Introduction
Gemcitabine (GCB) is an anticancer agent widely used
alone or in combination with other cytotoxics in the treatment of
various malignancies including non-small cell lung cancer (NSCLC),
pancreatic, bladder, breast, ovarian, prostate cancer, and
cholangiocarcinoma (1). GCB toxicity
is generally mild, transitory and rarely dose limiting. Most common
side effects include laboratory alterations, such as
myelosupression, transaminase elevation, mild proteinuria and
hematuria, whereas symptomatic toxicities are usually well
controlled and not life threatening (2–4). Factors
increasing GCB toxicity include its combination with platinum
derivatives or taxanes, liver and kidney diseases, and alcohol
abuse (5–10). There are no evidence-based and
generally accepted recommendations for dose modifications of GCB,
and clinical decisions are typically made based on empirical
grounds.
The main enzyme involved in hepatic metabolism of
GCB is cytidine deaminase (CDA), encoded by the CDA gene
located in locus 1p36.2–35 (11–15). Data
regarding toxicity related to CDA polymorphisms are
inconsistent (16–18). Nevertheless, several studies
demonstrated that single nucleotide polymorphism (SNP) of
CDA c.79 A>C, c.208 G>A and c.435C>T, with
resulting decreased serum CDA concentration, may lead to severe
toxicity induced by GCB (18–31).
We describe here severe toxicity in four patients
treated with GCB used alone or in combination with cisplatin. For
each case, the probability of adverse drug reaction probability was
assessed using the Naranjo scale described in the study by Naranjo
et al (32). In the search of
potential toxicity causes, we performed in all cases evaluation of
CDA polymorphisms.
Case 1
A 67-year-old-woman was diagnosed with poorly
differentiated tubule-solid gallbladder adenocarcinoma invading the
liver and spreading to the greater omentum. The patient reported
recurrent pain in the upper abdomen, but was otherwise in a fairly
good condition [World Health Organization Performance Status (WHO
PS) 2], with body mass index of 18.6 and no apparent active
inflammatory symptoms. Serum alanine transaminase (ALT) and
aspartate transaminase (AST) levels were 55 and 100 U/l,
respectively, and the levels of bilirubin, serum creatinine and
glomerular filtration rate were within the normal values.
Biochemical abnormalities included elevated levels of C reactive
protein (CRP; 9 mg/dl), alkaline phosphatase (ALP; 1237 U/l),
gamma-glutamyl transpeptidase (GGT; 1,073 U/l) and white blood
cells (WBC; 10.4×109/l). Six months earlier she
underwent biliary stenting by percutaneous transhepatic
cholangiography, complicated by transient paralytic ileus. Braun
gastrointestinal bypass was performed one month before commencing
palliative chemotherapy. She was medicated with fentanyl patch (50
g/day every 3 days) and acetaminophen (500 mg orally three times
daily), morphine (20 mg daily) and low molecular weight heparin (60
mg once daily subcutaneously). Due to hypertension, for a few years
the patient had been administered enalapril, 5 mg twice daily.
Treatment plan included intravenous administration of GCB 1,000
mg/m2 on day 1 and 8, and cisplatin 25 mg/m2
on day 1, every 21 days. Two days after the first administration of
chemotherapy the patient developed a sudden deterioration of
general condition: weakness, severe pain in the right hypochondrium
and hypotension, accompanied by increased liver parameters: AST,
1876 U/l [Common Terminology Criteria for Adverse Events v. 4 Grade
(G)4]; ALT, 497 U/l (G3); bilirubin, 2.3 mg/dl (G2); anemia Hg, 8.9
g/dl (G2); leukopenia (2.0×109/l); and neutropenia
(1.44×109/l). There were no ECG signs of acute
myocardial ischemia, and the troponin level was 0.011 ng/ml (with a
level of ≥0.12 ng/ml corresponding to acute myocardial infarction).
Despite the treatment (hydrocortisone, isotonic solution, morphine)
the patient died within 6 h after onset of symptoms due to acute
cardio-pulmonary insufficiency. According to the will of the
family, the autopsy was not performed. Based on the Naranjo scale,
the drug causality of adverse reactions was considered probable
(total score, 5): Are there previous conclusive reports on this
reaction? Yes (1+). Did the adverse event appear after the
suspected drug was administered? Yes (2+). Are there alternative
causes that could on their own have caused the reaction? No
(2+).
Case 2
A 60-year-old woman presented with metastatic
squamous cell carcinoma of the right lung. She was a long-term
cigarette smoker, with accompanying chronic obstructive pulmonary
disease and carotid atherosclerosis, resulting in an episode of
stroke and epilepsy. Five years earlier the patient received
radical chemoradiation for laryngeal cancer and one year earlier
she underwent craniotomy for brain metastasis from lung cancer,
whole brain irradiation and three cycles of vinorelbine with
cisplatin. At admission WHO PS was 1 and body mass index 26. There
were no overt infections, apart from asymptomatic bacteriuria. The
CT scan showed multiple metastatic lesions in both lungs, a large
metastatic mass in the right adrenal gland and enlarged abdominal
lymph nodes. She was administered tramadol (50 mg twice daily),
ketoprofen (100 mg daily), dexamethasone (4 mg daily) and
omeprazole (20 mg daily). The complete blood count (CBC), liver and
kidney function parameters were within normal values, and the only
laboratory abnormalities included leukocytosis related to chronic
steroid therapy.
The treatment plan included administration of GCB
1,250 mg/m2 on day 1 and 8, and cisplatin 80
mg/m2 intravenously, every 21 days. One day after the
first dose of GCB in combination with cisplatin, she developed a
sudden deterioration of the general status: symptoms of pulmonary
edema, liver failure and a shock. The patient was administered
ceftazidime, steroids, isotonic solution, furosemide, morphine,
dopamine, dobutamine, noradrenaline and synchronized intermittent
mandatory ventilation. Despite this, on day 4 after the initiation
of chemotherapy, she died with symptoms of acute cardio-pulmonary
insufficiency. The autopsy showed lung sarcomatoid carcinoma with
metastases to the right adrenal gland and paraaortic lymph nodes.
There was also chronic hypertrophy of the left and right heart
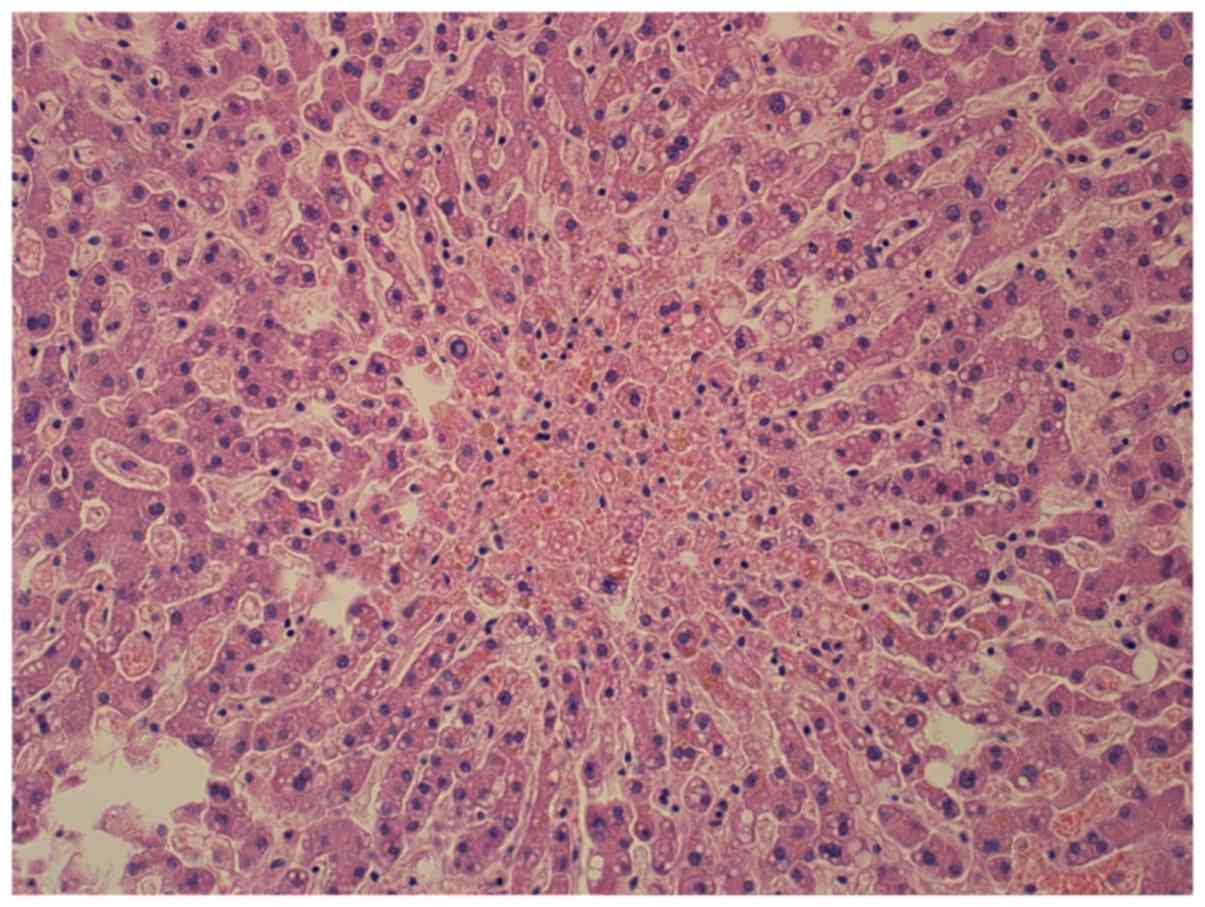
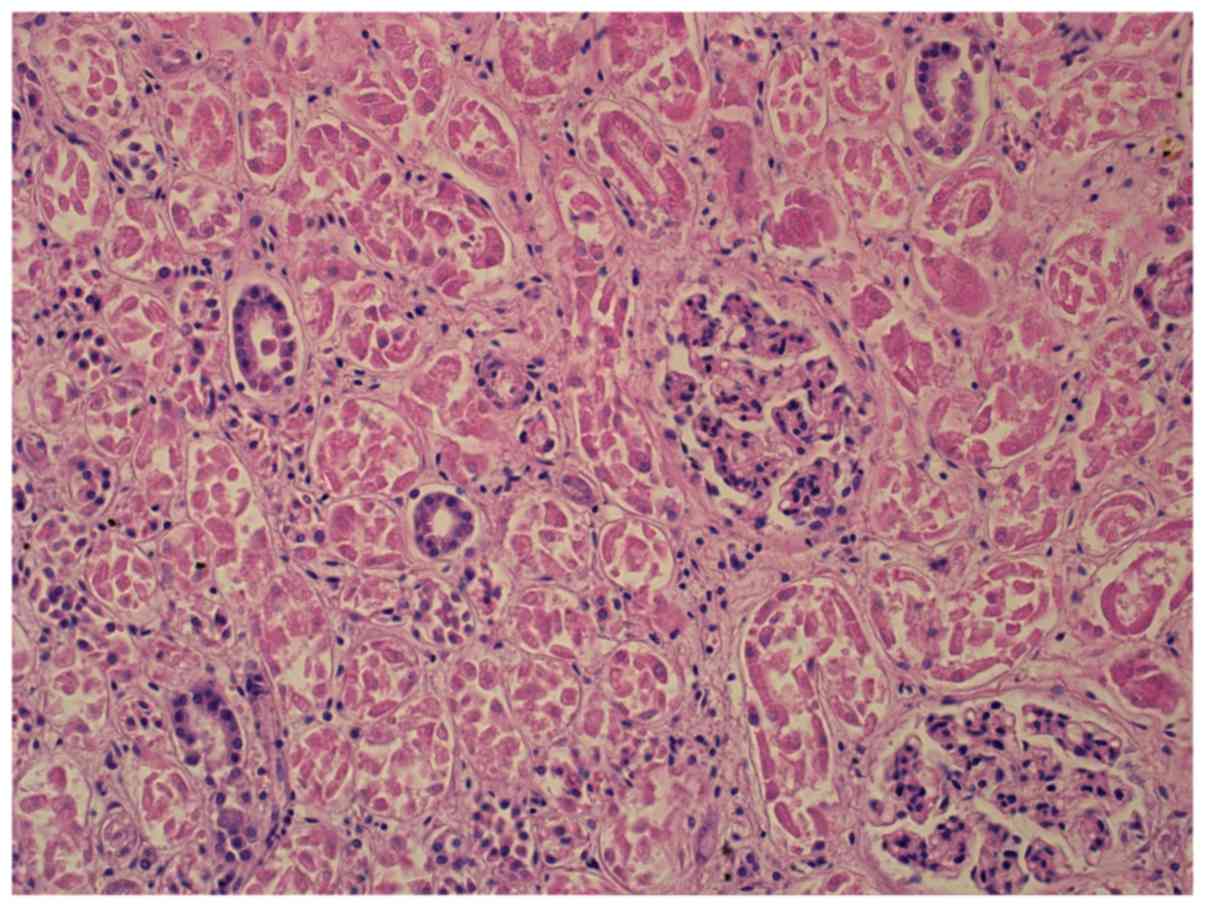
ventricles, liver and kidney damage, brain edema (Figs. 1 and 2),
lung emphysema, atelectasis, passive congestion and bilateral
bronchopneumonia. The probable cause of death was septic shock and
multiple organ failure caused by severe purulent lung inflammation.
Based on the Naranjo scale the drug causality of adverse reactions,
were considered possible (total score, 2): Are there previous
conclusive reports on this reaction? Yes (1+). Did the adverse
event appear after the suspected drug was administered? Yes (2+).
Are there alternative causes that could on their own have caused
the reaction? Yes (−1).
Case 3
A 68 year old man was diagnosed with tumor of the
pancreatic head. He presented with obstructive jaundice, with a
total serum bilirubin level of 12.8 mg/dl. CT scan and ultrasound
showed only a stricture in common bile duct, confirmed by
endoscopic retrograde cholangiopancreatography. Laparotomy revealed
an unresectable tumor of pancreatic head (adenocarcinoma G2)
infiltrating surrounding vessels, and Roux-Y
hepaticojejunoanastomosis was performed. The patient was in good
general status (WHO PS 1), with hypertension controlled with
combined therapy. Due to exocrine pancreatic insufficiency, he was
treated with pancreatine. Laboratory abnormalities before
chemotherapy commencement included thrombocytopenia (89 G/l) and
increased Ca 19-9 level (879 U/ml). Other CBC parameters, kidney
and liver functions were normal. Planned chemotherapy included GCB
at a dose of 1,000 mg/m2 on day 1, 8 and 15, with 30%
dose reduction due to baseline thrombocytopenia. One week after the
first GCB administration, platelet level decreased to 32 G/l (G3).
The next drug administration, was possible only after three weeks,
also with 30% dose reduction. Again, a week later a significant
thrombocytopenia (60 G/l) occurred (G2). The third GCB
administration was delayed by a week, and the dose was further
reduced to 50% of the due dose. Despite this, he developed a G2
thrombocytopenia (67×109/l) with accompanying G2
neutropenia (1.4×109/l) and G2 anemia (8.7 g/dl).
Abdominal ultrasound showed disease progression and a increasing Ca
19-9 serum level (1,707 U/ml). Owing only to the local tumor
extension, the patient received radiotherapy at a dose of 50.4 Gy
in 28 fractions without concurrent chemotherapy. Based on the
Naranjo scale, the drug causality of the adverse reactions was
considered probable (total score, 6): Are there previous conclusive
reports on this reaction? Yes (1+). Did the adverse event appear
after the suspected drug was administered? Yes (2+). Did the
adverse reaction improve when the drug was discontinued? Yes (1+).
Did the adverse event reappear when the drug was re-administered?
Yes (2+). Are there alternative causes that could on their own have
caused the reaction? Yes (−1). Was the reaction more severe when
the dose was increased or less severe when the dose was decreased?
Yes (1+).
Case 4
A 58 year old woman was diagnosed with a tumor of
the pancreatic head. The first symptom was mechanical jaundice,
with serum bilirubin level of 13, 5 mg/dl. Abdominal CT revealed a
2 cm mass of the pancreatic head. Endoscopic retrograde
cholangiopancreatography showed a stenosis of the common bile and
pancreatic ducts, which was managed by stent insertion. A month
later a radical pancreatoduedenectomy was performed. Pathology
examination showed G1 ductal adenocarcinoma staged pT3N0M0. There
was blood and lymphatic vessels invasion and no tumor-free margins
were obtained. After the surgery the patient was in a general good
condition (WHO PS 1), with hypertension treated with amlodypine.
CBC parameters, kidney and liver functions were within normal
ranges. The patient received single-agent adjuvant GCB at a dose of
1,000 mg/m2 on days 1, 8 and 15. Two days after the
third administration of GCB she developed a sudden deterioration of
general status: weakness, severe pain in the right hypochondrium,
vomiting (G2) and fever (G2). Blood tests showed increased
bilirubin level (1.9 mg/dl), leukocytosis (14×109/l),
neutrocytosis (11×109/l), anemia (Hg, 8.1 g/dl),
elevated CRP (228 mg/l) and procalcitonin (1.0 ng/ml).
Microbiological tests were negative. The patient was administered
antifungals, antipyretics, setrons, intravenous fluids and
electrolyte supplementation, the general condition improved, and
after three weeks the patient began a second chemotherapy cycle.
Shortly after the first GCB administration she developed a severe
pain in the upper abdomen, accompanied by a headache and fever
(G2). The patient developed G3 neutropenia (0.68×109/l)
and was treated with oral antibiotics. Due to repeating toxicity,
chemotherapy was discontinued. Abdominal CT showed a few small
liver metastases. She received five cycles of chemotherapy
including oxaliplatin, 5-fluorouracil and leucovorine (OFF), which
was well tolerated and resulted in a complete remission. After 2.5
years, due to local progression, OFF chemotherapy was reinstituted
and now, 3.5 years after the initial treatment, the patient
developed a metastatic lesion in sigmoid colon, and was
administered palliative radiotherapy. Despite this, she remains in
good general condition (PS 1). Based on the Naranjo scale, the drug
causality of adverse reactions was considered probable (total
score, 5) Are there previous conclusive reports on this reaction?
Yes (1+). Did the adverse event appear after the suspected drug was
administered? Yes (2+). Did the adverse reaction improve when the
drug was discontinued? Yes (+1). Did the adverse event reappear
when the drug was re-administered? Yes (2+). Are there alternative
causes that could on their own have caused the reaction? Yes
(−1).
Polymorphism of CDA gene
assessment
In a search for the cause of the severe toxicity, in
all cases we assessed three SNPs of CDA gene related to GCB
metabolism: c.79A>C (rs2072671), c.208G>A (rs60369023) and
c.435C>T (rs1048977). In the two deceased cases DNA was obtained
from paraffin embedded healthy tissue material. Paraffin was
removed with xylene and isolation was done using the Cobas DNA
Sample Preparation Kit, according to the procedure specified by the
manufacturer (Roche Diagnostic, Warsaw, Poland). In another two
patients DNA was isolated from peripheral blood samples using
Genomic Midi Ax (A&A Biotechnology, Gdynia, Poland). All CDA
polymorphisms were analyzed by polymerase chain reaction (PCR)
followed by bidirectional Sanger sequencing. Sequences were
analyzed using the reference sequence CDA (NM_001785.2) and
Sequencher software version 4.10.1 (Gene Codes Corporation, Ann
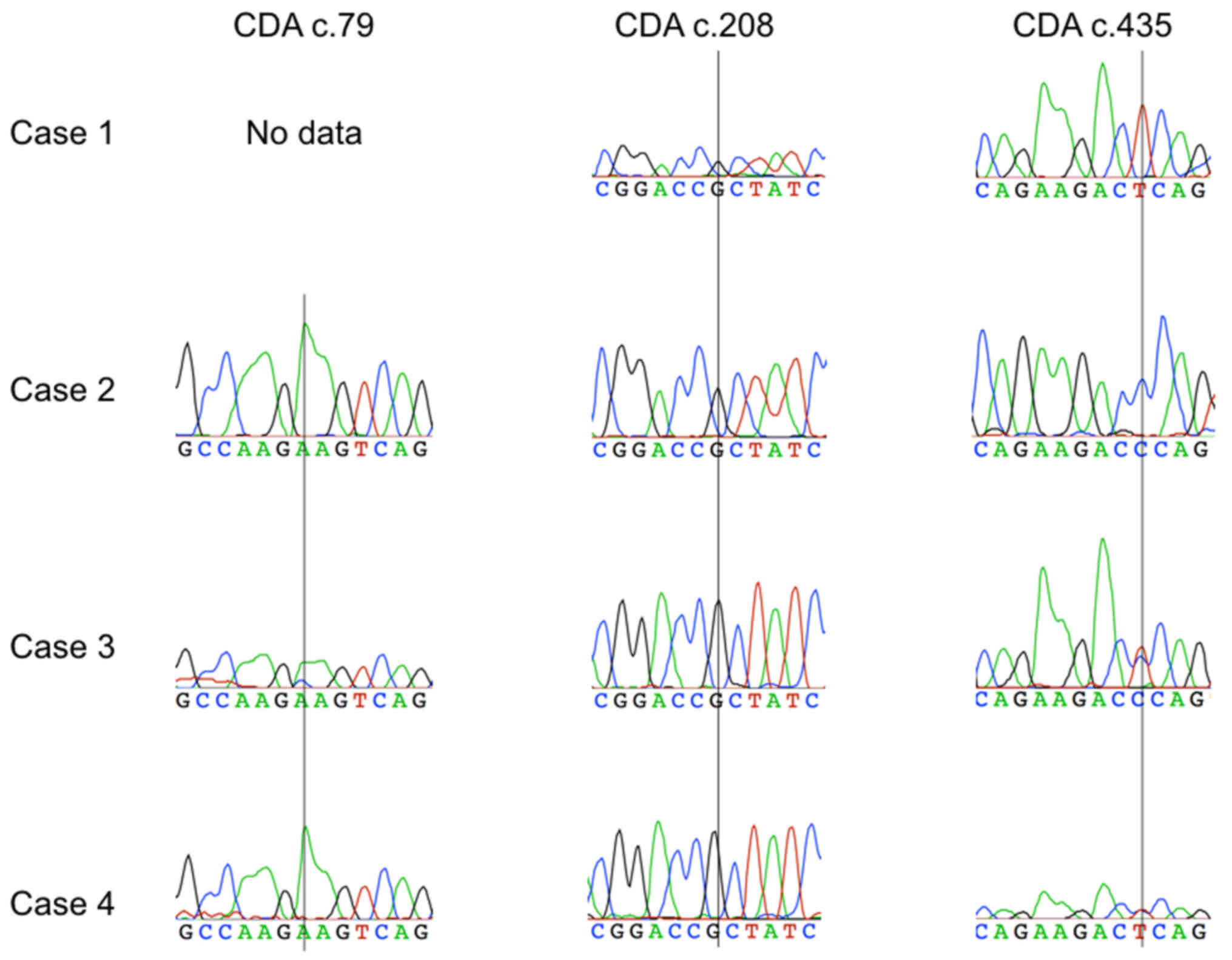
Arbor, MI, USA). The genotyping results are presented in Fig. 3. In the case 1, the presence of a
homozygous CDA variant c.435TT was found while the reaction
product PCR was not obtained for amplicon including polymorphism
c.79A>C. Cases 3 and 4 showed a heterozygous variant CDA
c.435CT, accompanied in case 3 by a heterozygotic c.79A>C
variant. In no patient the c.208G>A variant was diagnosed.
Discussion
We report here four severe toxicity cases, including
two fatal, following the administration of GCB alone or in
combination with cisplatin. In three of these cases the causative
role of GCB therapy was considered probable and in another one as
possible. In the differential diagnosis all risk factors present in
patients at start of therapy were included. Patient 1, 6 months
earlier underwent biliary stenting by percutaneous transhepatic
cholangiography, complicated by transient paralytic ileus, with
persistently elevated but stable ALP and gamma-glutamyl
transpeptidase. In patients 2 and 4 an infection, although not
diagnosed, could be the main cause of sudden deterioration, but
with strong association with GCB administration. In patient 4, good
tolerance of other cytotoxic agents suggest causative role of GCB.
Patient 3 presented with initial trombocytopenia, which contributed
to subsequent toxicity.
The two fatal cases in this series have some common
features: concomitant administration of GCB and cisplatin, and
sudden onset after the start of chemotherapy (two days; case 1, and
one day; case 2). The combination of GCB and cisplatin is routinely
used in various malignancies including NSCLC and advanced biliary
cancer, and is considered safe. However there are data indicating
that this regimen may occasionally induce oxidative stress leading
to multi-organ failure (2,5,33,34). Cisplatin-induced liver toxicity has
been attributed to the enhanced expression of cytochrome P450 2E1
(CYP2E1), and is exacerbated in patients with diabetes, obesity,
nicotine addiction or alcohol abuse (34,35).
Several preclinical studies have investigated possibilities of
hepatic mitochondrial oxidative damage protection using selenium,
vitamin E, a hydroxyl radical scavenger dimethylthiourea (DMTU) and
a polyphenolic flavonoid daidzein (36–38), but
none has yet found its application in clinical practice.
Data on the relationship between CDA
polymorphisms and GCB toxicity are inconsistent (15–31).
Severe GCB toxicity in NSCLC patients was reported in cases with
heterozygous c.437CT variant and, to a lesser degree, in those with
homozygous c.435TT variant (25,26).
Interestingly, the latter was also found to be related to better
response to GCB treatment, owing to reduced serum CDA concentration
and higher exposure to active GCB metabolites (27,28).
Heterozygous c.79A>C polymorphism was reported to cause severe
leukopenia and neutropenia (14,19–22).
In our series, c.435TT variant was found in one
patient, and c.435CT variant in two (in one accompanied by
c.79A>C variant). In one case the presence of c.79A>C could
not be excluded, due to inability of performing the PCR reaction.
No case showed the presence of c.208G>A variant, which is
typical for the Asian population (15,23,24).
Importantly, our analysis included only three most common
CDA polymorphisms, therefore the presence of other, less
frequent variants with potential impact on GCB metabolism cannot be
excluded (15,21,39).
Previous studies suggested that CDA deficiency associated with
CDA gene polymorphisms may affect more patients treated with
GCB, but the lack of population data does not allow for the
estimation of real CDA polymorphisms frequency. In patients
with decreased CDA activity in the serum, the risk of serious side
effects is in the range of 5–10% for GCB alone and 15–30% for GCB
combinations (27,28). The evaluation of gene polymorphisms is
relatively simple and non-invasive (swabbing the inner site of the
cheek), yet currently there are no recommendations for routine
pretreatment assessment of CDA polymorphisms or CDA serum
level (18,40,41).
In conclusion, determining the cause of acute GCB
toxicity, including both baseline clinical conditions and genetic
susceptibilities, may inform clinical decisions. In patients with
clinical factors predisposing to increased risk of toxicity,
assessment of polymorphic variants related to pyrimidine metabolism
may increase treatment safety.
Acknowledgements
The authors thank Ms. Claudia Wiewiora for
linguistic check.
References
|
1
|
Toschi L, Finocchiaro G, Bartolini S,
Gioia V and Cappuzzo F: Role of gemcitabine in cancer therapy.
Future Oncol. 1:7–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aapro MS, Martin C and Hatty S:
Gemcitabine-a safety review. Anticancer Drugs. 9:191–201. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tonato M, Mosconi AM and Martin C: Safety
profile of gemcitabine. Anticancer Drugs. 6 Suppl 6:S27–S32. 1995.
View Article : Google Scholar
|
|
4
|
Wong A, Soo RA, Yong WP and Innocenti F:
Clinical pharmacology and pharmacogenetics of gemcitabine. Drug
Metab Rev. 41:77–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Moorsel CJ, Pinedo HM, Veerman G,
Bergman AM, Kuiper CM, Vermorken JB, van der Vijgh WJ and Peters
GJ: Mechanisms of synergism between cisplatin and gemcitabine in
ovarian and non-small-cell lung cancer cell lines. Br J Cancer.
80:981–990. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shord SS, Faucette SR, Gillenwater HH,
Pescatore SL, Hawke RL, Socinski MA and Lindley C: Gemcitabine
pharmacokinetics and interaction with paclitaxel in patients with
advanced non-small-cell lung cancer. Cancer Chemother Pharmacol.
51:328–336. 2003.PubMed/NCBI
|
|
7
|
Jiang X, Galettis P, Links M, Mitchell PL
and McLachlan AJ: Population pharmacokinetics of gemcitabine and
its metabolite in patients with cancer: Effect of oxaliplatin and
infusion rate. Br J Clin Pharmacol. 65:326–333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teusink AC and Hall PD: Toxicities of
gemcitabine in patients with severe hepatic dysfunction. Ann
Pharmacother. 44:750–754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Felici A, Di Segni S, Milella M,
Colantonio S, Sperduti I, Nuvoli B, Contestabile M, Sacconi A,
Zaratti M, Citro G and Cognetti F: Pharmacokinetics of gemcitabine
at fixed-dose rate infusion in patients with normal and impaired
hepatic function. Clin Pharmacokinet. 48:131–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ciccolini J, Serdjebi C, Peters GJ and
Giovannetti E: Pharmacokinetics and pharmacogenetics of gemcitabine
as a mainstay in adult and pediatric oncology: An EORTC-PAMM
perspective. Cancer Chemother Pharmacol. 78:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demontis S, Terao M, Brivio M, Zanotta S,
Bruschi M and Garattini E: Isolation and characterization of the
gene coding for human cytidine deaminase. Biochim Biophys Acta.
1443:323–333. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gilbert JA, Salavaggione OE, Ji Y,
Pelleymounter LL, Eckloff BW, Wieben ED, Ames MM and Weinshilboum
RM: Gemcitabine pharmacogenomics: Cytidine deaminase and
deoxycytidylate deaminase gene resequencing and functional
genomics. Clin Cancer Res. 12:1794–1803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kocabas NA, Aksoy P, Pelleymounter LL,
Moon I, Ryu JS, Gilbert JA, Salavaggione OE, Eckloff BW, Wieben ED,
Yee V, et al: Gemcitabine pharmacogenomics: Deoxycytidine kinase
and cytidylate kinase gene resequencing and functional genomics.
Drug Metab Dispos. 36:1951–1959. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu J, Zhou Y, Zhang J, Chen Y, Zhuang R,
Liu T and Cai W: High incidence of severe neutropenia after
gemcitabine-based chemotherapy in Chinese cancer patients with CDA
79A>C mutation. Clin Chim Acta. 413:1284–1287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Micozzi D, Carpi FM, Pucciarelli S,
Polzonetti V, Polidori P, Vilar S, Williams B, Costanzi S and
Vincenzetti S: Human cytidine deaminase: A biochemical
characterization of its naturally occurring variants. Int J Biol
Macromol. 63:64–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Serdjebi C, Milano G and Ciccolini J: Role
of cytidine deaminase in toxicity and efficacy of nucleosidic
analogs. Expert Opin Drug Metab Toxicol. 11:665–672. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon KA, Woo SM, Hong EK, Jung MK, Park
WS, Bae K, Han SS, Kim TH, Koh YH, Park SJ and Lee WJ: Cytidine
deaminase as a molecular predictor of gemcitabine response in
patients with biliary tract cancer. Oncology. 89:345–350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Evrard A and Mbatchi L: Genetic
polymorphisms of drug metabolizing enzymes and transporters: The
long way from bench to bedside. Curr Top Med Chem. 12:1720–1729.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joerger M, Burgers SA, Baas P, Smit EF,
Haitjema TJ, Bard MP, Doodeman VD, Smits PH, Vincent A and Huitema
AD: Germline polymorphisms in patients with advanced nonsmall cell
lung cancer receiving first-line platinum-gemcitabine chemotherapy:
A prospective clinical study. Cancer. 118:2466–2475. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka M, Javle M, Dong X, Eng C,
Abbruzzese JL and Li D: Gemcitabine metabolic and transporter gene
polymorphisms are associated with drug toxicity and efficacy in
patients with locally advanced pancreatic cancer. Cancer.
116:5325–5335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tibaldi C, Giovannetti E, Vasile E, Mey V,
Laan AC, Nannizzi S, Di Marsico R, Antonuzzo A, Orlandini C,
Ricciardi S, et al: Correlation of CDA ERCC1, and XPD polymorphisms
with response and survival in gemcitabine/cisplatin-treated
advanced non-small cell lung cancer patients. Clin Cancer Res.
14:1797–1803. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Farrell JJ, Bae K, Wong J, Guha C, Dicker
AP and Elsaleh H: Cytidine deaminase single-nucleotide polymorphism
is predictive of toxicity from gemcitabine in patients with
pancreatic cancer: RTOG 9704. Pharmacogenomics J. 12:395–403. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yomemori K, Ueno H, Okusaka T, Yamamoto N,
Ikeda M, Saijo N, Yoshida T, Ishii H, Furuse J, Sugiyama E, et al:
Severe drug toxicity associated with a single-nucleotide
polymorphism of the cytidine deaminase gene in a Japanes cancer
patient treated with gemcytabine plus cisplatin. Clin Cancer Res.
11:2620–2624. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sugiyama E, Kaniwa N, Kim SR,
Kikura-Hanajiri R, Hasegawa R, Maekawa K, Saito Y, Ozawa S, Sawada
J, Kamatani N, et al: Pharmacokinetics of gemcitabine in Japanese
cancer patients: The impact of a cytidine deaminase polymorphism. J
Clin Oncol. 25:32–42. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ludovini V, Floriani I, Pistola L, Minotti
V, Meacci M, Chiari R, Garavaglia D, Tofanetti FR, Flacco A,
Siggillino A, et al: Association of cytidine deaminase and
xeroderma pigmentosum group D polymorphisms with response,
toxicity, and survival in cisplatin/gemcitabine-treated advanced
non-small cell lung cancer patients. J Thorac Oncol. 6:2018–2026.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okazaki T, Javle M, Tanaka M, Abbruzzese
JL and Li D: Single nucleotide polymorphisms of gemcitabine
metabolic genes and pancreatic cancer survival and drug toxicity.
Clin Cancer Res. 16:320–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ciccolini J, Dahan L, André N, Evrard A,
Duluc M, Blesius A, Yang C, Giacometti S, Brunet C, Raynal C, et
al: Cytidine deaminase residual activity in serum is a predictive
marker of early severe toxicities in adults after gemcitabine-based
chemotherapies. J Clin Oncol. 28:160–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mercier C, Raynal C, Dahan L, Ortiz A,
Evrard A, Dupuis C, Blesius A, Duluc M, Franceschini F, Giacometti
S, et al: Toxic death case in a patient undergoing
gemcitabine-based chemotherapy in relation with cytidine deaminase
downregulation. Pharmacogenet Genomics. 17:841–844. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding X, Chen W, Fan H and Zhu B: Cytidine
deaminase polymorphism predicts toxicity of gemcitabine-based
chemotherapy. Gene. 559:31–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou M, Ding YJ, Feng Y, Zhang QR, Xiang Y
and Wan HY: Association of xeroderma pigmentosum group D
(Asp312Asn, Lys751Gln) and cytidine deaminase (Lys27Gln, Ala70Thr)
polymorphisms with outcome in Chinese non-small cell lung cancer
patients treated with cisplatin-gemcitabine. Genet Mol Res.
13:3310–3318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li H and Wang X and Wang X: The impact of
CDA A79C gene polymorphisms on the response and hematologic
toxicity in gemcitabine-treated patients: A meta-analysis. Int J
Biol Markers. 29:e224–e232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Naranjo CA, Busto U, Sellers EM, Sandor P,
Ruiz I, Roberts EA, Janecek E, Domecq C and Greenblatt DJ: A method
for estimating the probability of adverse drug reactions. Clin
Pharmacol Ther. 30:239–245. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Waseem M, Bhardwaj M, Tabassum H,
Raisuddin S and Parvez S: Cisplatin hepatotoxicity mediated by
mitochondrial stress. Drug Chem Toxicol. 38:452–459. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu Y and Cederbaum AI: Cisplatin-induced
hepatotoxicity is enhanced by elevated expression of cytochrome
P450 2E1. Toxicol Sci. 89:515–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karale S and Kamath JV: Effect of daidzein
on cisplatin-induced hematotoxicity and hepatotoxicity in
experimental rats. Indian J Pharmacol. 49:49–54. 2017.PubMed/NCBI
|
|
37
|
Naziroglu M, Karaoğlu A and Aksoy AO:
Selenium and high dose vitamin E administration protects
cisplatin-induced oxidative damage to renal, liver and lens tissues
in rats. Toxicology. 195:221–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
dos Santos NA, Martins NM, Curti C, Pires
Bianchi Mde L and dos Santos AC: Dimethylthiourea protects against
mitochondrial oxidative damage induced by cisplatin in liver of
rats. Chem Biol Interact. 170:177–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Raynal C, Ciccolini J, Mercier C, Boyer
JC, Polge A, Lallemant B, Mouzat K, Lumbroso S, Brouillet JP and
Evrard A: High-resolution melting analysis of sequence variations
in the cytidine deaminase gene (CDA) in patients with cancer
treated with gemcitabine. Ther Drug Monit. 32:53–60. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Petros WP and Evans WE: Pharmacogenomics
in cancer therapy: Is host genome variability important? Trends
Pharmacol Sci. 25:457–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Evrard A, Lacarelle B and Ciccolini J:
Severe or lethal toxicities with nucleosidic analogs: Time for
action. Pharmacogenomics. 14:227–230. 2013. View Article : Google Scholar : PubMed/NCBI
|