Introduction
Uterine cervical cancer (CC) is the second most
common cancer among women, worldwide (1). The annual global incidence of CC in 2012
was 528,000 cases, and the annual global mortality rate was 266,000
deaths, with 85% of cases occurring in developing countries, where
CC is a leading cause of cancer-related death in women (2). Oncogenic type human papillomavirus (HPV)
infection is a major etiologic risk factor (3) associated with carcinogenesis (4). More than 100 HPV types have been
identified, with a subset of these being classified as high risk.
HPV 16 and HPV 18 are the most commonly detected genotypes
occurring in 71% of invasive CCs (5).
Current detection methods have uncovered a HPV prevalence of
95–100% in women with CC (6,7).
The primary treatment strategy for uterine CC
consists of surgery or radiotherapy (RT). Surgery is typically
reserved for early-stage disease and small lesions including stage
IA, IB1, and selected IIA1 diseases (8). Based on the results of randomized
clinical trials, concurrent chemoradiotherapy (CCRT) has become the
primary treatment for stage IB2 to IVA disease (9,10). Only a
few studies have assessed specific treatments for cervical
adenocarcinomas, but they are typically treated in a similar manner
to cervical squamous cell carcinomas (11).
Several studies have demonstrated a relationship
between HPV subtype and outcome in patients who underwent primary
surgery (12–15). These studies showed that, of all HPV
genotype infections, HPV 18 infection was associated with more
aggressive CC. By contrast, some studies showed that patients with
HPV 33-related tumors had favorable outcomes (16,17). Few
reports have investigated the relationships between HPV genotype
and outcome in patients with uterine CC after RT.
Oncogenic HPV types can be classified
phylogenetically according to their L1 open reading frame (18). When HPVs share 60–70% nucleotide
identity, they are clustered into the same species. Two HPV
species, α-7 (HPV 18, 39, 45, 59, and 68) and α-9 (HPV 16, 31, 33,
35, 52, and 58), account for >80% of all CCs (19). Wang et al reported that
patients with HPV α-7-positive CC had worse local control (LC)
after RT compared with HPV α-9-positive patients (20). However, by performing assays in clonal
CC cell lines, Hall et al revealed that poor prognosis
associated with HPV species might not be explained by intrinsic
radiosensitivities because cells harboring the HPV α-9 and α-7
species had similar radiosensitivities (21). In the present study, we investigated
the frequencies of HPV genotypes and species distribution in
Japanese CC patients who underwent RT at our institution. We then
evaluated the relationships between LC after RT and HPV species in
these patients.
Materials and methods
Patient characteristics
Between November 2001 and August 2006, 157 patients
with uterine CC were treated with RT or CCRT with curative intent
in our institution. Pretreatment, formalin-fixed, paraffin-embedded
biopsies were obtained from 83 patients. All 83 patients provided
written informed consent according to the institutional
regulations. The concept of the present study was approved by the
Institutional Review Board (ID: NIRS-06-004). The patients'
characteristics are listed in Table
I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics
(n=83) | Number (%) |
|---|
| Age, years
(range) | 59 (32–83) |
| Histology |
|
Squamous cell carcinoma | 61 (73.5) |
|
Adenocarcinoma | 21 (25.3) |
| Small
cell carcinoma | 1 (1.2) |
| FIGO stage |
| IB | 8 (9.6) |
| II | 13 (15.7) |
|
III | 45 (54.2) |
| IV | 17 (20.5) |
| Pelvic LN
metastasis |
|
Negative | 40 (48.2) |
|
Positive | 43 (51.8) |
| PALN
metastasis |
|
Negative | 69 (83.1) |
|
Positive | 14 (16.9) |
| Concurrent
chemotherapy |
| No | 36 (43.4) |
|
Yes | 47 (56.6) |
HPV genotyping
DNA was extracted from formalin-fixed
paraffin-embedded tumors using the DEXPATTM system (Takara Bio,
Inc., Otsu, Japan). Extracted DNA were further purified using the
QIAamp DNA Micro kit (Qiagen GmbH, Hilden, Germany) according to
the manufacturer's protocol. Genomic DNA was also isolated from
biopsies frozen in RNAlater (Ambion; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) using the Genomic-tip 100/G kit (Qiagen
GmbH). HPV genotypes were determined using the polymerase chain
reaction (PCR) method (22) and the
Linear Array HPV Genotyping test according to the manufacturer's
instruction (Roche Molecular Diagnostics, Pleasanton, CA, USA)
(23,24). Samples that contained more than about
70% of tumor cells were used in our study (22). The genomic DNA samples analyzed for
the presence of HPV DNA were also used for searching structural
variations of tumor suppressor gene candidates including p53. The
DNA samples were either analyzed directly or after mixing with
equal amounts of reference DNA, which was obtained commercially
(Promega Corporation, Madison, WI, USA). This reference DNA that
did not contain HPV genome but had p53 gene could be used to test
the HPV detection manners used here. More specifically, mixing the
reference DNA can provide us information about how the DNA sample
is amplifiable or the DNA sample does not contain any inhibitor for
the reactions. The reference DNA also work as a negative control of
the detection of HPV genome. We used some cervical cancer patients'
tumor DNA containing HPV genome, which were detected in our
previous experiments, as positive controls. The data indicated that
the DNA samples used here showed good quality for PCR (24). Sixteen oncogenic HPV genotypes were
evaluated including HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58,
59, 66, 68, 71, and 82 (23,24).
Treatment
Patients were treated using a combination of
external beam RT and high-dose rate brachytherapy. External whole
pelvis irradiation was performed using the
anteroposterior-posteroanterior field or box techniques. The median
external beam RT dose was 50.0 Gy with 1.8–2.0 Gy/fraction. Central
shielding (3-cm width) was used, yielding total doses of 19.8–20.0
Gy for stage IB1 and II disease (tumor diameter ≤4 cm) or 30.0–30.6
Gy (or 39.6–40.0 Gy for bulky cases) for stage IB2 and II, IIIB,
and IVA disease (tumor diameter >4 cm). Pelvic irradiation with
central shielding was performed to a total dose of 49.8–50.6 Gy. In
patients with gross lymph node metastases, an additional 6.0–10.0
Gy boost was applied to the lesion.
High-dose rate brachytherapy was performed using the
Ir-192 remote after loading system (microSelectron HDR; Elekta
Instrument AB, Stockholm, Sweden). A Fletcher-Suit Asian Pacific
applicator set (tandem and half-size ovoids) was used in the
majority of the patients. If a patient had severe vaginal invasion,
a vaginal cylinder was used in place of the tandem and ovoids. An
in-room CT on-rail brachytherapy system was installed in 2001 at
our institution, and CT-based brachytherapy was introduced for
advanced cases that year. According to dose distribution generated
by radiography-based 2D planning, the dose was administrated to
Point A in 4 fractions with 6.0 Gy/fraction. Dose adaptation was
performed based on dose changes at Point A for advanced cases. We
modified the dose at Point A so that a 6 Gy isodose line could
cover the tumor. For patients with FIGO stage IB-II or III–IVA
tumors >4 cm in diameter or those with pelvic lymph node
metastasis, cisplatin-based chemotherapy was administered
concurrently during RT. Exclusion criteria for chemotherapy
included being >70 years old or having severe concomitant
diseases.
Follow-up
Patients were followed-up every 1–3 months for the
first 2 years and every 3–6 months for the subsequent 3 years.
Disease status was assessed at every follow-up examination by a
physical examination, with or without appropriate laboratory and
radiologic tests. Suspected recurrent CC was confirmed by biopsy
whenever possible.
Statistical analyses
LC was measured from the date of therapy initiation
to the date of the first local recurrence or the most recent
follow-up. Distant metastasis-free survival (DMFS) was measured
from the date of therapy initiation to the date of detection of the
first distant metastasis. Disease-free survival (DFS) was measured
from the date of initiation of therapy to the date of the first
recurrence was detected regardless of recurrent site. Overall
survival (OS) was measured from the date of the therapy initiation
to the date of death from any cause or the most recent follow-up.
The actuarial rates of LC, DMFS, DFS, and OS were calculated using
the Kaplan-Meier method and compared using the log-rank test. The
Mann-Whitney U test was used to evaluate associations with and
between clinicopathological variables. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using SPSS 23.0 for Mac (SPSS, Inc.,
Chicago, IL, USA).
Results
HPV genotype frequencies
Among 83 patients, 14 patients (16.9%) had
HPV-negative tumors. For the 69 HPV-positive patients, 16 HPV
genotypes were detected. HPV genotypes are summarized in Table II. The 5 most prevalent genotypes
included HPV 16, 58, 18, 52, and 31. The patients were categorized
into the HPV α-7 (HPV 18, 39, 45, 59, and 68 genotypes), HPV α-9
(HPV 16, 31, 33, 35, 52, and 58 genotypes), or ‘other’ (HPV 51 and
56) groups. Patients with multiple HPV infections, but who had at
least one HPV α-7 or α-9 species were categorized into the HPV α-7
or α-9 groups, respectively. Fifty-four patients comprised the HPV
α-9 group, 13 comprised the HPV α-7 group, and 2 were included in
the ‘other’ group.
 | Table II.Frequency of HPV genotypes in 83
patients. |
Table II.
Frequency of HPV genotypes in 83
patients.
| Parameter | Type | n | % |
|---|
| Single HPV | 16 | 26 | 32.3 |
|
| 18 | 6 |
7.2 |
|
| 31 | 5 |
6.0 |
|
| 33 | 2 |
2.4 |
|
| 35 | 1 |
1.2 |
|
| 39 | 2 |
2.4 |
|
| 45 | 2 |
2.4 |
|
| 51 | 1 |
1.2 |
|
| 52 | 6 |
7.2 |
|
| 56 | 1 |
1.2 |
|
| 58 | 10 | 12.0 |
|
| 59 | 1 |
1.2 |
|
| 68 | 1 |
1.2 |
| Multiple HPV | 16, 58 | 1 |
1.2 |
|
| 16, 66 | 1 |
1.2 |
|
| 18, 71 | 1 |
1.2 |
|
| 56, 58 | 1 |
1.2 |
|
| 58, 82 | 1 |
1.2 |
| HPV negative |
| 14 | 16.9 |
Associations between HPV genotype and
RT outcomes
The median follow-up time after treatment initiation
was 52 months (range, 2–161 months). There were no significant
differences between the HPV α-9 and α-7 groups regarding age, FIGO
stage, the lymph node metastases rate at diagnosis, or concurrent
chemotherapy administration, whereas CC histology was significantly
different between the two groups (P<0.01). The comparison of
patients' characteristics between the HPV α-9 and α-7 groups are
shown in Table III.
 | Table III.Comparison of patients'
characteristics between HPV α-9 and α-7. |
Table III.
Comparison of patients'
characteristics between HPV α-9 and α-7.
| Characteristics
(n=83) | HPV α-7 (n=13) | HPV α-9 (n=54) | P-value |
|---|
| Age, years
(range) | 52 (38–74) | 61 (36–82) | 0.10 |
| Histology |
|
| <0.01 |
|
Squamous cell carcinoma | 7 | 48 |
|
|
Adenocarcinoma | 5 | 6 |
|
| Small
cell carcinoma | 1 | 0 |
|
| FIGO stage |
|
| 0.22 |
| IB | 0 | 5 |
|
| II | 1 | 12 |
|
|
III | 9 | 26 |
|
| IV | 3 | 11 |
|
| Pelvic LN
metastasis |
|
| 0.19 |
|
Negative | 3 | 28 |
|
|
Positive | 10 | 26 |
|
| PALN
metastasis |
|
| 0.09 |
|
Negative | 10 | 46 |
|
|
Positive | 3 | 8 |
|
| Concurrent
chemotherapy |
|
| 0.09 |
| No | 3 | 30 |
|
|
Yes | 10 | 24 |
|
By the end of the study, among all 83 patients, 40
(48.2%) had no recurrence, and 43 (51.8%) had experienced treatment
failure including 4 local failures and 34 distant relapses, and 5
patients had both. Forty patients were alive without disease, 6
patients were alive after successful salvage, and 37 patients were
dead. Of the 37 patients who died, 34 (91.8%) died due to CC. The
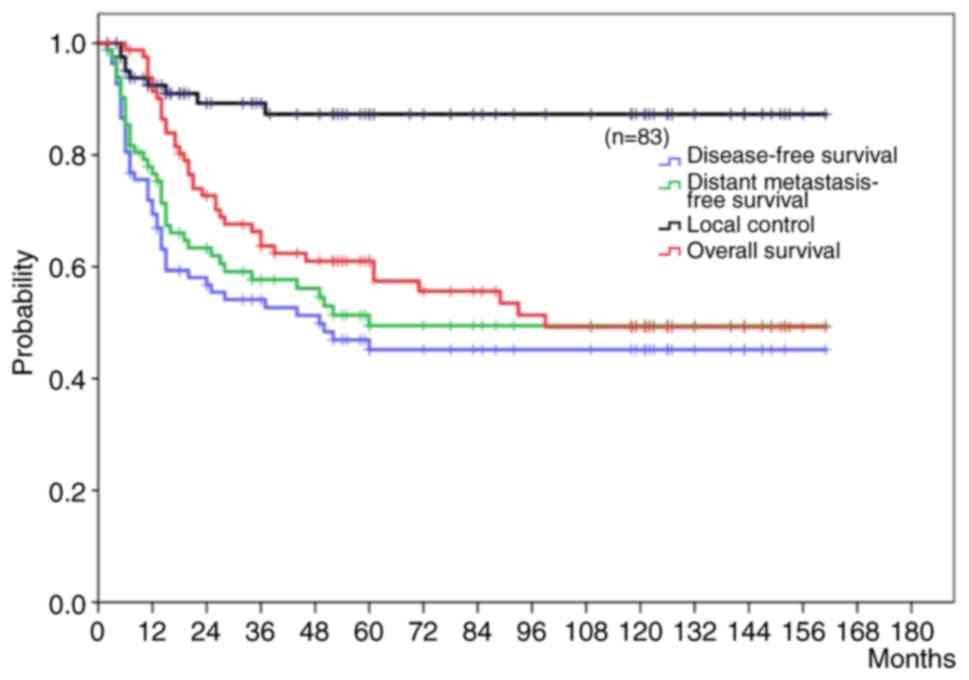
5-year LC, DFS, DMFS, and OS rate in all cases were 97.3, 45.2,
49.5, and 61.0%, respectively (Fig.
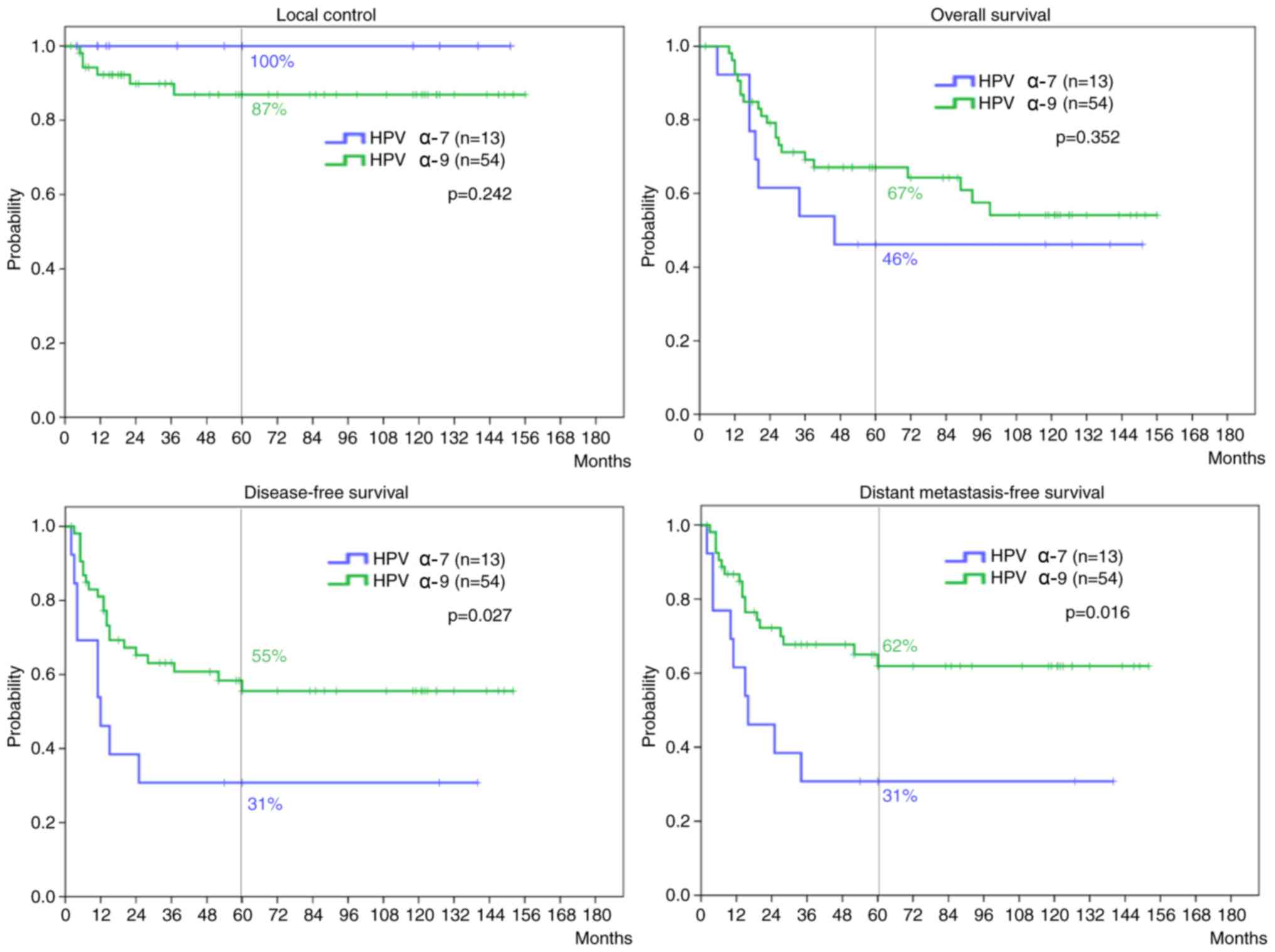
1). There were no significant differences in the 5-year LC
(P=0.242) and OS rates (P=0.352) between the HPV α-7 and α-9 groups
(Fig. 2). By contrast, the HPV α-7
group had significantly inferior 5-year DFS and DMFS rates compared
with the HPV α-9 group (P=0.027 and 0.016, respectively). The
5-year DMFS in patients with squamous cell CC showed a tendency
toward inferiority in the HPV α-7 group compared with the HPV α-9
group, although the difference was not significant (P=0.108).
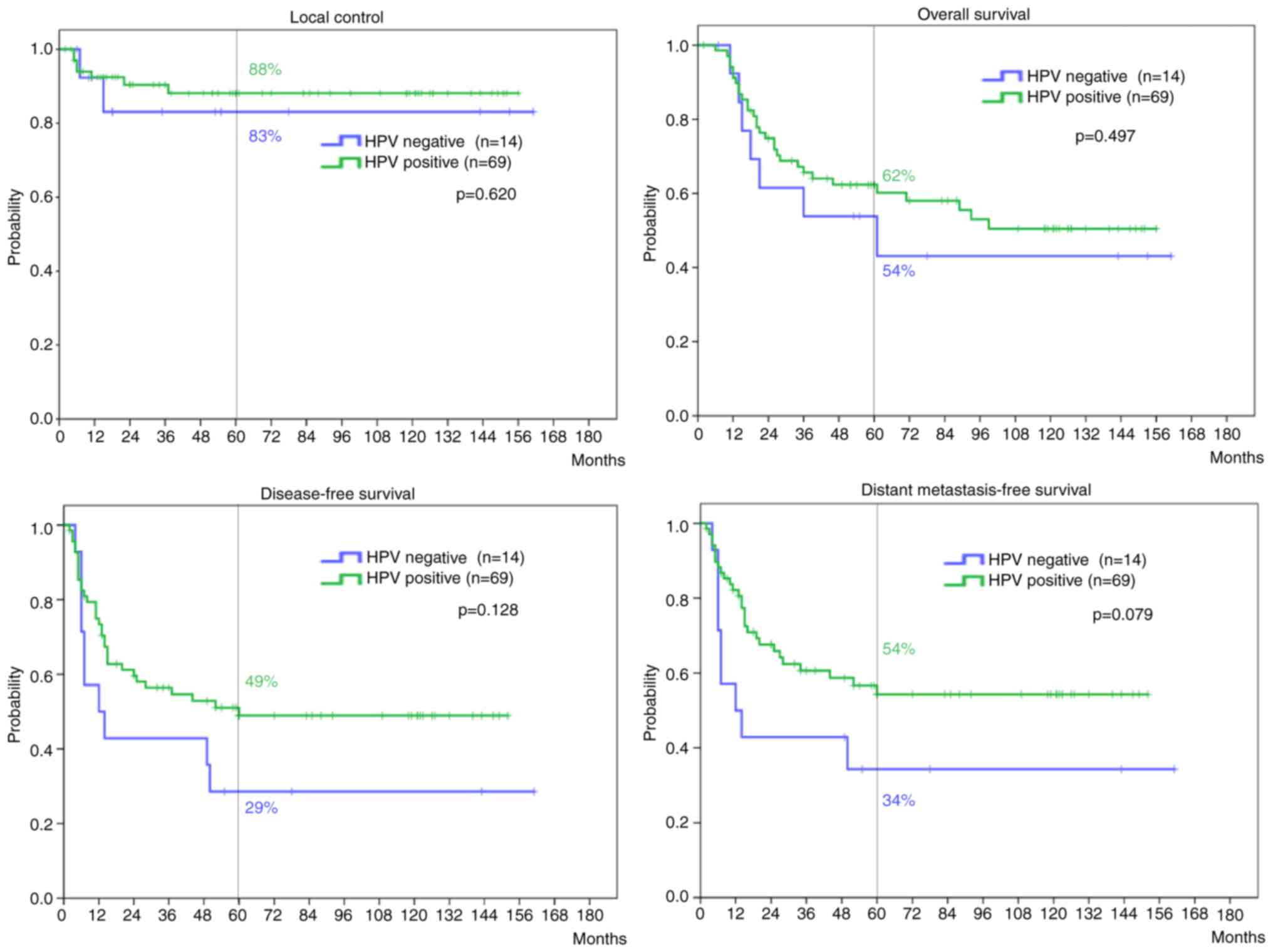
The comparison of patients' characteristics between
the HPV positive and negative groups are shown in Table IV. There were no statistical
differences in age, FIGO stage, presence of LN metastases, or
administration of chemotherapy. However, there was statistical
difference in histology; more than 70% patients who were judged as
HPV negative had adenocarcinoma. Although relatively poorer
outcomes in the HPV negative group were observed, there were no
significant differences between the HPV positive and negative
groups in the 5-year LC (P=0.620), OS (P=0.497), DFS (P=0.128), or
DMFS rates (P=0.079) (Fig. 3).
 | Table IV.Comparison of patients'
characteristics between HPV positive and HPV negative. |
Table IV.
Comparison of patients'
characteristics between HPV positive and HPV negative.
| Characteristics
(n=83) | HPV positive
(n=69) | HPV negative
(n=14) | P-value |
|---|
| Age, years
(range) | 60 (36–82) | 58 (37–83) | 0.33 |
| Histology |
|
| <0.01 |
|
Squamous cell carcinoma | 57 | 4 |
|
|
Adenocarcinoma | 11 | 10 |
|
| Small
cell carcinoma | 1 | 0 |
|
| FIGO stage |
|
|
0.20 |
| IB | 5 | 3 |
|
| II | 13 | 1 |
|
|
III | 36 | 7 |
|
| IV | 15 | 3 |
|
| Pelvic LN
metastasis |
|
| 0.66 |
|
Negative | 32 | 8 |
|
|
Positive | 37 | 6 |
|
| PALN
metastasis |
|
| 0.91 |
|
Negative | 58 | 11 |
|
|
Positive | 11 | 3 |
|
| Concurrent
chemotherapy |
|
| 0.11 |
| No | 34 | 3 |
|
|
Yes | 35 | 11 |
|
Discussion
The present study is the first to show the
relationship between HPV genotypes and clinical outcomes after RT
in Japanese patients with uterine CC. The majority of patients in
the present study were HPV-positive, with high proportions of HPV
16, 58, 18, 52. Although the number of patients in the present
study was small, the findings were consistent with previous reports
regarding the global incidence of HPV genotypes in uterine CC
(5). De Sanjose et al found
that HPV 16 and 18 were the most common genotypes for uterine CC
worldwide (5). However, Salehi-Vaziri
et al reported that the most common HPV genotypes in Iranian
women were HPV 16 and 53 (25).
García Muentes et al reported that the most common HPV
genotypes in Ecuadorian women were HPV 16 and HPV 33 (26). Wang et al indicated that the
most common HPV genotypes in Chinese women were HPV 16, 52, and 58
(27). Moreover, a high prevalence of
HPV 52 and 58 genotypes in Southeast Asian countries has been
reported previously (28–30). Therefore, HPV genotype distribution in
uterine CC varies geographically.
Out of 83 cervical cancers analyzed, 14 (16.9%) were
found to be HPV-negative. This fig. is higher than previously
reported (20,21). Kusanagi et al reported that HPV
has been rarely detected in some types of adenocarcinoma of the
uterus cervix (31). The present
study included a larger number of patients (25.3%) with
adenocarcinoma. Therefore, relatively higher proportions of
HPV-negative patients may contribute to the higher proportion of
adenocarcinoma in the present study. However, there was no
significant clinical effect of HPV presence on the outcomes of
patients with CC who underwent RT.
Meanwhile, the present study showed the significant
clinical effect of HPV genotypes on outcome in patients with CC
patients who underwent RT. HPV α-7-positive patients had
significantly inferior DFS and DMFS rates compared with
α-9-positive patients. By contrast, HPV species had no impact on LC
or OS rates in patients with uterine CC. These findings contrast
with those of Wang et al who found a significant effect of
HPV species on LC and local progression-free survival rates,
respectively (20). Meanwhile, Hall
et al assessed that the HPV α-9 and α-7 species had similar
radiosensitivity by performing assays in clonal CC cell lines
(21). Taken together, HPV genotype
may affect the DFS and DMFS rates, but not the LC or OS in patients
with uterine cervical cancer after RT. Our present study showed
superior local control rates when compared to Wang et al and
Hall et al (20,21). The main reason for a higher local
control rate could be attributed to our use of CT-based
brachytherapy. In general, if the local control rate improves, DFS
or DMFS should also improve. Nonetheless, statistically significant
differences were still found in DFS or DMFS between HPV
α-7-positive patients and α-9-positive patients in the present
study. This fact supports that HPV genotype affected the distant
metastatic rate. However, the underlying mechanisms associated with
increased metastatic rates in patients with the α-7 species are
unknown. The findings of Hall et al (21) in clonal CC cell lines, which, although
preclinical, suggest that these species do not differ regarding
radiosensitivities, implied than another, as yet undetermined
factor could be responsible for outcome differences according to
HPV species. Therefore, further studies are needed to identify
these potential factors and determine the mechanisms involved.
HPV α-7 species, in particular, the HPV 18 and HPV
45 genotypes, are commonly associated with adenocarcinoma of the
cervix; there is a significantly higher association of HPV 18 and
HPV 45 with adenocarcinoma (44%) compared with squamous cell
carcinoma (14%) (5). In the present
study, the distribution of HPV α-7 and α-9 was similar between
patients with adenocarcinoma, although the number of patients with
adenocarcinoma was small. By contrast, there was a significant
difference in the distribution of HPV species in patients with
squamous cell carcinoma, for whom the HPV α-9 species was
especially prevalent. A previous study showed that patients with
adenocarcinoma had significantly poorer prognoses after radical RT
compared to those with squamous cell carcinoma (32). However, in the present study, in
patients with squamous cell carcinoma, although HPV α-7 was
associated with inferior DMFS compared with HPV α-9, the difference
was not observed in patients with squamous cell carcinoma. This
suggested that higher metastatic rates in patients with HPV α-7
cannot be explained by tumor histology alone.
In vitro studies have shown that several
features of HPV 18 infection that differ from those of HPV 16
infection, including enhanced E7 phosphorylation and increased
transformation (33,34). Furthermore, in uterine CC cells, HPV
18 was associated with significantly lower levels of apoptosis
compared with HPV 16 (35). E6
protein activities in CC also differ between HPV 18 and HPV 16
infections (36). E6 protein plays a
pivotal role in carcinogenesis by targeting the host proteins, such
as p53 and PDZ domain proteins that are involved in
proteasome-mediated degradation (36). The PDZ domains play a vital role in
organizing and maintaining complex scaffolding formations (37). This difference also exists between the
HPV α-9 and α-7 species. In uterine CC cells, these molecular
effects mediated by HPV infection are thought to play a key role in
carcinogenesis. In addition, it has been suggested recently that
scaffolding proteins might regulate invasion and metastasis in some
cancer types (38–40), which affords a possible explanation
for the more aggressive nature of CC associated with the HPV α-7
species.
In conclusion, HPV species affected the DMFS and DFS
rates but not the LC or OS rates in patients with uterine CC after
RT. However, further large population studies on the usefulness HPV
genotype information in Japanese patients with uterine CC are
needed to determine if HPV genotypes could be considered a
prognostic marker in patients with uterine CC after RT.
Acknowledgements
This study was supported by the Research Project
with Heavy Ions at the National Institute of Radiological
Sciences.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cervical cancer: Estimated incidence,
mortality and prevalence worldwide in 2012. International Agency
for Research on Cancer and World Health Organization. 2012,
http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspxDecember
25–2016
|
|
3
|
Bouvard V, Baan R, Straif K, Grosse Y,
Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C,
Galichet L, et al: A review of human carcinogens-Part B: Biological
agents. Lancet Oncol. 10:321–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Sanjose S, Quint WG, Alemany L, Geraets
DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin
HR, et al: Human papillomavirus genotype attribution in invasive
cervical cancer: A retrospective cross-sectional worldwide study.
Lancet Oncol. 11:1048–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Villiers EM, Wagner D, Schneider A,
Wesch H, Miklaw H, Wahrendorf J, Papendick U and zur Hausen H:
Human papillomavirus infections in women with and without abnormal
cervical cytology. Lancet. 2:703–706. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parkin DM and Bray F: Chapter 2: The
burden of HPV-related cancers. Vaccine. 3(24 Suppl): S11–S25. 2006.
View Article : Google Scholar
|
|
8
|
American College of Obstetricians and
Gynecologists: ACOG practice bulletin. Diagnosis and treatment of
cervical carcinomas. Number 35, May 2002. American College of
Obstetricians and Gynecologists. Int J Gynaecol Obstet. 78:79–91.
2002.PubMed/NCBI
|
|
9
|
Gaffney DK, Erickson-Wittmann BA, Jhingran
A, Mayr NA, Puthawala AA, Moore D, Rao GG, Small W Jr, Varia MA,
Wolfson AH, et al: ACR Appropriateness Criteria® on
advanced cervical cancer expert panel on radiation
oncology-gynecology. Int J Radiat Oncol Biol Phys. 81:609–614.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Monk BJ, Tewari KS and Koh WJ:
Multimodality therapy for locally advanced cervical carcinoma:
State of the art and future directions. J Clin Oncol. 25:2952–2965.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gien LT, Beauchemin MC and Thomas G:
Adenocarcinoma: A unique cervical cancer. Gynecol Oncol.
116:140–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai CH, Chang CJ, Huang HJ, Hsueh S, Chao
A, Yang JE, Lin CT, Huang SL, Hong JH, Chou HH, et al: Role of
human papillomavirus genotype in prognosis of early-stage cervical
cancer undergoing primary surgery. J Clin Oncol. 25:3628–3634.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rose BR, Thompson CH, Simpson JM, Jarrett
CS, Elliott PM, Tattersall MH, Dalrymple C and Cossart YE: Human
papillomavirus deoxyribonucleic acid as a prognostic indicator in
early-stage cervical cancer: A possible role for type 18. Am J
Obstet Gynecol. 173:1461–1468. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burger RA, Monk BJ, Kurosaki T,
Anton-Culver H, Vasilev SA, Berman ML and Wilczynski SP: Human
papillomavirus type 18: Association with poor prognosis in early
stage cervical cancer. J Natl Cancer Inst. 88:1361–1368. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lombard I, Vincent-Salomon A, Validire P,
Zafrani B, de la Rochefordière A, Clough K, Favre M, Pouillart P
and Sastre-Garau X: Human papillomavirus genotype as a major
determinant of the course of cervical cancer. J Clin Oncol.
16:2613–2619. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang LW, Chao SL and Hwang JL: Human
papillomavirus-31-related types predict better survival in cervical
carcinoma. Cancer. 100:327–334. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lai HC, Sun CA, Yu MH, Chen HJ, Liu HS and
Chu TY: Favorable clinical outcome of cervical cancers infected
with human papilloma virus type 58 and related types. Int J Cancer.
84:553–557. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Villiers EM, Fauquet C, Broker TR,
Bernard HU and zur Hausen H: Classification of papillomaviruses.
Virology. 324:17–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doorbar J: Molecular biology of human
papillomavirus infection and cervical cancer. Clin Sci (Lond).
110:525–541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang CC, Lai CH, Huang HJ, Chao A, Chang
CJ, Chang TC, Chou HH and Hong JH: Clinical effect of human
papillomavirus genotypes in patients with cervical cancer
undergoing primary radiotherapy. Int J Radiat Oncol Biol Phys.
78:1111–1120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hall JS, Iype R, Armenoult LS, Taylor J,
Miller CJ, Davidson S, de Sanjose S, Bosch X, Stern PL and West CM:
Poor prognosis associated with human papillomavirus α7 genotypes in
cervical carcinoma cannot be explained by intrinsic
radiosensitivity. Int J Radiat Oncol Biol Phys. 85:e223–e229. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishikawa H, Mitsuhashi N, Sakurai H,
Maebayashi K and Niibe H: The effects of p53 status and human
papillomavirus infection on the clinical outcome of patients with
stage IIIB cervical carcinoma treated with radiation therapy alone.
Cancer. 91:80–89. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Woo YL, Damay I, Stanley M, Crawford R and
Sterling J: The use of HPV linear array assay for multiple HPV
typing on archival frozen tissue and DNA specimens. J Virol
Methods. 142:226–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakamura E, Iwakawa M, Furuta R, Ohno T,
Satoh T, Nakawatari M, Ishikawa K, Imadome K, Michikawa Y, Tamaki
T, et al: Villin1, a novel diagnostic marker for cervical
adenocarcinoma. Cancer Biol Ther. 8:1146–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salehi-Vaziri M, Sadeghi F, Hashemi FS,
Haeri H, Bokharaei-Salim F, Monavari SH and Keyvani H: Distribution
of human papillomavirus genotypes in iranian women according to the
severity of the cervical lesion. Iran Red Crescent Med J.
18:e244582016.PubMed/NCBI
|
|
26
|
García Muentes GD, García Rodríguez LK,
Galarraga RI Burgos, Carpio F Almeida and Cabezas JC Ruiz:
Genotypes distribution of human papillomavirus in cervical samples
of Ecuadorian women. Rev Bras Epidemiol. 19:160–166. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Wu B, Li J and Chen L: Prevalence
of human papillomavirus and its genotype among 1336 invasive
cervical cancer patients in Hunan province, central south China. J
Med Virol. 87:516–521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin QQ, Yu SZ, Qu W, Cruz Y and Burk RD:
Humanpapillomavirus types 52 and 58. Int J Cancer. 75:484–485.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin H, Ma YY, Moh JS, Ou YC, Shen SY and
ChangChien CC: High prevalence of genital human papillomavirus type
52 and 58 infection in women attending gynecologic practitioners in
South Taiwan. Gynecol Oncol. 101:40–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lai CH, Huang HJ, Hsueh S, Chao A, Lin CT,
Huang SL, Chao FY, Qiu JT, Hong JH, Chou HH, et al: Human
papillomavirus genotype in cervical cancer: A population-based
study. Int J Cancer. 120:1999–2006. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kusanagi Y, Kojima A, Mikami Y, Kiyokawa
T, Sudo T, Yamaguchi S and Nishimura R: Absence of high-risk human
papillomavirus (HPV) detection in endocervical adenocarcinoma with
gastric morphology and phenotype. Am J Pathol. 177:2169–2175. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fyles AW, Pintilie M, Kirkbride P, Levin
W, Manchul LA and Rawlings GA: Prognostic factors in patients with
cervix cancer treated by radiation therapy: Results of a multiple
regression analysis. Radiother Oncol. 35:107–117. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Werness BA, Levine AJ and Howley PM:
Association of human papillomavirus types 16 and 18 E6 proteins
with p53. Science. 248:76–79. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Villa LL and Schlegel R: Differences in
transformation activity between HPV-18 and HPV-16 map to the viral
LCR-E6-E7 region. Virology. 181:374–377. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arends MJ, Wyllie AH and Bird CC: Human
papillomavirus type 18 is associated with less apoptosis in
fibroblast tumours than human papillomavirus type 16. Br J Cancer.
72:646–649. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hampson L, El Hady ES, Moore JV, Kitchener
H and Hampson IN: The HPV16 E6 and E7 proteins and the radiation
resistance of cervical carcinoma. FASEB J. 15:1445–1447.
2001.PubMed/NCBI
|
|
37
|
Harris BZ and Lim WA: Mechanism and role
of PDZ domains in signaling complex assembly. J Cell Sci.
114:3219–3231. 2001.PubMed/NCBI
|
|
38
|
Izumchenko E, Singh MK, Plotnikova OV,
Tikhmyanova N, Little JL, Serebriiskii IG, Seo S, Kurokawa M,
Egleston BL, Klein-Szanto A, et al: NEDD9 promotes oncogenic
signaling in mammary tumor development. Cancer Res. 69:7198–7206.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim M, Gans JD, Nogueira C, Wang A, Paik
JH, Feng B, Brennan C, Hahn WC, Cordon-Cardo C, Wagner SN, et al:
Comparative oncogenomics identifies NEDD9 as a melanoma metastasis
gene. Cell. 125:1269–1281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kondo S, Iwata S, Yamada T, Inoue Y,
Ichihara H, Kichikawa Y, Katayose T, Souta-Kuribara A, Yamazaki H,
Hosono O, et al: Impact of the integrin signaling adaptor protein
NEDD9 on prognosis and metastatic behavior of human lung cancer.
Clin Cancer Res. 18:6326–6338. 2012. View Article : Google Scholar : PubMed/NCBI
|