Introduction
Lung cancer has remained a global leading cause of
cancer mortality in both men and women (1–3). In 2013,
this malignant disorder constituted approximately 1.8 million new
cancer cases and accounted for 1.6 million cancer deaths worldwide
(2). Moreover, the number of lung
cancer deaths is expected to grow up to 3 million for the year 2035
(3). Lung cancer is generally divided
into two differently growing histological types, i.e., small-cell
lung cancer and non-small cell lung cancer that accounts for the
most of lung cancer cases (around 85%) and includes adenocarcinoma,
squamous cell carcinoma and large cell carcinoma (1,3,4). Adenocarcinoma is the most common
histological subtype that represents about 40% of all lung cancer
cases (1,4). Although men are more likely to be
affected by this malignancy than women, with 1 in 18 men and 1 in
51 women diagnosed with lung cancer at some point in their lives,
its incidence in women is globally increasing (3). Potential male-female differences have
been demonstrated also in other aspects of lung cancer, including
better prognosis, higher treatment responses and survival in women
as compared to men (5–8). Nevertheless, the mean 5-year survival
rate of lung cancer is estimated to be less than 18%, showing an
urgent need for more effective treatment choices (2,4). Recent
bibliometric analysis revealed that international research level of
lung cancer lags substantially behind the publication outputs for
other malignancies. Despite the poor prognosis, high mortality rate
and huge economic costs, in 2013 the research in lung cancer
accounted only a small proportion, i.e., 5.6% of all oncology
research (2). Consequently, further
in vitro and in vivo investigations using different
lung cancer models are highly needed to develop novel treatment
strategies and improve the survival rate of lung cancer
patients.
In the recent years, several epidemiological studies
have demonstrated that higher intake of fruits and vegetables can
be beneficial for prevention of different types of human cancers,
including lung tumors (9–13). As anticarcinogenic components of these
plant products, polyphenolic flavonoids have been proposed with
numerous experimental investigations to display various antitumoral
activities (14–16). Indeed, these polyphenols can express
antiproliferative, cytotoxic, proapoptotic, antiinvasive,
antimetastatic, antiangiogenic and antiinflammatory properties in
different cancer cell lines or animal models (17). However, it is well known that in the
human body, flavonoids undergo an extensive metabolism and as a
result of this conversion only different metabolic conjugates enter
the circulatory system and can reach target malignant tissue
(18,19). Differently from parent flavonoids,
current knowledge about the possible anticancer action of their
metabolites is still rather limited making the prediction of
bioactive behavior of flavonoids in the human body complicated.
One of the most important metabolic pathways that
flavonoids undergo in the small intestine and liver is their
methylation catalyzed by catechol-O-methyltransferase (COMT)
(20). This phase II enzyme catalyzes
the transfer of a methyl moiety from S-adenosylmethionine donor
substance to a catecholic substrate, such as flavonoids (21). As the methylated flavonoids formed in
this way may potentially reveal substantially different biological
properties than the parent compounds, we focus in this study on the
anticancer effects and mechanisms of two methylated quercetin
molecules, i.e., 3′-O-methylquercetin or isorhamnetin and
4′-O-methylquercetin or tamarixetin in human non-small cell lung
cancer (adenocarcinoma) lines, A549 and HCC-44, and investigate
their antiproliferative activities compared to the parent
quercetin. In addition to studying the cell growth inhibitory
effects of these methylated flavonoids by the MTT assay, their
ability to trigger apoptotic pathways (i.e., intrinsic vs extrinsic
routes) is also under examination by determining caspase-9 and −8
activities. This investigation shows that flavonoid metabolites can
be considered as leading compounds for further development of lung
cancer chemotherapeutics possibly supplementing the available
treatment arsenal in the future.
Materials and methods
Reagents
All flavonoids (genistein, daidzein, fisetin,
quercetin, hesperetin, luteolin, chrysin, baicalein) and methylated
derivatives of quercetin (isorhamnetin, tamarixetin) were purchased
from Santa Cruz Biotechnology (Dallas, TX, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and L-glutamine were the products of Sigma-Aldrich (St. Louis, MO,
USA). Dimethyl sulfoxide (DMSO) was from Mediatech, Inc. (Manassas,
VA, USA). Phosphate-buffered saline (PBS) was obtained from Lonza
(Verviers, Belgium).
Cell lines and culture conditions
A549 and HCC-44 human lung adenocarcinoma cell lines
were obtained from the Leibniz Institute DSMZ-German Collection of
Microorganisms and Cell Cultures (Leibniz-Institut DSMZ-Deutsche
Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig,
Germany).
A549 and HCC-44 are cell lines derived from the
adenocarcinoma of a 58-year-old Caucasian man (22) and a 54-year-old woman, respectively
(23). To exclude clinically distinct
disease entity-oncogene addicted lung cancer, these cell lines were
further tested in our laboratory for epidermal growth factor
receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK)
translocation. Both cell lines were wild type and did not have
these genetic alterations (data not shown).
A549 cells were cultivated in Dulbecco's modified
Eagle's medium (DMEM) (Life Technologies Corporation, Grand Island,
NY, USA) supplemented with 10% of heat-inactivated fetal bovine
serum (FBS; Invitrogen™, Auckland, NZ, USA). HCC-44
cells were cultivated in RPMI 1640 medium (Life Technologies
Corporation) supplemented with 10% of heat-inactivated FBS. Cells
were maintained in a 5% CO2 incubator at 37°C with and
passaged 1–2 times per week.
Measurement of cell viability by MTT
assay
The cell growth inhibitory effects of flavonoids
against human lung cancer cell lines were tested by the MTT
colorimetric assay first described by Mosmann in 1983 (24). In detail, the cells were plated on to
96-well U shaped bottom plates at concentration of 1×105
cells/ml of medium, putting 100 µl of suspension to each well.
Cells were counted in Bürker counting chamber. As phenol red can
interfere with the reading of absorbance (25), the cells were seeded in the phenol
red-free RPMI-1640 medium (Mediatech, Inc.). After overnight
culturing, cells were treated with varying doses of flavonoids (10
nM-500 µM) for 48 h at 37°C and 5% CO2. At the end of
the incubation, 50 µl of MTT solution in PBS was added to the wells
with the final concentration of MTT of 5 mg/ml. Plates were further
incubated for 4 h followed by centrifugation at 1,000 rpm for 10
min and removing of the supernatant. To dissolve the purple
formazan crystals 150 µl of DMSO was added and the plates were
shaken for 30 min. Absorbance was measured at 540 nm using a LED
based microplate reader (Ledetect 96; Labexim Products, Lengau,
Austria). To calculate the proportion of surviving cells, the
following formula was used: (OD of drug-treated sample - OD of
blank)/(OD of control - OD of blank) ×100%, where OD of blank
represents the absorbance reading of wells containing the buffer
only (without cells) and OD of control represents the reading value
of wells without any added test compounds. Dose-response curves
were constructed to evaluate the half-maximal inhibitory
concentrations (IC50 values). All separate tests were
carried out 2–3 times in different days, performing the experiments
in triplicates.
Measurement of caspase activities
To study the cell death mechanisms triggered by the
treatment of human lung cancer cell lines with methylated quercetin
derivatives, the Caspase Colorimetric Protease Assay Sampler kit
(Invitrogen Corporation, Frederick, MD, USA) was used according to
the protocol provided by the manufacturer. As this kit uses
para-nitroaniline-labeled synthetic peptides as substrates of
different caspases, absorption of cleaved para-nitroaniline was
spectrophotometrically quantified at 405 nm.
Statistics
Data were treated using the GraphPad Prism
statistical software (version 4.0; GraphPad Software, Inc., La
Jolla, CA, USA). The Kolmogorov-Smirnov test for normality was
applied and the one-way analysis of variance (ANOVA) was performed
to determine whether the differences between means were
statistically significant. P-value <0.05 were considered as
statistically significant and all values were expressed as mean ±
standard deviation (SD).
Results
Cytotoxicity profiles of flavonoids in
human lung adenocarinoma cells A549 and HCC-44
Among the tested panel of flavonoids, isoflavones
genistein and daidzein and flavanone hesperetin had no growth
inhibitory effects on both lung cancer cell lines up to 100 µM
concentration. Flavonols fisetin and quercetin as well as flavones
luteolin, chrysin and baicalein revealed low antiproliferative
efficiency with half maximal inhibitory constants (IC50)
more than 100 µM, except quercetin in A549 cells with
IC50 of 72.2 µM, and fisetin and chrysin in HCC-44 cells
with IC50 values of 78.7 and 79.6 µM, respectively
(Table I).
 | Table I.Antiproliferative effects of
flavonoids on human lung adenocarcinoma cell lines A549 and
HCC-44. |
Table I.
Antiproliferative effects of
flavonoids on human lung adenocarcinoma cell lines A549 and
HCC-44.
|
| IC50,
µM |
|---|
|
|
|
|---|
| Variable | A549 | HCC-44 |
|---|
| Isoflavones |
|
|
|
Genistein | >500 | >500 |
|
Daidzein | >500 | >500 |
| Flavonols and their
methylated metabolites |
|
|
|
Fisetin | 127.9±1.9 | 78.7±2.0 |
|
Quercetin | 72.2±2.3 | 107.6±2.2 |
|
3′-O-Methylquercetin or
isorhamnetin | 26.6±1.7 | 15.9±1.7 |
|
4′-O-Methylquercetin or
tamarixetin | 19.6±1.3 | 20.3±1.4 |
| Flavanones |
|
|
|
Hesperetin | >500 | >500 |
| Flavones |
|
|
|
Luteolin | 155.6±1.6 | 123.0±1.6 |
|
Chrysin | 135.8±2.3 | 79.6±1.6 |
|
Baicalein | 307.6±3.1 | 194.5±1.9 |
Growth inhibition of human lung
adenocarcinoma cells A549 and HCC-44 by methylated metabolites of
quercetin
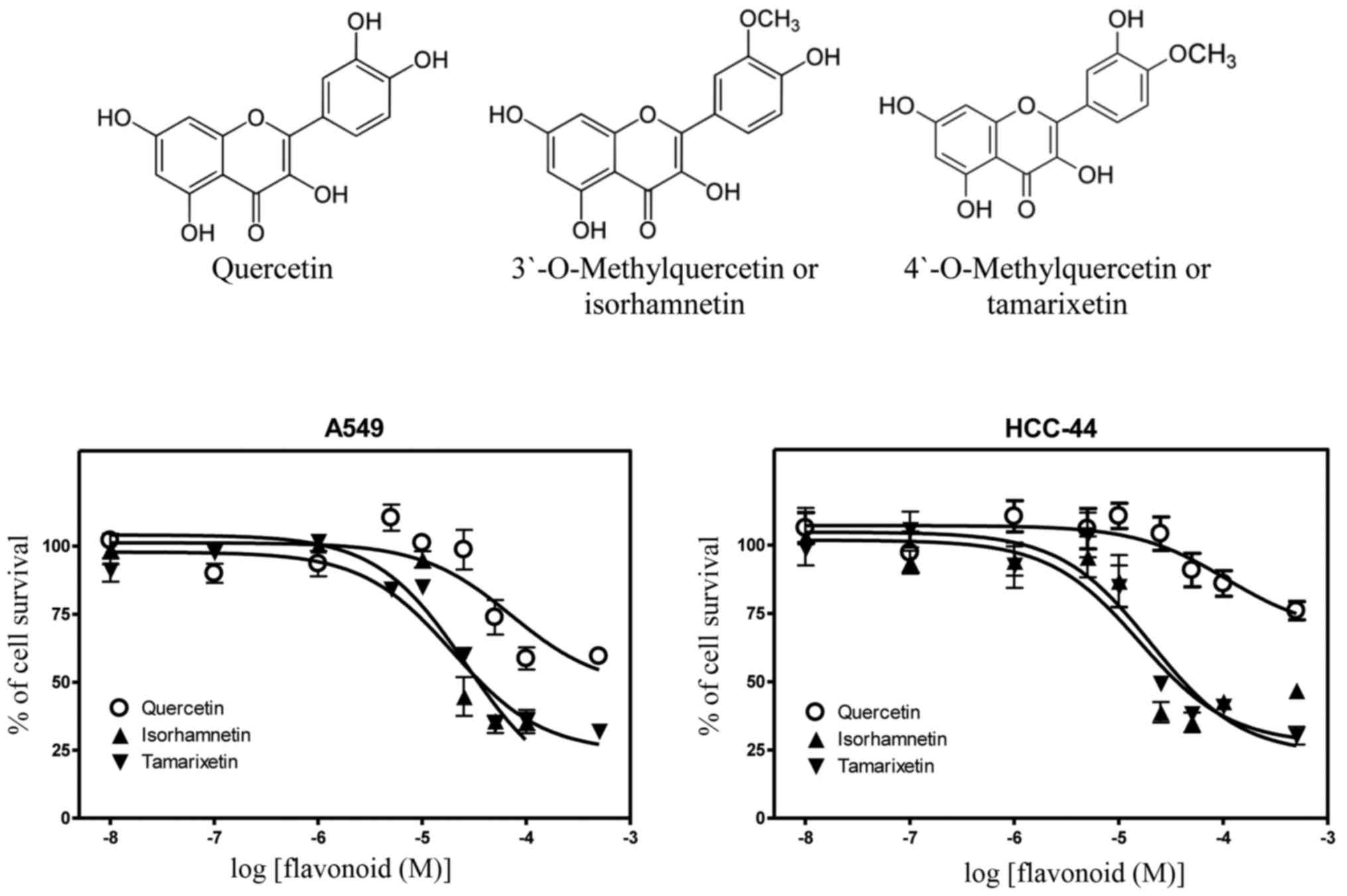
Differently from the parent quercetin, its
metabolites with one methyl group in the different positions of
B-ring revealed much higher efficiency in inhibition of growth of
lung cancer cells. In detail, the derivative containing a methyl
moiety in the 3′-position, i.e., 3′-O-methylquercetin or
isorhamnetin, displayed the inhibitory constants of 2.7- and
6.8-fold lower compared to the parent quercetin in A549
(IC50, 26.6 µM) and HCC-44 cells (IC50, 15.9
µM). The respective increases in cytotoxic potencies were 3.7- and
5.3-fold for 4′-O-methylquercetin or tamarixetin in A549
(IC50, 19.6 µM) and HCC-44 cells (IC50, 20.3
µM) (Table I). Chemical structures of
these methylquercetins with the dose-response curves in both lung
adenocarcinoma cell lines are presented in Fig. 1.
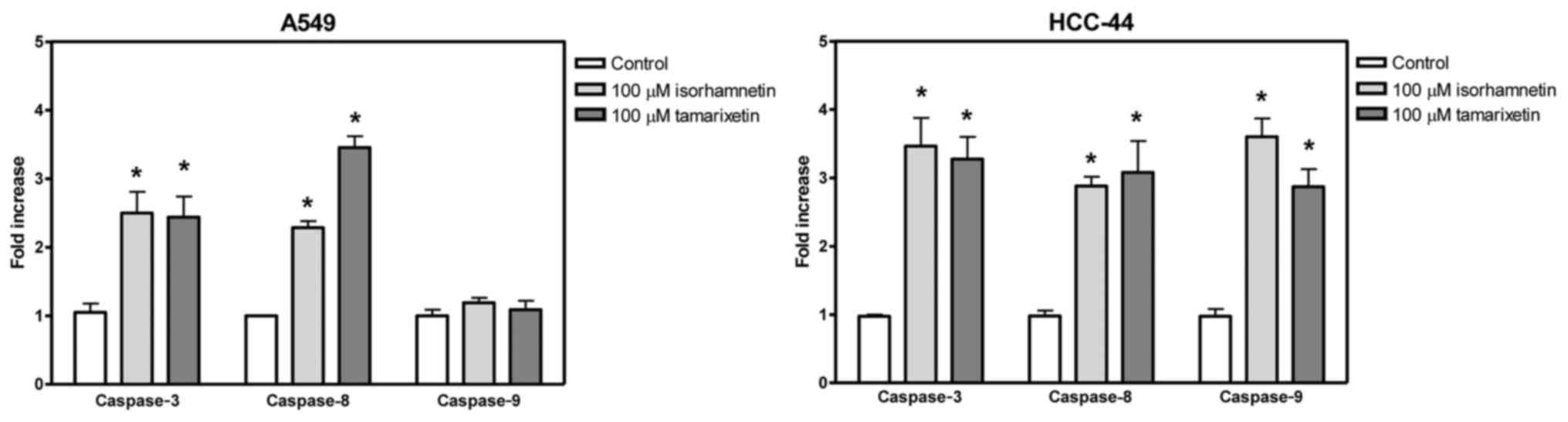
Effect of methylquercetins on
activation of caspase family members
The effect of methylated quercetins on lung
adenocarcinoma cell lines was further estimated by analysis of
activity of caspase family members. In both A549 and HCC-44 cells,
isorhamnetin increased caspase-3 activity by 2.5- and 3.5-fold,
respectively, indicating induction of apoptosis. Similar results
were measured for tamarixetin with the respective increases of 2.3-
and 3.3-fold in A549 and HCC-44 cells. Furthermore, both
derivatives elevated caspase-8 activity in both cell lines pointing
to the occurrence of apoptosis via extrinsic pathway. In HCC-44,
but not in A549 cells, also the activity of caspase-9 was increased
demonstrating that in these cells the apoptosis is induced through
both intrinsic and extrinsic routes (Fig.
2).
Discussion
A549 and HCC-44 cell lines are two non-small cell
lung cancer lines derived from the adenocarcinoma of a 58-year-old
Caucasian man (22) and a 54-year-old
woman, respectively (23). The
current work is the first study to describe the inhibitory effects
of flavonoids on the viability of HCC-44 cells. As A549 line has
been widely used as a model system to study human alveolar
carcinoma, cytotoxic profile of flavonoids in these cells was
previously characterized with the results very similar to those
measured in our work. Indeed, the IC50 value of 72.2 µM
for quercetin is close to the inhibitory constants published by
Loizzo et al (26), Tan et
al (27–29), Robaszkiewicz et al (30), and Chan et al (31); and IC50 values more than
100 µM were previously reported for fisetin (32), hesperetin (33–35),
luteolin (32,36), chrysin (35), baicalein (34), daidzein (37), and genistein (38). Based on our results presented in this
article, cytotoxic activity profiles of flavonoids were rather
similar for both A549 and HCC-44 lung cancer lines, despite the
gender difference of initial origin of these cells.
However, the data clearly show that the growth
inhibitory effects of tested flavonoids on lung cancer cells
revealed only at very high micromolar doses that are
physiologically unachievable. Indeed, the maximum serum
concentrations of daidzein and genistein were measured to be less
than 0.5 µM following to consumption of 100 ml of untreated soymilk
(39). The baseline plasma
concentration of quercetin was reported to be generally about 50–80
nM reaching 0.63 µM after supplementation with 80 mg quercetin per
day for one week or 1.5 µM after supplementation with >1 g
quercetin per day for 4 weeks (40).
Although bioavailability of flavonoids depends on food sources (for
instance, quercetin is somewhat better bioavailable from onions
than apples) and there is also high interindividual variability
(40,41), it is rather impossible to achieve
plasma doses exceeding some micromolar level by oral ingestion of
flavonoids-rich food items or dietary supplements.
Despite numerous experimental works performed with
bioactivity of parent flavonoids, the knowledge about possible
antiproliferative effects of their metabolites is still rather
sparse today. One reason for this is the very limited commercial
availability of metabolites for experimental testing. There are
three major types of metabolic derivatives of flavonoids formed as
a consequence of enzymatic conjugation with methyl-, sulfate- or
glycuronyl groups in the small intestine and liver catalyzed by
COMT, sulfotransferase (SULT) or UDP-glucuronosyltransfrease (UGT),
respectively (19,20,42).
Considering some structural modification of initial compounds
changes in their bioactivities could also be expected. Our results
with two methyl conjugates of quercetin indeed confirm this
standpoint indicating significantly higher antiproliferative
potencies of both isorhamnetin and tamarixetin in HCC-44 and A549
lung adenocarcinoma cells compared to the parent flavonol. As the
growth inhibitory effects of isorhamnetin have been previously
reported in A549 cells (43–45), to the best knowledge of the authors
this is the first study at all to describe the action of
tamarixetin in human lung cancer cells. The results demonstrate
that methylated metabolites of quercetin have considerably stronger
anticancer activity than quercetin itself, whereas the potency does
not depend on whether the methyl group is located in
3′-(isorhamnetin) or 4′-position (tamarixetin) of the B-ring in
quercetin skeleton. In the future, it would be interesting to test
the cytotoxic activity of other methylated derivatives of quercetin
in lung cancer cell lines to study the possible structure-activity
relationships. Furthermore, testing the potential antiproliferative
action of other types of quercetin conjugates, i.e., sulfates and
glucuronidates, would be equally important to better understand the
anticarcinogenic role of flavonoids in the human body.
The two main mechanisms of cytotoxic action of
flavonoids in malignant cells involve cell cycle arrest and
induction of apoptosis. At that, apoptosis is largely mediated by
two major routes: The intrinsic or mitochondrial signaling and
extrinsic death receptor pathway. The former way is triggered by
the release of cytochrome c to the cytoplasm, cleavage of
caspase-9 and activation of caspase-3. The latter pathway includes
the interaction with death receptor and sequential activation of
caspase-8 and caspase-3. In this study, we demonstrated that both
methyl conjugates of quercetin, isorhamnetin and tamarixetin,
induced apoptotic cell death in A549 and HCC-44 cells,
characterized by the activation of effector caspase-3. However, as
the cell death was predominantly mediated by extrinsic pathway in
A549 cells, both extrinsic and intrinsic pathways were activated in
HCC-44 cells by both methylated quercetin metabolites. The
differences in activated caspase cascades in the tested cellular
models can probably be caused by the different cellular signaling
pathways triggered by quercetin derivatives. However, as A549 and
HCC-44 lines were initially derived from a male and female lung
adenocarcinoma patient, respectively, it is also possible that
differences in the induced apoptotic pathways might involve some
gender-specific aspects. Interestingly, the increase in
cytotoxicity of methylquercetins compared to the parent quercetin
molecule was significantly stronger (6.8-fold for isorhamnetin and
5.3-fold for tamarixetin) in female origin line HCC-44 than in male
origin line A549 (2.7- and 3.7-fold increases, respectively).
Therefore, it is possible that lung adenocarcinoma cells derived
from men and women can behave differently to the treatment with
methylated quercetins, a situation similar to the higher responses
of female patients to the current therapeutic modalities in
clinical use (7). These aspects
clearly need further exploration. Moreover, considering that
apoptosis has emerged as an important molecular mechanism for the
anticancer action of standard chemotherapeutic drugs and novel
candidate agents, further experiments for investigation of
signaling pathways activated by methylated quercetins in different
lung cancer cell lines are highly needed. In addition, the
potential effects as well as possible toxicity issues of these
compounds in xenograft rodent models also wait for testing.
Although the main aim of this work was to study the
role of structural modification with adding a methyl group to
quercetin molecule on its cytotoxic activity, this study has also
several limitations. Among these, rate of apoptosis was not
evaluated by flow cytometry and expression of pro- and
anti-apoptotic proteins, such as Bax or Bcl-2, were not detected.
Also, the level of apoptotic proteins, such as caspase-3 and
cleaved caspase-3, were not detected. Moreover, the effect of
quercetin, isorhamnetin and tamarixetin on the cell cycle
progression of A549 and HCC-44 lung adenocarcinoma cells as well as
potential triggering of necrosis needs further unraveling in the
future studies.
In conclusion, we showed that two methylated
quercetin metabolites, isorhamnetin and tamarixetin,
dose-dependently decreased the viability of A549 and HCC-44 lung
adenocarcinoma cells at doses many-fold lower than those
cytotoxically active for parent quercetin. Both metabolites also
induced the apoptotic cell death in tested lung cancer experimental
models revealing methylated quercetins as potential novel drug
candidates for future treatment of non-small cell lung cancer.
Acknowledgements
This study was supported by the Estonian Society of
Clinical Oncologists.
References
|
1
|
Lemjabbar-Alaoui H, Hassan OU, Yang YW and
Buchanan P: Lung cancer: Biology and treatment options. Biochim
Biophyc Acta. 1856:189–210. 2015.
|
|
2
|
Aggarwal A, Lewison G, Idir S, Peters M,
Aldige C, Boerckel W, Boyle P, Trimble EL, Roe P, Sethi T, et al:
The state of lung cancer research: A global analysis. J Thorac
Oncol. 11:1040–1050. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McIntyre A and Ganti AK: Lung cancer-A
global perspective. J Surg Oncol. 115:550–554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zappa C and Mousa SA: Non-small cell lung
cancer: Current treatment and future advances. Transl Lung Cancer
Res. 5:288–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alberg AJ, Wallace K, Silvestri GA and
Brock MV: Invited commentary: The etiology of lung cancer in men
compared with women. Am J Epidemiol. 177:613–616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu JB, Kau TY, Severson RK and Kalemkerian
GP: Lung cancer in women: Analysis of the national surveillance,
epidemiology and end results database. Chest. 127:768–777. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cerfolio RJ, Bryant AS, Scott E, Sharma M,
Robert F, Spencer SA and Garver RI: Women with pathologic stage I
II, and III non-small cell lung cancer have better survival than
men. Chest. 130:1796–1802. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boffetta P, Couto E, Wichmann J, Ferrari
P, Trichopoulos D, Bueno-de-Mesquita HB, van Duijnhoven FJ, Büchner
FL, Key T, Boeing H, et al: Fruit and vegetable intake and overall
cancer risk in the European Prospective Investigation into Cancer
and Nutrition (EPIC). J Natl Cancer Inst. 102:529–537. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miller AB, Altenhurg HP, Bueno-de-Mesquita
B, Boshuizen HC, Agudo A, Berrino F, Gram IT, Janson L, Linseisen
J, Overvad K, et al: Fruits and vegetables and lung cancer:
Findings from the European Prospective Investigation into Cancer
and Nutrition. Int J Cancer. 108:269–276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Linseisen J, Rohrmann S, Miller AB,
Bueno-de-Mesquita HB, Büchner FL, Vineis P, Agudo A, Gram IT,
Janson L, Krogh V, et al: Fruit and vegetable consumption and lung
cancer risk: Updated information from the European Prospective
Investigation into Cancer and Nutrition (EPIC). Int J Cancer.
121:1103–1114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Büchner FL, Bueno-de-Mesquita HB, Ros MM,
Overvad K, Dahm CC, Hansen L, Tjønneland A, Clavel-Chapelon F,
Boutron-Ruault MC, Touillaud M, et al: Variety in fruit and
vegetable consumption and the risk of lung cancer in the European
prospective investigation into cancer and nutrition. Cancer
Epidemiol Biomarkers Prev. 19:2278–2286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang M, Qin S, Zhang T, Song X and Zhang
S: The effect of fruit and vegetable intake on the development of
lung cancer: A meta-analysis of 32 publications and 20,414 cases.
Eur J Clin Nutr. 69:1184–1192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen
YM and Li HB: Natural polyphenols for prevention and treatment of
cancer. Nutrients. 8:pii: E5152016. View Article : Google Scholar
|
|
15
|
Amararathna M, Johnston MR and Rupasinghe
HP: Plant polyphenols as chemopreventive agents for lung cancer.
Int J Mol Sci. 17:pii: E13522016. View Article : Google Scholar
|
|
16
|
Patil BS, Jayaprakasha GK, Chidambara
Murthy KN and Vikram A: Bioactive compounds: Historical
perspectives, opportunities, and challenges. J Agric Food Chem.
57:8142–8160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sak K: Cytotoxicity of dietary flavonoids
on different human cancer types. Pharmacogn Rev. 8:122–146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cassidy A and Minihane AM: The role of
metabolism (and the microbiome) in defining the clinical efficacy
of dietary flavonoids. Am J Clin Nutr. 105:10–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Zheng S, Li L and Jiang H:
Metabolism of flavonoids in human: A comprehensive review. Curr
Drug Metab. 15:48–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sak K: The Val158Met polymorphism in COMT
gene and cancer risk: Role of endogenous and exogenous catechols.
Drug Metab Rev. 49:56–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao Y, Chen ZJ, Jiang HD and Chen JZ:
Computational studies of the regioselectivities of COMT-catalyzed
meta-/para-O methylations of luteolin and quercetin. J Phys Chem B.
118:470–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lieber M, Smith B, Szakal A, Nelson-Rees W
and Todaro G: A continuous tumor-cell line from a human lung
carcinoma with properties of type II alveolar epithelial cells. Int
J Cancer. 17:62–70. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gazdar AF, Girard L, Lockwood WW, Lam WL
and Minna JD: Lung cancer cell lines as tools for biomedical
discovery and research. J Natl Cancer Inst. 102:1310–1321. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kupcsik L: Estimation of cell number based
on metabolic activity: The MTT reduction assay. Methods Mol Biol.
740:13–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loizzo MR, Said A, Tundis R, Hawas UW,
Rashed K and Menichini F, Frega NG and Menichini F: Antioxidant and
antiproliferative activity of Diospyros lotus L. extract and
isolated compounds. Plant Foods Hum Nutr. 64:264–270. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan J, Wang B and Zhu L: DNA binding,
cytotoxicity, apoptotic inducing activity, and molecular modeling
study of quercetin zinc(II) complex. Bioorg Med Chem. 17:614–620.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan J, Wang B and Zhu L: DNA binding and
oxidative DNA damage induced by a quercetin copper(II) complex:
Potential mechanism of its antitumor properties. J Biol Inorg Chem.
14:727–739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan J, Zhu L and Wang B: From GC-rich DNA
binding to the repression of survivin gene for quercetin nickel(II)
complex: Implications for cancer therapy. Biometals. 23:1075–1084.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robaszkiewicz A, Balcerczyk A and Bartosz
G: Antioxidative and prooxidative effects of quercetin on A549
cells. Cell Biol Int. 31:1245–1250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan ST, Yang NC, Huang CS, Liao JW and
Yeh SL: Quercetin enhances the antitumor activity of trichostatin A
through upregulation of p53 protein expression in vitro and in
vivo. PLoS One. 8:e542552013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Katalinic M, Rusak G, Domaćinović Barović
J, Sinko G, Jelić D, Antolović R and Kovarik Z: Structural aspects
of flavonoids as inhibitors of human butyrylcholinesterase. Eur J
Med Chem. 45:186–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawaii S, Tomono Y, Katase E, Ogawa K and
Yano M: Antiproliferative activity of flavonoids on several cancer
cell lines. Biosci Biotechnol Biochem. 63:896–899. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim DH, Jung EA, Sohng IS, Han JA, Kim TH
and Han MJ: Intestinal bacterial metabolism of flavonoids and its
relation to some biological activities. Arch Pharm Res. 21:17–23.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hernandez J, Goycoolea FM, Quintero J,
Acosta A, Castañeda M, Dominguez Z, Robles R, Vazquez-Moreno L,
Velazquez EF, Astiazaran H, et al: Sonoran propolis: Chemical
composition and antiproliferative activity on cancer cell lines.
Planta Med. 73:1469–1474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Said A, Tundis R, Hawas UW, El-Kousy SM,
Rashed K, Menichini F, Bonesi M, Huefner A, Loizzo MR and
Menichinib F: In vitro antioxidant and antiproliferative activities
of flavonoids from Ailanthus excelsa (Roxb.) (Simaroubaceae)
leaves. Z Naturforsch C. 65:180–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han BJ, Li W, Jiang GB, Lai SH, Zhang C,
Zeng CC and Liu YJ: Effects of daidzein in regards to cytotoxicity
in vitro, apoptosis, reactive oxygen species level, cell cycle
arrest and the expression of caspase and Bcl-2 family proteins.
Oncol Rep. 34:1115–1120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Y, Zang A, Jia Y, Shang Y, Zhang Z,
Ge K, Zhang J, Fan W and Wang B: Genistein inhibits A549 human lung
cancer cell proliferation via miR-27a and MET signaling. Oncol
Lett. 12:2189–2193. 2016.PubMed/NCBI
|
|
39
|
Kano M, Takayanagi T, Harada K, Sawada S
and Ishikawa F: Bioavailability of isoflavones after ingestion of
soy beverages in healthy adults. J Nutr. 136:2291–2296.
2006.PubMed/NCBI
|
|
40
|
Manach C, Williamson G, Morand C, Scalbert
A and Rémésy C: Bioavailability and bioefficacy of polyphenols in
humans. I. Review of 97 bioavailability studies. Am J Clin Nutr.
81(1Suppl): 230S–242S. 2005.PubMed/NCBI
|
|
41
|
Lee J and Mitchell AE: Pharmacokinetics of
quercetin absorption from apples and onions in healthy humans. J
Agric Food Chem. 60:3874–3881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sak K and Everaus H: Sulfotransferase 1A1
as a biomarker for susceptibility to carcinogenesis: From molecular
genetics to the role of dietary flavonoids. Curr Drug Metab.
17:528–541. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ruan Y, Hu K and Chen H: Autophagy
inhibition enhances isorhamnetin-induced mitochondria-dependent
apoptosis in non-small cell lung cancer cells. Mol Med Rep.
12:5796–5806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang BY, Wang YM, Gong G, Zhao H, Lv XY,
Yuan GH and Han SR: Isorhamnetin flavonoid synergistically enhances
the anticancer activity and apoptosis induction by cisplatin and
carboplatin in non-small cell lung carcinoma (NSCLC). Int J Clin
Exp Pathol. 8:25–37. 2015.PubMed/NCBI
|
|
45
|
Li Q, Ren FQ, Yang CL, Zhou LM, Liu YY,
Xiao J, Zhu L and Wang ZG: Anti-proliferation effects of
isorhamnetin on lung cancer cells in vitro and in vivo. Asian Pac J
Cancer Prev. 16:3035–3042. 2015. View Article : Google Scholar : PubMed/NCBI
|