Introduction
Combined small cell lung carcinoma (SCLC) is
categorized as a histopathological variant of SCLC, and defined as
an admixture of SCLC and non-SCLC components (1). The incidence of combined SCLC is
0.2%-1.3% of the surgically resected primary lung cancers (2,3), and
comprises up to 28% of the surgically resected SCLC cases (4). Adenocarcinoma and squamous cell
carcinoma are the common components of non-SCLC (2–5). In the
case series reported by Yamada et al adenocarcinoma is the
most common histopathological subtype of the non-SCLC component
(6/9 cases), followed by adenocarcinoma and squamous cell carcinoma
(2 cases) and squamous cell carcinoma (1 case) (2). Any histopathological subtypes can be
present as a non-SCLC component (1).
However, the occurrence of a pleomorphic (giant cell) carcinoma
component in combined SCLC is extremely rare (6–9). We have
already reported on the clinical and histological characteristics
of this case (10), and this report
we described the detailed cytological features of combined SCLC
with giant cell carcinoma component in order to draw attention to a
potential cause of diagnostic error.
Case report
A 50-year-old Japanese female was referred to Kansai
Medical University Hospital because of an abnormal chest shadow,
which had been detected by a chest X-ray examination at an
out-patient clinic. She was a heavy smoker (30 cigarettes daily
over 30 years). Chest computed tomography (CT) demonstrated a
relatively well-circumscribed mass lesion, measuring 23.8×20.8 mm
in diameter, in S6 of the left lung. Her serum tumor markers were
elevated [neuron specific enolase, 21.0 ng/ml (range, <12) and
ProGRP, 84.4 pg/ml (range, <46)].
CT-guided fine-needle aspiration cytology
examination and needle biopsy were performed for this lesion.
Bronchoscpoic biopsy was not performed due to the location of the
tumor. Subsequently, lobectomy of the left lower lobe and lymph
node dissection were performed.
The post-operative course was uneventful, and no
tumor recurrence has been observed during 13 months of medical
follow-up.
The CT-guided fine-needle aspiration cytology
specimens were stained conventionally with Papanicolaou stain.
Formalin-fixed and paraffin-embedded CT-guided
needle biopsy and surgically resected specimens of the lung were
processed for routine histological examination and
immunohistochemical analyses.
In this report, immunohistochemical analyses were
performed using an autostainer (XT System Benchmark; Roche
Diagnostics, Basel, Switzerland; or Autostainer link 48;
DakoCytomation, Glostrup, Denmark) according to the manufacturer's
instructions. The primary antibodies used in this report were a
mouse monoclonal antibody against chromogranin A (LK2H10; Cell
Marque, Rocklin, CA, USA), a mouse monoclonal antibody against CD56
(123c3; DakoCytomation), a mouse monoclonal antibody against
E-cadherin (NCH-38; DakoCytomation), a mouse monoclonal antibody
against p40 (BC28; Novocastra Laboratories, Ltd., Newcastle upon
Tyne, UK), a mouse monoclonal antibody against synaptophysin
(27G12; Nichirei Bioscience, Tokyo, Japan), a mouse monoclonal
antibody against thyroid transcription factor (TTF)-1 (8G7G3;
DakoCytomation), and a mouse monoclonal antibody against vimentin
(1D2C3; DakoCytomation).
Cytological findings of the CT-guided
fine-needle aspiration specimen
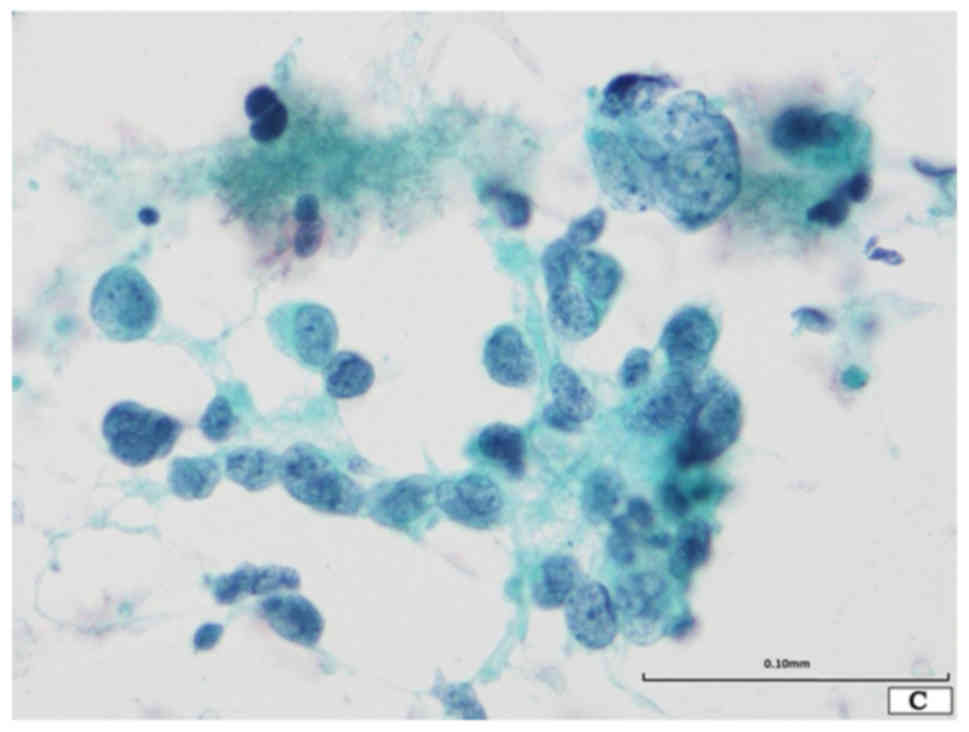
The Papanicolaou smear revealed the presence of two
distinct neoplastic components in a necrotic background (Fig. 1A). One component was composed of
small-sized neoplastic cells showing nuclear molding and loose
aggregates (Fig. 1A and B). These
neoplastic cells were round to oval in shape, and had scant
cytoplasm and a high nuclear/cytoplasmic ratio and round to oval
nuclei with finely dispersed granular nuclear chromatin and absent
or inconspicuous nucleoli (Fig. 1A and
B). Although apparent mitotic figures were not observed,
apoptotic bodies were noted. This component was typical for SCLC.
The other component was composed of loose aggregates or single
discohesive giant cells (Fig. 1A-C).
These cells were round to oval in shape, and had large and
irregularly lobulated hyperchromatic nuclei, which were
approximately 7 to 10 times larger than those of SCLC, with
conspicuous single or multiple nucleoli (Fig. 1A-C). The latter component corresponded
to giant cell carcinoma. No spindle cell, squamous cell carcinoma,
or adenocarcinoma components were noted.
Accordingly, combined SCLC with giant cell carcinoma
component was suspected.
Histopathological findings of the
CT-guided needle biopsy specimen
Microscopic examination revealed proliferation of
small round cells with a high nuclear/cytoplasmic ratio and scant
cytoplasm. These neoplastic cells had round to oval nuclei with
dispersed granular nuclear chromatin without nucleoli. Mitotic
figures were easily observed. These histopathological features were
typical for SCLC. No giant cell carcinoma component, which was
observed in the cytological specimen, was present.
Histopathological findings of the
lobectomy specimen
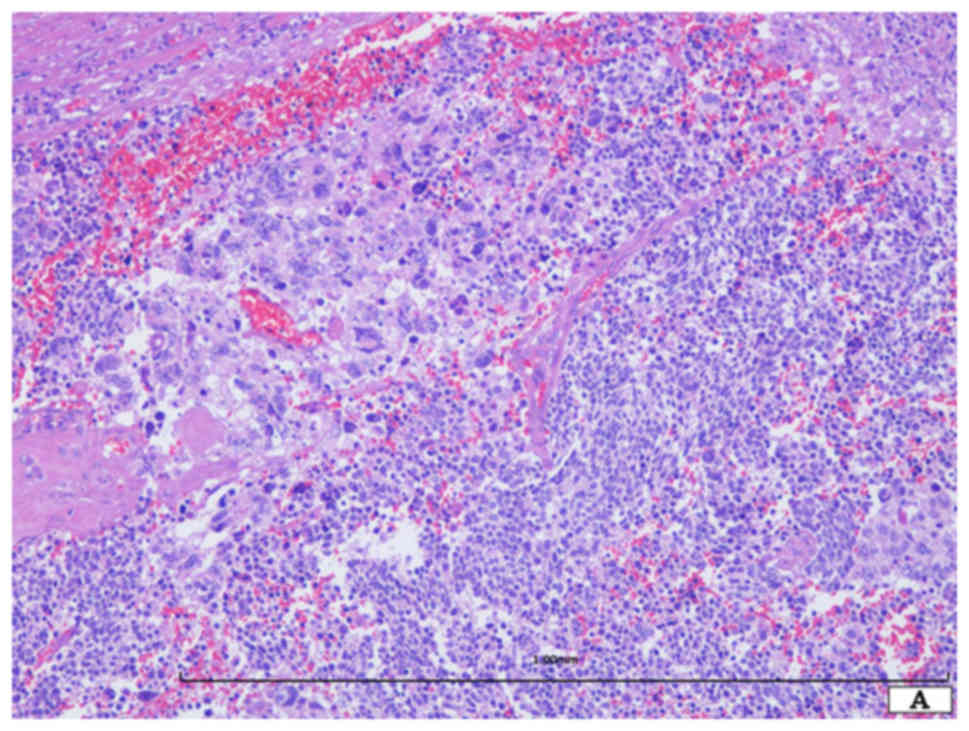
Histopathological study demonstrated the presence of
two distinct neoplastic components (Fig.
2A). One component was SCLC (approximately 60% of the tumor),
which was composed of a sheet-like proliferation of small round
cells with scant cytoplasm (Fig. 2B).
These neoplastic cells had oval nuclei with dispersed granular
chromatin without nucleoli (Fig. 2B).
Mitotic figures and apoptotic bodies were easily observed (Fig. 2B). The other component was giant cell
carcinoma (approximately 40% of the tumor) (Fig. 2A). The neoplastic cells were round to
oval in shape and had relatively rich eosinophilic cytoplasm and
single or multiple large nuclei with coarse chromatin (Fig. 2C). The nuclei of this component were
more than 7–10 times larger than those of the SCLC cells (Fig. 2C). Mitotic figures and apoptotic
bodies were easily observed. Neither differentiated carcinoma,
including adenocarcinoma and squamous cell carcinoma, nor spindle
cell carcinoma component was present. Lymph node metastases (SCLC
component) were observed (6/23).
Immunohistochemical findings of the
lobectomy specimen
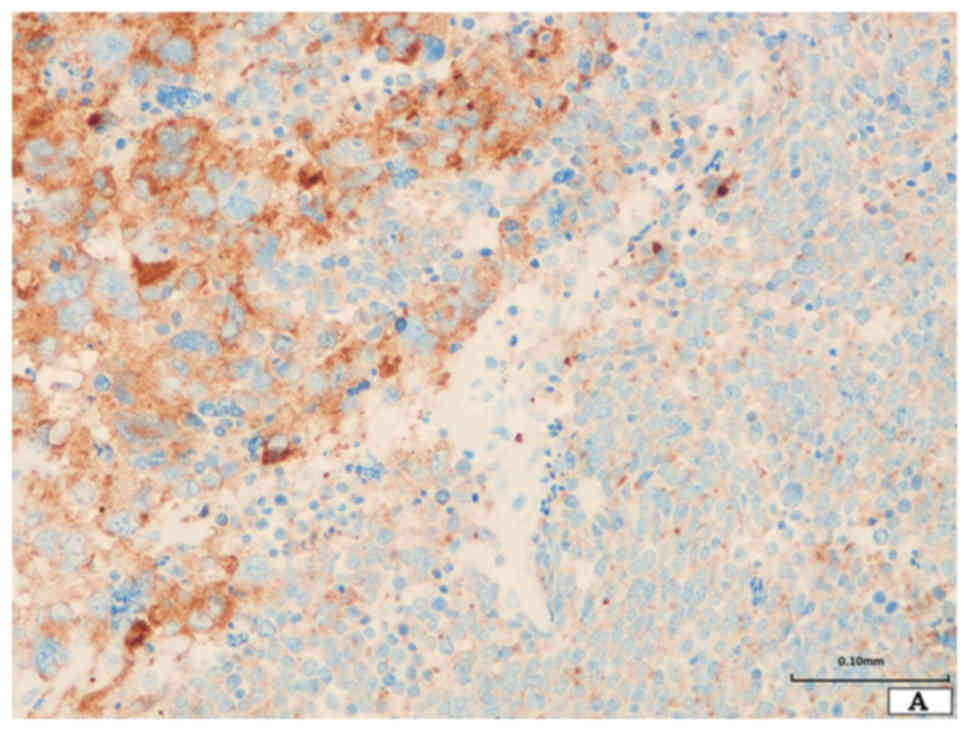
The neoplastic cells of SCLC were diffusely positive
for chromogranin A, synaptophysin, and CD56 (Fig. 3A). TTF-1 and E-cadherin were also
expressed. However, p40 and vimentin were not expressed (Fig. 3B and C).
The giant neoplastic cells were positive for CD56
and synapto-physin, and TTF-1 was focally expressed (Fig. 3A). Chromo-granin A and p40 were not
expressed. E-cadherin was not expressed, however, vimentin was
diffusely expressed (Fig. 3B and
C).
According to these results, a final diagnosis of
combined SCLC with giant cell carcinoma component (pT1b N2 M0,
stage IIIA) was made.
Discussion
Herein, we describe the first reported cytological
case of combined SCLC with giant cell carcinoma component. The
present case carries an important message to consider in
cytodiagnosis of carcinoma with neoplastic giant cell component in
respiratory cytological specimens because neoplastic giant cells
are occasionally observed in various types of tumors, and some
neoplastic conditions-such as SCLC and giant cell carcinoma-must be
included in differential diagnostic considerations.
Pleomorphic carcinoma is a relatively rare, highly
aggressive histopathological variant of lung cancer, and is defined
as a poorly differentiated non-SCLC that contains at least 10%
neoplastic spindle and/or giant cells (11). Most pleomorphic carcinomas contain
both non-SCLC and spindle cell and/or giant cell components
(11,12), and SCLC with giant cell carcinoma
component is extremely rare. Nicholson et al reported the
largest case series of combined SCLC, and non-SCLC component was
present in 28 of 100 cases, but none with giant cell carcinoma
(4). According to the other case
series of combined SCLC, none case with giant cell carcinoma was
observed (2). Moreover, Fishback
et al reported that only 1 of 48 cases of giant cell
carcinoma had SCLC component (13).
Only a few reports regarding the cytological features of
pleomorphic carcinoma have been published (14–17).
The characteristic cytological features of
pleomorphic carcinoma are summarized in Table I. The cytological features of v) in
Table I are characteristic for giant
cell carcinoma, and the cytological features of the present case
corresponded to these features.
 | Table I.The cytological features of
pleomorphic carcinoma. |
Table I.
The cytological features of
pleomorphic carcinoma.
| i) | The tumor cells are
arranged in monolayer, 3-dimentional clusters, or as scattered
single cells in a necrotic (with or without inflammation)
background. |
| ii) | The tumor cells are
large and epithelioid, spindle, or pleomorphic in shape, and show
marked pleomorphism. |
| iii) | The sizes of the
tumor cells vary more than fivefold. |
|
| iv) | The chromatin is
unevenly distributed and there is a prominent single nucleolus; the
tumor cells have abundant thick cytoplasm. |
| v) | Mono-, bi-, and
multi-nucleated giant cells can be present, and the nucleus of the
giant cells are hyperchromatic to vesicular, bizarre in shape, and
more than 5 times the size of the nucleus of the small lymphocytes,
and often much larger. |
The histopathological diagnosis of the lung tumors
with giant cells may be straightforward because presence of
neoplastic non-giant cell component is easily detectable. However,
cytological diagnosis may be challenging because in respiratory
cytological specimens, the neoplastic giant cells can be found in
various types of tumors, including giant cell carcinoma, non-SCLC,
and pulmonary blastoma (18,19). Poorly differentiated non-SCLC
occasionally contains neoplastic giant cells, however, a
conventional non-giant cell carcinoma component, such as
adenocarcinoma and squamous cell carcinoma, may be present in a
cytological specimen. Pulmonary blastoma may have bizarre giant
cells of a mesenchymal component, however, this type of rare tumor
typically has sheets of cohesive epithelial cells as a glandular
component (19).
Moreover, pure SCLC must be included in the
differential diagnostic consideration of a lung tumor with giant
cells because it is well recognized that SCLC occasionally shows
cytological pleomorphism in the form of scattered or clustered
multinucleated giant cells as the tumor size increases (4). Therefore, the present case must be
differentiated from SCLC with giant cells. However, the current
case contained abundant loose aggregates or single discohesive
giant cells (the nuclear size was more than 7 to 10 times larger
than those of SCLC), in contrast, scattered giant cells are
typically observed in SCLC. Therefore, a cytodiagnosis of combined
SCLC with giant cell carcinoma component was made.
In the present case, the giant cell carcinoma
component was only present in the CT-guided fine-needle aspiration
cytological specimen, but not in the needle biopsy specimen. Thus,
the CT-guided fine-needle aspiration cytological examination aided
the accurate pre-operative diagnosis of combined SCLC.
The giant cell carcinoma component of the present
tumor was immunohistochemically positive for vimentin, but
E-cadherin and p40 were not expressed. This immunohistochemical
profile corresponded to that of giant cell carcinoma (11), which may suggest occurrence of
epithelial-mesenchymal transition in giant cell carcinoma.
Because of rarity of combined SCLC with giant cell
carcinoma component, the prognosis and therapeutic strategy for
this type of tumor has not been determined. Therefore, the
significance of pre-operative diagnosis of this type of tumor has
not been concluded. However, accurate pre-operative diagnosis is
important for further analyses, and additional studies are needed
to clarify this issue.
In conclusion, we describe the first reported case
of combined SCLC with giant cell carcinoma component successfully
diagnosed in a CT-guided fine-needle aspiration cytological
specimen. Both SCLC and giant cell carcinoma show the
characteristic cytological features, therefore, albeit extremely
rare, careful observation can lead to detection of giant cell
carcinoma as well as SCLC in cytological specimens.
References
|
1
|
Brambilla E, Beasley MB, Austin JHM,
Capellozzi VL, Chirieac LR, Devesa SS, Frank GA, Gazdar A, Ishikawa
Y, et al: Small cell carcinomaWHO Classification of Tumours of the
Lung, Pleura, Thymus and Heart. Travis WD, Brambillia E, Burke AP,
Marx A and Nicholson AG: IARC; Lyon: pp. 63–68. 2015
|
|
2
|
Yamada K, Maeshima AM, Tsuta K and Tsuda
H: Combined high-grade neuroendocrine carcinoma of the lung:
Clinicopathological and immunohistochemical study of 34 surgically
resected cases. Pathol Int. 64:28–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruffini E, Rena O, Oliaro A, Filosso PL,
Bongiovanni M, Arslanian A, Papalia E and Maggi G: Lung tumors with
mixed histologic pattern. Clinico-pathologic characteristics and
prognostic significance. Eur J Cardiothorac Surg. 22:701–707. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nicholson SA, Beasley MB, Brambilla E,
Hasleton PS, Colby TV, Sheppard MN, Falk R and Travis WD: Small
cell lung carcinoma (SCLC): A clinicopathologic study of 100 cases
with surgical specimens. Am J Surg Pathol. 26:1184–1197. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaharopoulos P, Wong JY and Stewart GD:
Cytomorphology of the variants of small-cell carcinoma of the lung.
Acta Cytol. 26:800–808. 1982.PubMed/NCBI
|
|
6
|
Tsubota YT, Kawaguchi T, Hoso T, Nishino E
and Travis WD: A combined small cell and spindle cell carcinoma of
the lung: Report of a unique case with immunohistochemical and
ultrastructural studies. Am J Surg Pathol. 16:1108–1115. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gotoh M, Yamamoto Y, Huang CL and Yokomise
H: A combined small cell carcinoma of the lung containing three
components: Small cell, spindle cell and squamous cell carcinoma.
Eur J Cadiothorac Surg. 26:1047–1049. 2004. View Article : Google Scholar
|
|
8
|
Fujiwara M, Horiguchi M, Inage Y,
Horiguchi H, Satoh H and Kamma H: Combined small cell carcinoma in
the peripheral lung: Importance of appropriate sampling. Acta
Cytol. 49:575–578. 2005.PubMed/NCBI
|
|
9
|
Purkait S, Jain D, Madan K, Mathur S and
Iyer VK: Combined small cell carcinoma of the lung: A case
diagnosed on bronchoscopic wash cytology and bronchial biopsy.
Cytopathology. 26:197–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saito T, Tsuta K, Fukumoto KJ, Matsui H,
Konobu T, Torii Y, Yokoi T, Kurata T, Kurokawa H, Uemura Y, et al:
Combined small cell lung carcinoma and giant cell carcinoma: A case
report. Surg Case Rep. 3:522017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kerr KM, Pelosi G, Austin JHM, Brambilla
E, Geisinger K, Jambhekar NA, Jett J, Koss MN, Nicholson AG, et al:
Pleomorphic, spindle cell and giant cell carcinomaWHO
Classification of Tumours of the Lung, Pleura, Thymus and Heart.
Travis WD, Brambillia E, Burke AP, Marx A and Nicholson AG: IARC;
Lyon: pp. 88–90. 2015
|
|
12
|
Mochizuki T, Ishii G, Nagai K, Yoshida J,
Nishimura M, Mizuno T, Yokose T, Suzuki K and Ochiai A: Pleomorphic
carcinoma of the lung: Clinicopathologic characteristics of 70
cases. Am J Surg Pathol. 32:1727–1735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fishback NF, Travis WD, Moran CA, Guinee
DG Jr, McCarthy WF and Koss MN: Pleomorphic (spindle/giant cell)
carcinoma of the lung. A clinicopathologic correlation of 78 cases.
Cancer. 73:2936–2945. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi HS, Seol H, Heo IY, Jung CW, Cho SY,
Park S, Koh JS and Lee SS: Fine-needle aspiration cytology of
pleomorphic carcinomas of the lung. Korean J Pathol. 46:576–582.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zafar N and Johns CD: Pleomorphic
(sarcomatoid) carcinoma of lung-cytohistologic and
immunohistochemical features. Diagn Cytopathol. 39:115–116. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hiroshima K, Dosaka-Akita H, Usuda K,
Ogura S, Kusunoki Y, Kodama T, Saito Y, Sato M, Tagawa Y, Baba M,
et al: Cytological characteristics of pulmonary pleomorphic and
giant cell carcinomas. Acta Cytol. 55:173–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hummel P, Cangiarella JF, Cohen JM, Yang
G, Waisman J and Chhieng DC: Transthoracic fine-needle aspiration
biopsy of pulmonary spindle cell and mesenchymal lesions: A study
of 61 cases. Cancer. 93:187–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alasio TM, Sun W and Yang GC: Giant cell
carcinoma of the lung impact of diagnosis and review of cytological
features. Diagn Cytopathol. 35:555–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahon BM, Placido JB and Gattuso P:
Fine-needle aspiration of classic biphasic pulmonary blastoma.
Diagn Cytopathol. 38:427–429. 2010.PubMed/NCBI
|