Introduction
Tumor hypoxia is recognized as a well-known obstacle
to radiotherapy due to the limitation of oxygen effect, i.e.,
oxygen-dependent sensitization of radiation efficiency (1). To reduce the radioresistance of hypoxic
tumors, several strategies, such as fractionated radiotherapy,
increased oxygen delivery, hypoxia inducible factor (HIF)-1
inhibitors, and radiosensitizers, have been developed (2). Since the introduction of misonidazole in
the 1970s, numerous hypoxic cell radiosensitizers have been
screened; several have undergone clinical evaluation (3). However, clinical trials with
nitroimidazole derivatives have demonstrated their limited
therapeutic benefit and remain inconclusive. Furthermore,
misonidazole and etanidazole, the well-known nitroimidazole
derivatives, induced undesirable side effects such as neurotoxicity
(4). Additionally, the low tolerable
dose produced an inconclusive outcome and restricted the clinical
use of these drugs. In contrast to these results, a Danish trial of
nimorazole, another radiosensitizer, significantly improved the
radiotherapy of squamous head and neck cancers without major side
effects (5,6). In these trials, clinically relevant
hypoxia markers allowed the identification of patients that benefit
from the combination of nimorazole and radiotherapy.
Furthermore, doranidazole
[1-(1′,3′,4′-trihydroxy-2′-butoxy)-methyl-2-nitroimidazole, PR-350;
Fig. 1] was established in Japan; its
structure is intended to reduce neurotoxicity owing to its
impermeability across the blood-brain barrier (BBB) (7). The radiosensitizing effect of this
compound under hypoxia has been clarified in vitro (8–10) and
in vivo (8,9,11,12). Recently, our group demonstrated the
radiosensitizing effect of doranidazole on C6 rat intracranial
glioma, which shows wide range of hypoxia and disruption of the BBB
(13). Based on these studies, a
phase I/II trial against locally advanced non-small-cell lung
cancer (14) and phase III trials of
doranidazole against advanced pancreatic cancer (15,16) were
performed. Some studies demonstrated that doranidazole treatment
after irradiation significantly improved the local tumor control
and overall survival. These recent clinical reports have encouraged
the further development and improvement of nitroimidazoles.
In China, sodium glycididazole, a nitroimidazole
derivative with a chemical structure containing a dimer of
metronidazole (Fig. 1), was designed,
screened, and studied by the Second Military Medical University
(17). There are several literature
reports in Chinese that indicate the radiation-enhancing potential
of glycididazole. In addition, glycididazole was reported to have
radiosensitizing and chemosensitizing effects in clinical trials
(17–19) and is marketed and used clinically.
However, there are few preclinical studies and no investigations
have been performed to evaluate the radiosensitizing efficacy of
glycididazole in comparison with that of other nitroimidazole
derivatives. Therefore, in this study, we aimed to evaluate the
radiosensitizing effects of glycididazole and doranidazole on tumor
cells with different oxygenation conditions in vitro and on
the tumor growth of subcutaneous xenografts in vivo.
Materials and methods
Reagents
Glycididazole was purchased from Luye Pharma Group
Co., Ltd, (Beijing, China). Doranidazole was supplied by Pola
Pharma Inc. (Tokyo, Japan). Ultrapure N2 gas (99.999%)
was obtained from Air Water Technical Supply (Ishikari, Japan). All
other chemicals were purchased from Wako Pure Chemical Industries,
Ltd. (Tokyo, Japan), unless otherwise stated.
Cell culture
Murine squamous cell carcinoma SCCVII cells were
grown in α-MEM (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Filtron Pty.
Ltd., Brooklyn, Australia). Cells were maintained at 37°C in an
atmosphere of 5% CO2 and 95% air.
Drug treatment, hypoxic incubation,
and X-irradiation in vitro
Tumor cells were allowed to adhere to a 6-cm plastic
dish and treated with 10 mM glycididazole or doranidazole before
hypoxic incubation. To establish the hypoxic conditions (oxygen
concentration ≤10 mmHg [1.3%]; unpublished data) for tumor cells,
the dish was placed in a gas-exchangeable chamber and ultrapure
N2 gas was continuously passed over the dish, which was
placed on ice, for 25 min. The cells were then exposed to the
indicated doses of X-rays while maintaining the gas flow.
X-irradiation was performed with a Shimadzu PANTAK HF-350 X-ray
generator (1.0 mm Al filter; 200 kVp; 20 mA; Shimadzu, Kyoto,
Japan).
Clonogenic survival assay
At 5 h before hypoxic induction, 100–20,000 of
SCCVII cells were seeded on a 6-cm plastic dish. After drug
treatment, hypoxic induction, and X-irradiation as described above,
the cells were incubated with fresh medium supplemented with 10%
FBS under normoxia at 37°C for 8 days. The cells were subsequently
fixed with methanol, stained with Giemsa solution, and scored under
a microscope. Only colonies that contained more than 50 cells were
scored as surviving cells. After the surviving fraction at each
dose was calculated with respect to the plating efficiency of the
non-irradiated control, the mean ± standard error of the surviving
fractions obtained from three experiments was plotted. The survival
curves were fitted to a linear-quadratic (LQ) model: SF=exp
(−αD-βD2), where SF is the surviving
fraction and D is the physical dose, by using the data
analysis software Origin Pro 7 (OriginLab Co., Northampton, MA,
USA).
Animal model
All animal experiments in this study were conducted
according to the guidelines of the Law for The Care and Welfare of
Animals in Japan and approved by the Animal Experiment Committee of
the Graduate School of Veterinary Medicine, Hokkaido University.
Male C3H/HeN mice were purchased from Japan SLC (Hamamatsu, Japan).
SCCVII solid tumors were established via s.c. injection of
2×105 cells into the limbs of micle.
Drug treatment and X-irradiation in
vivo
The experiment was initiated when the tumor volume
(V=0.5 × length × width2) reached 250–450
mm3. The body weights measured before the experiments
were in the range from 20–25 g. The animals were randomized into
six groups of 9–10 animals per group: i) saline only; ii) saline
and X-irradiation (30 Gy); iii) glycididazole only; iv)
glycididazole and X-irradiation; v) doranidazole only; and vi)
doranidazole and X-irradiation. Glycididazole and doranidazole were
intravenously injected into mice at a dose of 200 mg/kg, 20 min
before X-irradiation. For the irradiation of transplanted tumors,
the mice were shielded with lead panels, except for the
tumor-bearing limbs. X-irradiation was performed with an X-Rad
iR-225 (1.0 mm Al + 0.5 mm Cu filters; 250 kVp; 16.7 mA; Precision
X-Ray; North Branford, CT, USA) at a dose rate of 1.7 Gy/min. The
tumor volume was measured three times per week and the tumor growth
was monitored for a maximum of 47 days.
high performance liquid chromatography
(HPLC) analysis
At 20 min after i.v. injection of the compounds, the
mice were sacrificed and blood and tumor tissues were sampled.
Serum was obtained via centrifugation of the blood sample at 5,000
× g for 15 min and passed through an Ultrafree-MC filtration device
(EMD Millipore, Billerica, MA, USA). Tumor samples were homogenized
with Polytron (Kinematica, Luzern, Switzerland) and centrifuged at
3,000 × g for 5 min. The supernatant was diluted with two volumes
of methanol and centrifuged at 2,500 × g for 10 min. The obtained
supernatant was mixed with two volumes of ethanol, which was
subsequently evaporated completely. Aqueous serum and resolved
tumor samples were analyzed using an HPLC system comprising a
gradient pump (LC-10ADvp), an autosampler (SIL-10ADvp), an in-line
degasser (DGU-20A3), a column oven (CTO-10ACvp), and a UV detector
(SPD-10Avp) (all from Shimadzu). System control and data analysis
were performed by using LCsolution (Shimadzu). Separation was
performed by using a reversed phase column (4.6×250 mm) of TSK gel
ODS-80Tm (TOSOH).
Statistical analysis
The Kaplan-Meier method was used to analyze the
doubling times. Log-rank test was used to compare the doubling
times between any 2 treatment groups.
Results
Glycididazole radiosensitized cancer
cells under hypoxic conditions to a similar extent as doranidazole
did in vitro
To investigate the effects of glycididazole and
doranidazole on the radiosensitivity of cancer cells, colony
formation assay was performed. The clonogenic survival curves of
SCCVII cells irradiated in vitro under normoxic and hypoxic
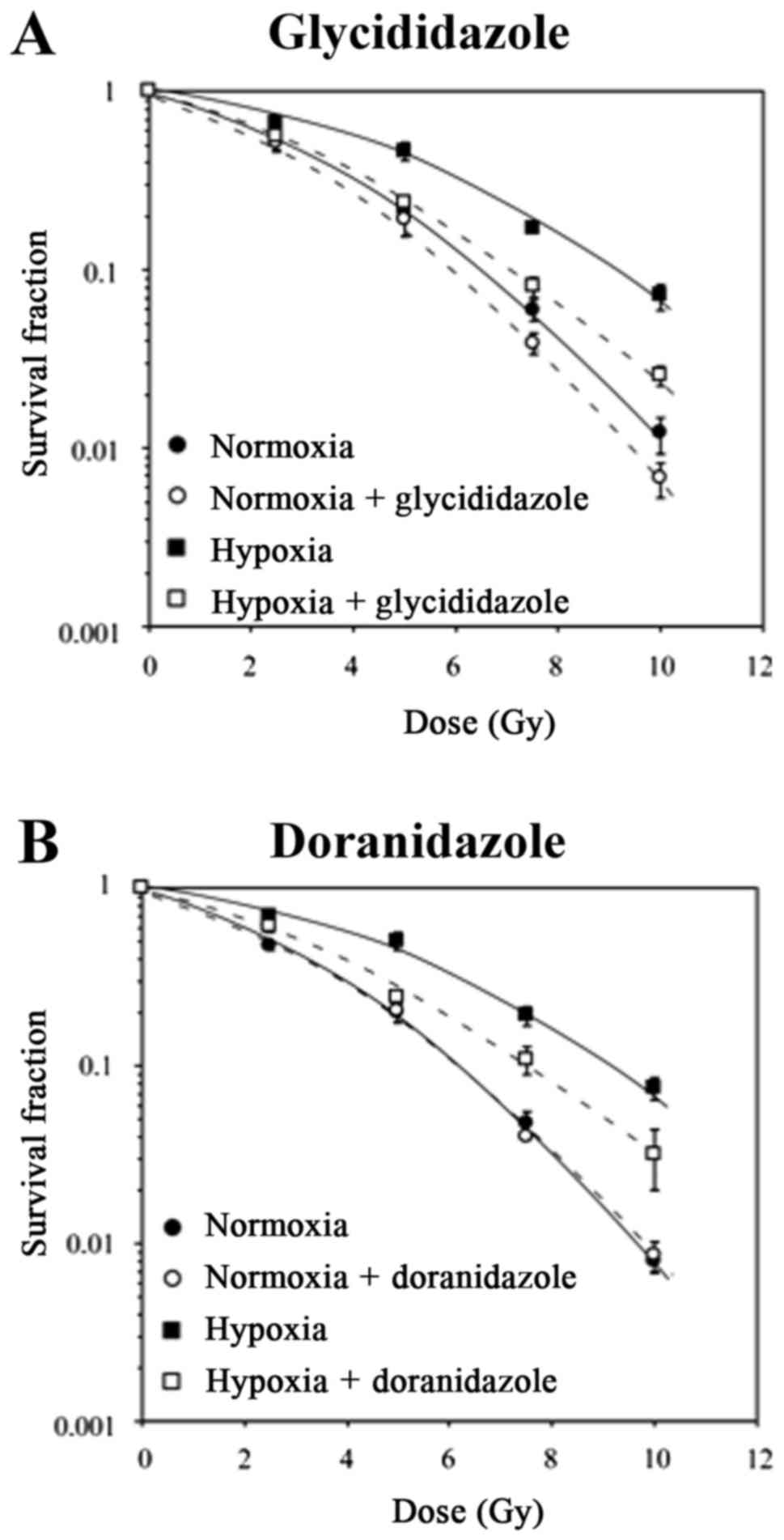
conditions, with or without these drugs, are shown in Fig. 2A. In the absence of glycididazole,
X-irradiation under hypoxia reduced the radiosensitivity of SCCVII
cells. Under both normoxia and hypoxia without irradiation, 10 mM
glycididazole was not toxic to SCCVII cells. The survival curves of
SCCVII cells irradiated under normoxia show that glycididazole
decreased the clonogenic ability. The dose that reduced cell
survival to 10% (D10) obtained from the normoxic cell
survival curve was 6.55 Gy, which decreased to 6.08 Gy when the
cells were irradiated in the presence of glycididazole (Table I). The enhancement of
radiation-inducing cell death was more pronounced in hypoxic
condition; the D10 values were 9.18 and 7.09 in the
absence and presence of glycididazole, respectively. The
sensitizing enhancement ratio (SER) for glycididazole was 1.08 and
1.29 for normoxic and hypoxic conditions, respectively, which
suggested that glycididazole sensitized both hypoxic and normoxic
cells to radiation in vitro.
 | Table I.Survival parameters and sensitizer
enhancement ratios for SCCVII cells treated with X-irradiation
combining with glycididazole or doranidazole. |
Table I.
Survival parameters and sensitizer
enhancement ratios for SCCVII cells treated with X-irradiation
combining with glycididazole or doranidazole.
| Treatment | Oxygen condition | Drug | α
(Gy−1) | β
(Gy−2) | D10 |
SERD10 |
|---|
| Glycididazole |
Normoxia | − | 0.183 | 0.026 | 6.55 | 1.08 |
|
|
| + | 0.186 | 0.032 | 6.08 |
|
|
| Hypoxia | − | 0.088 | 0.018 | 9.18 | 1.29 |
|
|
| + | 0.214 | 0.016 | 7.09 |
|
| Doranidazole |
Normoxia | − | 0.173 | 0.031 | 6.29 | 1.02 |
|
|
| + | 0.191 | 0.030 | 6.16 |
|
|
| Hypoxia | − | 0.052 | 0.021 | 9.38 | 1.24 |
|
|
| + | 0.189 | 0.015 | 7.53 |
|
Alternatively, although 10 mM doranidazole exerted
no sensitizing effect when combined with irradiation in aerobic
conditions, a significant sensitizing activity was observed in
combination with irradiation under hypoxic conditions (Fig. 2B). The SER for doranidazole, which was
calculated from the D10 values in each condition, was
1.02 and 1.24 for normoxic and hypoxic conditions, respectively
(Table I). These results suggested
that glycididazole exerted a radiosensitizing effect on hypoxic
cells that was comparable to that of doranidazole.
Glycididazole showed weak
radiosensitizing effect on tumor growth compared with that of
doranidazole in vivo
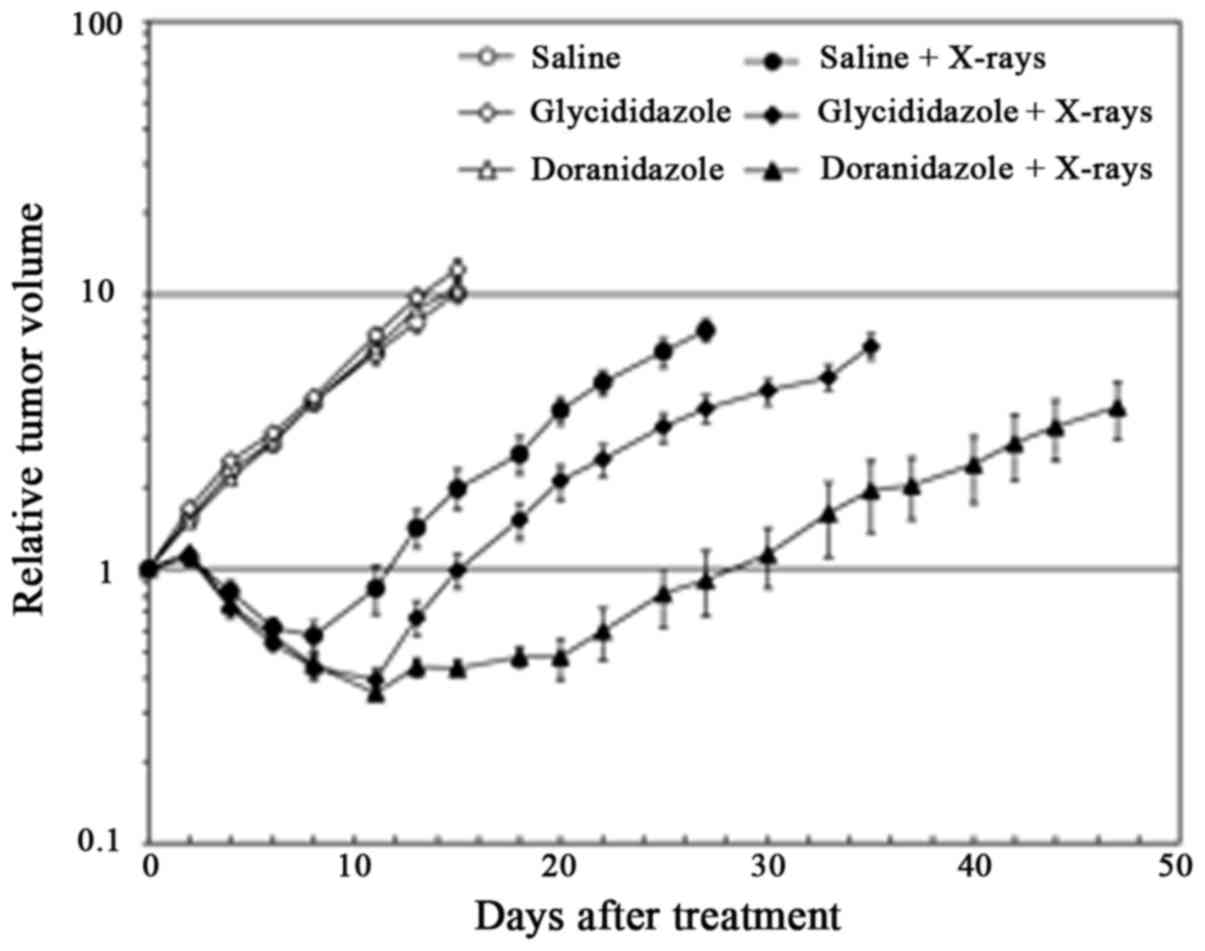
To evaluate the radiosensitizing effects of
glycididazole and doranidazole on squamous carcinoma in
vivo, SCCVII tumor-bearing mice were treated with 30 Gy
X-irradiation 20 min after the i.v. administration of 200 mg/kg
glycididazole or doranidazole. As shown in Fig. 3 and Table
II, a single administration of either glycididazole or
doranidazole exerted little effect on tumor growth. X-irradiated
tumors exhibited delayed growth and the doubling time of tumor
volume after X-irradiation was 18 days. The treatment with 200
mg/kg doranidazole significantly enhanced the inhibition of
radiation-induced growth and extended the doubling time to 42 days
(P<0.001). Although treatment with glycididazole at the same
dose extended the doubling time to 20 days significantly
(P<0.05), the enhancement rate was clearly smaller than that of
doranidazole.
 | Table II.Tumor doubling time. |
Table II.
Tumor doubling time.
| Treatment | Days (lower, upper
limits) |
|---|
| Saline | 4 (4, 8) |
| Glycididazole | 4 (2, 6) |
| Doranidazole | 4 (4, 6) |
| Saline +
X-rays | 18 (8, 20) |
| Glycididazole +
X-rays | 20 (18,
33)a |
| Doranidazole +
X-rays | 42 (27,
47)b,c |
Glycididazole was decomposed to
metronidazole and its concentration was relatively low compared
with that of doranidazole
To explain why glycididazole did not enhance
radiation-induced tumor suppression, we assessed the chemical forms
and tissue concentrations after the administration of glycididazole
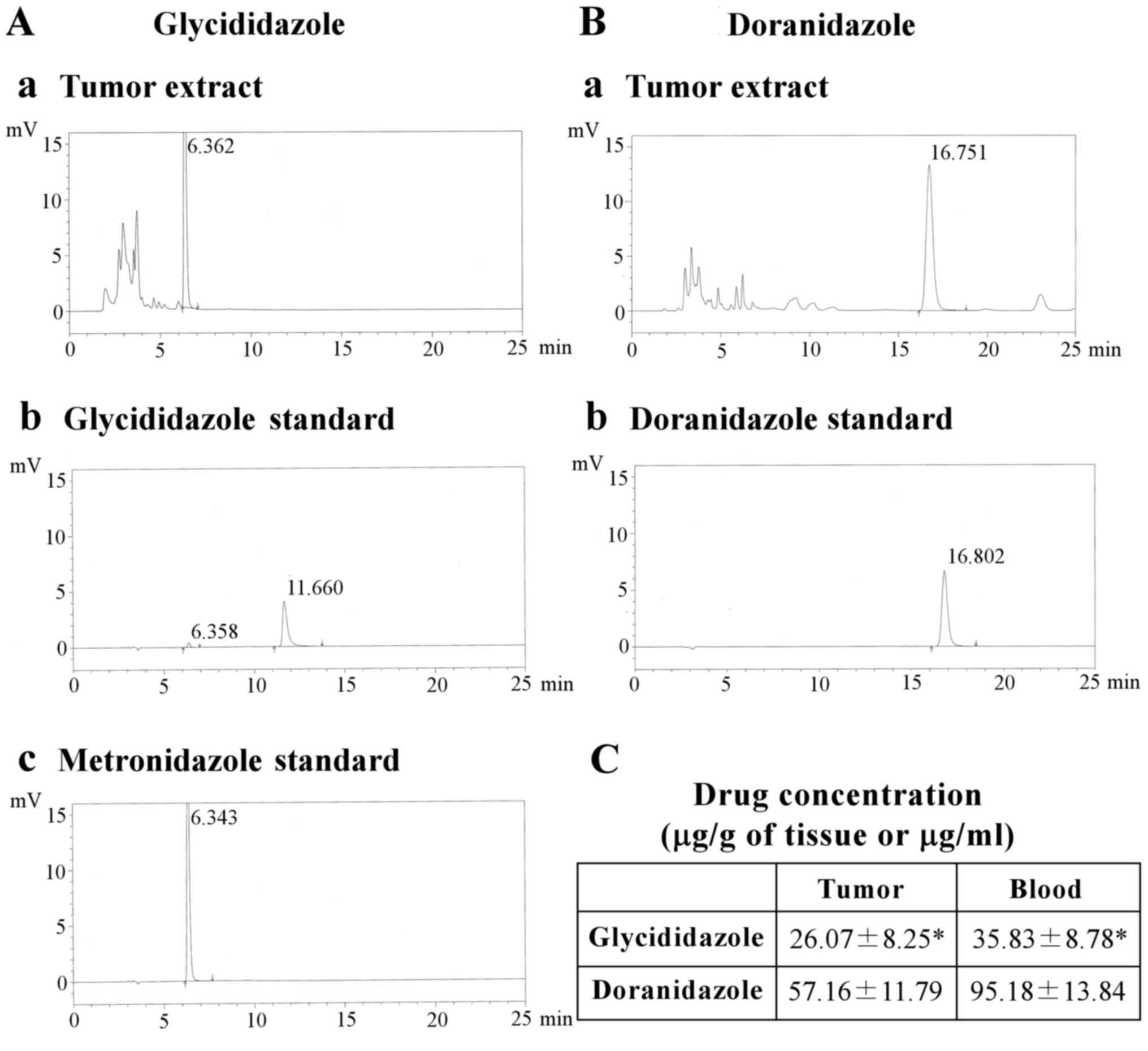
and doranidazole in vivo. The results of the HPLC analysis
showed that the incorporated glycididazole was barely detected in
its original form within the tumor and that the only detectable
metabolite was metronidazole, as shown in Fig. 4A. In contrast, the administered
doranidazole was detected as the undecomposed form (Fig. 4B). The concentrations of these drugs
in the tumor and blood serum were also examined. As shown in
Fig. 4C, the amount of glycididazole
incorporated into tumor in the form of metronidazole was
significantly lower (26.07±8.25 µg/g of tissue) than that of
doranidazole (57.16±11.79 µg/g of tissue). This trend was also
observed in blood serum.
Discussion
The purpose of this study was to re-evaluate the
radiosensitizing effect of glycididazole, which is widely used in
China for clinical treatment, in vitro and in vivo.
As shown in Fig. 2, treatment with 10
mM glycididazole improved the radiosensitivity of SCCVII cells
under hypoxia, similar to doranidazole. Interestingly, the
enhancement of radiation-induced cell death by glycididazole was
observed even in normoxia. A recent report by Zeng et al
demonstrated the similar effect of glycididazole on normoxic
laryngeal cancer cells and revealed significant downregulation of
ataxia-telangiectasia mutated (ATM), p-ATM, CHK2, and p53 and the
upregulation of MDM2 and Cdk2 after the combined treatment with
glycididazole and X-irradiation, not only in hypoxic conditions,
but also in normoxic conditions, and concluded that glycididazole
has the potential to inhibit the ATM signaling response after
X-irradiation (20). Although
glycididazole is composed of a metronidazole dimer, to the best of
our knowledge, there are no reports to demonstrate that
metronidazole exerted radiation modifying effects, except for its
oxygen-mimetic properties owing to the electron affinity.
Therefore, glycididazole may have unknown physiological activity
and further investigation is necessary.
In contrast to the in vitro study that showed
that the radiosensitizing effect of glycididazole (SER=1.29) was
similar to that of doranidazole (SER=1.24) under hypoxic
conditions, 200 mg/kg glycididazole exhibited a relatively weak
sensitizing effect compared with that of doranidazole in
SCCVII-transplanted tumor cells (Fig.
3). Only one study has examined the antitumor effect of
glycididazole using the growth delay assay in a transplanted tumor
model (20). The authors demonstrated
the significant radiosensitizing potential of glycididazole in a
xenografted Hep-2 tumor mouse model, but they employed a high dose
of glycididazole (3×700 mg/m2/day). Combined with our
results, glycididazole did not show as strong a radiosensitizing
effect as expected from the in vitro study. The
inconsistencies between the in vitro and in vivo
studies may be explained by the results of the HPLC analysis
(Fig. 4). In tumor lysate obtained 20
min after glycididazole administration, glycididazole was not
detected in the HPLC spectra, but metronidazole was detected. These
results suggested that the glycididazole incorporated into the
tumor was rapidly decomposed and bound to biomolecules as
metronidazole adducts. This hydrolysis is thought to occur in the
presence of esterase or ester hydrolase. Mahfouz and Hassan
examined the hydrolysis kinetics of a series of metronidazole amino
acid ester prodrugs (21). In an
aqueous phosphate solution of pH 7.4, the prodrugs exhibited
adequate chemical stability (half-life, t1/2,
4–16 h). However, in 80% human plasma, the drugs were hydrolyzed to
metronidazole within a few min. Metronidazole benzoate, an internal
remedy, also underwent rapid hydrolysis; however, no significant
hydrolysis of the ester in gastric fluid and intestinal fluid was
observed, but only metronidazole was reported in the sera and urine
of patients administered metronidazole benzoate (22,23). In
addition, it was reported that a twin ester prodrug, which
consisted of a dimer of metronidazole and twin esters, was
chemically stable at the physiological pH buffer solution
(t1/2, 13–40 h) but the release of metronidazole
from the prodrug ensued rapidly in 80% human plasma
(t1/2, 10–150 min) (24). These reports suggest that
glycididazole, in which two metronidazoles are linked by the ester
bond, can be easily decomposed to the parental form in vivo.
Unfortunately, a high-dose amount of metronidazole is required to
achieve radiosensitization; at this level, metronidazole causes
severe nausea and vomiting (25).
Therefore, to increase the bioavailability of glycididazole and to
obtain a sufficient response in the tumor, it was necessary to
increase its stability in blood through modification of the linker
bond that connected the two metronidazoles. For example, the twin
ester prodrug derived from phthalic acid is not a substrate for the
enzyme esterase and shows a slow release of metronidazole
(t1/2, 12.4 h) (24).
We think two advantages will be obtained if the
stability of such a twin drug is improved as follows. First is that
the availability of 2-nitroimidazole moiety for radiosensitization
against hypoxic cells can be increased. Because 2-fold of
2-nitroimidazole moieties per mole are provided, the chance of
oxidation of radiation-induced free radicals is thought to be
increased, leading to the induction of much more cell killing. This
effect may be more pronounced in a condensed microenvironment in
vivo than a monolayer culture in vitro. Second, to
establish the method to stabilize the linker bond that connected
the two metronidazoles is important as a toehold to develop the
stable polymeric nitroimidazole compounds. Thus far, few attempts
to develop and evaluate the polymeric compounds have been achieved.
The promising compounds with multiple 2-nitoroimidazole moieties
have a potential not only to exhibit stronger radiation-enhancing
effect as a hypoxic radiosensitizer, but also to accumulate in
hypoxic region more efficiently as a hypoxic imaging probe.
In conclusion, glycididazole efficiently sensitized
cancer cells to radiation under both hypoxia and normoxia in
vitro. However, the expected extent of tumor regression could
not be achieved by the combination of X-irradiation and
glycididazole. The modification of the chemical structure to reduce
the decomposition of glycididazole to metronidazole is necessary to
improve the cure rate of the combined therapy with X-irradiation in
clinic.
Acknowledgements
This study was partially supported by the JSPS
KAKENHI, Japan [nos. 25861045 (H.Y.), 15K09983 (H.Y.), 26461875
(T.Y.) and 24659551 (O.I.)] and by the Takeda Science Foundation
(H.Y.).
References
|
1
|
Harada H: How can we overcome tumor
hypoxia in radiation therapy? J Radiat Res. 52:545–556. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown JM: Tumor hypoxia in cancer therapy.
Methods Enzymol. 435:297–321. 2007.PubMed/NCBI
|
|
3
|
Horsman MR, Lindegaard JC, Grau C,
Nordsmark M, Alsner J and Overgaard J: Chapter 3-dose-response
modifiers in radiation therapy A2-gunderson, leonard LClinical
radiation oncology. Tepper JE: 4th. Elsevier; Philadelphia, PA: pp.
51–62. 2016, View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaanders JH, Bussink J and van der Kogel
AJ: Clinical studies of hypoxia modification in radiotherapy. Semin
Radiat Oncol. 14:233–240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Overgaard J, Eriksen JG, Nordsmark M,
Alsner J and Horsman MR; Danish Head and Neck Cancer Study Group, :
Plasma osteopontin, hypoxia and response to the hypoxia sensitiser
nimorazole in radiotherapy of head and neck cancer: Results from
the DAHANCA 5 randomised double-blind placebo-controlled trial.
Lancet Oncol. 6:757–764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Overgaard J, Hansen HS, Overgaard M,
Bastholt L, Berthelsen A, Specht L, Lindeløv B and Jørgensen K: A
randomized double-blind phase III study of nimorazole as a hypoxic
radiosensitizer of primary radiotherapy in supraglottic larynx and
pharynx carcinoma. Results of the Danish Head and Neck Cancer Study
(DAHANCA) Protocol 5–85. Radiother Oncol. 46:135–146. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oya N, Shibamoto Y, Sasai K, Shibata T,
Murata R, Takagi T, Iwai H, Suzuki T and Abe M: Optical isomers of
a new 2-nitroimidazole nucleoside analog (PR-350 series):
Radiosensitization efficiency and toxicity. Int J Radiat Oncol Biol
Phys. 33:119–127. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shibamoto Y, Kubota T, Kishii K and
Tsujitani M: Radiosensitivity of human pancreatic cancer cells in
vitro and in vivo and the effect of a new hypoxic cell sensitizer,
doranidazole. Radiother Oncol. 56:265–270. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yahiro T, Masui S, Kubota N, Yamada K,
Kobayashi A and Kishii K: Effects of hypoxic cell radiosensitizer
doranidazole (PR-350) on the radioresponse of murine and human
tumor cells in vitro and in vivo. J Radiat Res. 46:363–372. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Gong A, Ji J, Wu Y, Zhu X, Lv S,
Lv H and Sun X: The radiosensitizing effect of doranidazole on
human colorectal cancer cells exposed to high doses of irradiation.
BMC cancer. 7:1882007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuoka H, Shibamoto Y, Kubota T,
Tsujitani M and Majima T: In vivo efficacy and pharmacokinetics of
a new hypoxic cell radiosensitizer doranidazole in SUIT-2 human
pancreatic cancer xenografted in mouse pancreas. Oncol Rep.
7:23–26. 2000.PubMed/NCBI
|
|
12
|
Murata R, Tsujitani M and Horsman MR:
Enhanced local tumour control after single or fractionated
radiation treatment using the hypoxic cell radiosensitizer
doranidazole. Radiother Oncol. 87:331–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yasui H, Asanuma T, Kino J, Yamamori T,
Meike S, Nagane M, Kubota N, Kuwabara M and Inanami O: The
prospective application of a hypoxic radiosensitizer, doranidazole
to rat intracranial glioblastoma with blood brain barrier
disruption. BMC Cancer. 13:1062013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishimura Y, Nakagawa K, Takeda K, Tanaka
M, Segawa Y, Tsujino K, Negoro S, Fuwa N, Hida T, Kawahara M, et
al: Phase I/II trial of sequential chemoradiotherapy using a novel
hypoxic cell radiosensitizer, doranidazole (PR-350), in patients
with locally advanced non-small-cell lung Cancer (WJTOG-0002). Int
J Radiat Oncol Biol Phys. 69:786–792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karasawa K, Sunamura M, Okamoto A, Nemoto
K, Matsuno S, Nishimura Y and Shibamoto Y: Efficacy of novel
hypoxic cell sensitiser doranidazole in the treatment of locally
advanced pancreatic cancer: Long-term results of a
placebo-controlled randomised study. Radiother Oncol. 87:326–330.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sunamura M, Karasawa K, Okamoto A, Ogata
Y, Nemoto K, Hosotani R, Nishimura Y, Matsui K and Matsuno S;
PR-350 Study Group, : Phase III trial of radiosensitizer PR-350
combined with intraoperative radiotherapy for the treatment of
locally advanced pancreatic cancer. Pancreas. 28:330–334. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng YC, Wu R, Xu ZG, Zhang XY, Fan GL, Wu
LN, Wang YM, Hao SH, Zheng W, Chen XD, et al: Safety and
radiation-enhancing effect of sodium glycididazole in
locoregionally advanced laryngeal cancers previously treated with
platinum-containing chemotherapy regimens: A preliminary report.
Cancer Radiother. 14:59–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li MY, Liu JQ, Chen DP, Qi B, Liang YY and
Yin WJ: Glycididazole sodium combined with radiochemotherapy for
locally advanced nasopharyngeal carcinoma. Asian Pac J Cancer Prev.
15:2641–2646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeng YC, Wu R, Xing R, Chi F, Wang SL,
Chen XD, Xuan Y, Wu LN, Duan QY, Tang MY, et al:
Radiation-enhancing effect of sodium glycididazole in patients
suffering from non-small cell lung cancer with multiple brain
metastases: A randomized, placebo-controlled study. Cancer
Radiother. 20:187–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng YC, Xing R, Zeng J, Xue M, Chi F, Xin
Y, Fan GL, Wang HM, Duan QY, Sun YN, et al: Sodium glycididazole
enhances the radiosensitivity of laryngeal cancer cells through
downregulation of ATM signaling pathway. Tumour Biol. 37:5869–5878.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahfouz NM and Hassan MA: Synthesis,
chemical and enzymatic hydrolysis and bioavailability evaluation in
rabbits of metronidazole amino acid ester prodrugs with enhanced
water solubility. J Pharm Pharmacol. 53:841–848. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alestig K, Freij L and Arnold E:
Absorption and excretion of metronidazole after administration of
metronidazole benzoate mixture. Scand J Infect Dis. 12:149–152.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mathew M, Das Gupta V and Bethea C:
Stability of metronidazole benzoate in suspensions. J Clin Pharm
Ther. 19:31–34. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mahfouz NM, Aboul-Fadl T and Diab AK:
Metronidazole twin ester prodrugs: Synthesis, physicochemical
properties, hydrolysis kinetics and antigiardial activity. Eur J
Med Chem. 33:675–683. 1998. View Article : Google Scholar
|
|
25
|
Urtasun R, Feldstein ML, Partington J,
Tanasichuk H, Miller JD, Russell DB, Agboola O and Mielke B:
Radiation and nitroimidazoles in supratentorial high grade gliomas:
A second clinical trial. Br J Cancer. 46:101–108. 1982. View Article : Google Scholar : PubMed/NCBI
|