Introduction
Microglia has been categorized as quiescent ramified
microglia with small round cells and numerous branching processes,
ameboid or reactive microglia devoid of branching processes,
macrophages and perivascular microglia (1). They can be intrinsic to the central
nervous system (CNS) as resident microglia, or blood-borne from the
monocytic phagocyting system of the blood. Both derive from the
yolk sac, the resident microglia earlier and later the cells
destined to bone marrow for hematopoiesis and migrating to the CNS
crossing the blood-brain barrier (BBB) in case of lesions.
In normal CNS, resident microglia is 5–10% of cells
(2,3).
Microglia/macrophages increase in low-grade gliomas (LGG) (4), especially in pilocytic astrocytomas
(5), and progressively with the
glioma grades (6), reaching 30% of
cells in high grade gliomas (HGG), closely correlating with
vascular density (7–11), developing around and inside the tumors
and clustering around vessels and necroses.
Contrary to what is known from other CNS diseases
and from microglia assimilation to peripheral macrophages,
glioma-associated M/M (GAMs) are today prevailingly interpreted as
favoring glioma cell migration and growth (12–15). GAM
polarization into the two functional profiles M1 (classically
activated, pro-inflammatory) and M2 (alternatively activated,
immunosuppressive) has long been discussed (12,13–19).
Recently, GAMs have been shown to overlap only partial with the M1
and M2 phenotypes (20). Glioma cells
would establish an immunosuppressive tumor environment, promoting
GAM recruitment and proliferation, polarizing them toward the M2
phenotype and remaining unaffected by their phagocyting and
anti-tumorigenic functions (16,19–29). In
this way they would be ‘friends’ and not ‘foes’ to gliomas
(16,30). GAMs are recruited by glioma cells
through chemoattractant factors and, in turn, they stimulate tumor
growth (26,31,32). Both
glioma cells and GAMs promote angiogenesis (33,34).
In tissue, four antibodies are currently used to
demonstrate GAMs, i.e., allograft inflammatory factor 1 (Iba1),
CD16, CD68, and CD163. They reveal different antigens with
different final reaction products (FRP) and with different
localization in the cells: In cytoplasms for Iba1, in lysosomes for
CD68 and on cell membranes for CD16 and CD163. Various GAM forms
can be demonstrated, going from ramified microglia to granular
macrophage-like cells, which correspond to different antigen
expression. Given the uncertainties still existing on the
significance of GAMs, one wonders to which stimuli so various cell
forms and immunohistochemical behaviors respond in the different
tumor districts. The aim of this work is to clarify their
functional significance after the systematic study of a broad
series of human gliomas and to interpret them on the basis of the
information available on their functions.
Materials and methods
Brain tumor specimens
The study has been carried out on 98 adult glioma
specimens from our archive and operated on at different
Neurosurgery Units of the Piedmont Region and at the Istituto
Neurologico ‘Carlo Besta’ IRCCS Foundation, Milan, Italy. The
series was composed of 3 pilocytic astrocytomas, 20 diffuse
astrocytomas (16 isocitrate dehydrogenase (IDH)-mutant and 4
IDH-wild-type), 4 anaplastic astrocytomas (1 IDH-mutant and 3
IDH-wild-type), 26 oligodendrogliomas (20 IDH-mutant and
1p/19q-codeleted and 6 not otherwise specified (NOS)), 10
anaplastic oligodendrogliomas (6 IDH-mutant and 1p/19q-codeleted
and 4 NOS) and 35 glioblastomas (GBs), IDH-wild-type. The
histological diagnosis was made according to the current World
Health Organization (WHO) guidelines (35). As controls, 10 tumor-free tissue
samples were used. Surgical tumor samples were formalin fixed,
paraffin embedded (FFPE) and cut in 5 µm-thick sections. The study
was in compliance with the local institutional review board and
Committee on Human Research and with the ethical human subject
principles of the World Medical Association Declaration of Helsinki
Research. Written informed consent of patients was obtained after
the Ethics Committee approval.
Immunohistochemistry (IHC)
Beside haematoxylin and eosin (H&E) staining,
immunohistochemical analyses were performed using a Ventana Full
BenchMark® XT automated immunostainer (Ventana Medical
Systems Inc., Tucson, AZ, USA) and the UltraView™ Universal DAB
Detection Kit (Ventana Medical Systems Inc.) as detection system.
Heat-induced epitope retrieval (HIER) was performed in Tris-EDTA,
pH 8. Primary antibodies are listed in Table I. Double immunostainings for
c-MAF/CD68, c-MAF/CD163, Iba1/CD34 and CD68/CD34 were performed
with the ultraView™ Universal Alkaline Phosphatase Red Detection
Kit (Ventana). Negative controls were obtained by omitting the
primary antibodies. Observations were made on a Zeiss Axioskop
fluorescence microscope (Carl Zeiss, Oberkochen, Germany) equipped
with an AxioCam5 MR5c and coupled to an Imaging system (AxioVision
Release 4.5; Carl Zeiss).
 | Table I.List of primary antibodies used for
immunohistochemistry. |
Table I.
List of primary antibodies used for
immunohistochemistry.
| Antibody
(Clone) | Source | Dilution | Code | Manufacturer |
|---|
| Ki-67 (MIB-1) | Mouse | 1:100 | M7240 | Dako |
| GFAP | Mouse | 1:200 | M0761 | Dako |
| CD34 | Mouse | Pre-diluted | 790–2927 | Ventana |
| Iba1 | Rabbit | 1:500 | 019–19741 | Wako Chemicals |
| CD68 (KP-1) | Mouse | Pre-diluted | 790–2931 | Ventana |
| CD16 (SP175) | Rabbit | Pre-diluted | 760–4863 | Ventana |
| CD163(MRQ-26) | Mouse | Pre-diluted | 760–4437 | Ventana |
| c-MAF (M-153) | Rabbit | 1:50 | sc-7866 | Santa Cruz
Biotech. |
| CD45 (LCA) | Mouse | Pre-diluted | 760–2505 | Ventana |
| CD11b | Rabbit | 1:100 | AB52478 | Abcam |
| CD98 | Rabbit | 1:100 | AB108300 | Abcam |
Molecular genetics
Genomic DNA (gDNA) from FFPE tumor samples was
isolated using the QIAamp DNA Mini kit (Qiagen NV, Venlo, The
Netherlands). Search for somatic point mutations in IDH1
Arg132 (exon 4) (GenBank sequence NM_005896) and IDH2 Arg172
(exon 4) (GenBank sequence NM_002168) hot-spot codons was performed
by Sanger direct sequencing on an ABI® 3130 Genetic
Analyzer (Thermo Fisher Scientific, Inc., Waltham, MA, USA), as
published (36). The 1p/19q
chromosomal status was assessed by Multiplex Ligation-dependent
Probe Amplification (MLPA) using the SALSA-MLPA Kit P088-B2
(MRC-Holland, Amsterdam, The Netherlands), according to the
manufacturer's instructions. Fragment analysis was performed on an
ABI® 3130 Genetic Analyzer (Thermo Fisher Scientific,
Inc.).
GAM frequency and survival
analysis
The frequency of GAMs was quantified by counting the
number of immunopositive cells for each antibody and for each cell
form (see Results) in five randomly selected microscopic high power
fields (HPF) at ×400 magnification per section and by calculating
the mean values. Only cells were counted the nucleus of which was
visible in counterstained sections. The number of GAMs was compared
with the three grades (II–IV) of malignancy. CD163
immunohistochemical expression was evaluated according to the
average frequency of positive cells (<10, 20–50, 50–100,
>100) and distribution (perivascular or scattered in the
parenchyma) after examining 5 randomly selected fields per
tumor section at ×400 high-power magnification, with a
semi-quantitative scoring system including four categories: 0, 1, 2
and 3 (Table II) and compared with
the overall survival (OS) of patients. OS was defined as the time
from date of diagnosis until the time of death or last follow-up of
the patient. The comparison was made also by scoring the CD163
staining in two categories of expression: Low (including the above
0 and 1 categories) and high (including the above 2 and 3
categories) (Table II). The
correlation between CD163 expression and OS was investigated in the
tumor series also after stratification for IDH1/2 mutation
status.
 | Table II.Four and two-score system for
evaluation of CD163 immunostaining. |
Table II.
Four and two-score system for
evaluation of CD163 immunostaining.
| Score | Frequency of
positive cells (per ×400 HPF) | Distribution |
|---|
| 0a | <10 | Only
perivascular |
| 1a | 20–50 | Perivascular and
scattered in the parenchyma |
| 2b | 50–100 | Perivascular and
scattered in the parenchyma |
| 3b | >100 | Perivascular and
scattered in the parenchyma |
Statistical analysis
The correlation between tumor grade and
immunohistochemical expression of CD68 or CD163 was analyzed by
investigating differences among the groups with a one-way ANOVA and
Tukey's post hoc test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Survival curves were estimated using the Kaplan-Meier method and
comparison between them were performed by the log-rank test
(Mantel-Cox). Data were analyzed with the SPSS v23.0 software
(SPSS, Inc., Chicago, IL, USA).
Results
Immunohistochemical analysis
Four groups of GAM forms were identified
immunohistochemically, that differed not only for positive or
negative staining, but also for staining intensity: i) normal
ramified microglia with a small elongated nucleus and polar
processes with a radial or longitudinal branching; ii) reactive
microglia (RM); iii) cells with a roundish and granular macrophagic
form (M); and iv) cells of intermediate form (IF), with very thick
and short processes, or simply roundish bumpy cell (BC) forms. The
first two cell types stained prevailingly with Iba1 and CD16, the
third group with CD68 and CD163 and the last group showed a
variable staining with the 4 antibodies.
Normal cortex. Iba1 stained diffusely 10–15 cells
per ×400 HPF with thin processes and the typical branching
(Fig. 1A). CD68 revealed 7–8 cells
per ×400 HPF with a FRP of fine granules in the cytoplasms and/or
short processes. CD16 showed the same finding as Iba1. Ischemic
neurons showed CD16-positive nuclei with a round or elongated form.
CD163 was almost negative.
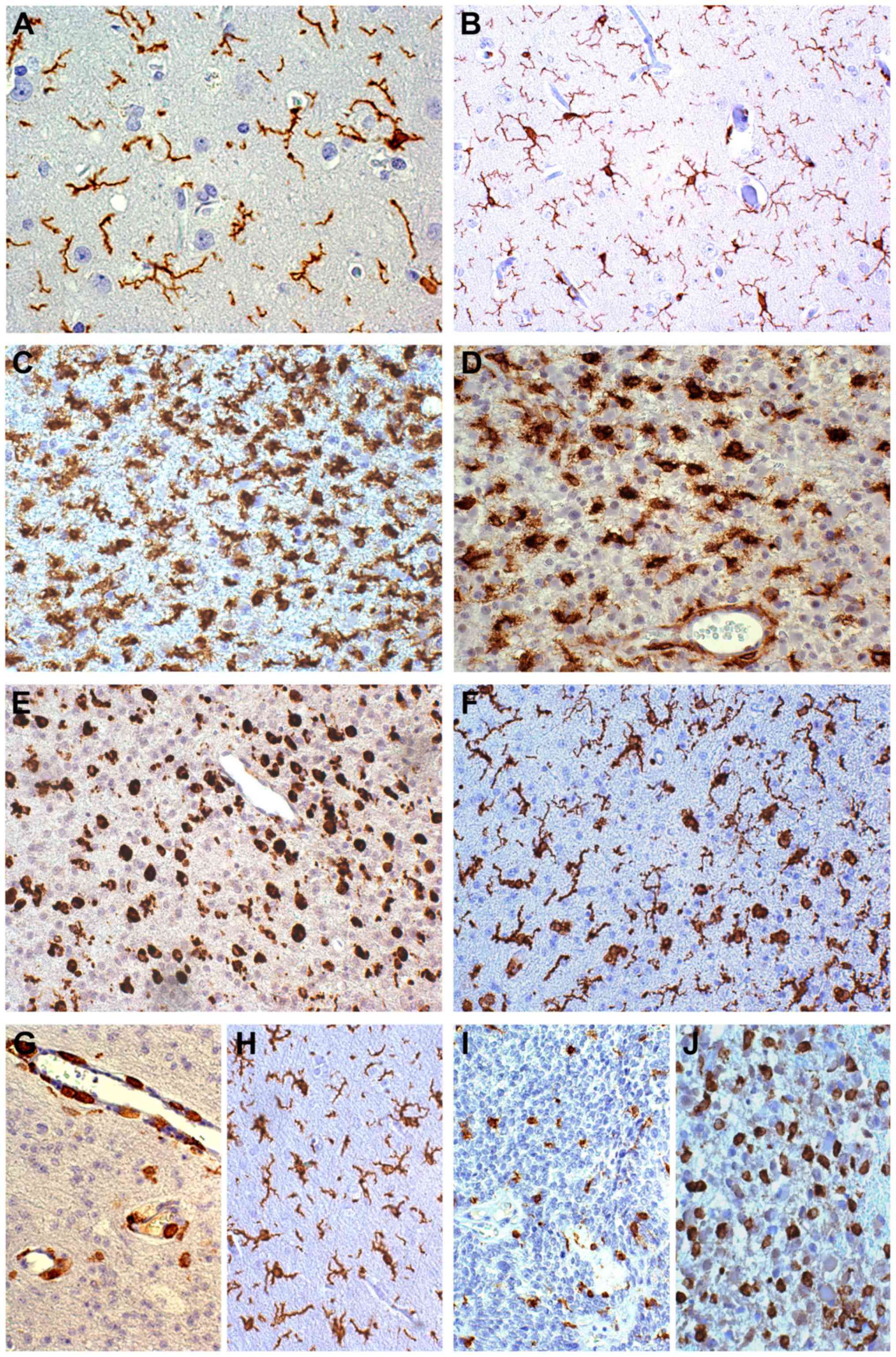 | Figure 1.Immunohistochemical characterization
of microglia/macrophages. (A) Resident ramified microglia in the
normal cortex, Iba1 staining, ×400. (B) Diffuse astrocytoma.
Reactive ramified microglia in mild tumor infiltration, Iba1
staining, ×200. (C) Diffuse astrocytoma. Reactive ramified
microglia in high tumor infiltration, Iba1 staining, ×200. (D)
Diffuse astrocytoma. Intermediate and macrophagic forms, CD16
staining, ×200. (E) Oligodendroglioma. Cells with a macrophage-like
aspect in solid tumor, CD68 staining, ×200. (F) Oligodendroglioma.
Reactive ramified microglia and macrophagic forms, Iba1 staining,
×200. (G) Oligodendroglioma. CD163+ cells were mainly
found around vessels, CD163 staining, ×200. (H) Oligodendroglioma.
In MIA and HIA Iba1+ RM did not crowd around small
vessels, Iba1 staining, ×200. (I) Anaplastic oligodendroglioma.
Intermediate and macrophagic forms in solid tumor, Iba1 staining,
×200. (J) Anaplastic astrocytoma. Intermediate and macrophagic
forms in solid tumor, CD163 staining, ×200. All stained with DAB.
Iba1, allograft inflammatory factor 1; DAB, 3,3′-diaminobenzidine;
CD, cluster of differentiation; MIA, mild infiltration area; HIA,
high infiltration area; RM, reactive ramified microglia. |
Gliomas. Gliomas are heterogeneous tumors,
therefore, selected areas with defined cell distribution patterns
were chosen from all the cases for comparison, corresponding to
mild (MIA) and high (HIA) tumor infiltration areas, solid tumor
(ST) and regressive areas (RA). In LGG form types 2 and 4 and in
HGG types 3 and 4 prevailed. In ST and RA of HGG most part of GAMs
were, therefore, positive for CD68 and CD163.
Diffuse astrocytomas. Iba1. In MIA, cells appeared
as elegant RM with thin processes, longer than in normal cortex, in
number of 20–25 positive cells per ×400 HPF (Fig. 1B). In HIA, they increased
progressively with the increase of tumor cell number until to
100–200 cells per ×400 HPF (Fig. 1C).
In ST the number of positive cells decreased to 80–120 cells per
×400 HPF and IF and M phenotypes with and without processes
prevailed, not only around vessels, but also scattered in the
tissue.
CD68. In MIA, RM cells with positive granules in the
cytoplasm or short processes occurred in number of 15–20 per ×400
HPF. In HIA, the positive cells increased to 50–100 per ×400 HPF
with coarser granules and broader short branching. They assumed the
IF or M aspects approaching ST. In the latter, the density of
positive cells decreased to 40–50 per ×400 HPF, assuming
prevailingly the roundish form of M. Perivascular M clusters could
be observed with a frequency depending on the vessel density as
well as rare scattered forms. Some IF and most M forms stained more
intensely than with Iba1.
CD16. The distribution was similar to that of Iba1
(Fig. 1D). Ischemic neurons entrapped
in the tumor appeared as ball-like positive figures.
CD163. Positive cells with a M-like or with an IF or
BC appearance were rare and mostly with an intraluminal or
perivascular localization; positive cells scattered in ST were even
rarer. CD163 was positive only in a quota of CD68+ cells.
Oligodendrogliomas. The tumor growth could be
diffuse or nodular. In the first type, GAMs were diffusely
increased in number, whereas in the second one GAMs were limited to
a thin strip immediately close to the tumor and in ST they were
less numerous and mainly IFs or BCs. The findings were similar to
those of diffuse astrocytomas, but with a great difference between
MIA and HIA: 30–40 vs. > 200 positive cells. In ST (nodular
growth), IFs and M forms (Fig. 1E)
prevailed on RM phenotype (Fig. 1F),
both in perivascular position and scattered in the tissue. CD16 and
CD163 behaved as in diffuse astrocytomas. In MIA and HIA most
CD163+ cells are perivascular (Fig. 1G). In the same areas, Iba1+
RM did not crowd around small vessels (Fig. 1H).
Anaplastic astrocytomas and oligodendrogliomas. The
only difference was that in astrocytomas there was no increase of
vessels and, therefore, the number of perivascular M cells did not
vary; in anaplastic oligodendrogliomas the vessels increased as
well as microvascular proliferations and M forms and IFs increased
correspondingly (Fig. 1I). In grade
III gliomas an increase of CD163-positive cells was observed
(Fig. 1J).
Pilocytic astrocytomas. GAMs showed the same
frequency as in diffuse and anaplastic astrocytomas with a
prevalence of M forms and IFs.
Glioblastomas. In MIA and HIA the findings were
similar to those described in II and III grade gliomas. In ST the
number of RM cells strongly reduced and
Iba1+/CD16+ IFs and mainly M forms increased
(Fig. 2A), especially in perinecrotic
or perivascular clusters as CD163+ cells (Fig. 2B). Iba1+ M forms can show a
density >200 cells per ×400 HPF, representing >40% of tumor
cells (Fig. 2C). In the transition
from ST to RA, M-like forms increased. They stained more intensely
with CD68 and CD163 (Fig. 2D) than
with Iba1 and CD16. In necroses larger than the microscopic field
at ×400 HPF, almost all cells could be represented by M forms
reaching, therefore, the 100% of cells. IFs stained more intensely
with Iba1 and CD16 whereas M forms with CD68 and CD163. GAMs
crowded in glomerular structures (Fig.
2E). M cells showed in variable number a nuclear positivity for
c-MAF, and this was well evident in c-MAF/CD163 (Fig. 2F) and c-MAF/CD68 double
immunostainings.
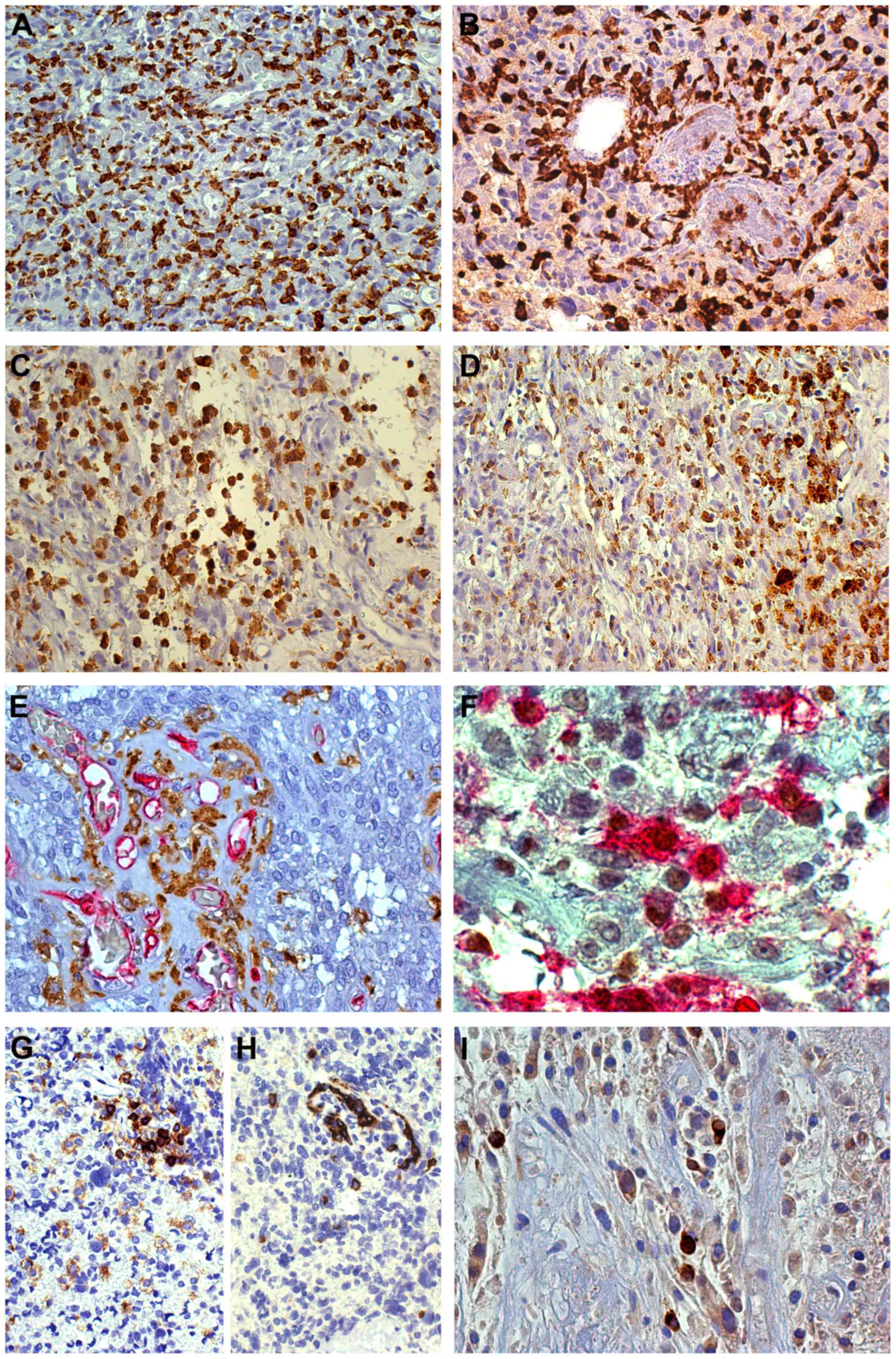 | Figure 2.Immunohistochemical characterization
of microglia/macrophages. (A) GB; High frequency of
CD16+ intermediate forms in solid tumor, CD16 staining,
×200, DAB. (B) GB; Perivascular CD163+ macrophagic
forms, CD163 staining, ×200, DAB. (C) GB; Iba1+ macrophagic forms,
Iba1 staining, ×200, DAB. (D) GB; Passage from a solid tumor to a
regressive area: CD163+ cells are rare in the former and strongly
positive and frequent as macrophagic forms in the latter, CD163
staining, ×200, DAB. (E) GB; Crowding of GAMs in glomerular
structures, Iba1 (DAB) and CD34 (RED) stainings, ×200. (F) GB;
Macrophagic forms with c-MAF+ nuclei and
CD163+ cytoplasms, c-MAF (DAB) and CD163 (RED)
stainings, ×630. (G) GB; Perivascular CD45+ M forms,
CD45 staining, ×200, DAB. (H) GB; Perivascular CD163+ M
forms, CD163 staining, ×200, DAB. (I) GB; CD98+
macrophage-like cells, CD98 staining, ×400, DAB. CD, cluster of
differentiation; DAB, 3,3′-diaminobenzidine; HPF, high power
fields; GB, Glioblastoma; Iba1, allograft inflammatory factor 1;
GAMs, glioma-associated microglia/macrophages. |
CD11b and CD45. The former was weakly expressed in a
quota of GAMs, whereas the latter was positive in a quota of
CD68+ and CD163+ cells. Roughly
CD45+ cells corresponded to CD163+ cells
(Fig. 2G, H).
In some GBs, CD98 was co-expressed with GFAP in
scattered cells in ST areas (Fig.
2I).
Correlation of GAMs with histological
grade and with survival
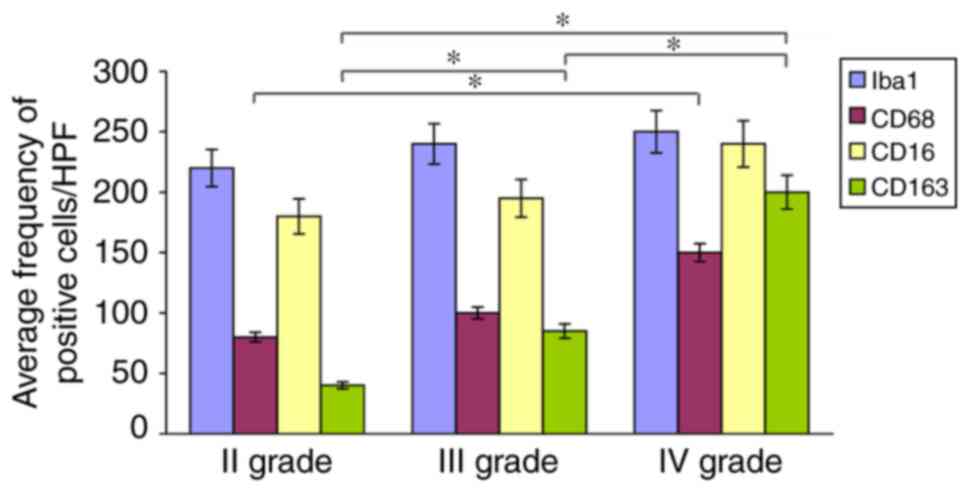
Iba1+ and CD16+ cells
prevailed in LGG, whereas CD68+ and CD163+
cells prevailed in HGG. If all GAM forms (RM, BC, IF and M),
positive to one of the 4 antibodies were considered, there was no
correlation with the histologic grade, because the high number of
RM in LGG paralleled that of M forms in HGG. Iba1+ cells
did not show a significant difference in number among WHO II–IV
grade gliomas, nor CD16+ cells. On the contrary,
expression levels of CD68+ and especially
CD163+ cells increased with malignancy grade with a
statistically significant difference between II and IV grade
(Fig. 3). CD163-positive BC and M
were definitely in a higher number in HGG than in LGG.
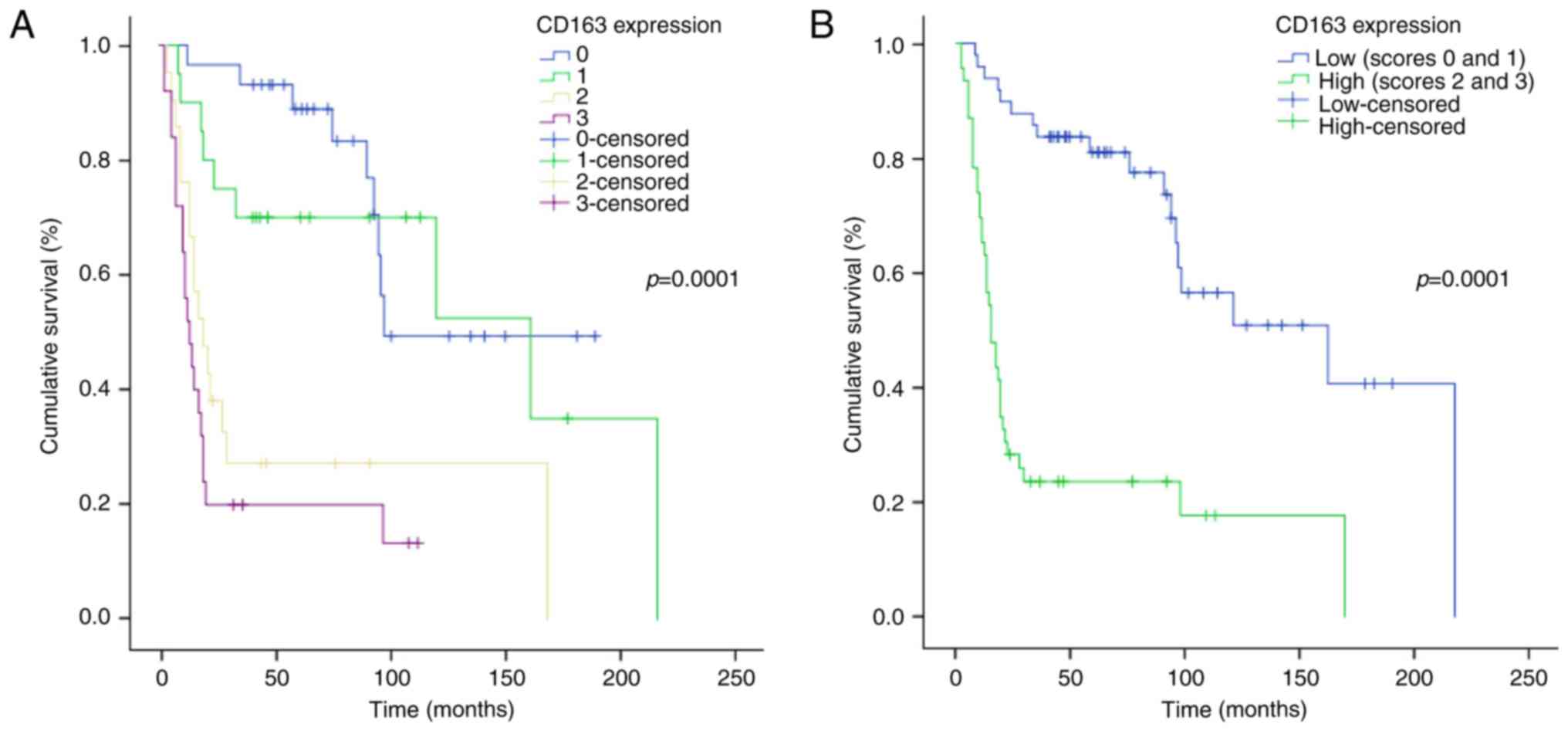
As for survival analysis, considering the whole
series of gliomas (WHO grade II–IV), CD163 immunohistochemical
expression significantly correlated with a shorter OS for patients,
both applying the 4-score system (log-rank test, P=0.0001) and
using a 2-score system (log-rank test, P=0.0001) for CD163
(Fig. 4A and B).
Evaluating the correlation between OS and CD163
within each single tumor type and grade, no statistically
significant correlation was found. Also considering the group of II
grade tumors (astrocytomas and oligodendrogliomas), no correlation
emerged, whereas a significant correlation was found in the group
of III grade gliomas, astrocytomas + oligodendrogliomas (log-rank
test, P=0.028) (Fig. 5A). Considering
separately astrocytomas (II and III grade) and oligodendrogliomas
(II and III grade), a significant correlation come out (log-rank
test, P=0.007 and P=0.008, respectively) (curves not shown). These
findings suggested that CD163, evaluated on the whole series of
grade II–IV gliomas, has a prognostic value, however, completely
absorbed by that of histological grade.
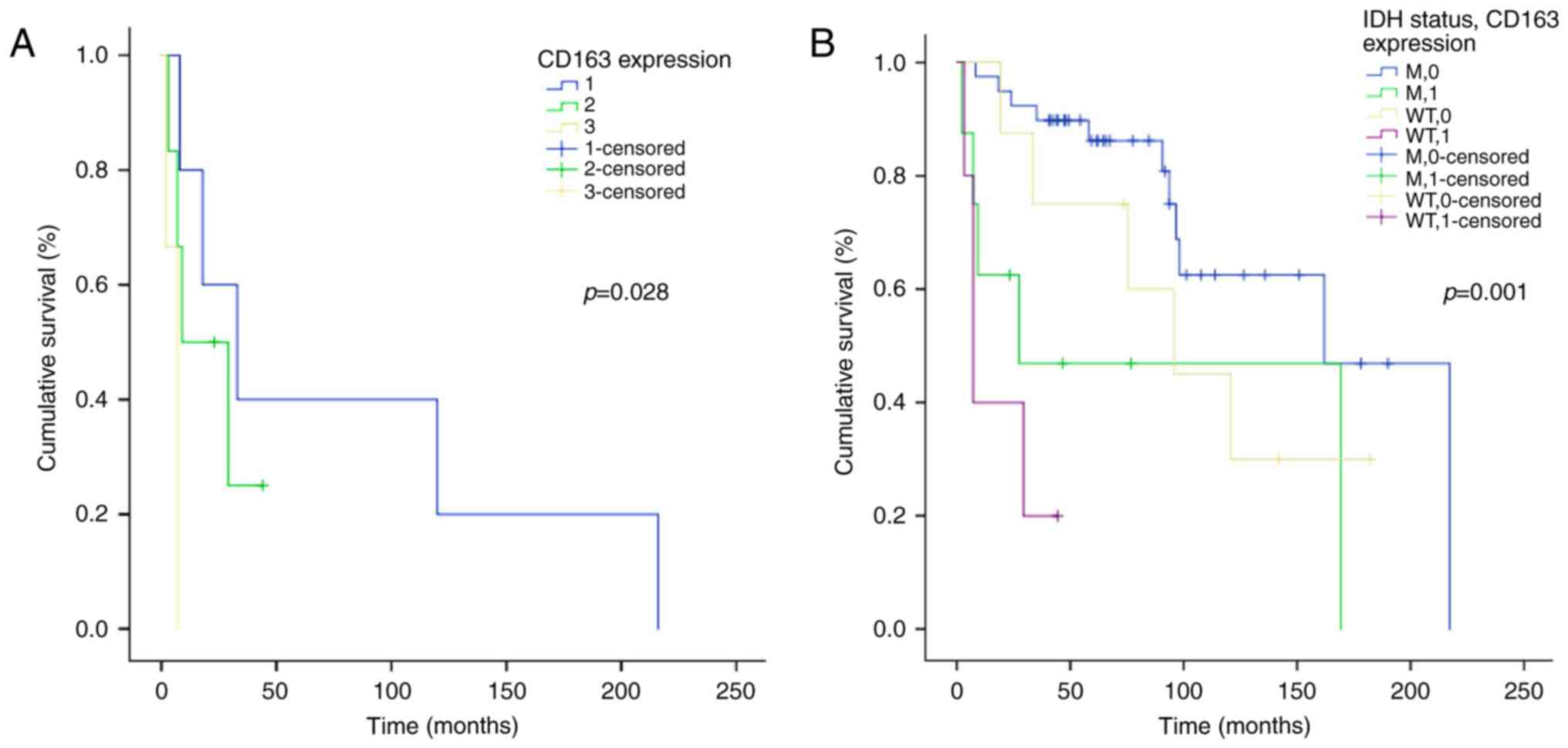 | Figure 5.Kaplan-Meier survival analysis
according to the CD163 immunohistochemical expression. (A)
Correlation of overall survival (OS) with CD163 levels according to
a four-category (0, 1, 2, 3) scoring system in WHO III grade glioma
patients. (B) Correlation of OS with the IDH gene status and the
CD163 expression (0=low, 1=high) in 60 patients of WHO II–III grade
gliomas. The log-rank test was performed to determine statistical
significance, and P<0.05 was considered significant. CD, luster
of differentiation; M, mutated; WT, wildtype; WHO, World Health
Organization; IDH, isocitrate dehydrogenase. |
After stratification of patients for IDH1/2 mutation
status, no significant correlation between OS and CD163 was found
within the LGG series, whereas including III grade tumors a
significant correlation was observed, but it reflected that of the
histological grade (log-rank test, P=0.001) (Fig. 5B). It is to be remarked that in our
series IDH-wild-type II and III grade tumors were very few.
Microglia relationship with the tumor
vasculature
In MIA and HIA of LGG, where only in
oligodendrogliomas an increase of small vessels occurred in
comparison with normal tissue, RM forms tremendously increased,
without crowding on single vessels, but showing a high frequency in
areas rich in vessels. On the contrary, adherence of single
microglia cells to capillaries was frequent. In HGG, GAMs
accumulated around vessels either as M forms or IFs and BCs or as
unidentifiable forms, because secondary to pathoplastic influences,
such as those of cells stretched around vessels.
Discussion
The greatest information about microglia and GAMs
derives from studies on myeloid cells in tissues, in vitro
cultures, in animal experimentation and recently from the
Two-photon laser scanning microscopy (TPLSM) (37–39). The
GAM types identified by the four relevant antibodies, more or less
correspond to those described in GL-261 glioma model by TPLSM
(38). In infiltration areas, there
was no doubt on the origin of RM from resident microglia (27), more intensely stained with Iba1 and
CD16 than with CD68 and CD163 and almost no macrophage was present
(33).
The transition from HIA to ST was marked by an
almost disappearance of RM forms, that could be reduced, destroyed
or switched to serve a different function. In ST, IFs with thick
short processes or BCs and round M forms without processes were
found, but in a less number than RM of infiltration areas, with the
exception of their clustering around necroses or in perivascular
position. They stained more intensely with CD68 and CD163. A quota
of M forms were CD45high (11,19,40) and
stained with c-MAF (41) and roughly
corresponded to CD163+ cells. They correspond,
therefore, to blood-borne macrophages, unless resident microglia
upregulates CD45 becoming thus responsible for most macrophages in
gliomas (27). IFs and BCs can
originate either from resident microglia or represent blood-borne
macrophages, except that they correspond to the phagocyting cells
described by TPLSM (38). In HGG,
CD68+ and CD163+ GAMs with M-like morphology prevailed.
Contrary to the impression that they represent an immune or
cytotoxic defense against the tumor, a pro-tumor function has been
attributed to these cells, recruited and stimulated to proliferate
by the glioma cells through TGF-β, prostaglandine E2,
CCL2, CX3CL1 and colony stimulating factor 1 (CSF-1) (23,26,42). Where
the boundary between tumor and normal tissue was sharp, RM cells
were limited to a narrow peritumor strip and this supports the idea
that migrating neoplastic cells are the main motive of RM
proliferation.
In HGG, despite GAMs show complement (CR1, CR3, CR4)
and Fc-γ receptors, their phagocytic capacity is considered reduced
(23), based on the relief that
CR3+ microglia cells were never observed to kill glioma
cells (43), but correlated with
tumor proliferation rate (44). GAMs
don't express cytokines for tumoricidal activity (21); in contrast, glioma-released factors
induce in microglia cells, via Toll-like receptors (TLRs), the
expression of membrane type 1 matrix metalloprotease (MT1-MMP) that
promotes glioma expansion (12,45).
From a neuropathological point of view, one wonders
what else CD68+ and CD163+ perinecrotic and
perivascular cells could be if not cells phagocyting tumor debris
in regressive areas. The same could be true for macrophagic forms
scattered in tumor proliferating areas. Here apoptosis occurs
correlating with mitoses (46) and
its decomposition products are known to be phagocyted in few hours
by macrophages (47).
M2 polarized cells are deduced to represent the
greatest part of GAMs, independently of their phenotypes, including
the rare CD163+ M forms of LGG and the tremendous amount
of them in HGG, where it could be secondary to the BBB breakdown.
Were it not for the increase of this cell component in GB, it could
not be said that GAMs increase in HHG in comparison with LGG, for
the great richness of the latter in RM forms. It was observed that
M2 GAMs stain by CD163 (48–51), or express c-MAF (41), not exclusively, but prevailingly. We
confirm this findings, but, at the same time, we remark that the
number of CD11b+ cells was too low, so that it could indicate only
a quota of cells originating from resident microglia.
The relationship between vessels and GAMs is
controversial. They increased near vessels or in infiltration areas
in LGG and cluster in perivascular spaces in HGG, but, even if
showing direct contacts with capillaries in both tumor types, they
did not crowd around the small vessels in infiltration areas. In
LGG, the finding of intra- and perivascular GAMs depended on the
vessel number, that was higher in oligodendroglioma than in
astrocytoma and, together with GAMs scattered in the parenchyma,
prevailed in solid tumor upon its periphery. It has been reported
that VEGF produced by glioma cells has a chemotactic effect on
them, inducing their migration and proliferation in vitro
(52) and in vivo (53). GAMs are attracted by vessels and, in
turn, promote angiogenesis (54,55). It
must be remarked that perivascular IFs and M forms are mainly
CD163+.
Despite the literature considers CD16 as indicating
M1 (56) and CD163 M2 (31,48–50,57–59)
polarization profile, IFs and BCs, independently of their origin
from resident microglia or blood-borne macrophages, are not
strictly recognizable as M1 or M2 polarized. It has been rather
hypothesized a continuum M1-M2 functional state with the
possibility that, upon different stimulations (60), both or neither one is expressed by
GAMs (18–20,26). It
must be considered that microglia cells undergo rapid changes
(38), passing cell forms quickly
into one another under the influence of the microenvironment;
moreover, in compact tumor areas they can be mechanically
modified.
The tumorigenetic function of GAMs is supported
in vitro by the interaction with glioblastoma stem cells
(GSCs), that induce in them an immunosuppressive phenotype
(31); GAMs, in turn, enhance the
invasiveness of the glioma initiating cells (GICs) (32). In GB, an indirect proof is the
correlation of GAMs not only with Ki-67/MIB-1, but also with the
stem cell markers Nestin, SOX2 and CD133 (61). Recently, much attention is given to
the relationship between GAMs, GSCs and vessels, through CCL2/CCR2,
CXCL12/CXCR4, CX3CL1/CX3CR1, CSF-1 and periostin signalings;
however, our knowledge on the relations between microglia, tumor
cells and other components cannot be considered exhaustive
(26).
Finally, in GB, it has been hypothesized a fusion
between tumor cells and macrophages, denounced by CD98 positivity
(62). This would be in line with the
phagocytic capacity maintained in glioma cell lines and fetal rat
normal glia (63,64). In our GBs, one wonders whether
CD98+ cells are nothing else than macrophages that
phagocyted tumor cells, as it happens in non-neoplastic conditions,
for example, where GFAP and myelin debris can be found in
macrophages.
The inter-grade analysis didn't show a correlation
of GAMs with survival if all the microglia forms demonstrated by
the four antibodies were considered, because in LGG RM forms can
reach the same frequency as M in HGG (4). The correlation with the tumor grade and
with survival came out if only CD68+ and
CD163+ M forms were considered. In GB, however,
CD163+ M forms could not be directly related to
malignancy, because they are associated to tumor structures that
are typical of the malignant phenotype. The occurrence of
CD163+ M forms in perivascular position should mean that
they derived from the peripheral blood, because of the BBB
disrupture, which already by itself points out malignancy.
CD163+ M or IF forms may have different interpretations;
it is known that they increase from II to IV tumor grade.
Considering together II grade astrocytomas and oligodendrogliomas,
no correlation of CD163 expression with OS was found, whereas, in
the group of III grade gliomas, a correlation was evident
(P=0.028). Also putting together II and III grade astrocytomas or
II and III grade oligodendrogliomas, a significant correlation was
found. This is quite comprehensible in oligodendrogliomas, where an
increase of vessels occurs, but not in astrocytomas, where the
number of vessels does not increase. IDH status does not seem to
have any correlation with GAMs in II grade gliomas; considering
together II and III grade tumors a correlation came out, but also
in this case it can be attributed to the short survival of
anaplastic oligodendrogliomas.
It must be remarked that the number of
CD163+ cells was higher than that of CD45+
cells; this means that M2 polarization could be independent of the
origin of microglia.
Today most observations on GAMs demonstrate their
favouring function on tumor progression. This conclusion is mainly
derived from experiments on animal models and in vitro
cultures. In the neuropathological practice, however, uncertainty
still exists and the evaluation of GAMs must be given directly on
tumor tissue and be part of the prognosis/diagnosis. The answer to
the question if GAMs are ‘friends’ or ‘foes’ cannot be so sharp as
in experimental condition, also because the increase of
CD163+ forms might be secondary and not primary to
malignancy.
Acknowledgements
This study was supported by Cassa di Risparmio di
Vercelli Foundation, Vercelli, Italy.
Glossary
Abbreviations
Abbreviations:
|
BBB
|
blood-brain barrier
|
|
BC
|
bumpy cell
|
|
CD
|
cluster of differentiation
|
|
CNS
|
central nervous system
|
|
FFPE
|
formalin fixed, paraffin embedded
|
|
FRP
|
final reaction products
|
|
GAMs
|
glioma-associated
microglia/macrophages
|
|
GB
|
glioblastoma
|
|
gDNA
|
genomic DNA
|
|
GICs
|
glioma initiating cells
|
|
H&E
|
haematoxylin and eosin
|
|
HGG
|
high grade gliomas
|
|
HIA
|
high infiltration area
|
|
HIER
|
heat-induced epitope retrieval
|
|
HPF
|
high power fields
|
|
Iba1
|
allograft inflammatory factor 1
|
|
IDH
|
isocitrate dehydrogenase
|
|
IF
|
intermediate form
|
|
IHC
|
immunohistochemistry
|
|
LGG
|
low-grade gliomas
|
|
M
|
macrophagic form
|
|
MIA
|
mild infiltration area
|
|
MM
|
microglia/macrophages
|
|
NOS
|
not otherwise specified
|
|
RA
|
regressive area
|
|
RM
|
reactive ramified microglia
|
|
ST
|
solid tumor
|
|
TLRs
|
Toll-like receptors
|
|
TPLSM
|
two-photon laser scanning
microscopy
|
|
WHO
|
World Health Organization
|
References
|
1
|
Graeber MB and Streit WJ: Microglia:
Biology and pathology. Acta Neuropathol. 119:89–105. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawson LJ, Perry VH, Dri P and Gordon S:
Heterogeneity in the distribution and morphology of microglia in
the normal adult mouse brain. Neuroscience. 39:151–170. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kettenmann H, Hanisch UK, Noda M and
Verkhratsky A: Physiology of microglia. Physiol Rev. 91:461–553.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simmons GW, Pong WW, Emnett RJ, White CR,
Gianino SM, Rodriguez FJ and Gutmann DH: Neurofibromatosis-1
heterozygosity increases microglia in a spatially-and
temporally-restricted pattern relevant to mouse optic glioma
formation and growth. J Neuropathol Exp Neurol. 70:51–62. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klein R and Roggendorf W: Increased
microglia proliferation separates pilocytic astrocytomas from
diffuse astrocytomas: A double labeling study. Acta Neuropathol.
101:245–248. 2001.PubMed/NCBI
|
|
6
|
Komohara Y, Ohnishi K, Kuratsu J and
Takeya M: Possible involvement of the M2 anti-inflammatory
macrophage phenotype in growth of human gliomas. J Pathol.
216:15–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shinonaga M, Chang CC, Suzuki N, Sato M
and Kuwabara T: Immunohistological evaluation of macrophage
infiltrates in brain tumors. Correlation with peritumoral edema. J
Neurosurg. 68:259–265. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roggendorf W, Strupp S and Paulus W:
Distribution and characterization of microglia/macrophages in human
brain tumors. Acta Neuropathol. 92:288–293. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishie A, Ono M, Shono T, Fukushi J,
Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, et al:
Macrophage infiltration and heme oxygenase-1 expression correlate
with angiogenesis in human gliomas. Clin Cancer Res. 5:1107–1113.
1999.PubMed/NCBI
|
|
10
|
Badie B and Schartner JM: Flow cytometric
characterization of tumor-associated macrophages in experimental
gliomas. Neurosurgery. 46:957–962. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watters JJ, Schartner JM and Badie B:
Microglia function in brain tumors. J Neurosci Res. 81:447–455.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Markovic DS, Vinnakota K, Chirasani S,
Synowitz M, Raguet H, Stock K, Sliwa M, Lehmann S, Kälin R, van
Rooijen N, et al: Gliomas induce and exploit microglial MT1-MMP
expression for tumor expansion. Proc Natl Acad Sci USA. 106:pp.
12530–12535. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhai H, Heppner FL and Tsirka SE:
Microglia/macrophages promote glioma progression. Glia. 59:472–485.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaminska B: Microglia in Gliomas: Friend
or Foe?Glioma Cell Biology. Sedo A and Mentlein R: Springer-Verlag;
Berlin: pp. 241–270. 2014
|
|
15
|
Schiffer D, Mellai M, Bovio E and
Annovazzi L: The neuropathological basis to the functional role of
microglia/macrophages in gliomas. Neurol Sci. 2017. View Article : Google Scholar
|
|
16
|
Gabrusiewicz K, Ellert-Miklaszewska A,
Lipko M, Sielska M, Frankowska M and Kaminska B: Characteristics of
the alternative phenotype of microglia/macrophages and its
modulation in experimental gliomas. PLoS One. 6:e239022011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Durafourt BA, Moore CS, Zammit DA, Johnson
TA, Zaguia F, Guiot MC, Bar-Or A and Antel JP: Comparison of
polarization properties of human adult microglia and blood-derived
macrophages. Glia. 60:717–727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perry VH and Teeling J: Microglia and
macrophages of the central nervous system: The contribution of
microglia priming and systemic inflammation to chronic
neurodegeneration. Semin Immunopathol. 35:601–612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Glass R and Synowitz M: CNS macrophages
and peripheral myeloid cells in brain tumours. Acta Neuropathol.
128:347–362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szulzewsky F, Pelz A, Feng X, Synowitz M,
Markovic D, Langmann T, Holtman IR, Wang X, Eggen BJ, Boddeke HW,
et al: Glioma-associated microglia/macrophages display an
expression profile different from M1 and M2 polarization and highly
express Gpnmb and Spp1. PLoS One. 10:e01166442015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hussain SF, Yang D, Suki D, Aldape K,
Grimm E and Heimberger AB: The role of human glioma-infiltrating
microglia/macrophages in mediating antitumor immune responses.
Neuro Oncol. 8:261–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parney IF, Waldron JS and Parsa AT: Flow
cytometry and in vitro analysis of human glioma-associated
macrophages. Laboratory investigation. J Neurosurg. 110:572–582.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li W and Graeber MB: The molecular profile
of microglia under the influence of glioma. Neuro Oncol.
14:958–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei J, Gabrusiewicz K and Heimberger A:
The controversial role of microglia in malignant gliomas. Clin Dev
Immunol. 2013:2852462013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prinz M, Tay TL, Wolf Y and Jung S:
Microglia: Unique and common features with other tissue
macrophages. Acta Neuropathol. 128:319–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hambardzumyan D, Gutmann DH and Kettenmann
H: The role of microglia and macrophages in glioma maintenance and
progression. Nat Neurosci. 19:20–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Müller A, Brandenburg S, Turkowski K,
Müller S and Vajkoczy P: Resident microglia, and not peripheral
macrophages, are the main source of brain tumor mononuclear cells.
Int J Cancer. 137:278–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou W, Ke SQ, Huang Z, Flavahan W, Fang
X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, et al: Periostin
secreted by glioblastoma stem cells recruits M2 tumour-associated
macrophages and promotes malignant growth. Nat Cell Biol.
17:170–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szulzewsky F, Arora S, de Witte L, Ulas T,
Markovic D, Schultze JL, Holland EC, Synowitz M, Wolf SA and
Kettenmann H: Human glioblastoma-associated microglia/monocytes
express a distinct RNA profile compared to human control and murine
samples. Glia. 64:1416–1436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kennedy BC, Showers CR, Anderson DE,
Anderson L, Canoll P, Bruce JN and Anderson RC: Tumor-associated
macrophages in glioma: Friend or foe? J Oncol 2013. 4869122013.
|
|
31
|
Wu A, Wei J, Kong LY, Wang Y, Priebe W,
Qiao W, Sawaya R and Heimberger AB: Glioma cancer stem cells induce
immunosuppressive macrophages/microglia. Neuro Oncol. 12:1113–1125.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen
L, Xiao HL, Wang B, Yi L, Wang QL, et al: Tumor-associated
microglia/macrophages enhance the invasion of glioma stem-like
cells via TGF-β1 signaling pathway. J Immunol. 189:444–453. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brandenburg S, Müller A, Turkowski K,
Radev YT, Rot S, Schmidt C, Bungert AD, Acker G, Schorr A, Hippe A,
et al: Resident microglia rather than peripheral macrophages
promote vascularization in brain tumors and are source of
alternative pro-angiogenic factors. Acta Neuropathol. 131:365–378.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Coniglio S, Miller I, Symons M and Segall
JE: Coculture assays to study macrophage and microglia stimulation
of glioblastoma invasion. J Vis Exp. 20:1162016.
|
|
35
|
Louis DN, Ohgaki H, Wiestler OD and
Cavenee WK: WHO classification of tumours of the Central Nervous
System. 4th. IARC Press; Lyon: pp. 1–408. 2016
|
|
36
|
Mellai M, Piazzi A, Caldera V, Monzeglio
O, Cassoni P, Valente G and Schiffer D: IDH1 and IDH2 mutations,
immunohistochemistry and associations in a series of brain tumors.
J Neurooncol. 105:345–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kondo S and Okabe S: In vivo two-photon
microscopy of microglia. Methods Mol Biol. 1041:319–335. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bayerl SH, Niesner R, Cseresnyes Z,
Radbruch H, Pohlan J, Brandenburg S, Czabanka MA and Vajkoczy P:
Time lapse in vivo microscopy reveals distinct dynamics of
microglia-tumor environment interactions-a new role for the tumor
perivascular space as highway for trafficking microglia. Glia.
64:1210–1226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nimmerjahn A: Two-photon imaging of
microglia in the mouse cortex in vivo. Cold Spring Harb Protoc.
5:pii: pdb.prot069294. 2012.
|
|
40
|
Greter M, Lelios I and Croxford AL:
Microglia versus myeloid cell nomenclature during brain
inflammation. Front Immunol. 6:2492015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barros MH, Hauck F, Dreyer JH, Kempkes B
and Niedobitek G: Macrophage polarisation: An immunohistochemical
approach for identifying M1 and M2 macrophages. PLoS One.
8:e809082013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
da Fonseca AC and Badie B: Microglia and
macrophages in malignant gliomas: Recent discoveries and
implications for promising therapies. Clin Dev Immunol.
2013:2641242013.PubMed/NCBI
|
|
43
|
Morioka T, Baba T, Black KL and Streit WJ:
Immunophenotypic analysis of infiltrating leukocytes and microglia
in an experimental rat glioma. Acta Neuropathol. 83:590–597. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morimura T, Neuchrist C, Kitz K, Budka H,
Scheiner O, Kraft D and Lassmann H: Monocyte subpopulations in
human gliomas: Expression of Fc and complement receptors and
correlation with tumor proliferation. Acta Neuropathol. 80:287–294.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vinnakota K, Hu F, Ku MC, Georgieva PB,
Szulzewsky F, Pohlmann A, Waiczies S, Waiczies H, Niendorf T,
Lehnardt S, et al: Toll-like receptor 2 mediates microglia/brain
macrophage MT1-MMP expression and glioma expansion. Neuro Oncol.
15:1457–1468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schiffer D: Pathology and
neuroepidemiology of the brain and nervous system. Curr Opin Oncol.
3:449–458. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hochreiter-Hufford A and Ravichandran KS:
Clearing the dead: Apoptotic cell sensing, recognition, engulfment,
and digestion. Cold Spring Harb Perspect Biol. 5:a0087482013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kamper P, Bendix K, Hamilton-Dutoit S,
Honoré B, Nyengaard JR and d'Amore F: Tumor-infiltrating
macrophages correlate with adverse prognosis and Epstein-Barr virus
status in classical Hodgkin's lymphoma. Haematologica. 96:269–276.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zaki MA, Wada N, Ikeda J, Shibayama H,
Hashimoto K, Yamagami T, Tatsumi Y, Tsukaguchi M, Take H, Tsudo M,
et al: Prognostic implication of types of tumor-associated
macrophages in Hodgkin lymphoma. Virchows Arch. 459:361–366. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki
M, Kosuge T, Kanai Y and Hiraoka N: Immune cell infiltration as an
indicator of the immune microenvironment of pancreatic cancer. Br J
Cancer. 108:914–923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Herrera M, Herrera A, Domínguez G, Silva
J, García V, García JM, Gómez I, Soldevilla B, Muñoz C, Provencio
M, et al: Cancer-associated fibroblast and M2 macrophage markers
together predict outcome in colorectal cancer patients. Cancer Sci.
104:437–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Forstreuter F, Lucius R and Mentlein R:
Vascular endothelial growth factor induces chemotaxis and
proliferation of microglial cells. J Neuroimmunol. 132:93–98. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kerber M, Reiss Y, Wickersheim A, Jugold
M, Kiessling F, Heil M, Tchaikovski V, Waltenberger J, Shibuya M,
Plate KH and Machein MR: Flt-1 signaling in macrophages promotes
glioma growth in vivo. Cancer Res. 68:7342–7351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li Y, Liu DX, Li MY, Qin XX, Fang WG, Zhao
WD and Chen YH: Ephrin-A3 and ephrin-A4 contribute to
microglia-induced angiogenesis in brain endothelial cells. Anat Rec
(Hoboken). 297:1908–1918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rymo SF, Gerhardt H, Wolfhagen Sand F,
Lang R, Uv A and Betsholtz C: A two-way communication between
microglial cells and angiogenic sprouts regulates angiogenesis in
aortic ring cultures. PLoS One. 6:e158462011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ding P, Wang W, Wang J, Yang Z and Xue L:
Expression of tumor-associated macrophage in progression of human
glioma. Cell Biochem Biophys. 70:1625–1631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mantovani A, Allavena P and Sica A:
Tumour-associated macrophages as a prototypic type II polarised
phagocyte population: Role in tumour progression. Eur J Cancer.
40:1660–1667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lan C, Huang X, Lin S, Huang H, Cai Q, Wan
T, Lu J and Liu J: Expression of M2-polarized macrophages is
associated with poor prognosis for advanced epithelial ovarian
cancer. Technol Cancer Res Treat. 12:259–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Edin S, Wikberg ML, Dahlin AM, Rutegård J,
Öberg Å, Oldenborg PA and Palmqvist R: The distribution of
macrophages with a M1 or M2 phenotype in relation to prognosis and
the molecular characteristics of colorectal cancer. PLoS One.
7:e470452012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lisi L, Stigliano E, Lauriola L, Navarra P
and Dello Russo C: Proinflammatory-activated glioma cells induce a
switch in microglial polarization and activation status, from a
predominant M2b phenotype to a mixture of M1 and M2a/B polarized
cells. ASN Neuro. 6:171–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Noorani I, Petty G, Grundy PL, Sharpe G,
Willaime-Morawek S, Harris S, Thomas GJ, Nicoll JA and Boche D:
Novel association between microglia and stem cells in human
gliomas: A contributor to tumour proliferation? J Pathol Clin Res.
1:67–75. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
62
|
Takeuchi H, Kubota T, Kitai R, Nakagawa T
and Hashimoto N: CD98 immunoreactivity in multinucleated giant
cells of glioblastomas: An immunohistochemical double labeling
study. Neuropathology. 28:127–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Huysentruyt LC, Akgoc Z and Seyfried TN:
Hypothesis: Are neoplastic macrophages/microglia present in
glioblastoma multiforme? ASN Neuro. 3(pii): e000642011.PubMed/NCBI
|
|
64
|
Bjerknes R, Bjerkvig R and Laerum OD:
Phagocytic capacity of normal and malignant rat glial cells in
culture. J Natl Cancer Inst. 78:279–288. 1987.PubMed/NCBI
|