Cell proliferation and apoptosis are key cellular
events in the development of organisms (1). A number of biological processes, such as
tissue development and homeostasis, require a balance between cell
proliferation and apoptosis, dysregulation of which would result in
different types of human diseases (1). In eukaryotes, the cell cycle consists of
four stages: G1/G0, S, G2 and M. Each stage needs to be monitored
by specific checkpoint to ensure that the genetic information of
the cell is faithfully transmitted to the next generation. The G2/M
checkpoint (DNA damage checkpoint) is an important cell cycle
checkpoint to prevent the cell from entering the mitosis with DNA
damage (2). In other words, cells
will be arrested at G2/M phase when genomic DNA is damaged and
needs to be repaired. All somatic cells proliferate via a mitotic
process determined by successful progression of the cell cycle
(3).
Programmed cell death 5 (PDCD5) is a one of the
members of programmed cell death protein family. The gene
PDCD5, alternatively named TFAR19, was originally
cloned from human leukemia cell line TF-1 (4). This gene is localized on chromosome
19q12-q1311 and spans around 6 kb of genomic DNA that contains 5
introns and 6 exons. The open reading frame of PDCD5 encodes
a 125-aa protein that is highly conserved ranging from yeast to
human (4). PDCD5 is
ubiquitously expressed in different tissues and involved in the
regulation of apoptosis in different cell types (4–8). The
apoptotic potential of PDCD5 may be partially resulted from its
phosphorylation at serine 118 by CK2, which is required for the
nuclear translocation of PDCD5 in response to genotoxic stress
(9,10). Recently, it was shown that PDCD5 is
also an important regulator of the non-apoptotic programmed cell
death (PCD), designated ‘paraptosis’ (11). More recently, it was reported that
PDCD5 also regulates autophagy to protect against cardiac
remodeling (12). Dysregulation of
PDCD5 has been found to be involved in different type of
tumors (13–22). The antitumor activity of PDCD5 has
been also proposed (23–29) and low expression level of PDCD5 has
been suggested to be a prognostic indicator for cancers (30). PDCD5 was also indicated to have the
therapeutic potential in the treatment of rheumatoid arthritis and
other autoimmune diseases because of its inflammatory effects
(31,32). Knockout of PDCD5 can also
protect the brain from ischemic injury by inhibiting the PDCD5-VHL
pathway (33).
PDCD5 is downregulated in the lung adenocarcinoma
patients compared to the healthy controls, which indicates PDCD5 is
a tumor suppressor gene associated with lung cancer (34). Single nucleotide polymorphism in the
PDCD5 gene locus was also found to be associated with
non-small cell lung cancers (35).
Recently, a few important interacting partners of PDCD5 have been
discovered, including Tip60, CK2, CTT, p53, tumor suppressor
protein pVHL and YY1-associated factor 2 (YAF-2) (9,36–41). In the genotoxic conditions, PDCD5
selectively mediates HDAC3 dissociation from p53, and induces HDAC3
degradation through the ubiquitin-dependent proteasomal pathway,
which subsequently activates p53 as a result in response to the
stress (42,43). The promoter activity of PDCD5
is activated by the transcription factor NF-κB p65 (44) and the protein stability of
PDCD5 are positively regulated by YAF2 and OTUD5 (41,45), and
negatively regulated by DNAJB1 (46).
In the present study, we investigate the roles of
PDCD5 in cell proliferation, cell cycle progression and apoptosis
by using a PDCD5 stably overexpressing A431 cell line. We further
examine whether these changes of cellular processes caused by
overexpression of PDCD5 are related to the P53 signaling
pathway.
DMEM [10% fetal bovine serum (FBS), 2 mM glutamine,
1% penicillin/streptomycin]. The A431 cells were cultured at 37°C
incubator supplemented with 5% CO2. dNTP (10 mM) and One
Step SYBR® PrimeScript™ RT-PCR kit were purchased from
Takara Bio (Dalian, China); Primers were synthesized by GeneCreate
Biological Engineering Co., Ltd. (Wuhan, China); TRIzol was
purchased from Invitrogen (Carlsbad, CA, USA); MTT was purchased
from Sigma (St. Louis, MO, USA; cat. no. m5655); FBS was purchased
from Gibco; PI and Annexin V-FITC were purchased from Beyotime.
Antibodies were purchased from Cusabio. The PDCD5 overexpressing
A431 cell line was established by GeneCreate Biological Engineering
Co., Ltd. (Wuhan, China). The cell line stably transfected empty
vector was used a control.
Cells splitted into each well of 96-well plate with
the cell density ~1000–10000 cells/well. 180 µl of diluted cells
was added into each well. 5 different time points including 12, 24,
48, 72 and 96 h were set-up and each time point has 5 replicates
for PDCD5 overexpressing and control cells. The cells were cultured
in the 37°C incubator supplemented with 5% CO2. 20 µl of
MTT (5 mg/ml, 0.5% MTT) was added to each well and the cells were
cultured for additional 4 h. The culture media were carefully
removed and 100 µl DMSO was added into each well. The plate was
gently shaken on the shaker at low speed for 10 min to dissolve the
crystal completely. The absorbance of each well was measured at
OD490 nm by using the ELISA reader.
The cells were synchronized with serum withdrawal
(media without serum) for 24 h and then replenished with 10% FBS
containing DMEM media for additional 48 h. The cells were then
trypsinized and transferred to the collection tube. Cells were
pelleted by centrifuge at 1000 rpm for 5 min. The supernatant was
removed and cells were resuspended with ice-cold PBS and then
transferred into 1.5 ml tube, and repelleted by centrifugation. The
cells were then fixed in 1 ml of 70% cold ethanol for >2 h. The
cells were pelleted again by centrifugation at 1000 rpm for 5 min.
The supernatant was carefully aspirated and the cells were
suspended with 1 ml ice-cold PBS. The cells were then repelleted
and the supernatant was carefully removed. 0.5 ml PI solution (PI 5
mg, RNase 2 mg, 1.0% Triton X-100 0.25 ml, saline 65 ml, sodium
citrate 100 mg, ddH2O was added to bring total volume to 100 ml and
the pH value was adjusted to 7.2–7.6; Stored at 4°C in brown bottle
and keep away from light) was added into the cells and incubated at
37°C for 30 min without light exposure. The cells can be stored at
4°C or kept on ice. Flow experiments should proceed within 24 h
after PI staining. Flow cytometer (Beckman Moflo XDP) was set at
488 nm (excitation wavelength) to detect red fluorescence and light
scattering. DNA content and light scattering analyses were
performed by using Modfit software.
Total RNA was extracted by using TRIzol method
(Invitrogen) according to the instructions of the manual. 1 ml
TRIzol was added to the cell pellet (containing ~1×107
cells), mixed well and incubated at RT for 5 min. 0.2 ml chloroform
was then added, votexed for 15 sec and incubated for 3 min. The
lysates were centrifuged at 12,000 rpm, 4°C for 10 min. The
supernatant was removed and mixed well with 0.5 ml cold
isopropanol, and kept on ice for 20~30 min. The mixture was then
centrifuged at 12,000 rpm, 4°C for 10 min to pellet the RNA. The
supernatant was removed and the pellet was washed with 1 ml 75%
ethanol. The RNA/ethanol mixture was centrifuged again at 7,500 g
for 5 min, and the supernatant was discarded. The RNA was air-dried
and dissolved in ddH2O. The qPCR reaction was as
follows: RNA (template): 2 µl; SYBR® PrimeScript Master
Mix (2×), 12.5 µl; forward primer (20 µM): 0.5 µl; reverse primer
(20 µM): 0.5 µl; ddH2O: 11.5 µl; total volume, 25 µl.
GAPDH or β-actin was used as an internal control. Primer sequences
are presented below in Table I.
qRT-PCR program was as follows: 45°C, 15 min; 95°C, 5 min; 95°C, 20
sec; 60°C, 20 sec; 72°C, 30 sec; 40 cycles. The data were analyzed
by using 2−ΔΔCq method.
A431 is a cell line derived from human epidermoid
carcinoma and has been widely used in the studies on cell cycle
progression and tumor-related signaling pathways. Therefore, we
introduced this cell line to our studies. The cells were
transfected with PDCD5 construct and its empty vector,
respectively. To confirm whether the stable cell line was
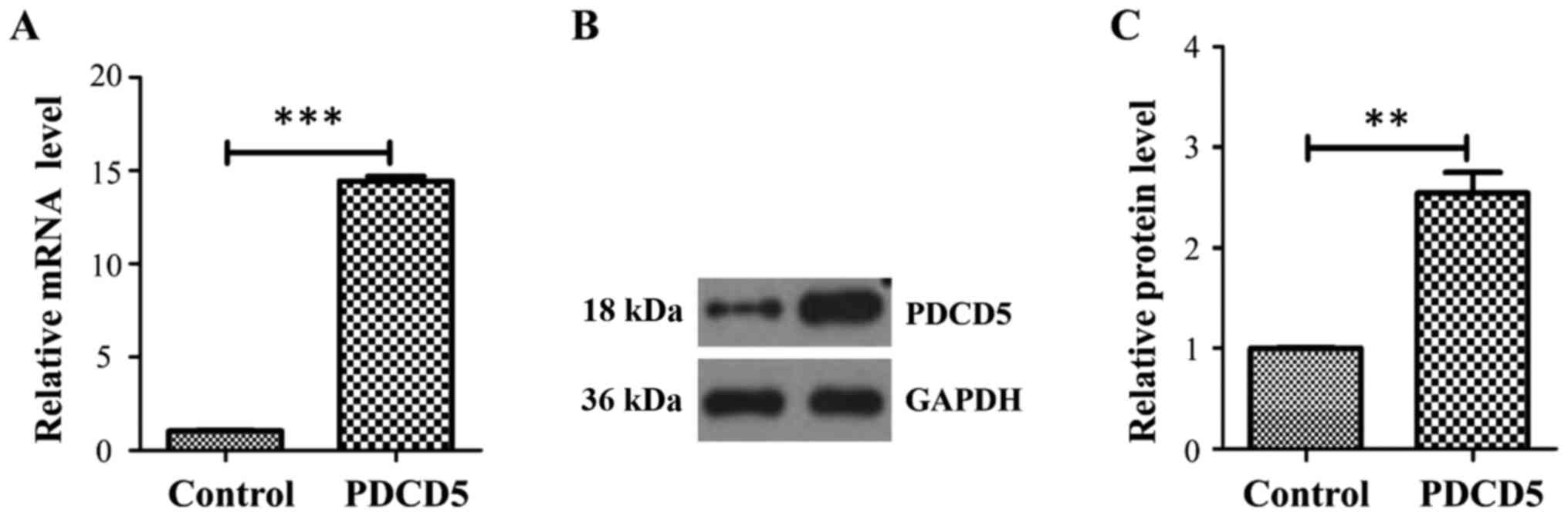
successfully established, we performed quantitative RT-PCR and
western blot. At the transcript level, PDCD5 was increased ~15-fold
in the PDCD5 stably overexpressing A431 cells compared with the
empty vector control (Fig. 1A). At
the protein level, PDCD5 was also strikingly increased in the PDCD5
overexpressing cells compared with the control (Fig. 1B and C).
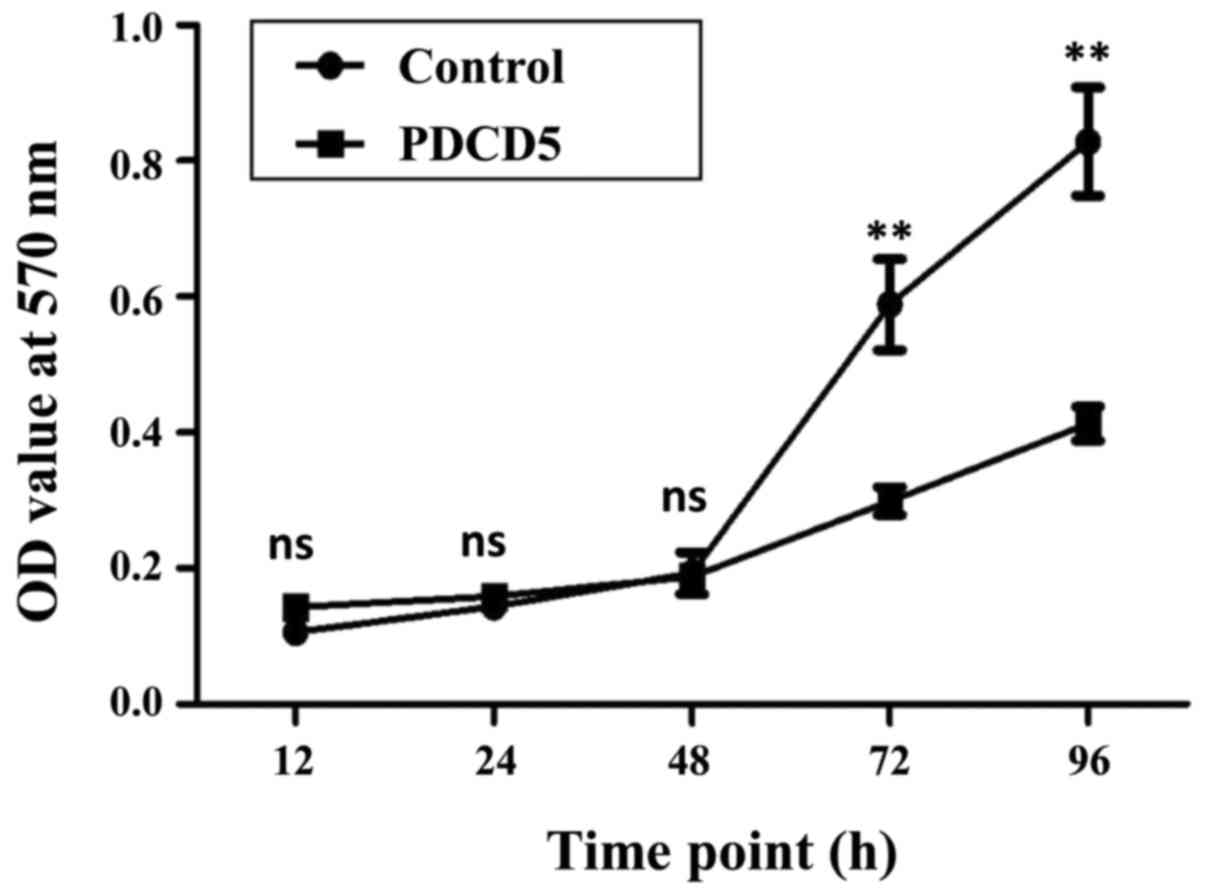
To examine whether overexpression of PDCD5 affects
cell proliferation, MTT assay was performed for the PDCD5
overexpressing cells and control cells at different time points,
including 12, 24, 48, 72 and 96 h. The growth curves for these two
cell lines were generated according to the OD values at these time
points. The data indicated that at the time points of 72 and 96 h,
cell proliferation in the PDCD5 stably overexpressing cells was
significantly slower than that in the empty vector control cells
(Fig. 2).
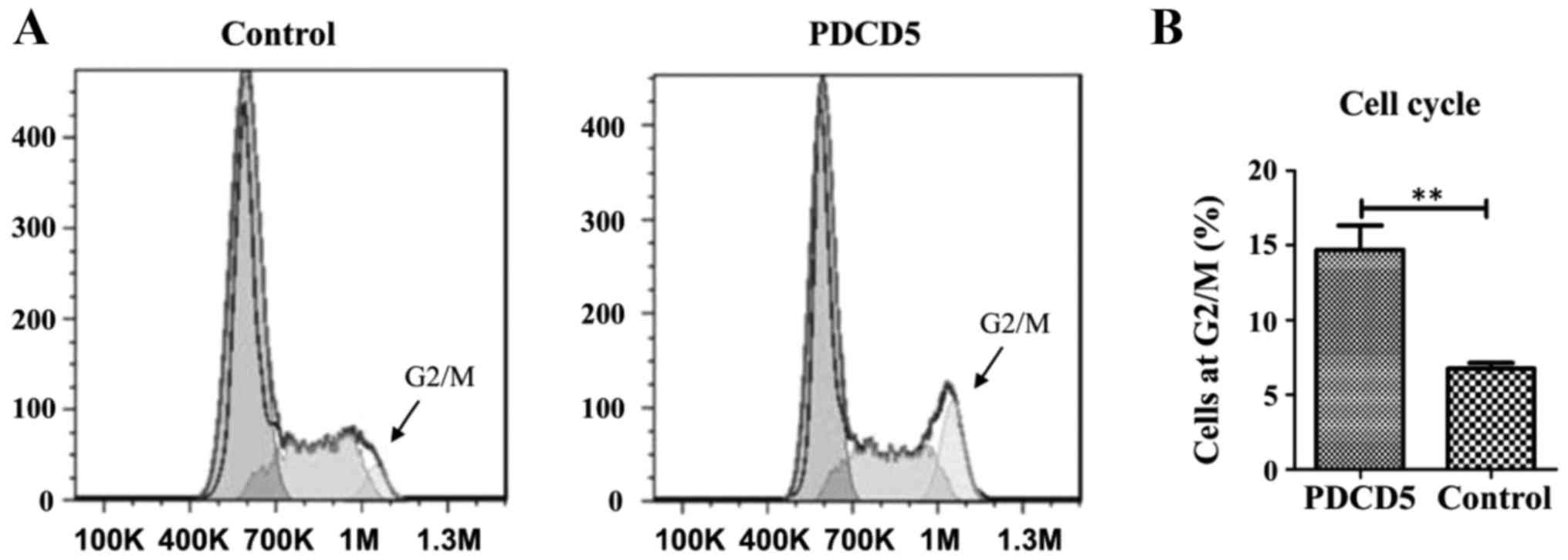
To further understand the inhibitory effect of PDCD5
on cell proliferation, we performed flow cytometry to investigate
the distribution of specific phases of cell cycle in the PDCD5
stably overexpressing A431 cells and its empty vector control. The
results showed that PDCD5 overexpressing cells were strikingly
arrested at the G2/M phase of the cell cycle, compared with the
control cells (Fig. 3A and B).
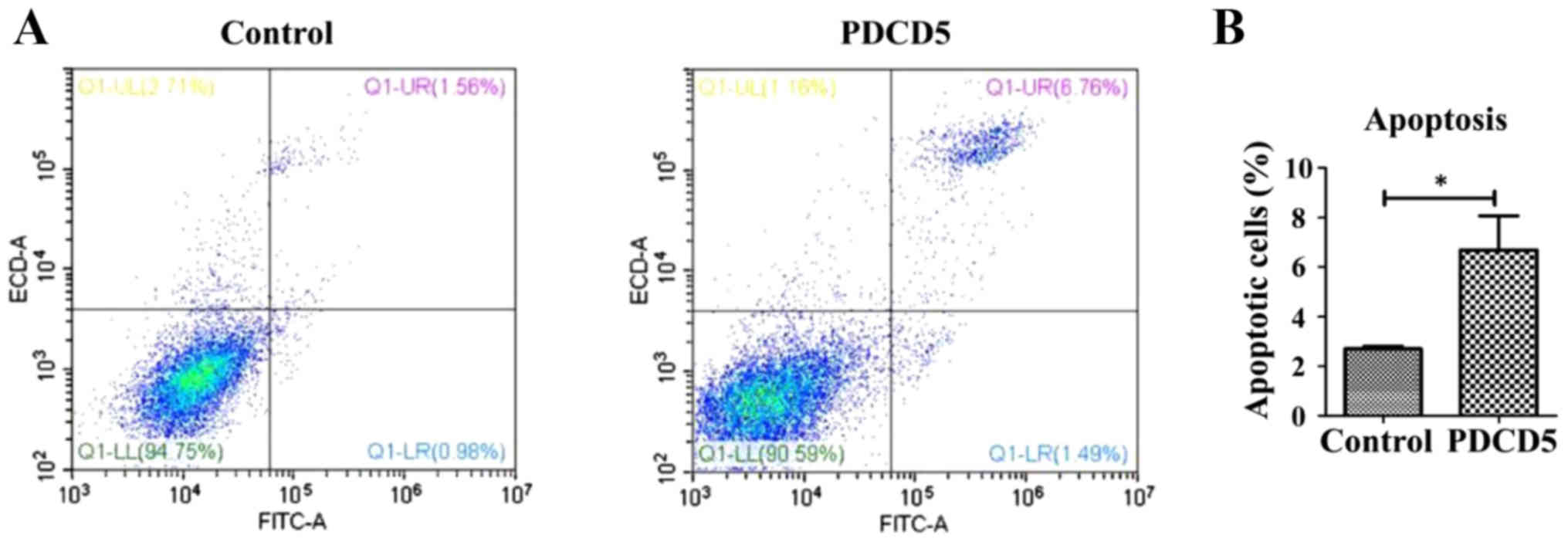
Cell cycle arrest at G2/M is generally resulted from
DNA damage. To investigate whether the PDCD5 overexpressing cells
with DNA damage underwent apoptosis, we performed flow cytometric
analysis as well. The data indicated that overexpression of PDCD5
strikingly induces apoptosis, compared with the control cells
(Fig. 4A and B).
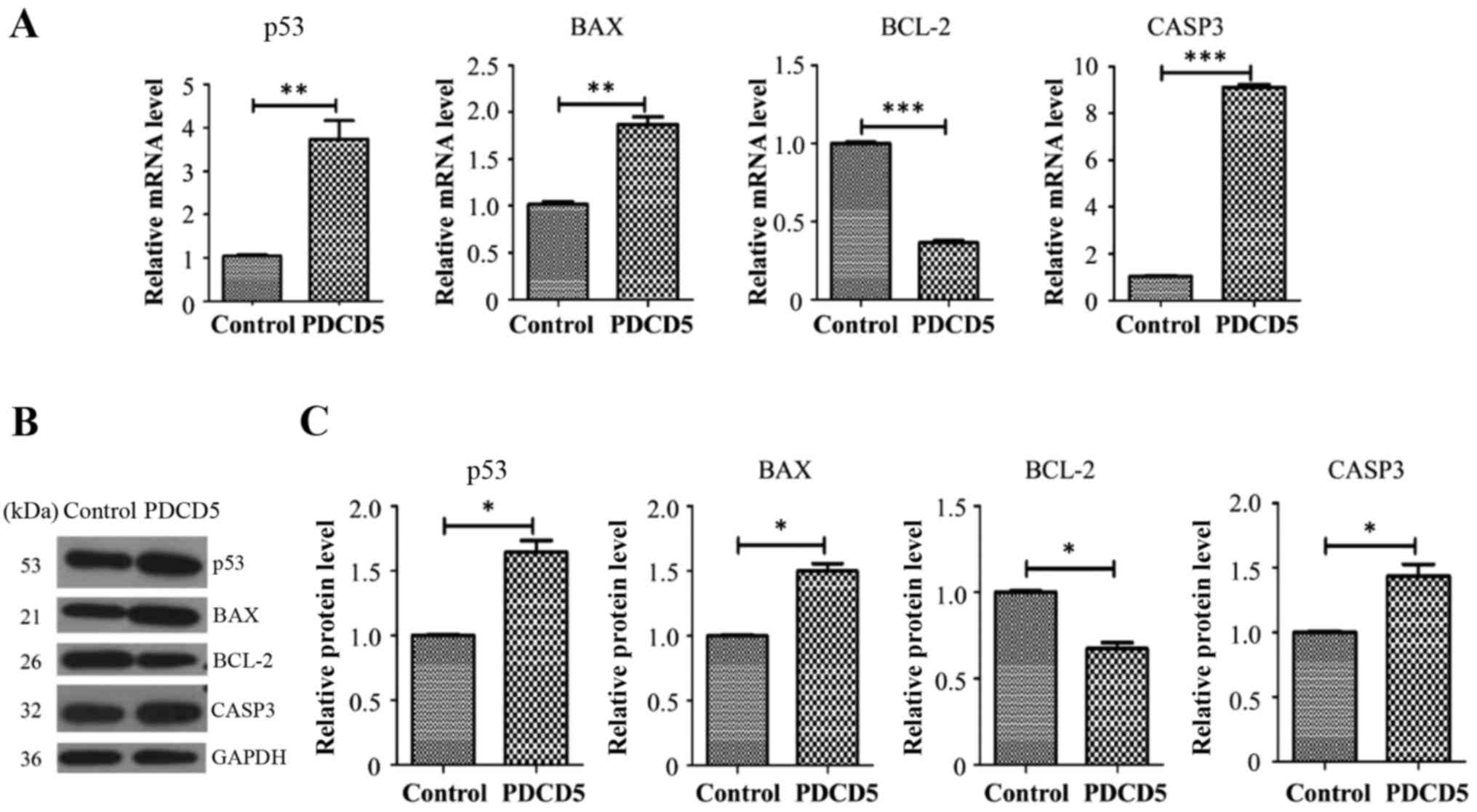
To further understand the molecular underpinnings
that might be relevant to decreased proliferation, increased
apoptosis and G2/M arrest in the PDCD5 overexpressing A431 cells,
we examined the transcript and protein levels of tumor suppressor
P53 and key molecules of apoptosis including BCL-2, BAX and CAPS3.
Real-time RT-PCR results showed that P53, BAX and
CAPS3 were all upregulated in the PDCD5 stably
overexpressing A431 cells while BCL-2 is downregulated,
compared with the empty vector control cells (Fig. 5A). Western blot by using specific
antibodies against these proteins was then performed to further
confirm RT-PCR results (Fig. 5B and
C). The data showed that the pattern of protein dysregulations
was basically consistent with that observed in the qRT-PCR
data.
Programmed cell death (PCD) is the death of a cell
mediated by a serial of intracellular programs. There are three
forms of PCD: Apoptosis, autophagy and programmed necrosis
(47). It is well known that
apoptosis is an orchestrated cellular process that can occur in
physiological and pathological conditions (48). Cell proliferation is a cellular event
that causes an increase of cell number. In human cancers, cell
proliferation is out of control and apoptosis is suppressed
(49). Cell proliferation is
decreased when cell cycle arrest occurs. In the condition of DNA
damage, cell cycle arrest will be initiated as an attempt to repair
the damage, however, if the damage is too extensive to be repaired,
the cell will undergo cell death in a way of apoptosis (50).
According to the NCBI database, there are currently
12 members in total in the PDCD protein family, namely
PDCD1~PDCD12. Among them, PDCD8 and PDCD9 are officially known as
AIFM1 and MRPS30, respectively. PDCD1, often known as PD-1, is the
member that has been most extensively studied and shown to
negatively regulate T cell responses, in collaboration with its two
ligands, PD-L1 and PD-L2 (51–53). In
addition to PDCD5, other programmed cell death proteins are also
known to play important roles in apoptosis and/or cell cycle
progression (54–57), and are also dysregulated in many types
of human cancers (13,16,58–63).
Opposite to what we observed for PDCD5, depletion of PDCD2 in human
acute leukemia cells impairs their proliferation, induces cell
cycle arrest and p53 activation while overexpression of PDCD2
facilitates cell growth in cancers (55,64).
However, in gastric cancer cells, expression of PDCD2 seems to
induce cell cycle arrest and apoptosis, which are also found to be
p53-dependent (54). This suggests
the connection between PDCD2 and cell cycle arrest might be tissue
and cancer type-dependent.
In the present study, we used A431 cells as a cell
model to investigate the role of PDCD5 in cell proliferation, cell
cycle progression and apoptosis. As a human model epidermoid
carcinoma cell line, A431 has been widely used in studies on the
cell cycle and tumor related cell signaling pathways because
epidermal growth factor receptor (EGFR) is known to be strikingly
upregulated in these cells (65–68). In
this study, we found that in the A431 cell, overexpression of PDCD5
inhibits cell proliferation, induces cell cycle arrest at G2/M
phase and apoptosis. We next attempted to examine the molecular
underpinnings of such dysregulations in these cellular events
described above. Some key molecules involved in cell proliferation,
cell cycle progression and apoptosis, including P53, BAX,
BCL-2 and CASP-3, were found to be dysregulated when
PDCD5 was stably overexpressed in A431 cells.
It is noted that all our data in this study were
generated by using a PDCD5 stably overexpressing cell model. In our
future study, we might need to establish a PDCD5 knockdown cell
model and knockout animal model to further confirm the roles of
PDCD5 in cell proliferation, cell cycle progression and apoptosis.
Moreover, the detailed molecular mechanism underlying the
regulation of cell proliferation, cell cycle progression and
apoptosis by PDCD5 also needs to be further investigated.
Thie present study was supported by the Project of
Department of Education of Heilongjiang Province (grant no.
12531797).
|
1
|
Hipfner DR and Cohen SM: Connecting
proliferation and apoptosis in development and disease. Nat Rev Mol
Cell Biol. 5:805–815. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Löbrich M and Jeggo PA: The impact of a
negligent G2/M checkpoint on genomic instability and cancer
induction. Nat Rev Cancer. 7:861–869. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alenzi FQ: Links between apoptosis,
proliferation and the cell cycle. Br J Biomed Sci. 61:99–102. 2004.
View Article : Google Scholar
|
|
4
|
Liu H, Wang Y, Zhang Y, Song Q, Di C, Chen
G, Tang J and Ma D: TFAR19, a novel apoptosis-related gene cloned
from human leukemia cell line TF-1, could enhance apoptosis of some
tumor cells induced by growth factor withdrawal. Biochem Biophys
Res Commun. 254:203–210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang N, Lu HS, Guan ZP, Sun TZ, Chen YY,
Ruan GR, Chen ZK, Jiang J and Bai CJ: Involvement of PDCD5 in the
regulation of apoptosis in fibroblast-like synoviocytes of
rheumatoid arthritis. Apoptosis. 12:1433–1441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen LN, Wang Y, Ma DL and Chen YY: Short
interfering RNA against the PDCD5 attenuates cell apoptosis and
caspase-3 activity induced by Bax overexpression. Apoptosis.
11:101–111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YH, Zhao M, Li WM and Lu YY, Chen YY,
Kang B and Lu YY: Expression of programmed cell death 5 gene
involves in regulation of apoptosis in gastric tumor cells.
Apoptosis. 11:993–1001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruan GR, Zhao HS, Chang Y, Li JL, Qin YZ,
Liu YR, Chen SS and Huang XJ: Adenovirus-mediated PDCD5 gene
transfer sensitizes K562 cells to apoptosis induced by idarubicin
in vitro and in vivo. Apoptosis. 13:641–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salvi M, Xu D, Chen Y, Cabrelle A, Sarno S
and Pinna LA: Programmed cell death protein 5 (PDCD5) is
phosphorylated by CK2 in vitro and in 293T cells. Biochem Biophys
Res Commun. 387:606–610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li G, Ma D and Chen Y: Cellular functions
of programmed cell death 5. Biochim Biophys Acta. 1863:572–580.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Li X, Wang L, Ding P, Zhang Y, Han
W and Ma D: An alternative form of paraptosis-like cell death,
triggered by TAJ/TROY and enhanced by PDCD5 overexpression. J Cell
Sci. 117:1525–1532. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang S, Li G, Fu X, Qi Y, Li M, Lu G, Hu
J, Wang N, Chen Y, Bai Y and Cui M: PDCD5 protects against cardiac
remodeling by regulating autophagy and apoptosis. Biochem Biophys
Res Commun. 461:321–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H, Wang Q, Gao F, Zhu F, Wang X, Zhou
C, Liu C, Chen Y, Ma C, Sun W and Zhang L: Reduced expression of
PDCD5 is associated with high-grade astrocytic gliomas. Oncol Rep.
20:573–579. 2008.PubMed/NCBI
|
|
14
|
Du YJ, Xiong L, Lou Y, Tan WL and Zheng
SB: Reduced expression of programmed cell death 5 protein in tissue
of human prostate cancer. Chin Med Sci J. 24:241–245. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen C, Zhou H, Xu L, Liu X, Liu Z, Ma D,
Chen Y and Ma Q: Prognostic significance of downregulated
expression of programmed cell death 5 in chondrosarcoma. J Surg
Oncol. 102:838–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Wang X, Song X, Wei Z, Zhou C,
Zhu F, Wang Q, Ma C and Zhang L: Clinical and prognostic
significance of lost or decreased PDCD5 expression in human
epithelial ovarian carcinomas. Oncol Rep. 25:353–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Wang GH and Zhang QY:
Determination of PDCD5 in peripheral blood serum of cancer
patients. Chin J Cancer Res. 23:224–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao M, Gao W, Wang Z, Liu Y, Li Y, Wei C,
Sun Y, Guo C, Zhang L, Wei Z and Wang X: The reduced PDCD5 protein
is correlated with the degree of tumor differentiation in
endometrioid endometrial carcinoma. Springerplus. 5:9882016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Zou Z, Xu A, Liu Y, Pan H and Jin
L: Serum programmed cell death protein 5 (PDCD5) levels is
upregulated in liver diseases. J Immunoassay Immunochem.
34:294–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu F, Wu K, Zhao M, Qin Y and Xia M:
Expression and clinical significance of the programmed cell death 5
gene and protein in laryngeal squamous cell carcinoma. J Int Med
Res. 41:1838–1847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang D, Wang W, Song CL and Xia P: The
roles of serum PDCD5 in circulating CD133 positive cells of the
patients with gastric cancer. Tumour Biol. 37:11799–11804. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang W, Song XW and Zhao CH: Roles of
programmed cell death protein 5 in inflammation and cancer
(Review). Int J Oncol. 49:1801–1806. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi L, Song Q, Zhang Y, Lou Y and Wang Y,
Tian L, Zheng Y, Ma D, Ke X and Wang Y: Potent antitumor activities
of recombinant human PDCD5 protein in combination with chemotherapy
drugs in K562 cells. Biochem Biophys Res Commun. 396:224–230. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin A, Jiang Y, Zhang X, Zhao J and Luo H:
Transfection of PDCD5 sensitizes colorectal cancer cells to
cisplatin-induced apoptosis in vitro and in vivo. Eur J Pharmacol.
649:120–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu HY, Chen ZW, Pan YM, Fan L, Guan J and
Lu YY: Transfection of PDCD5 effect on the biological behavior of
tumor cells and sensitized gastric cancer cells to
cisplatin-induced apoptosis. Dig Dis Sci. 57:1847–1856. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han XR, Sun Y and Bai XZ: The anti-tumor
role and mechanism of integrated and truncated PDCD5 proteins in
osteosarcoma cells. Cell Signal. 24:1713–1721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Zhou G, La L, Chi X, Cao Y, Liu J,
Zhang Z, Chen Y and Wu B: Transgenic human programmed cell death 5
expression in mice suppresses skin cancer development by enhancing
apoptosis. Life Sci. 92:1208–1214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu W, Li Y and Gao L: Cisplatin in
combination with programmed cell death protein 5 increases
antitumor activity in prostate cancer cells by promoting apoptosis.
Mol Med Rep. 11:4561–4566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu DZ, Cheng Y, He H, Liu HY and Liu YF:
Recombinant human PDCD5 exhibits an antitumor role in
hepatocellular carcinoma cells via clathrin-dependent endocytosis.
Mol Med Rep. 12:8135–8140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao L, Ye X, Ma RQ, Cheng HY, Han HJ, Cui
H, Wei LH and Chang XH: Low programmed cell death 5 expression is a
prognostic factor in ovarian cancer. Chin Med J (Engl).
128:1084–1090. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao J, Li G, Hu J, Qu L, Ma D and Chen Y:
Anti-inflammatory effects of recombinant human PDCD5 (rhPDCD5) in a
rat collagen-induced model of arthritis. Inflammation. 38:70–78.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiao J, Liu W, Chen Y and Deng W:
Recombinant human PDCD5 (rhPDCD5) protein is protective in a mouse
model of multiple sclerosis. J Neuroinflammation. 12:1172015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu J, Jiang Z, Chen Y, Zhou C and Chen C:
Knockout of programmed cell death 5 (PDCD5) gene attenuates neuron
injury after middle cerebral artery occlusion in mice. Brain Res.
1650:152–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Spinola M, Meyer P, Kammerer S, Falvella
FS, Boettger MB, Hoyal CR, Pignatiello C, Fischer R, Roth RB,
Pastorino U, et al: Association of the PDCD5 locus with lung cancer
risk and prognosis in smokers. J Clin Oncol. 24:1672–1678. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nanba K, Toyooka S, Soh J, Tsukuda K,
Yamamoto H, Sakai A, Ouchida M, Kobayashi N, Matsuo K, Koide N, et
al: The allelic distribution of a single nucleotide polymorphism in
the PDCD5 gene locus of Japanese non-small cell lung cancer
patients. Mol Med Rep. 1:667–671. 2008.PubMed/NCBI
|
|
36
|
Xu L, Chen Y, Song Q, Xu D, Wang Y and Ma
D: PDCD5 interacts with Tip60 and functions as a cooperator in
acetyltransferase activity and DNA damage-induced apoptosis.
Neoplasia. 11:345–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yao H, Feng Y, Zhou T, Wang J and Wang ZX:
NMR studies of the interaction between human programmed cell death
5 and human p53. Biochemistry. 51:2684–2693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu L, Hu J, Zhao Y, Hu J, Xiao J, Wang Y,
Ma D and Chen Y: PDCD5 interacts with p53 and functions as a
positive regulator in the p53 pathway. Apoptosis. 17:1235–1245.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tracy CM, Gray AJ, Cuéllar J, Shaw TS,
Howlett AC, Taylor RM, Prince JT, Ahn NG, Valpuesta JM and
Willardson BM: Programmed cell death protein 5 interacts with the
cytosolic chaperonin containing tailless complex polypeptide 1
(CCT) to regulate β-tubulin folding. J Biol Chem. 289:4490–4502.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Essers PB, Klasson TD, Pereboom TC, Mans
DA, Nicastro M, Boldt K, Giles RH and MacInnes AW: The von
Hippel-Lindau tumor suppressor regulates programmed cell death
5-mediated degradation of Mdm2. Oncogene. 34:771–779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park SY, Choi HK, Jo SH, Seo J, Han EJ,
Choi KC, Jeong JW, Choi Y and Yoon HG: YAF2 promotes TP53-mediated
genotoxic stress response via stabilization of PDCD5. Biochim
Biophys Acta. 1853:1060–1072. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Choi HK, Choi Y, Park ES, Park SY, Lee SH,
Seo J, Jeong MH, Jeong JW, Jeong JH, Lee PC, et al: Programmed cell
death 5 mediates HDAC3 decay to promote genotoxic stress response.
Nat Commun. 6:73902015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhuge C, Sun X, Chen Y and Lei J: PDCD5
functions as a regulator of p53 dynamics in the DNA damage
response. J Theor Biol. 388:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Murshed F, Farhana L, Dawson MI and
Fontana JA: NF-κB p65 recruited SHP regulates PDCD5-mediated
apoptosis in cancer cells. Apoptosis. 19:506–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park SY, Choi HK, Choi Y, Kwak S, Choi KC
and Yoon HG: Deubiquitinase OTUD5 mediates the sequential
activation of PDCD5 and p53 in response to genotoxic stress. Cancer
Lett. 357:419–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cui X, Choi HK, Choi YS, Park SY, Sung GJ,
Lee YH, Lee J, Jun WJ, Kim K, Choi KC and Yoon HG: DNAJB1
destabilizes PDCD5 to suppress p53-mediated apoptosis. Cancer Lett.
357:307–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pucci B, Kasten M and Giordano A: Cell
cycle and apoptosis. Neoplasia. 2:291–299. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Leng C, Li Y, Qin J, Ma J, Liu X, Cui Y,
Sun H, Wang Z, Hua X, Yu Y, et al: Relationship between expression
of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the
antitumor effects of CD8+T cells. Oncol Rep. 35:699–708. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dong Y, Sun Q and Zhang X: PD-1 and its
ligands are important immune checkpoints in cancer. Oncotarget.
8:2171–2186. 2017.PubMed/NCBI
|
|
53
|
Bardhan K, Anagnostou T and Boussiotis VA:
The PD1:PD-L1/2 pathway from discovery to clinical implementation.
Front Immunol. 7:5502016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang J, Wei W, Jin HC, Ying RC, Zhu AK
and Zhang FJ: Programmed cell death 2 protein induces gastric
cancer cell growth arrest at the early S phase of the cell cycle
and apoptosis in a p53-dependent manner. Oncol Rep. 33:103–110.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Granier CJ, Wang W, Tsang T, Steward R,
Sabaawy HE, Bhaumik M and Rabson AB: Conditional inactivation of
PDCD2 induces p53 activation and cell cycle arrest. Biol Open.
3:821–831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sun Z, Li S, Kaufmann AM and Albers AE:
miR-21 increases the programmed cell death 4 gene-regulated cell
proliferation in head and neck squamous carcinoma cell lines. Oncol
Rep. 32:2283–2289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xia L, Wen H, Han X, Tang J and Huang Y:
Luteinizing hormone inhibits cisplatin-induced apoptosis in human
epithelial ovarian cancer cells. Oncol Lett. 11:1943–1947.
2016.PubMed/NCBI
|
|
58
|
Gao F, Ding L, Zhao M, Qu Z, Huang S and
Zhang L: The clinical significance of reduced programmed cell death
5 expression in human gastrointestinal stromal tumors. Oncol Rep.
28:2195–2199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
McDermott DF and Atkins MB: PD-1 as a
potential target in cancer therapy. Cancer Med. 2:662–673.
2013.PubMed/NCBI
|
|
60
|
Mo Z, Liu J, Zhang Q, Chen Z, Mei J, Liu
L, Yang S, Li H, Zhou L and You Z: Expression of PD-1, PD-L1 and
PD-L2 is associated with differentiation status and histological
type of endometrial cancer. Oncol Lett. 12:944–950. 2016.PubMed/NCBI
|
|
61
|
Wen YH, Shi X, Chiriboga L, Matsahashi S,
Yee H and Afonja O: Alterations in the expression of PDCD4 in
ductal carcinoma of the breast. Oncol Rep. 18:1387–1393.
2007.PubMed/NCBI
|
|
62
|
Fassan M, Cagol M, Pennelli G, Rizzetto C,
Giacomelli L, Battaglia G, Zaninotto G, Ancona E, Ruol A and Rugge
M: Programmed cell death 4 protein in esophageal cancer. Oncol Rep.
24:135–139. 2010.PubMed/NCBI
|
|
63
|
González-Villasana V, Nieves-Alicea R,
McMurtry V, Gutiérrez-Puente Y and Tari AM: Programmed cell death 4
inhibits leptin-induced breast cancer cell invasion. Oncol Rep.
27:861–866. 2012.PubMed/NCBI
|
|
64
|
Barboza N, Minakhina S, Medina DJ, Balsara
B, Greenwood S, Huzzy L, Rabson AB, Steward R and Schaar DG: PDCD2
functions in cancer cell proliferation and predicts relapsed
leukemia. Cancer Biol Ther. 14:546–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zidovetzki R, Johnson DA, Arndt-Jovin DJ
and Jovin TM: Rotational mobility of high-affinity epidermal growth
factor receptors on the surface of living A431 cells. Biochemistry.
30:6162–6166. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wu SL, Taylor AD, Lu Q, Hanash SM, Im H,
Snyder M and Hancock WS: Identification of potential glycan cancer
markers with sialic acid attached to sialic acid and up-regulated
fucosylated galactose structures in epidermal growth factor
receptor secreted from A431 cell line. Mol Cell Proteomics.
12:1239–1249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang F, Wang S, Yin L, Yang Y, Guan Y,
Wang W, Xu H and Tao N: Quantification of epidermal growth factor
receptor expression level and binding kinetics on cell surfaces by
surface plasmon resonance imaging. Anal Chem. 87:9960–9965. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Stanton P, Richards S, Reeves J, Nikolic
M, Edington K, Clark L, Robertson G, Souter D, Mitchell R, Hendler
FJ, et al: Epidermal growth factor receptor expression by human
squamous cell carcinomas of the head and neck, cell lines and
xenografts. Br J Cancer. 70:427–433. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ozaki T and Nakagawara A: Role of p53 in
cell death and human cancers. Cancers (Basel). 3:994–1013. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Drosten M, Sum EY, Lechuga CG,
Simón-Carrasco L, Jacob HK, García-Medina R, Huang S, Beijersbergen
RL, Bernards R and Barbacid M: Loss of p53 induces cell
proliferation via Ras-independent activation of the Raf/Mek/Erk
signaling pathway. Proc Natl Acad Sci USA. 111:pp. 15155–15160.
2014; View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang HT, Wang YL, Zhang J and Zhang QX:
Artemisinin inhibits gastric cancer cell proliferation through
upregulation of p53. Tumour Biol. 35:1403–1409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ranganathan S, Joseph J and Mehta JL:
Aspirin inhibits human coronary artery endothelial cell
proliferation by upregulation of p53. Biochem Biophys Res Commun.
301:143–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Taylor WR and Stark GR: Regulation of the
G2/M transition by p53. Oncogene. 20:1803–1815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
Bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nakazawa K, Dashzeveg N and Yoshida K:
Tumor suppressor p53 induces miR-1915 processing to inhibit Bcl-2
in the apoptotic response to DNA damage. FEBS J. 281:2937–2944.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang H, Ye Y, Chui JH, Zhu GY, Li YW, Fong
DW and Yu ZL: Oridonin induces G2/M cell cycle arrest and apoptosis
through MAPK and p53 signaling pathways in HepG2 cells. Oncol Rep.
24:647–651. 2010.PubMed/NCBI
|
|
77
|
Ou X, Lu Y, Liao L, Li D, Liu L, Liu H and
Xu H: Nitidine chloride induces apoptosis in human hepatocellular
carcinoma cells through a pathway involving p53, p21, Bax and
Bcl-2. Oncol Rep. 33:1264–1274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhou Y and Ho WS: Combination of
liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell
death through upregulating p53 and p21 in the A549 non-small cell
lung cancer cells. Oncol Rep. 31:298–304. 2014. View Article : Google Scholar : PubMed/NCBI
|