ki67 index in the prognostication of canine cutaneous mast cell
tumours. Vet Comp Oncol. 13:143–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Miller RL, Van Lelyveld S, Warland J,
Dobson JM and Foale RD: A retrospective review of treatment and
response of high-risk mast cell tumours in dogs. Vet Comp Oncol.
14:361–370. 2016. View Article : Google Scholar : PubMed/NCBI
|