Introduction
Metastasis is responsible for the majority of
melanoma related deaths (1,2). The median survival time of patients with
metastatic melanoma is 8–9 months and the 3-year overall survival
rate is <15% (3). Therefore, there
is an urgent need to identify novel therapeutic strategies for
melanoma metastasis and the search for a new agent with low
toxicity that can inhibit metastasis with clear molecular targets
has attracted scientist's attention.
Epithelial-mesenchymal transition (EMT), the
transformation of epithelial cells into motile mesenchymal cell
phenotypes, is one of the essential events in tumor progression
particularly in tumor invasion and metastasis (4–6). During
EMT, epithelial cells lose polarity and cell-to-cell contacts,
followed by dramatic remodeling of the cytoskeleton and acquisition
of migratory, invasive, and stem cell-like properties (7,8). The most
well-characterized factor responsible for induction of EMT is
transforming growth factor-β1 (TGF-β1) (9). There are several signaling pathways
related to TGF-β1-induced EMT in cancer cells, including the
PI3K/Akt, Wnt/β-catenin and Smads-dependent pathways.
PI3K/Akt/GSK-3β signaling pathways are overactive in cancer cells,
thus reducing apoptosis, allowing proliferation, and promoting
invasion and metastasis (10–13). Therefore, inhibition of the
PI3K/Akt/GSK-3β signaling pathway-mediated EMT may exert beneficial
effects in treating cancer patients with advanced metastasis.
Traditional Chinese medicinal plants or their active
components have been widely and successfully used in treating human
cancers (14–16). Oridonin (ORI), an active diterpenoid
compound isolated from Rabdosia rubescens, is currently one
of the most important active Chinese medicinal components. Previous
studies have demonstrated that ORI possesses multiple biological
activities such as anti-inflammatory, neuroprotective,
anti-bacterial, and antitumor effects. ORI shows broad-spectrum
anti-proliferative activity in various types of cancer (16–21).
Notably, several studies reported that ORI also demonstrates
significant anticancer activity in skin melanoma. For instance, Gu
et al reported that ORI potently impairs the capability of
survival and proliferation of melanoma cells by induction of
apoptosis (22). Wang et al
reported ORI induces human melanoma A375-S2 cell death through
inhibiting insulin-like growth factor-1 (IGF-1) receptor signaling
(23).
However, the inhibiting effects of ORI in metastasis
of melanoma cells and the underlying mechanisms of such effects
remain unclear. Here, in this study, we demonstrated that ORI could
effectively inhibit the migration, invasion, adhesion, and
TGF-β1-induced EMT in A375 and B16-F10 melanoma cells. Our data
also demonstrated that the PI3K/Akt/GSK-3β pathway is involved in
the reversion of TGF-β1-induced EMT in A375 and B16-F10 cells when
engaged with ORI. Our findings indicate that ORI may be a promising
anti-metastasis candidate compound for melanoma therapy.
Materials and methods
Reagents and antibodies
ORI was acquired from Aladdin Biochemical (Shanghai,
China), and dissolved in 0.5% dimethyl sulfoxide (DMSO). To make
sure the ORI solution was sterile it was filtered using a 0.2 µm
filtration membrane before it was added to the culture medium for
the in vitro assays.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT), LY294002 (an inhibitor of PI3K), and DMSO were purchased
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Phosphatase
inhibitor cocktail tablets were purchased from Roche Molecular
Biochemicals (Indianapolis, IN, USA). TGF-β1 was purchased from
Millipore (Billerica, MA, USA). Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS) and penicillin/streptomycin were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). All other chemicals were of analytical reagent grade. The
antibodies used were as follows: Anti-E-cadherin was purchased from
BD Biosciences (Franklin Lakes, NJ, USA). Anti-vimentin was
purchased from Epitomics (Burlingame, CA, USA). Anti-Snail,
anti-Akt, and anti-phospho-Akt (Ser473) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Anti-PI3K,
anti-phospho-PI3K (Tyr458), anti-GSK-3β, anti-phospho-GSK-3β (Ser9)
and anti-β-catenin were purchased from Abcam (Cambridge, MA, USA).
Anti-β-actin, goat anti-mouse IgG-HRP, and goat anti-rabbit IgG-HRP
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). All antibodies were diluted at 1:1,000 except where
specified.
Cell lines and cell culture
A375 human melanoma cell line obtained from ATCC
(Manassas, VA, USA). B16-F10 (mouse melanoma) cell line was
purchased from the Cell Culture Center of Chinese Academy of
Medical Sciences (Beijing, China). The cells were cultured in DMEM
supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml
streptomycin. All cell cultures were maintained at 37°C in an
atmosphere containing 5% CO2.
Cell viability assay (MTT assay)
Cell viability was measured by MTT assay to evaluate
the effect of ORI on cell proliferation (24). A375 or B16-F10 cells in the logarithm
phase were seeded in 96-well plates at the density of
5×103 cells/well. When the cells were adherent to the
walls, the cells were treated with ORI at indicated concentrations
for 24 h followed by adding 100 µl of MTT (1 mg/ml) and incubating
for 4 h. Then, the medium was removed and 150 µl of DMSO was added
to each well. Absorbance of each well was detected under 490 nm by
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
The test was repeated three times. Inhibition rate (% of control) =
(1 - absorbance of test sample/absorbance of control) × 100%.
Wound-healing migration assay
Cell migration wounding assay was performed
according to the protocol described previously (25,26). A375
or B16-F10 cells were cultured in 60 mm dishes at the density of
8×105 cells/dish to 100% confluency. After wounding with
pipette tip, the cells were washed with PBS and serum free medium
to which indicated concentrations of ORI had been added. Then, the
cells were allowed to migrate for 24 h at 37°C in 5%
CO2. At predetermined time-points (0, 3, 6, 9, 12 and 24
h), the widths of wound were measured and images of cells were
taken at time 0 and 24 h with a microscope (IX50; Olympus, Tokyo,
Japan). The assay was carried out double blind to eliminate the
deviation induced by subjective factors. The test was repeated
three times.
Matrigel invasion assay
Cell invasion was detected by Transwell assay
(26,27). After pre-treatment with indicated
concentrations of ORI for 24 h, A375 or B16-F10 cells were
harvested and seeded to the upper chamber which was coated with
matrigel (BD Biosciences) at the density of 3×104
cells/well in serum free medium. The lower chambers were filled
with standard medium. The cells were allowed to invade for 24 h
incubated at 37°C in 5% CO2. The invading cells were
fixed with methanol. Cell numbers were counted in five separate
fields using the computer-based microcopy imaging system. The assay
was carried out double blind to eliminate the deviation induced by
subjective factors. The test was repeated three times.
Cell-matrix adhesion assay
After pre-treatment with indicated concentrations of
ORI for 24 h, A375 or B16-F10 cells were harvested, re-suspended in
serum free medium at the density of 2×105 cells/well and
seeded to the 24-well plates coated with fibronectin (10 ng/ml).
After further incubations for 5, 15 and 30 min, non-adherent cells
were removed by PBS washes. The adherent cells were fixed with
methanol and counted in five separate fields under a light
microscope (28,29). The assay was carried out double blind
to eliminate the deviation induced by subjective factors. The
experiment was repeated three times.
Quantitative real-time polymerase
chain reaction (RT-PCR)
After pre-treatment with indicated concentrations of
ORI for 24 h, The total RNA of A375 or B16-F10 cells was extracted
by TRIzol reagent (Roche, Suisse). Reverse transcription was
carried out with fast quant RT kit (Tiangen, Beijing, China). The
procedure was based on the protocol provided by Tiangen. The
real-time PCR mixture volume was 25 µl including 12.5 µl SYBR Green
mix, 0.2 µl cDNA, 1.5 µl primer per mix (10 µM each primer), and
10.8 µl RNAse-free H2O. The experiment was then set up
with the following PCR program on ABI 7500 (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA): 95°C for 15 min,
1 cycle; 40 cycles of 95°C for 10 sec, 60°C for 20 sec, 72°C for 30
sec. Specific primers were designed by gene runner software and
were synthesized by Beijing Aoke Biotechnology Co., Ltd. (Beijing,
China). The specific primers are reported in Table I. The Ct value was automatically
calculated by software, the Ct values were all normalized against
the quantity of the β-actin control RNA, and the relative
quantification of gene expression was calculated by the
2−ΔCt method according to the formula: ΔCt
(target gene) = Ct (target gene) - Ct (control gene). All assays
were performed in triplicate and independently repeated 3
times.
 | Table I.Primers sequences used in
quantitative polymerase chain reaction. |
Table I.
Primers sequences used in
quantitative polymerase chain reaction.
| Gene name | Primer sequence
(5′-3′) |
|---|
| E-cadherin | F:
GGATTGCAAATTCCTGCCATTC |
|
| R:
AACGTTGTCCCGGGTGTCA |
| Vimentin | F:
GCAGGAGGCAGAAGAATGGTA |
|
| R:
GGGACTCATTGGTTCCTTTAAGG |
| Snail | F:
TCTAGGCCCTGGCTGCTACAA |
|
| R:
CATCTGAGTGGGTCTGGAGGTG |
| β-catenin | F:
TGAGGACAAGCCACAAGATTAC |
|
| R: TCCACCAGAGTGAAAA
GAACG |
| β-actin | F:
CCACGAAACTACCTTCAACTCCA |
|
| R:
GTGATCTCCTTCTGCATCCTGTC |
Western blotting
Western blotting was used for detection of
expressions of E-cadherin, vimentin, Snail, PI3K, phospho-PI3K,
Akt, phospho-Akt, GSK-3β, and phospho-GSK-3β, anti-β-catenin in
A375 and B16-F10 cells. After pretreatment with indicated
concentrations of ORI and/or LY294002 (an inhibitor of PI3K) for 24
h, cells were then stimulated by 10 ng/ml TGF-β1 for 15 min. Then
the protein lysates from cultured cells were separated by 10%
sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE)
systems and transferred to polyvinyllidene difluoride (PVDF)
membranes (Millipore). After blocking with 5% skim milk in
Tris-buffered saline (TBS) containing 0.1% Tween-20 for 2 h, the
membranes were incubated with primary antibodies at 1:500-1:1,000
dilutions with 5% BSA in TBST overnight at 4°C. The antibodies were
as follows: E-cadherin, vimentin, Snail, PI3K, phospho-PI3K, Akt,
phospho-Akt, GSK-3β, phospho-GSK-3β, and anti-β-catenin. The blots
were washed and incubated with secondary antibodies conjugated with
horseradish peroxidase (HRP) and incubated for 1 h at room
temperature. Membranes were visualized using enhanced
chemiluminescence (immobilon ECL; Millipore) and were photographed
using G-BOX (Gene Company Ltd., Beijing, China). The bands were
analyzed by ImageJ software.
Statistical analysis
Each experiment was repeated at least three times.
All data were expressed as mean ± standard deviation (SD).
Statistical Product and Service Solutions (version 19.0; SPSS,
Inc., Chicago, IL, USA). P-value <0.05 was considered to
indicate a statistically significant difference using one-way
analysis of variance (ANOVA) test.
Results
Effects of ORI on the viability of
A375 and B16-F10 cells
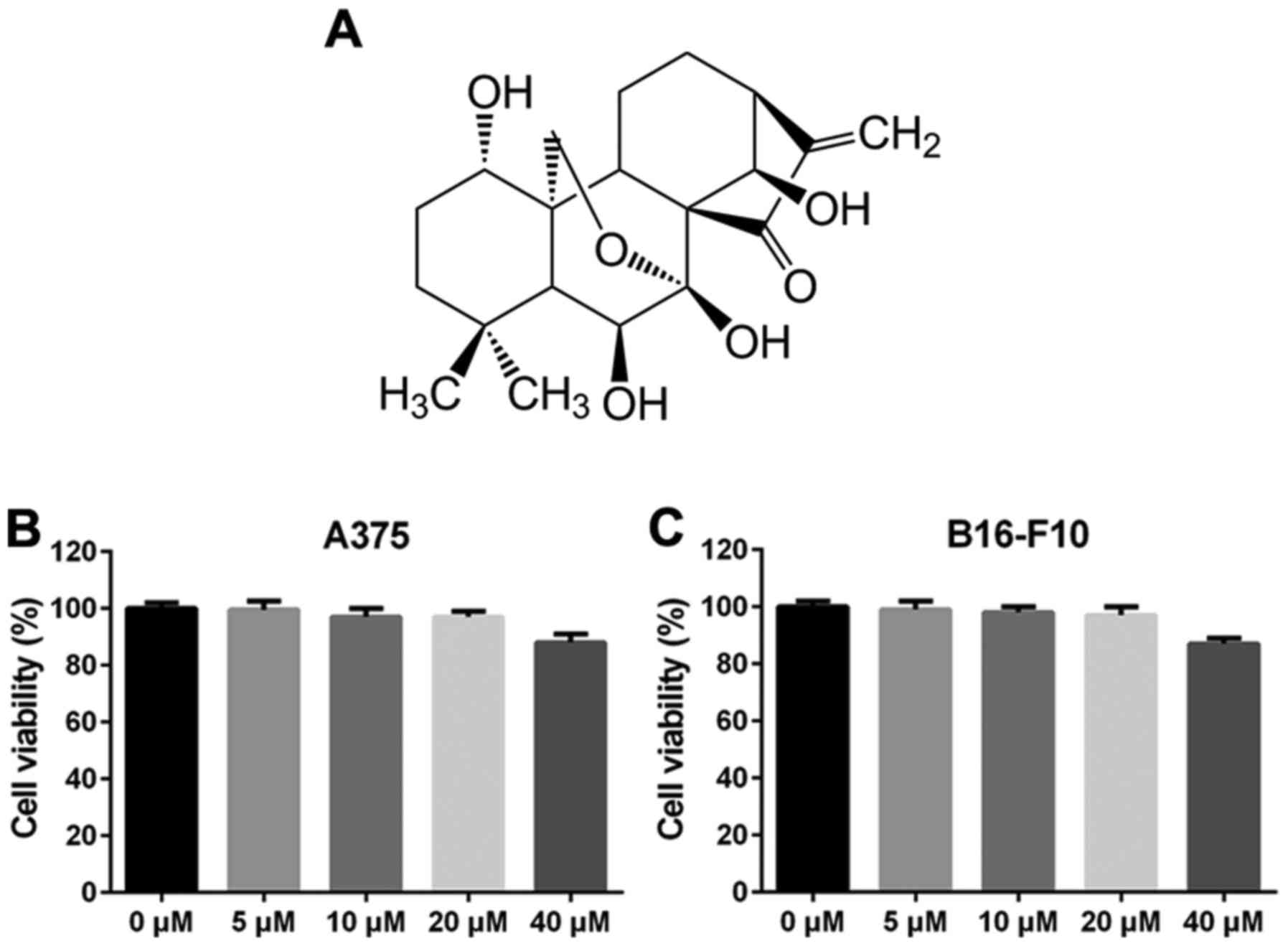
ORI, a diterpenoid purified from Rabdosia
rubescens, has a molecular weight of 364.44 g/mol and its
molecular structure is shown in Fig.
1A. The inhibitory effect of ORI on the proliferation of
melanoma cell lines A375 and B16-F10 were first detected by MTT
assay. As shown in Fig. 1B and C, the
survival rate of ORI (5–20 µM) treated groups were >90% at 48 h,
indicating that ORI at each of these concentrations alone had no
anti-proliferative effect and did not cause any apparently
cytotoxic effects in A375 and B16-F10 cells within 48 h. However,
when cells were treated with 40 µM of ORI for 48 h, the growth of
A375 and B16-F10 cells was inhibited by ORI, but the inhibition
rate of 40 µM for 48 h was only 15% (Fig.
1B and C). These data indicate that 48 h of ORI (5–20 µM)
exposure has no significantly influence on the cell viability of
A375 and B16-F10 cells. Thus, concentrations 5 and 10 µM of ORI
were used in the subsequent experiments.
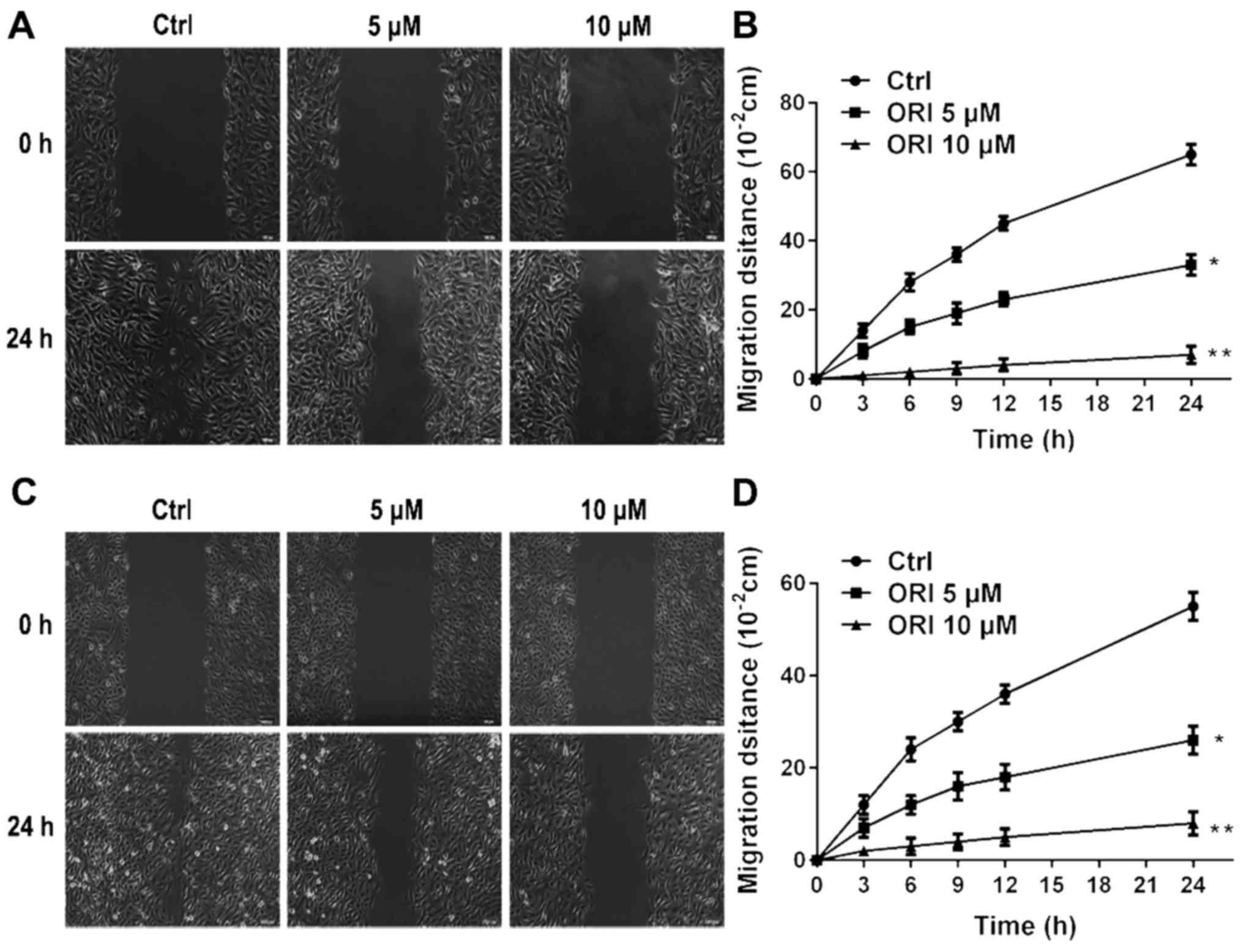
ORI inhibits migration and invasion of
A375 and B16-F10 cells
Cancer progression is associated with abrogation of
the normal controls that limit cell migration and invasion. Cell
invasion and migration are the initial and critical events in tumor
metastasis and enhance the ability of a cancer cell to enter and
exit the circulation to reach distant organs (30). The effect of ORI on cellular migration
was investigated using a classic in vitro wound healing
model. ORI was found to be effective in reducing cellular migration
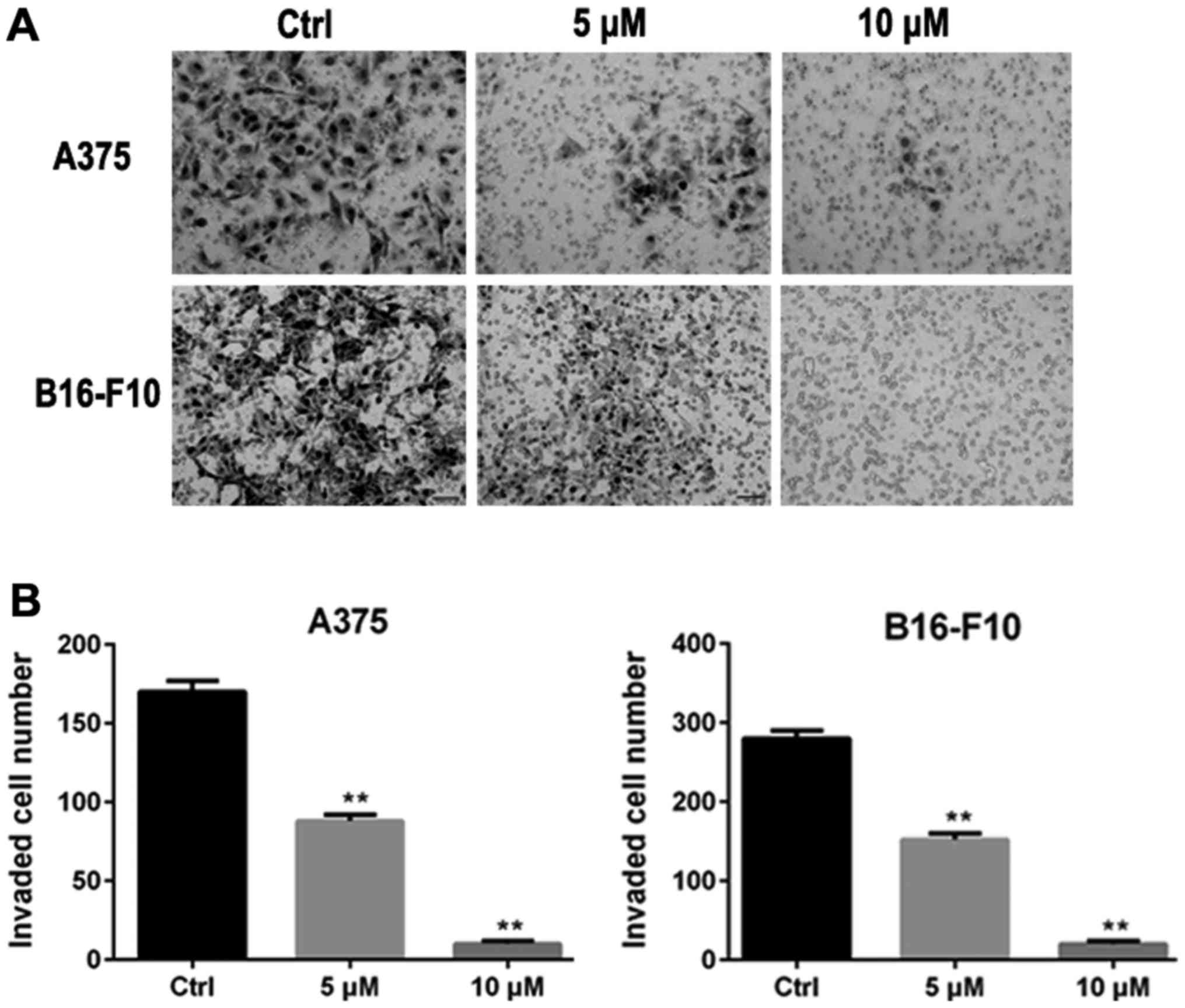
in A375 and B16-F10 cells (Fig. 2).
The inhibitory effect of ORI on invasion of A375 and B16-F10 cells
was examined using an invasion assay with matrigel-coated filters.
In the absence of ORI (control group), A375 and B16-F10 cells
displayed high invasive capability as indicated by being able to
completely penetrate through the matrigel-coated filters. Activity
of invasion of A375 and B16-F10 cells were markedly suppressed by
24 h exposure to ORI. At concentrations of 5 and 10 µM of ORI, the
number of cells able to penetrate through matrigel-coated filters
was significantly decreased compared with control group (Fig. 3).
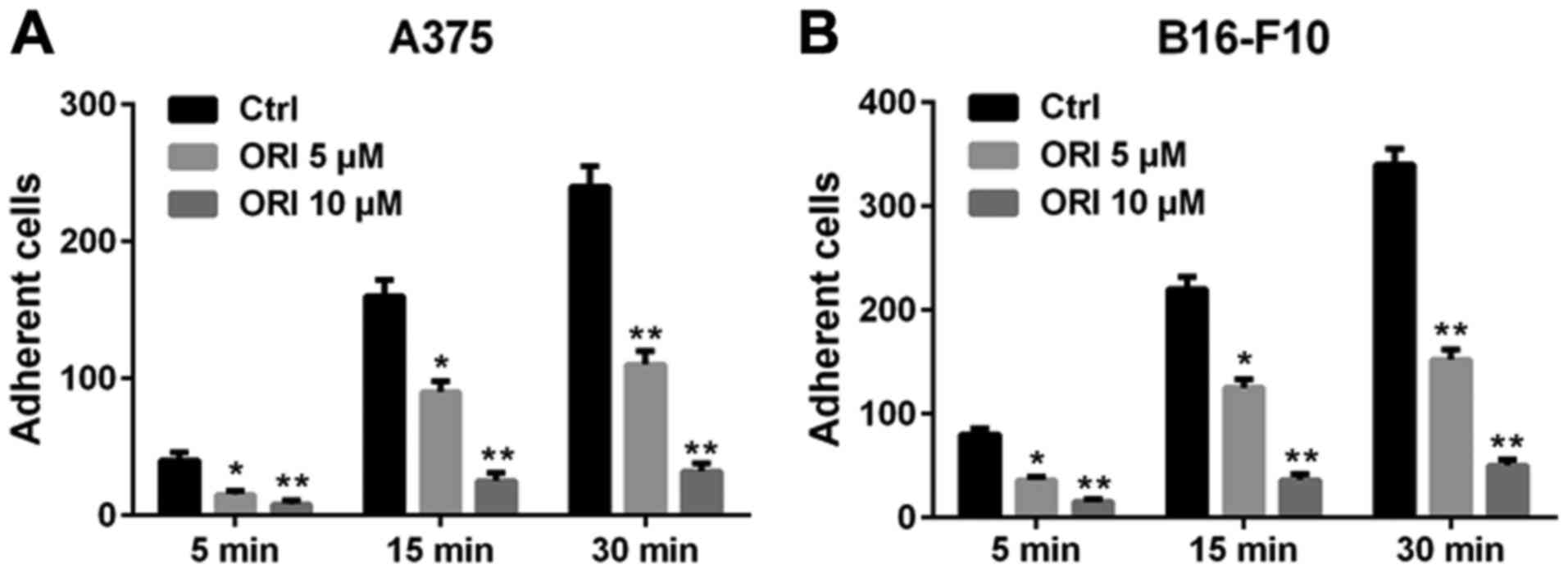
ORI inhibits adhesion in A375 and
B16-F10 cells
The altered adhesiveness of tumor cells plays an
important role in the formation of distal foci (31). The extracellular matrix (ECM) is a
powerful regulator of cancer progression, which promotes
transformation and regulates the tumor metastasis (32). Hence, we used fibronectin as the
basement membrane to mimic the adhesion of A375 and B16-F10 cells.
After the pre-treatment with indicated concentrations of ORI, the
number of cells adhering to the fibronectin significantly decreased
compared with the control group. Additional quantitative data are
shown in Fig. 4. These data indicate
that ORI inhibits the adhesiveness of A375 and B16-F10 cells
adhering to fibronectin.
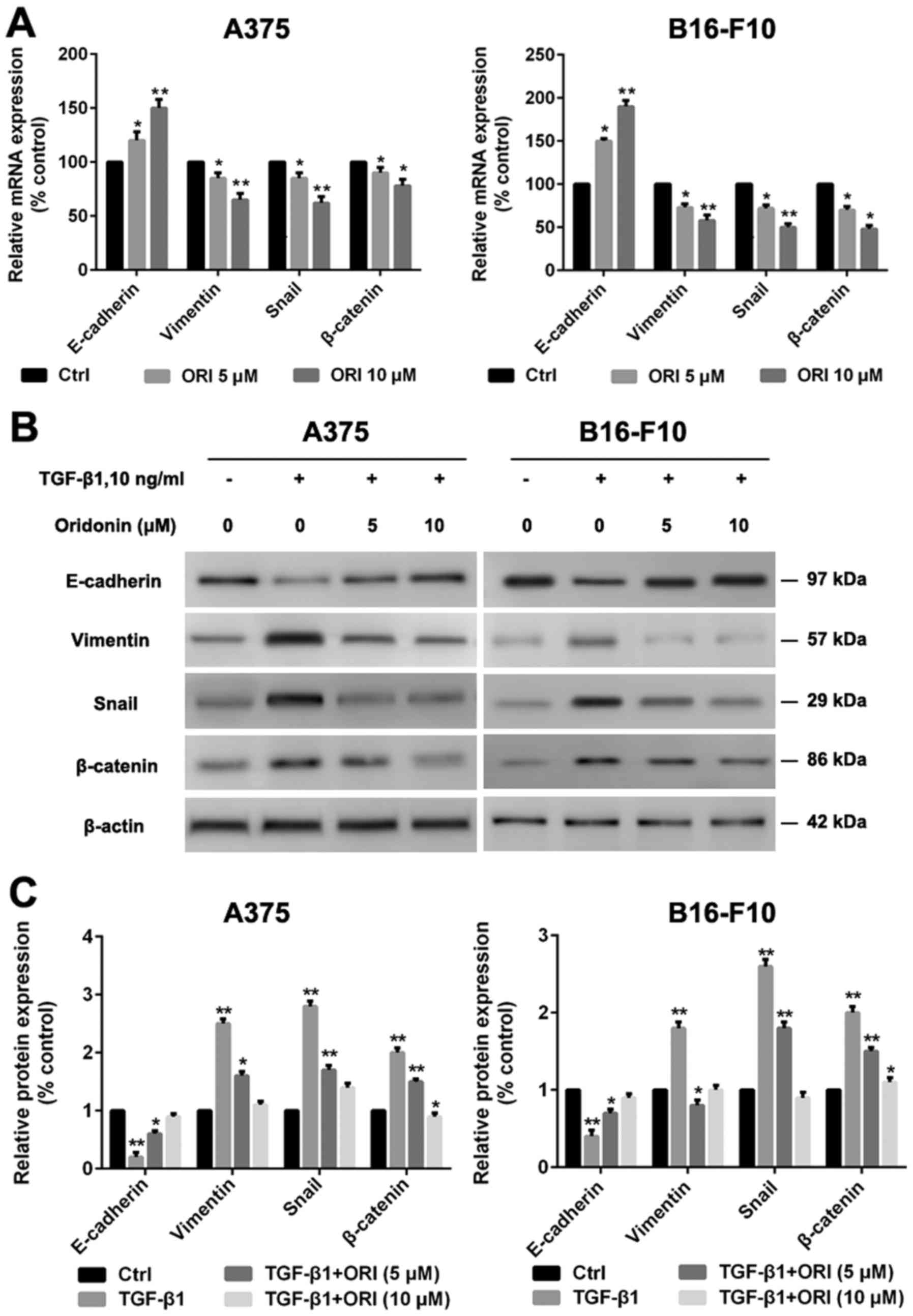
ORI regulates the expression of EMT
markers in A375 and B16-F10 cells
EMT plays a key role in cancer progression. Enhanced
cell migration and invasion properties are important consequences
of EMT (33). The most well
characterized factor responsible for induction of EMT is TGF-β1.
Snail as one of the transcriptional factors has been reported to be
involved in the regulation of E-cadherin (34). The accumulation of cytoplasmic
β-catenin and its subsequent nuclear translocation is also a key
event in EMT (35). Here we
investigated the effect of ORI on TGF-β1-mediated EMT in A375 and
B16-F10 cells by qRT-PCR and western blotting. As shown in Fig. 5, TGF-β1 reduced the expression of the
epithelial marker E-cadherin and enhanced the expression of the
mesenchymal marker vimentin. Snail and β-catenin expression levels
were also enhanced, induced by TGF-β1. Our data demonstrate that
the effects of ORI are dose dependent increasing the expression of
E-cadherin and decreasing the expression of vimentinin, Snail and
β-catenin in A375 and B16-F10 cells compared with control
group.
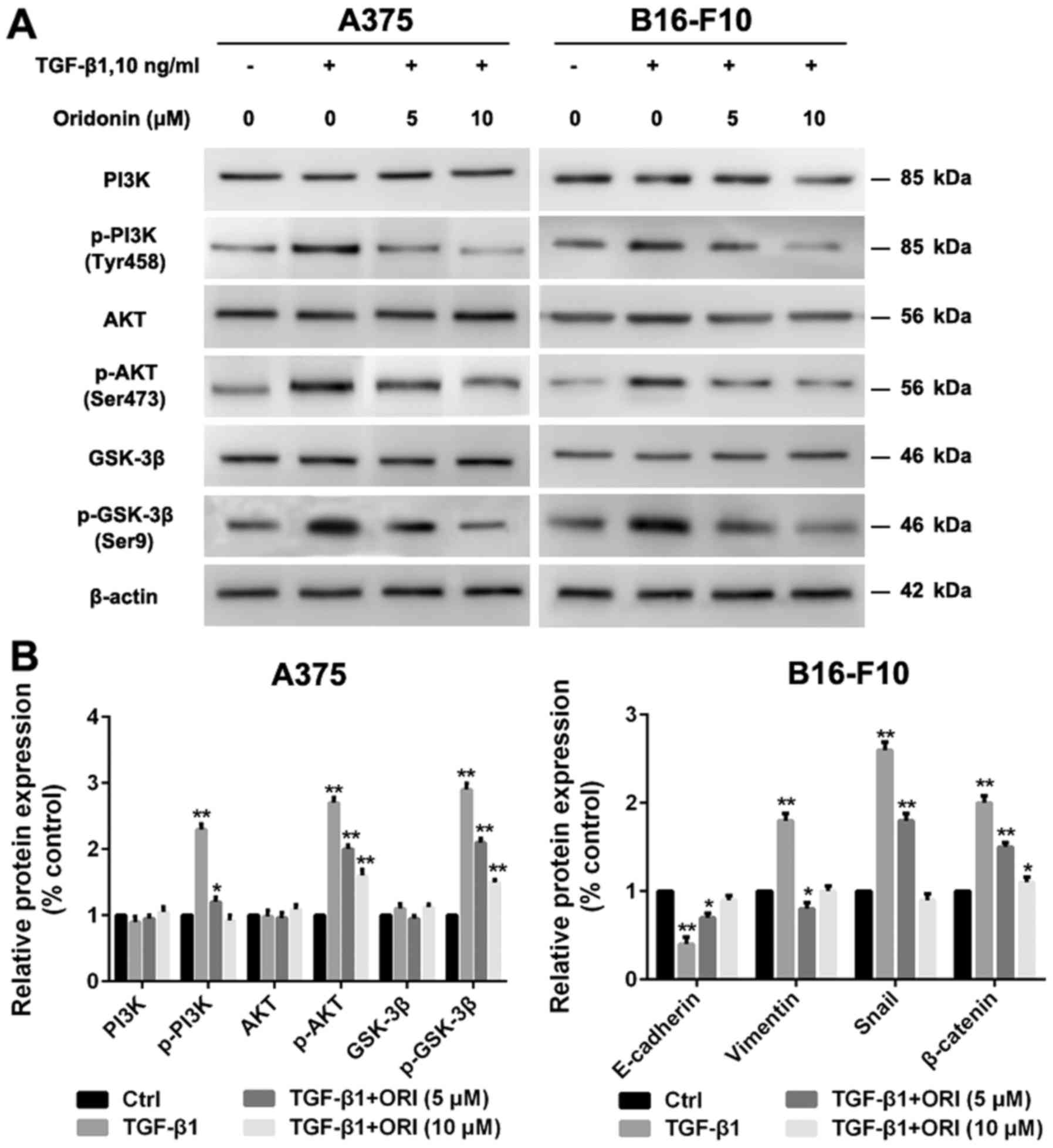
ORI suppresses the PI3K/Akt/GSK-3β
signaling pathway in A375 and B16-F10 cells
Recent studies demonstrated that aberration of
PI3K/Akt/GSK-3β signaling pathway is a very common mechanism for
many human cancers including melanoma, since it can mediate
survival, apoptosis, migration and invasion pathways (36). A growing number of studies showed that
inhibition of PI3K/Akt/GSK-3β pathway could inhibit the migration
and invasion of cancer cells (37,38).
Hence, we investigate the effects of ORI on the PI3K/Akt/GSK-3β
signaling pathway in A375 and B16-F10 cells. As shown in Fig. 6, TGF-β1 significantly increased the
protein expression levels of p-PI3K, p-Akt and p-GSK-3β in both
types of cells. Our studies have demonstrated that ORI has a
significant inhibitory effect on TGF-β1-mediated EMT, but the
detailed effect mechanism is unclear. In the present study, we
firstly examined the inhibitory activity of ORI against
TGF-β1-induced PI3K/Akt/GSK-3β pathway phosphorylation. The data
demonstrated that ORI could downregulate the levels of p-PI3K,
p-Akt and p-GSK-3β. It is necessary to verify whether ORI could
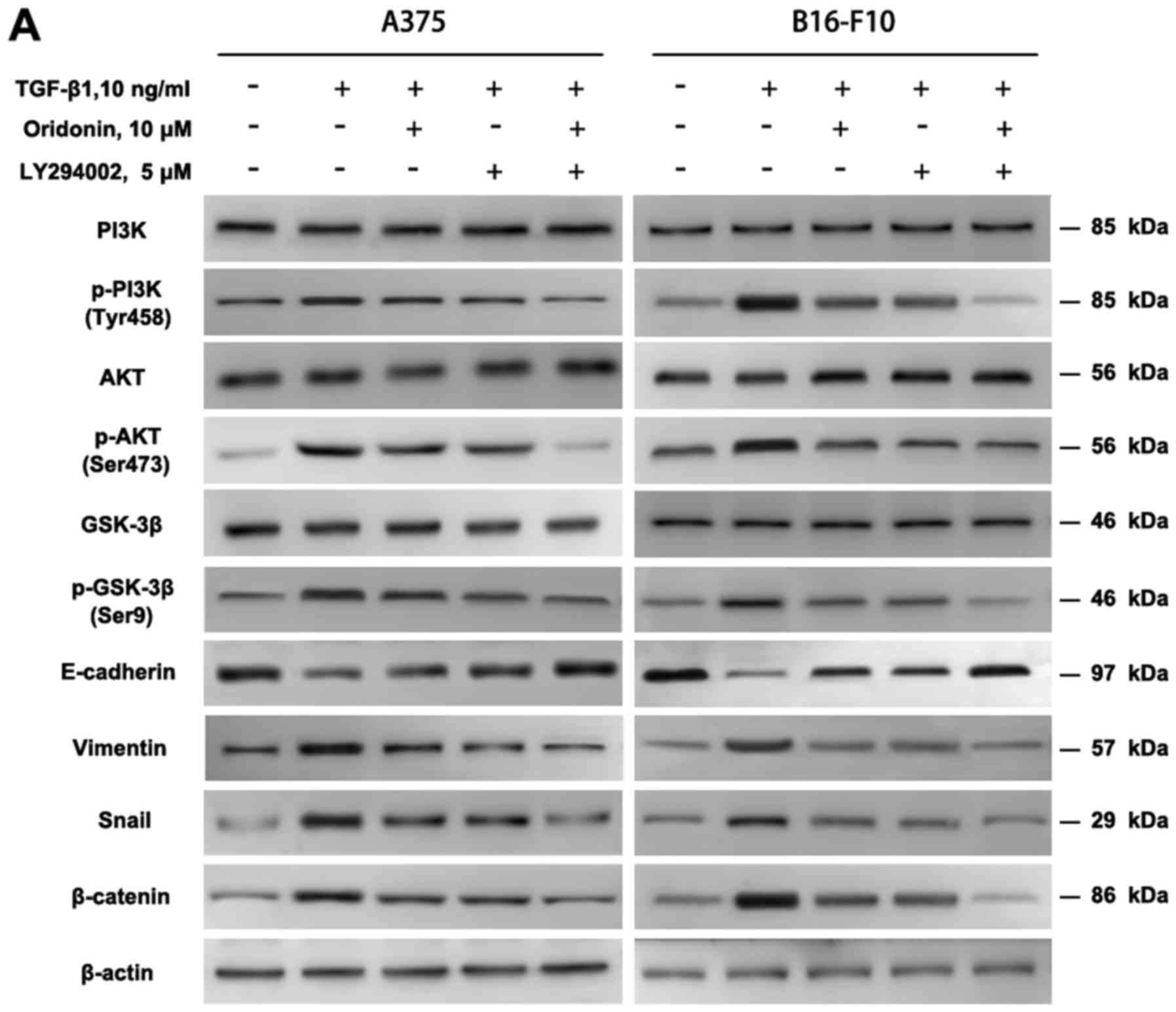
inhibit the EMT through TGF-β1 mediated PI3K/Akt/GSK-3β pathway. To
verify this hypothesis, PI3K inhibitor LY294002 was used to block
the PI3K/Akt pathway, then ORI and TGF-β1 were added. The data
demonstrated that, LY294002 and ORI abolished the TGF-β1-induced
decrease in E-cadherin and increase in vimentin, p-PI3K, p-Akt,
p-GSK-3β, Snail and β-catenin expression. ORI had little effect on
E-cadherin, Vimentin, p-PI3K, p-Akt, p-GSK-3β and β-catenin
(Fig. 7). There was no difference
between their effects. ORI combined with LY294002 synergistically
suppressed the related protein expression. The above data suggest
that ORI may regulate the expression of E-cadherin and vimentin may
work through PI3K/Akt/GSK-3β signaling pathway, thereby in
inhibiting TGF-β1-induced EMT, which may be one of the mechanisms
through which ORI inhibited the A375 and B16-F10 cells migration
and invasion.
Discussion
Melanoma is the most aggressive skin cancer and
well-known for its poor prognosis and low survival rate (39). The high rate of metastasis is the main
cause of death in patients with melanoma. Increased evidence
demonstrates that mechanisms linked to melanoma metastasis are the
induction of EMT, which is characterized by loss of epithelial
phenotype, and acquiring fibroblast-like properties (40). They also exhibit reduced cell-cell
adhesion and enhanced cell motility. Thus, exploring novel ways to
inhibit or even reverse EMT process has attracted more
attention.
Rabdosia rubescens is a well-known Chinese
folk herbal medicine. For centuries, Rabdosia rubescens has
been mainly used for inflammatory disorders such as sore throats
and tonsillitis. It is suggested that the extraction of Rabdosia
rubescens could delay tumor progress, improve patients quality
of life and survival rates (41).
ORI, an ent-kaurene diterpenoid, is the main active ingredient
found in Rabdosia rubescens. Recently, ORI has attracted
more and more attention due to its excellent antitumor activity
against human melanoma, cervical, ovarian and hepatocellular
cancers (21,42). The anticancer mechanisms are mainly
associated with triggering apoptosis, cell cycle arrest as well as
autophagy and producing reactive oxygen species (43). Thus, the molecular mechanisms of
anticancer activities of ORI are numerous and varied, depending on
the cancer cell type or cell environment. In this study, our
results first demonstrated the anti-metastatic capabilities of ORI
and provided possible mechanisms responsible for its effects in
melanoma cell lines.
In present study, ORI was dissolved in 0.5% DMSO
formed a stable and clear solution without micellar structure
formation. As the solution of ORI added into the culture medium,
the concentrations of DMSO was diluted 100 times (e.g., we added 30
µl ORI solution into the 3 ml culture medium for vitro migration
assay). Hence, the final volume ratio of DMSO in culture medium was
0.005%. As the MTT assay shown (Fig. 1B
and C) the solvent control (added into the culture medium with
0.5% DMSO) has no significantly influence on the cell viability of
A375 and B16-F10 cells. As the literature reported, the cytotoxic
activity of ORI varies a lot in different types of cancer cells
(42,44). Firstly, we evaluated the ORI's effect
on melanoma cells proliferation by MTT assay aiming to exclude
interference of cell proliferation on the results of motility
properties assays. The melanoma cells proliferation was slightly
affected by ORI at concentrations 5 and 10 µM for a 48-h treatment,
which is long enough for the subsequent motility properties assays.
The anti-proliferation effect of one compound have relationship
with many factors including the types of cells, the sensitivity of
cells, the density of cells seeded in the plates, the time of the
treatment and the reagents concentration used in the assay etc
(45,46). Our data showed that the viability of
A375 and B16-F10 melanoma cells were not allergic to ORI's
treatment compared with other cancer cell lines (47,48). As
the literature reported that ROS represents an initial and
independent apoptosis pathway in melanoma cells (49). So we speculate that ORI can not
trigger the ROS system inducing the apoptosis pathway in melanoma
cells at lower concentrations (5 and 10 µM). The metastatic
potential of cancer cells is mainly determined by their invasion,
migration and adhesion abilities, which are commonly tested
utilizing transwell systems with Matrigel-coated filters, wounding
healing and adhesion assay. In this study, our results demonstrated
that ORI at non-cytotoxic concentrations 5 and 10 µM could
significantly inhibit A375 and B16-F10 cells invasion, migration
and adhesion in vitro (Figs.
2–4).
Molecules involving migration, invasion and adhesion
of melanoma cells not only E-cadherin, vimentin, β-catenin and
Snail. As we known, cancer metastasis is a complex process. EMT is
positively correlated with the increased migration and invasion of
cancer cells. During EMT, the switch of the immobile
epithelial-like cancer cells to a motile mesenchymal-like phenotype
requires alterations in migration, invasion and adhesion. Cells
lose their epithelial traits and acquire mesenchymal
characteristics, such as loss of the epithelial markers
(E-cadherin, ZO-1, and β-catenin) and gain of the mesenchymal
markers (N-cadherin and vimentin) (50). Thus, generating a migratory phenotype.
Melanoma cells have morphological changes after EMT (51). It also has been noted that the
transcription factors, such as Snail, Slug and NF-κB etc. play
important roles in regulating EMT. Snail is able to downregulate
E-cadherin expression by binding to E-boxes in the E-cadherin
promoter (52). Accordingly, we chose
the classic EMT markers E-cadherin, β-catenin, vimentin and Snail
for evaluation of the effect of ORI on EMT. TGF-β1 is the most well
characterized factor responsible for induction of EMT (53,54). In
our preliminary experiment, without the addition of TGF-β1, the EMT
marker not changed obviously in A375 and B16-F10 cells, so we can
not well evaluated the ORI's effect on EMT (data not shown). As the
data shown in Figs. 5–7, the addition of TGF-β1 (10 ng/ml) induced
the EMT and activated the PI3K/Akt/GSK-3β1 signaling. Our results
demonstrated that ORI could inhibit the TGF-β1-induced EMT by
inhibiting the activity of PI3K/Akt/GSK-3β signaling pathway. We
did not design the group only exposure to ORI in this experiment.
In the further study, we will set one group only exposure to ORI.
The present data demonstrated that ORI markedly increase levels of
E-cadherin and decrease levels of vimentin, Snail and β-catenin in
A375 and B16-F10 cells (Figs. 5 and
6). These data indicate that ORI
negatively regulates EMT, and markedly attenuates melanoma cells
invasion, migration and adhesion in vitro.
To further understanding the cellular mechanisms
responsible for our findings, we examined the status of the
PI3K/Akt/GSK-3β signaling pathways associated with cancer cell
invasion and metastasis (55). The
PI3K/Akt signalling pathway plays an important role in several
cellular physiological activities including proliferation,
migration, invasion and adhesion; activation of this pathway by
TGF-β1 has been confirmed during the EMT process (56). Hyperactivation of PI3K/Akt pathway has
previously been reported in numerous human cancers. The PI3K/Akt
pathway exerts its effects in cells by phosphorylating a variety of
downstream molecules including GSK-3β in response to various
stimuli. GSK-3β, a major downstream target of Akt, is necessary for
the maintenance of epithelial architecture, and inhibition of
GSK-3β activity or expression results in a bona fide EMT (57). The active form of GSK-3β is in the
dephosphorylated status, when it is phosphorylated by Akt at
residue Ser-9, GSK-3β loses its activity. Furthermore, GSK-3β
negatively regulates the transcription of Snail, a repressor of
E-cadherin and an inducer of the EMT. Hence, inactivation of the
PI3K/Akt signaling pathway could promote GSK-3β activity and
suppress the expression of Snail, which in turn impedes the EMT
(58). β-catenin signaling plays
important roles in carcinogenesis and metastasis. Within the
nucleus, β-catenin binds to the nuclear transcription factors, T
cell factor/lymphoid enhancer factor (TCF/LEF), and regulates
expression of various downstream adhesion junction genes including
vimentin (59). In this study, our
data demonstrated that ORI significantly inhibits phosphorylation
of PI3K, phosphorylation of Akt, phosphorylation of GSK-3β, and
expression of Snail and β-catenin (Fig.
7). These results suggest that ORI may inhibit EMT in melanoma
cells through attenuating the PI3K/Akt/GSK-3β signaling
pathway.
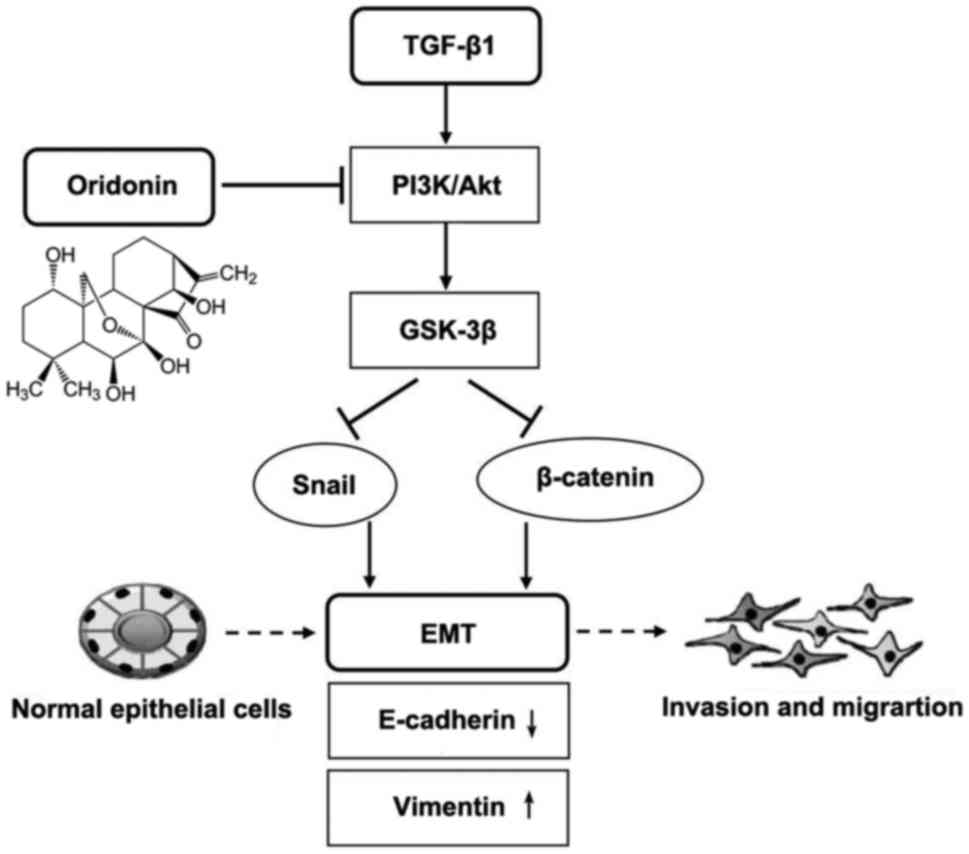
In conclusion, the findings of the present study
indicate that ORI could effectively inhibit migration, invasion and
adhesion by inhibiting EMT in melanoma cells through its inhibition
on the phosphorylation of PI3K, Akt, and GSK-3β in the presence of
TGF-β1. TGF-β1 promoted the phosphorylation of PI3K/Akt, and then
PI3K/Akt promoted the phosphorylation of GSK-3β, in agreement with
the results in Fig. 7A and B. As
shown in Fig. 8, signaling mechanisms
underline how ORI ameliorates TGF-β1-induced EMT. Owing to the
toxicity and attractive anticancer activity, ORI has become a
promising anti-metastasis candidate compound for melanoma therapy.
However, the effects and mechanisms of ORI need to be further
elucidated in animal models and clinical trials. In addition, our
results shed light on novel therapeutics in the prevention of
melanoma cancer recurrence and metastasis.
Acknowledgements
This study was supported by the Natural Science
Foundation of Tianjin Medical University (no. 2015KYZQ13),
Postdoctoral Science Foundation of China (no. 2016M591398) and
Basic Scientific Research Fund of Tianjin Medical University (no.
2016YD07).
References
|
1
|
Aviles-Izquierdo JA, Molina-Lopez I,
Rodriguez-Lomba E, Marquez-Rodas I, Suarez-Fernandez R and
Lazaro-Ochaita P: Who detects melanoma? Impact of detection
patterns on characteristics and prognosis of patients with
melanoma. J Am Acad Dermatol. 75:967–974. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bowyer S, Prithviraj P, Lorigan P, Larkin
J, McArthur G, Atkinson V, Millward M, Khou M, Diem S, Ramanujam S,
et al: Efficacy and toxicity of treatment with the anti-CTLA-4
antibody ipilimumab in patients with metastatic melanoma after
prior anti-PD-1 therapy. Br J Cancer. 114:1084–1089. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duan H, Ma L, Liu H, Zhang Y, Zhang Z, Yan
X and Li X: Tanshinone IIA attenuates epithelial-mesenchymal
transition to inhibit the tracheal narrowing. J Surg Res.
206:252–262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ling G, Ji Q, Ye W, Ma D and Wang Y:
Epithelial-mesenchymal transition regulated by p38/MAPK signaling
pathways participates in vasculogenic mimicry formation in SHG44
cells transfected with TGF-β cDNA loaded lentivirus in vitro and in
vivo. Int J Oncol. 49:2387–2398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma Y, Xu X and Luo M: CXCR6 promotes tumor
cell proliferation and metastasis in osteosarcoma through the Akt
pathway. Cell Immunol. 311:80–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le Coz V, Zhu C, Devocelle A, Vazquez A,
Boucheix C, Azzi S, Gallerne C, Eid P, Lecourt S and Giron-Michel
J: IGF-1 contributes to the expansion of melanoma-initiating cells
through an epithelial-mesenchymal transition process. Oncotarget.
7:82511–82527. 2016.PubMed/NCBI
|
|
8
|
Mao XY, Li QQ, Gao YF, Zhou HH, Liu ZQ and
Jin WL: Gap junction as an intercellular glue: Emerging roles in
cancer EMT and metastasis. Cancer Lett. 381:133–137. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Menezes ME, Shen XN, Das SK, Emdad L,
Sarkar D and Fisher PB: MDA-9/Syntenin (SDCBP) modulates small
GTPases RhoA and Cdc42 via transforming growth factor β1 to enhance
epithelial-mesenchymal transition in breast cancer. Oncotarget.
7:80175–80189. 2016.PubMed/NCBI
|
|
10
|
Da C, Liu Y, Zhan Y, Liu K and Wang R:
Nobiletin inhibits epithelial-mesenchymal transition of human
non-small cell lung cancer cells by antagonizing the TGF-β1/Smad3
signaling pathway. Oncol Rep. 35:2767–2774. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim YJ, Jeon Y, Kim T, Lim WC, Ham J, Park
YN, Kim TJ and Ko H: Combined treatment with zingerone and its
novel derivative synergistically inhibits TGF-β1 induced
epithelial-mesenchymal transition, migration and invasion of human
hepatocellular carcinoma cells. Bioorg Med Chem Lett. 27:1081–1088.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Yuan X, Li W, Cao Q and Shu Y:
Aspirin-triggered resolvin D1 inhibits TGF-β1-induced EMT through
the inhibition of the mTOR pathway by reducing the expression of
PKM2 and is closely linked to oxidative stress. Int J Mol Med.
38:1235–1242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Zhang C, Xu L, Zang K, Ning Z,
Jiang F, Chi H, Zhu X and Meng Z: Bufalin suppresses hepatocellular
carcinoma invasion and metastasis by targeting HIF-1α via the
PI3K/AKT/mTOR pathway. Oncotarget. 7:20193–20208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang CC, Ling XH, Hsu HF, Wu JM, Wang CP,
Yang JF, Fang LW and Houng JY: Siegesbeckia orientalis extract
inhibits TGFβ1-induced migration and invasion of endometrial cancer
cells. Molecules. 21:E10212016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng LX, Sun P, Mi T, Liu M, Liu W, Yao S,
Cao YM, Yu XL, Wu WY, Jiang BH, et al: Agglutinin isolated from
Arisema heterophyllum Blume induces apoptosis and autophagy in A549
cells through inhibiting PI3K/Akt pathway and inducing ER stress.
Chin J Nat Med. 14:856–864. 2016.PubMed/NCBI
|
|
16
|
Li D, Han T, Liao J, Hu X, Xu S, Tian K,
Gu X, Cheng K, Li Z, Hua H and Xu J: Oridonin, a promising
ent-Kaurane diterpenoid lead compound. Int J Mol Sci. 17:E13952016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li D, Han T, Xu S, Zhou T, Tian K, Hu X,
Cheng K, Li Z, Hua H and Xu J: Antitumor and antibacterial
derivatives of oridonin: A main composition of Dong-Ling-Cao.
Molecules. 21:E5752016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu J, Chen X, Qu S, Yao B, Xu Y, Wu J, Jin
Y and Ma C: Oridonin induces G2/M cell cycle arrest and apoptosis
via the PI3K/Akt signaling pathway in hormone-independent prostate
cancer cells. Oncol Lett. 13:2838–2846. 2017.PubMed/NCBI
|
|
19
|
Wang XH, Zhang SF, Bao JT and Liu FY:
Oridonin synergizes with Nutlin-3 in osteosarcoma cells by
modulating the levels of multiple Bcl-2 family proteins. Tumour
Biol. 39:10104283177016382017.PubMed/NCBI
|
|
20
|
Xia S, Zhang X, Li C and Guan H: Oridonin
inhibits breast cancer growth and metastasis through blocking the
Notch signaling. Saudi Pharm J. 25:638–643. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Wang L, Zi Y, Zhang L, Guo Y and
Huang Y: Oridonin effectively reverses the drug resistance of
cisplatin involving induction of cell apoptosis and inhibition of
MMP expression in human acute myeloid leukemia cells. Saudi J Biol
Sci. 24:678–686. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gu Z, Wang X, Qi R, Wei L, Huo Y, Ma Y,
Shi L, Chang Y, Li G and Zhou L: Oridonin induces apoptosis in
uveal melanoma cells by upregulation of Bim and downregulation of
fatty acid synthase. Biochem Biophys Res Commun. 457:187–193. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang HJ, Li D, Yang FY, Tashiro S, Onodera
S and Ikejima T: Oridonin induces human melanoma A375-S2 cell death
partially through inhibiting insulin-like growth factor 1 receptor
signaling. J Asian Nat Prod Res. 10:787–798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang G, Li J, Zhang L, Huang S and Zhao X
and Zhao X: Celecoxib induced apoptosis against different breast
cancer cell lines by down-regulated NF-κB pathway. Biochem Biophys
Res Commun. 490:969–976. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang JJ, Sanderson BJ and Zhang W:
Significant anti-invasive activities of α-mangostin from the
mangosteen pericarp on two human skin cancer cell lines. Anticancer
Res. 32:3805–3816. 2012.PubMed/NCBI
|
|
26
|
Cui S, Wang J, Wu Q, Qian J, Yang C and Bo
P: Genistein inhibits the growth and regulates the migration and
invasion abilities of melanoma cells via the FAK/paxillin and MAPK
pathways. Oncotarget. 8:21674–21691. 2017.PubMed/NCBI
|
|
27
|
Wu ZY, Lien JC, Huang YP, Liao CL, Lin JJ,
Fan MJ, Ko YC, Hsiao YP, Lu HF and Chung JG: Casticin inhibits
A375.S2 human melanoma cell migration/invasion through
downregulating NF-κB and matrix metalloproteinase-2 and −1.
Molecules. 21:3842016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abu R, Jiang Z, Ueno M, Isaka S, Nakazono
S, Okimura T, Cho K, Yamaguchi K, Kim D and Oda T: Anti-metastatic
effects of the sulfated polysaccharide ascophyllan isolated from
Ascophyllum nodosum on B16 melanoma. Biochem Biophys Res Commun.
458:727–732. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saviola AJ, Burns PD, Mukherjee AK and
Mackessy SP: The disintegrin tzabcanin inhibits adhesion and
migration in melanoma and lung cancer cells. Int J Biol Macromol.
88:457–464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao S, Wang J and Qin C: Blockade of
CXCL12/CXCR4 signaling inhibits intrahepatic cholangiocarcinoma
progression and metastasis via inactivation of canonical Wnt
pathway. J Exp Clin Cancer Res. 33:1032014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsu YY, Shi GY, Wang KC, Ma CY, Cheng TL
and Wu HL: Thrombomodulin promotes focal adhesion kinase activation
and contributes to angiogenesis by binding to fibronectin.
Oncotarget. 7:68122–68139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shih YL, Chou HM, Chou HC, Lu HF, Chu YL,
Shang HS and Chung JG: Casticin impairs cell migration and invasion
of mouse melanoma B16F10 cells via PI3K/AKT and NF-κB signaling
pathways. Environ Toxicol. 32:2097–2112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ruan JS, Liu YP, Zhang L, Yan LG, Fan FT,
Shen CS, Wang AY, Zheng SZ, Wang SM and Lu Y: Luteolin reduces the
invasive potential of malignant melanoma cells by targeting β3
integrin and the epithelial-mesenchymal transition. Acta Pharmacol
Sin. 33:1325–1331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng J, Cen J, Li J, Zhao R, Zhu C, Wang
Z, Xie J and Tang W: Histone deacetylase inhibitor valproic acid
(VPA) promotes the epithelial mesenchymal transition of colorectal
cancer cells via up regulation of Snail. Cell Adh Migr. 9:495–501.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pearlman RL, Montes de Oca MK, Pal HC and
Afaq F: Potential therapeutic targets of epithelial-mesenchymal
transition in melanoma. Cancer Lett. 391:125–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li F, Wang Y and Yan Y: Gambogenic acid
induces cell growth inhibition, cell cycle arrest and metastasis
inhibition in choroidal melanoma in a dose-dependent manner. Exp
Ther Med. 13:2456–2462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu Y, Cheng Y, Guo Y, Chen J, Chen F, Luo
R and Li A: Protein kinase D2 contributes to TNF-α-induced
epithelial mesenchymal transition and invasion via the
PI3K/GSK-3β/β-catenin pathway in hepatocellular carcinoma.
Oncotarget. 7:5327–5341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW,
Wang Z, Fan J, Dai Z and Zhou J: CXCR2/CXCL5 axis contributes to
epithelial-mesenchymal transition of HCC cells through activating
PI3K/Akt/GSK-3β/Snail signaling. Cancer Lett. 358:124–135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sadok A, McCarthy A, Caldwell J, Collins
I, Garrett MD, Yeo M, Hooper S, Sahai E, Kuemper S, Mardakheh FK
and Marshall CJ: Rho kinase inhibitors block melanoma cell
migration and inhibit metastasis. Cancer Res. 75:2272–2284. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Noguchi K, Dalton AC, Howley BV, McCall
BJ, Yoshida A, Diehl JA and Howe PH: Interleukin-like EMT inducer
regulates partial phenotype switching in MITF-low melanoma cell
lines. PLoS One. 12:e01778302017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Miao M, Yan X, Guo L and Shao S: Effects
of the Rabdosia rubescens total flavonoids on focal cerebral
ischemia reperfusion model in rats. Saudi Pharm J. 25:607–614.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ren CM, Li Y, Chen QZ, Zeng YH, Shao Y, Wu
QX, Yuan SX, Yang JQ, Yu Y, Wu K, et al: Oridonin inhibits the
proliferation of human colon cancer cells by upregulating BMP7 to
activate p38 MAPK. Oncol Rep. 35:2691–2698. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao J, Zhang M, He P, Zhao J, Chen Y, Qi
J and Wang Y: Proteomic analysis of oridonin-induced apoptosis in
multiple myeloma cells. Mol Med Rep. 15:1807–1815. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hao Y, Zhao F, Luo Y, Zhang M and Li S:
Inhibitory effect of oridonin on proliferation of RPMI8226 cells
and the possible underlying mechanism. J Tradit Chin Med.
36:225–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Staton CA, Reed MW and Brown NJ: A
critical analysis of current in vitro and in vivo angiogenesis
assays. Int J Exp Pathol. 90:195–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nakamura K, Peng Y, Utsumi F, Tanaka H,
Mizuno M, Toyokuni S, Hori M, Kikkawa F and Kajiyama H: Novel
intraperitoneal treatment with non-thermal plasma-activated medium
inhibits metastatic potential of ovarian cancer cells. Sci Rep.
7:60852017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu QX, Yuan SX, Ren CM, Yu Y, Sun WJ, He
BC and Wu K: Oridonin upregulates PTEN through activating p38 MAPK
and inhibits proliferation in human colon cancer cells. Oncol Rep.
35:3341–3348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xia R, Chen SX, Qin Q, Chen Y, Zhang WW,
Zhu RR and Deng AM: Oridonin suppresses proliferation of human
ovarian cancer cells via blockage of mTOR signaling. Asian Pac J
Cancer Prev. 17:667–671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bauer D, Werth F, Nguyen HA, Kiecker F and
Eberle J: Critical role of reactive oxygen species (ROS) for
synergistic enhancement of apoptosis by vemurafenib and the
potassium channel inhibitor TRAM-34 in melanoma cells. Cell Death
Dis. 8:e25942017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang Y, Sun Y, Wu Y and Zhang J:
Cucurbitacin E inhibits osteosarcoma cells proliferation and
invasion through attenuation of PI3K/AKT/mTOR signaling. Biosci
Rep. 36:e004052016. View Article : Google Scholar
|
|
51
|
Cha BK, Kim YS, Hwang KE, Cho KH, Oh SH,
Kim BR, Jun HY, Yoon KH, Jeong ET and Kim HR: Celecoxib and
sulindac inhibit TGF-β1-induced epithelial-mesenchymal transition
and suppress lung cancer migration and invasion via downregulation
of sirtuin 1. Oncotarget. 7:57213–57227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee YJ and Han HJ: Troglitazone
ameliorates high glucose-induced EMT and dysfunction of SGLTs
through PI3K/Akt, GSK-3β, Snail1, and β-catenin in renal proximal
tubule cells. Am J Physiol Renal Physiol. 298:F1263–F1275. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Baek SH, Ko JH, Lee JH, Kim C, Lee H, Nam
D, Lee J, Lee SG, Yang WM, Um JY, et al: Ginkgolic acid inhibits
invasion and migration and TGF-β-induced EMT of lung cancer cells
through PI3K/Akt/mTOR inactivation. J Cell Physiol. 232:346–354.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Petanidis S, Kioseoglou E, Domvri K,
Zarogoulidis P, Carthy JM, Anestakis D, Moustakas A and Salifoglou
A: In vitro and ex vivo vanadium antitumor activity in
(TGF-β)-induced EMT. Synergistic activity with carboplatin and
correlation with tumor metastasis in cancer patients. Int J Biochem
Cell Biol. 74:121–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Balakrishnan S, Mukherjee S, Das S, Bhat
FA, Raja Singh P, Patra CR and Arunakaran J: Gold
nanoparticles-conjugated quercetin induces apoptosis via inhibition
of EGFR/PI3K/Akt-mediated pathway in breast cancer cell lines
(MCF-7 and MDA-MB-231). Cell Biochem Funct. 35:217–231. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen XH, Lu LL, Ke HP, Liu ZC, Wang HF,
Wei W, Qi YF, Wang HS, Cai SH and Du J: The TGF-β-induced
up-regulation of NKG2DLs requires AKT/GSK-3β-mediated stabilization
of SP1. J Cell Mol Med. 21:860–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
He F, Chen H, Yang P, Wu Q, Zhang T, Wang
C, Wei J, Chen Z, Hu H, Li W and Cao J: Gankyrin sustains
PI3K/GSK-3β/β-catenin signal activation and promotes colorectal
cancer aggressiveness and progression. Oncotarget. 7:81156–81171.
2016.PubMed/NCBI
|
|
58
|
Qin CD, Ma DN, Ren ZG, Zhu XD, Wang CH,
Wang YC, Ye BG, Cao MQ, Gao DM and Tang ZY: Astragaloside IV
inhibits metastasis in hepatoma cells through the suppression of
epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin
pathway. Oncol Rep. 37:1725–1735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Guo H, Luo H, Yuan H, Xia Y, Shu P, Huang
X, Lu Y, Liu X, Keller ET, Sun D, et al: Litchi seed extracts
diminish prostate cancer progression via induction of apoptosis and
attenuation of EMT through Akt/GSK-3β signaling. Sci Rep.
7:416562017. View Article : Google Scholar : PubMed/NCBI
|