Introduction
In chronic lymphocytic leukemia (CLL), malignant B
lymphocytes, similar to other tumor cells, are in constant
cooperation with the tumor microenvironment, which is formed by
cancer cells through the recruitment and alteration of
non-malignant cells of the immune system (1). In their proximate surroundings,
neoplastic B cells interact with neighboring cells that include
mesenchymal stromal cells, monocytes, monocyte-derived nurse-like
cells, and T cells (2). These
interactions provide B cell clones with supporting factors that
inhibit or delay programmed apoptosis and thereby sustain disease
progression (3). The dialogue between
CLL B cells and interacting T lymphocytes may involve cytokines
(4). The present study focused on a
population of T lymphocytes, known as invariant natural killer T
cells (iNKT) (5). The role of iNKT in
tumor immunity is only partially understood and is poorly described
in CLL. This unique cell subset combines features of T lymphocytes
and NK cells (6). Unlike conventional
T lymphocytes, they express markedly less variable T cell receptors
(TCRαβ), and are formed by an invariant α chain (Vα24Jα18) combined
with a limited set of β chains, mainly containing Vβ11 (7–9). Using
such TCR receptors, iNKT cells recognize lipids and glycolipids,
presented by a non-classical MHC molecule known as CD1d (10). The agent most efficient in binding to
CD1d is a synthetic compound that originated from marine sponges,
known as α-galactosylceramide (α-GalCer). The discovery of this
potent agonist facilitated further understanding of the biology of
iNKT (11–13). Activated iNKT cells simultaneously
secrete interferon (IFN)-γ and interleukin (IL)-4 (14). In addition, they can release other
pro-inflammatory Th1 cytokines [such as tumor necrosis factor
(TNF)-α and IL-6] and anti-inflammatory Th2 cytokines [such as
IL-10, IL-13 and transforming growth factor (TGF)-β] (14–16). This
enables iNKT cells to influence other cells of the immune system,
including NK cells, cytotoxic T lymphocytes, helper T cells, B
cells and antigen presenting cells (APC) (17–19).
Certain pathological conditions may cause a change in the Th0-like
pattern of cytokines secreted by iNKT cells and polarize their
response in the Th1 or Th2 direction (10,20). Such
iNKT cells can modify the microenvironment and influence tumor
control (21). The role of these
cells in the pathogenesis and clinical course of CLL is not well
known. Understanding the function of iNKT cells in this specific
type of leukemia requires critical analysis of the cytokine release
profile. In the current study, the intracellular expression of
IFN-γ and IL-4 was analyzed by stimulating the iNKT cells.
Materials and methods
Patients and samples
Peripheral blood (PB) samples were obtained from 60
patients with CLL (29 females and 31 males; median age, 67 years;
range, 46–87 years) who met the diagnostic criteria of the
International Workshop on Chronic Lymphocytic Leukemia (IWCLL) 2008
(22). All samples were collected at
the time of diagnosis and prior to any anticancer therapy from
September 2014 to June 2016 in the Department of Hemato-Oncology
and Bone Marrow Transplantation of the Medical University of Lublin
(Lublin, Poland). According to the Rai classification (23), 24 patients were Stage 0, 17 patients
were Stage I, 7 patients were Stage II, 8 patients were Stage III
and 4 patients were Stage IV. Participants' characteristics at the
time of diagnosis are summarized in Table
I. Control PB samples were obtained from 28 healthy volunteers
(HVs; 12 females and 16 males, aged from 36–83 years, median, 57
years).
 | Table I.Clinical characteristics of patients
with CLL. |
Table I.
Clinical characteristics of patients
with CLL.
| A, Total number of
patients. |
|---|
|
|---|
| Variables | Patient no.
(%) |
|---|
| Sex |
|
| Female
(%) | 29 (48.30) |
| Male
(%) | 31 (51.70) |
| Rai stage |
|
| 0
(%) | 24 (40.00) |
| I
(%) | 17 (28.30) |
| II
(%) | 7 (11.70) |
| III
(%) | 8 (13.30) |
| IV
(%) | 4 (6.70) |
| ZAP-70 (cut-off
20%)a |
|
|
Positive (%) | 28 (46.70) |
|
Negative (%) | 32 (53.30) |
| CD38 (cut-off
20%)b |
|
|
Positive (%) | 26 (46.30) |
|
Negative (%) | 34 (56.70) |
| Cytogenetic
abnormalities |
|
|
del(17p13.1) (%) | 4 (6.70) |
|
del(11q22.3) (%) | 6 (10.00) |
| Without
del(17p13.1) and | 50 (83.30) |
|
del(11q22.3) (%) |
|
| Patients requiring
therapy | 12 (20.00) |
| Untreated
patients | 48 (80.00) |
|
| B, Median range
of patient data. |
|
|
Variables | Median
(range) |
|
| Age at diagnosis
(years) | 67 (46–87) |
| WBC count
(G/l) | 26.41
(11.96–280.46) |
| Lymphocyte count
(G/l) | 19.91
(5.62–269.13) |
| β2M (mg/dl) | 2.26
(1.36–8.10) |
| LDH (IU/l) | 387.00
(287.00–839.00) |
| Hemoglobin
(g/dl) | 14.20
(8.20–17.20) |
| Platelets
(G/l) | 183.00
(70.00–339.00) |
| %
CD19+/CD5+/ZAP-70+
cellsa | 17.02
(2.41–58.43) |
| %
CD19+/CD5+/CD38+
cellsb | 10.13
(0.72–87.72) |
| % iNKT cells
(Vα24-Jα18+/CD3+)c | 0.21
(0.01–1.51) |
Ethics statement
The current study was approved by the Ethics
Committee of the Medical University of Lublin (Lublin, Poland).
Written informed consent was obtained from all patients with
respect to the use of their blood for scientific purposes.
Activation of iNKT cells with α-GalCer
and analysis of intracellular IL-4 and IFN-γ expression
PB samples were collected into heparinized tubes. PB
samples were kept at room temperature and used within 1–2 h. Whole
blood samples were cultured in round-bottom FACS tubes. For
intracellular cytokine expression, iNKT cells in the PB were
activated using 100 ng/ml α-GalCer (KRN700; Enzo Life Sciences,
Inc., Farmingdale, NY, USA) for 24 h at 37°C in a 5% CO2
atmosphere, followed by the addition of the protein transport
inhibitor BD GolgiPlug™ (BD Biosciences, Franklin Lakes, NJ, USA)
for the last 6 h of activation. Furthermore, this procedure was
performed on non-activated lymphocytes using only BD GolgiPlug™ to
assess the level of residual IL-4 and IFN-γ synthesis from in
vivo activation. Cultured cells were then stained with
monoclonal antibodies (MoAbs) against cell-surface markers:
anti-iNKT cells FITC (TCR Vα24-Jα18, clone 6B11; cat. no. 558371,
20 µl/test) and anti-CD3 PE-Cy5 (clone HIT3a; cat. no. 555341, 20
µl/test) supplied by BD Biosciences; incubation was performed for
20 min at room temperature. Following membrane staining, cells were
fixed and permeabilized with Cytofix/Cytoperm™ solution and
Perm/Wash buffer (BD Biosciences), according to the manufacturer's
protocol. Cells were then intracellularly stained with anti-IL-4 PE
(clone 3010.211; BD Biosciences; cat. no. 340451, 20 µl/test, 1.25
µg/ml) or anti-IFN-γ PE (clone 25723.11, BD Biosciences; cat. no.
340452, 20 µl/test, 7.5 µg/ml) MoAbs (30 min at 4°C in the dark)
and washed twice in PBS. Finally, the cells were analyzed by flow
cytometry using FACSCalibur™ (BD Biosciences).
Flow cytometry analysis
Samples were analyzed by flow cytometry directly
following preparation. A FACSCalibur™ instrument (BD Biosciences)
and BD CellQuest Pro software version 6.0 (BD Biosciences) were
used. For each analysis, 200,000 events were acquired and analyzed.
In the experiment, the percentage of iNKT cells with IL-4 or IFN-γ
expression was determined. iNKT were defined as
Vα24-Jα18+/CD3+ cells. Dot plots illustrating
the analysis method for the identification of iNKT cells expressing
IL-4 and IFN-γ are presented in Fig.
1A-K. An acquisition gate was put on lymphocytes according to
the forward scatter (FSC) and side scatter (SSC) properties
(Fig. 1A). iNKT cells were defined
and gated on a dot plot of iNKT FITC (TCR Vα24-Jα18) vs. CD3 PE-Cy5
(Fig. 1B). Within those cells, the
cytokine expressing cells were identified. To establish the gating
strategy, a fluorescence minus one (FMO) control was used. The FMO
control tube included all antibodies that were used for iNKT cell
staining (anti-TCR Vα24-Jα18 FITC and anti-CD3 PE-Cy5), except for
the antibody (IL-4 PE or IFN-γ PE) that was measured. The FMO
control allowed the consideration of any spread of fluorochromes
into the unlabeled channel, and the placing of gates in the correct
place. The results are expressed as the percentage of iNKT cells
with intracellular IL-4 or IFN-γ expression. Specificity of
anti-IL-4 PE and anti-IFN-γ PE MoAbs was evaluated through the
estimation of unpermeabilized cells (Fig.
1C-E). Staining of unstimulated (24-h culture only with BD
GolgiPlug™; Fig. 1F-H) as well as
stimulated iNKT cells was performed (Fig.
1I-K).
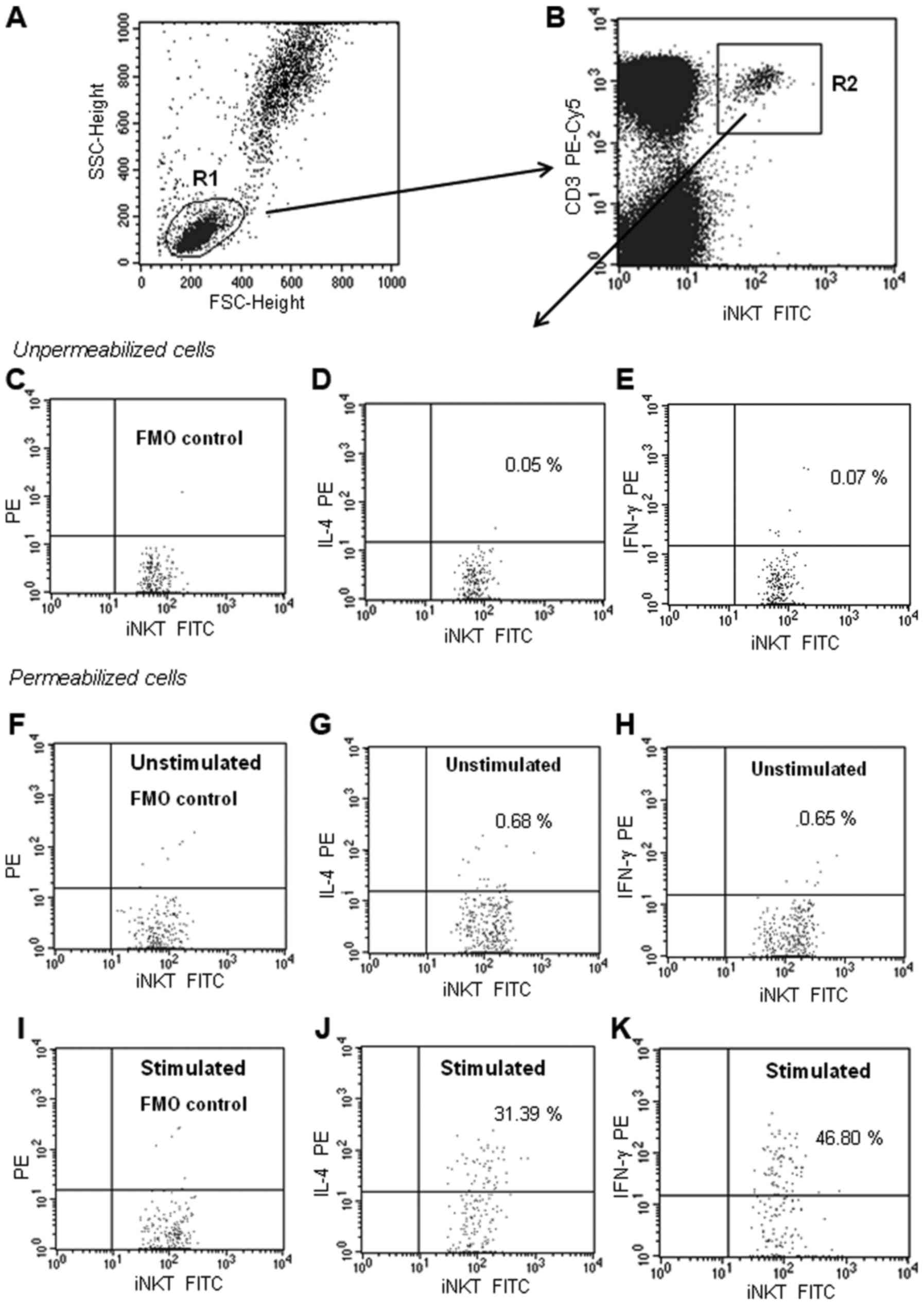 | Figure 1.Representative dot plots illustrating
the analysis method for the identification of iNKT+
cells with IL-4 or IFN-γ expression. (A) An acquisition gate was
established based on FSC and SSC that included mononuclear cells.
The R1 region was drawn around the lymphocytes. (B) The R1 gated
events were analyzed for TCR Vα24-Jα18 FITC (anti-iNKT) and CD3
PE-Cy5 staining, and the positive cells were gated (region R2). The
dot plots C-K were established by the combined gating of events
using R1 and R2 regions. Three dot plots (C-E) indicate
no-permeabilization control (checking whether the antibody binds to
the antigen only after permeabilization). Six dot plots (F-K)
indicate the identification of iNKT cells with intracellular IL-4
or IFN-γ (after permeabilization). (C, F and I) Dot plots
indicating the FMO control, which contains all the fluorochromes in
a panel, except for the one (IL-4 PE or IFN-γ PE) that was
measured. The final dot plots indicate iNKT cells (TCR Vα24-Jα18
FITC+CD3PE-Cy5+) cells positive for (D, G and
J) IL-4 or (E, H and K) IFN-γ. (D and E) The number in the upper
right quadrant represents the percentage of iNKT cells that bound
the anti-IL-4 or anti-IFN-γ MoAbs without permeabilization
(non-specific binding). (G and H) The two dot-plots represent IL-4
and IFN-γ expression in the unstimulated iNKT cells (non-activated
cells from 24-h culture only with BD GolgiPlug™). (J and K) The
number in the upper right quadrant represents the percentage of
iNKT cells with intracellular IL-4 or IFN-γ expression (after
α-GalCer stimulation). FSC, forward scatter; SCC side scatter; FMO,
Fluorescence Minus One Control; CLL, chronic lymphocytic leukemia;
iNKT, invariant natural killer T cells; IL, interleukin; IFN,
interferon; α-GalCer, α-galactosylceramide; MoAb, monoclonal
antibody. |
Sorting of iNKT cells for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
In 10 CLL cases and 5 HV cases (from 24-h culture
with and without α-GalCer stimulation), the iNKT cells were
purified. A BD FACSAria™ flow cytometer (BD Biosciences) was used
for iNKT cell sorting. In this case, the iNKT cells were labeled
with antibodies against TCR, Vα24-Jα18 PE and CD3 FITC (BD
Biosciences), following which the double-positive population was
selected. A standard whole-blood assay with erythrocyte cell lysis
was used for preparing the PB specimens. After sorting, the iNKT
cell purity was >97%.
RNA preparation and RT-qPCR) for IL-4
and IFN-γ in iNKT cells
Purified iNKT cells were used for RNA isolation.
Total RNA was isolated using the QIAamp® RNA Blood Mini
kit (Qiagen, Inc., Valencia, CA, USA; cat. no. 52304). RNA was
transcribed into cDNA using the QuantiTect® Reverse
Transcription kit (Qiagen, Inc.; cat. no. 205311), according to the
manufacturer's protocol. RT-qPCR was performed using TaqMan
reagents specific for human IL-4 and IFN-γ (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA; cat. no.
4331182), and β-actin was used as the internal control (Applied
Biosystems; Thermo Fisher Scientific, Inc.; cat. no. 4326315E).
RT-qPCR reactions were run for 40 cycles using universal cycling
conditions (95°C for 10 min followed by 40 cycles at 95°C for 15
sec and 60°C for 1 min) on an Applied Biosystems 7300 Real-Time PCR
System. Data were normalized to β-actin expression (endogenous
control), analyzed using the threshold cycle (Cq) and presented as
2ΔCq. ΔCq is the difference between the Cq of the target
gene (Cqt) and the reference gene (Cqr; DCq =
Cqt-Cqr) (24).
Analysis of CD1d expression on
monocytes and B cells
Flow cytometry analysis of CD19+ B cells
and CD14+ monocytes expressing CD1d was performed on
fresh PB samples stained with anti-CD1d PE (clone CD1d42; cat. no.
550255, 20 µl/test), anti-CD19 FITC (clone SJ25C1; cat. no. 34040,
20 µl/test, 6 µg/ml) and anti-CD14 FITC (clone MφP9; cat. no.
347493; 20 µl/test, 25 µg/ml) MoAbs from BD Biosciences. Cells were
incubated for 20 min at room temperature. A standard whole-blood
assay with erythrocyte cell lysis was used for preparing all PB
specimens. Samples were analyzed by flow cytometry directly
following preparation. In the experiment, the percentage of
CD1d+/CD19+ and
CD1d+/CD14+ cells, and the level of CD1d
expression on monocytes and B cells, indicated by the mean
fluorescence intensity (MFI), were analyzed. Dot plots illustrating
the analysis method for the identification of monocytes with
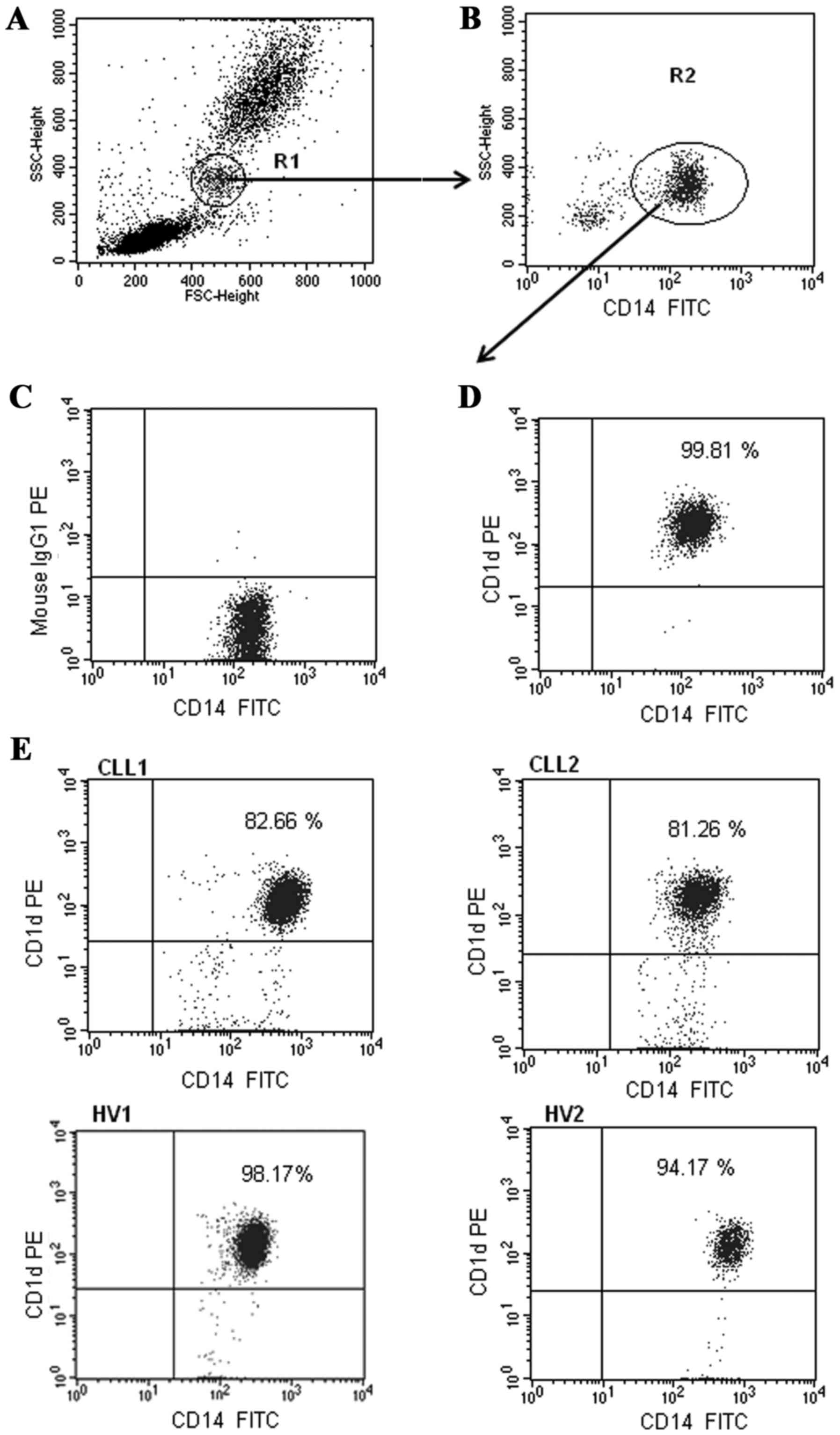
membrane expression of CD1d are presented in Fig. 2. The identification method of
CD1d-positive CD19+ B lymphocytes was exhibited in our
previous study (25).
Cells isolation for the determination
of apoptosis
Mononuclear cells were separated from PB samples by
density gradient centrifugation with Biocoll Separating Solution
(Biochrom, Ltd., Cambridge, UK) for 25 min at 400 × g and room
temperature. Interphase cells were removed, washed twice and
resuspended in PBS.
Determination of apoptosis by
MitoTracker® Red CMXRos
In 20 patients with CLL an apoptosis analysis within
the CD19+ cell population was performed. A previously
described method was used for flow cytometric examination of the
percentage of early apoptotic cells (ΔΨmlow) (26–28). The
level of apoptosis was measured by chloromethyl-X-rosamine staining
(MitoTracker® Red CMXRos; Molecular Probes; Thermo
Fisher Scientific, Inc.; cat. no. M7512). CMXRos is a cationic
lipophilic fluorochrome that can be used to detect disruptions in
the mitochondrial membrane potential (∆Ψm). CMXRos was used in
combination with an anti-CD19 FITC MoAb (BD Biosciences).
Mononuclear cells were incubated with CMXRos for 30 min at 37°C
and, after 15 min of incubation, the anti-CD19 MoAb was added. The
CD19+ cells that were determined to be apoptotic
exhibited a decrease in the mitochondrial membrane potential
following CMXRos staining (∆Ψmlow). The percentage of
apoptotic cells (∆Ψmlow/CD19+) was measured
at the time of diagnosis (ex vivo), and in vitro
after 24 h incubation at 37°C in RPMI-1640 supplemented with 2
mmol/l L-glutamine (Biochrom, Ltd., Cambridge, UK; cat. no.
FG1215), 10% fetal calf serum (Biochrom, Ltd.; cat. no. S0113), 100
U/ml penicillin and 100 µg/ml streptomycin (Biochrom, Ltd.; cat.
no. A2212), and with and without 100 ng/ml α-GalCer
stimulation.
Analysis of CD69 expression on iNKT
cells
For the assessment of CD69 expression on iNKT cells,
PB mononuclear cells were incubated with the following MoAbs: TCR
Vα24-Jα18 FITC (clone 6B11; BD Biosciences; cat. no. 558371; 20
m/test), CD3 PE-Cy5 (clone HIT3a; BD Biosciences; cat. no. 555341;
20 µl/test) and CD69 PE (clone FN50; BD Biosciences; cat. no.
555531, 20 µl/test). Samples were analyzed using flow cytometry
immediately following preparation.
Statistical analysis
Data are presented as the median and range. The
Mann-Whitney U test was applied for statistical comparisons between
the CLL and HV groups, as well as between the patient subgroups.
The Spearman's rank correlation coefficient was used for
correlation analysis. Statistica version 9.0 PL software (StatSoft,
Cracow, Poland) and GraphPad Prism software version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA) were used for statistical
procedures. P<0.05 was considered to indicate a statistically
significant difference.
Results
Intracellular IL-4 and IFN-γ
expression in iNKT cells
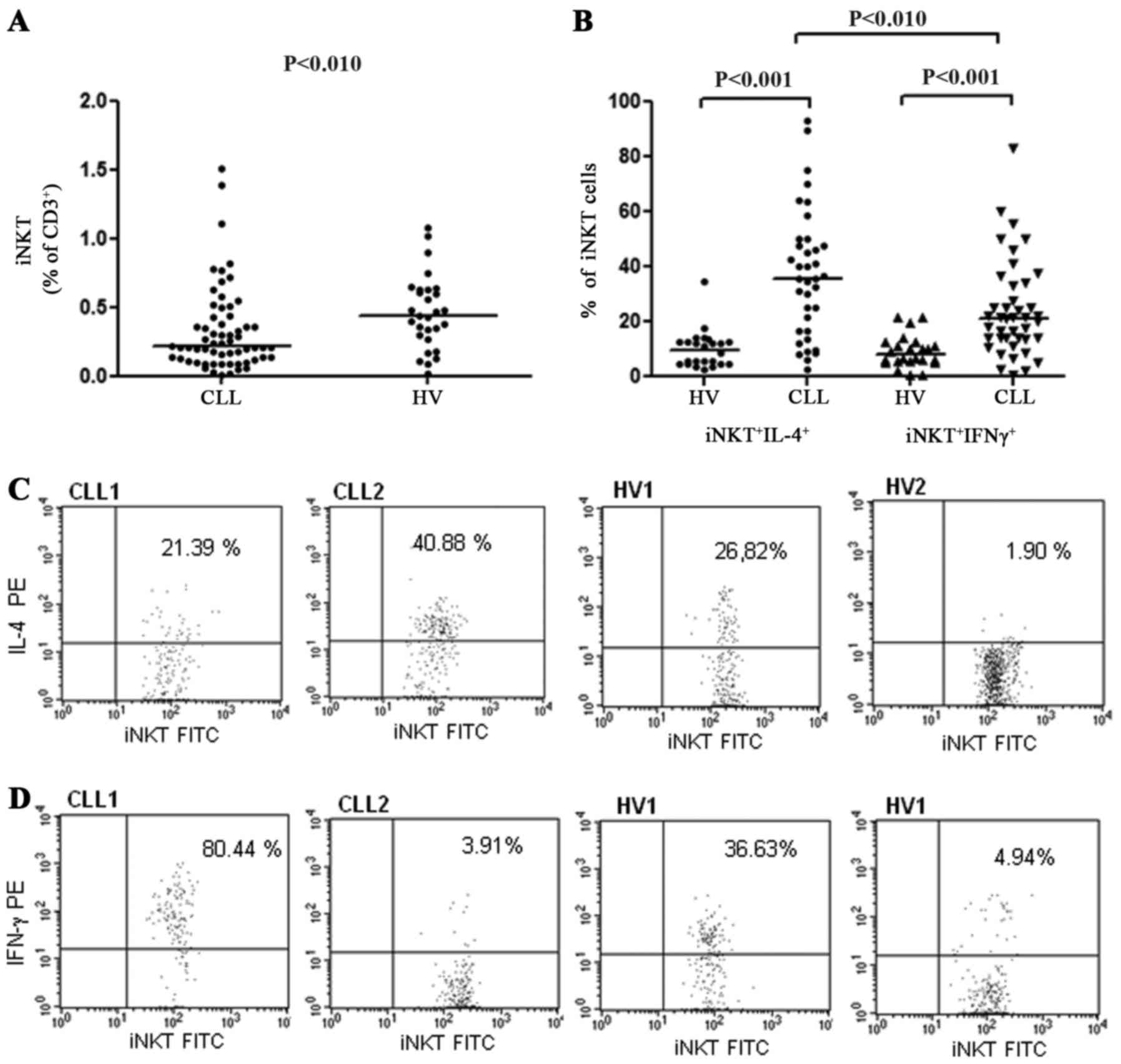
The percentage of iNKT cells within CD3+
T lymphocytes was significantly decreased in patients with CLL in
comparison to the HVs (median, 0.21 vs. 0.42%, P<0.01; Fig. 3A). The frequency of iNKT cells also
decreased with the disease stage. The median percentage of iNKT
cells in stage 0 was 0.25, 0.21% in stages I/II and 0.13% in stages
III/IV, according to the Rai classification. However, the
difference was not significant. In the group of patients with CLL
no significant association was identified between the frequency of
iNKT cells in the PB and the expression of ZAP-70 or CD38 (data not
presented).
The function of iNKT lymphocytes was investigated
through the analysis of intracellular cytokine expression following
stimulation with the iNKT-specific ligand α-GalCer. At the end of a
24-h stimulation period, the synthesis of IL-4 and IFN-γ in iNKT
cells was assessed by intracellular staining. In patients with CLL
as well as in HVs, the percentage of iNKT cells with intracellular
IL-4 or IFN-γ expression in non-activation assays was frequently
<1%, comparable with the level of auto-fluorescence (Fig. 1G-H). In vitro stimulation of
iNKT cells with α-GalCer resulted in CD69 upregulation, indicating
the activation of iNKT cells (median of
iNKT+CD69+ cells, 15.79% before and 29.32%
after α-GalCer). Higher percentages of iNKT with IL-4 expression
were noted in patients with CLL (median, 35.20%), as compared with
in the HV control group (median, 12.64%; P<0.001, Fig. 3B). There was also a statistically
significant difference between patients with CLL and HVs in terms
of the percentage of iNKT cells with IFN-γ expression (median,
10.96% vs. 24.50%; P<0.001; Fig.
3B). Representative plots of data from two patients with CLL
and two HVs with distinct IL-4 and IFN-γ expression profiles are
presented in Fig. 3C-D. In patients
with CLL, the percentage of iNKT+IL-4+ cells
was significantly higher when compared with the percentage of
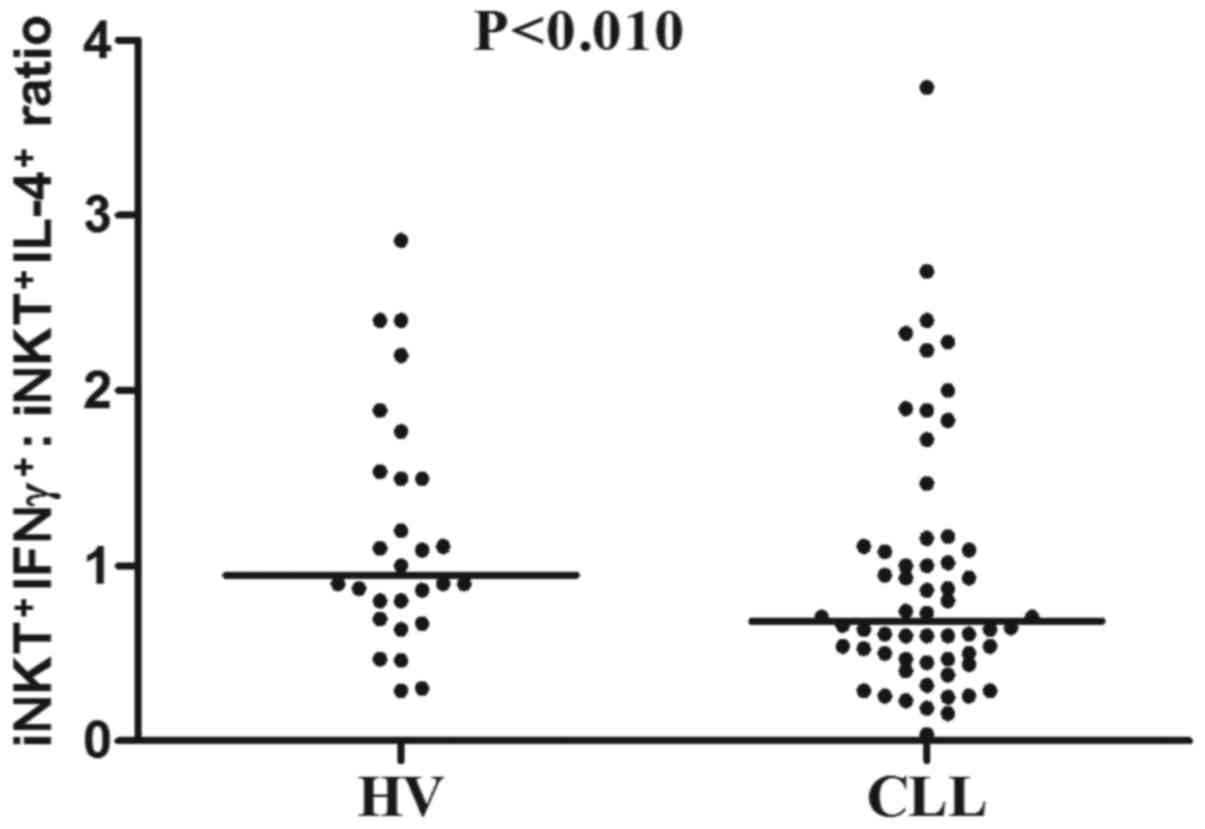
iNKT+IFN-γ+ cells (P<0.01; Fig. 3B). Further analysis revealed that the
ratio of iNKT+IFN-γ+ to
iNKT+IL-4+ was significantly decreased in the
CLL group compared with the HV group (median, 0.68 vs. 0.95;
P<0.01; Fig. 4).
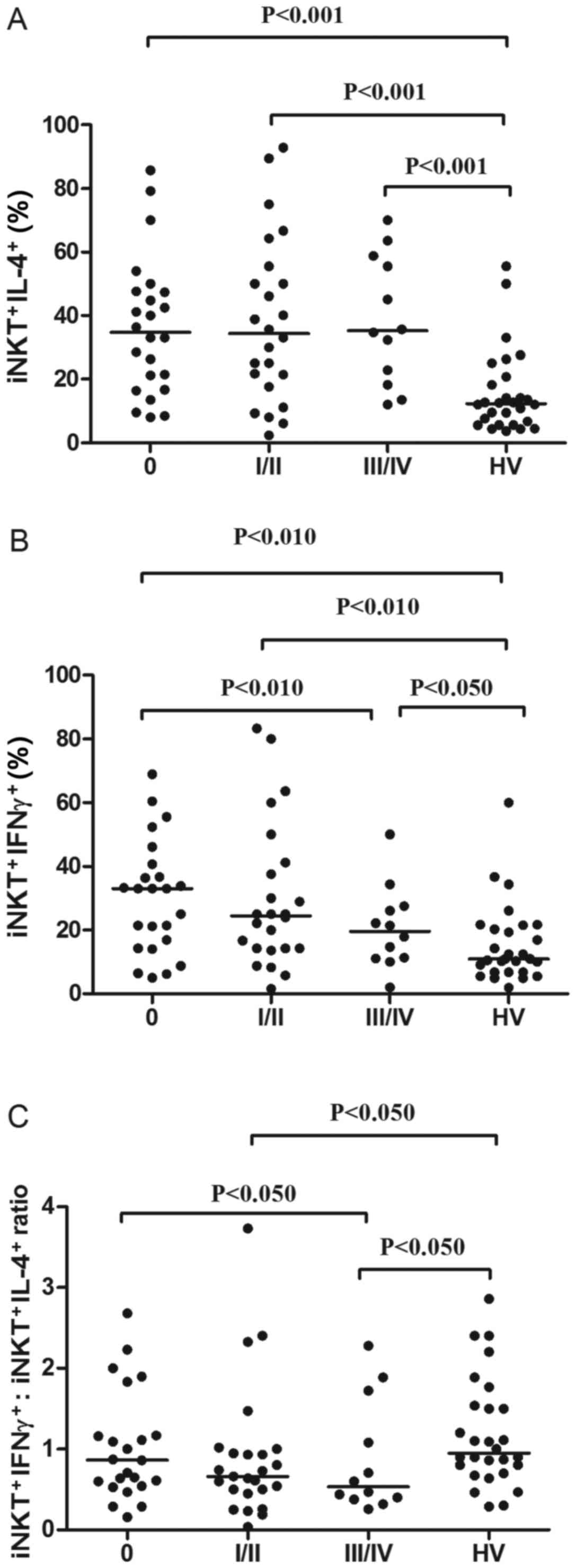
The CLL group was further divided into three risk
groups: Low risk (stage 0), intermediate-risk (stage I or II), and
high-risk (stage III or IV). Each of these groups exhibited a
significantly higher percentage of iNKT+IL-4+
cells in comparison with the HVs. However, there was no significant
difference between the three risk groups (Fig. 5A). Furthermore, each risk group
exhibited a significantly higher percentage of
iNKT+IFN-γ+ cells in comparison with the
control group (Fig. 5B). However,
when the risk groups were compared, the only significant finding
was that patients with CLL at Rai stage III–IV had a lower
percentage of iNKT+IFN-γ+ (median, 19.65%)
compared with those at Rai stage 0 (median, 33.0%; P<0.05;
Fig. 5B). Additional analysis
indicated that the ratio of iNKT+IFN-γ+ to
iNKT+IL-4+ decreased during disease
progression. The ratio was lower in patients at stages III–IV
(median, 0.39) than at stages I–II (median, 0.66) or 0 (median,
0.86; Fig. 5C). Nevertheless, the
difference was statistically significant only between the low and
high-risk groups (P<0.05). Only the
iNKT+IFN-γ+: iNKT+IL-4+
ratio of the intermediate-risk and high-risk groups was
significantly lower in comparison with the HVs (Fig. 5C).
The proportions of CLL group iNKT differed slightly
in the intracellular expression of cytokines, depending on the
ZAP-70 factor expression. The percentage of iNKT cells expressing
IL-4 was increased in ZAP-70-positive patients, as compared with in
ZAP-70-negative patients (Table II).
Additionally, a tendency towards an increased percentage of iNKT
cells expressing IL-4 in CD38-positive in comparison with
CD38-negative patients was noted, but this difference was not
statistically significant (Table
II). There was no significant difference in the
iNKT+IFN-γ+ percentage between the
ZAP-70-positive and ZAP-70-negative or the CD38-positive and
CD38-negative patients. However, there was a statistically
significant difference in the iNKT+IFN-γ+:
iNKT+IL-4+ ratio between ZAP-70-positive and
ZAP-70-negative patients (Table
II).
 | Table II.Percentage of iNKT cells with
expression of IL-4 or IFN-γ divided according to adverse prognostic
factors. |
Table II.
Percentage of iNKT cells with
expression of IL-4 or IFN-γ divided according to adverse prognostic
factors.
| Variable | ZAP-70-positive
patients | ZAP-70-negative
patients | P-value |
|---|
|
iNKT+IL-4+ (%) | 37.86
(8.00–92.86) | 30.19
(2.36–89.47) | 0.049a |
|
iNKT+IFN-γ+ (%) | 22.23
(2.00–68.97) | 25.00
(0.60–83.33) | 0.495 |
|
iNKT+IFN-γ+/iNKT+IL-4+
ratio | 0.60
(0.04–2.28) | 0.89
(0.16–3.73) | 0.046a |
|
|
| CD38-positive
patients | CD38-negative
patients |
|
|
|
iNKT+IL-4+ (%) | 36.71
(8.00–92.86) | 33.85
(2.36–89.47) | 0.462 |
|
iNKT+IFN-γ+ (%) | 21.43
(5.81–68.97) | 25.55
(0.60–83.33) | 0.382 |
|
iNKT+IFN-γ+/iNKT+IL-4+
ratio | 0.57
(0.16–2.68) | 0.77
(0.04–3.73) | 0.176 |
|
|
| del(17p13.1) and/or
del(11q22.3) | Without
del(17p13.1), del(11q22.3) |
|
|
|
iNKT+IL-4+ (%) | 32.13
(12.07–55.56) | 37.71
(2.36–92.86) | 0.548 |
|
iNKT+IFN-γ+ (%) | 16.08
(10.53–60.62) | 24.00
(0.60–83.33) | 0.064 |
|
iNKT+IFN-γ+/iNKT+IL-4+
ratio | 0.53
(0.32–2.29) | 0.71
(0.04–3.73) | 0.365 |
A higher percentage of
iNKT+IL-4+ cells and a lower percentage of
iNKT+IFN-γ+ cells were observed in patients
carrying unfavorable cytogenetic abnormalities (11q22.3 and/or
17p13.1 deletion), compared with in patients without these genetic
changes (Table II). Similarly,
patients with del (11q22.3) and/or del (17p13.1) exhibited a lower
ratio of iNKT+IFN-γ+ to
iNKT+IL-4+. However, these differences were
not statistically significant (Table
II).
The percentage of iNKT+IL-4+
cells correlated positively with the WBC count (R=0.387;
P<0.05), PB lymphocyte count (R=0.358; P<0.05) and
β2-microglobulin levels (R=0.474; P<0.01). There was
also an inverse correlation between the
iNKT+IFN-γ+: iNKT+IL-4+
ratio and the WBC count (R=−0.302; P<0.05) and
β2-microglobulin levels (R=0.507; P<0.01). However,
no significant association between the percentage of
iNKT+IFN-γ+ cells and other disease
parameters was identified.
In the present study, PB samples were obtained from
untreated patients with CLL diagnosed between September 2014 and
June 2016 (21 months of observations). During the follow-up period,
the treatment was initiated in 12 patients (20%). For these
patients, the median time to treatment (TTT) was 7 months (range,
0–12 months). TTT was defined as the interval from the date of
diagnosis to the date of first treatment. There was no significant
association between the percentage of
iNKT+IL-4+ or
iNKT+IFN-γ+ cells and the time to treatment.
No statistically significant differences were identified in the
percentage of iNKT+IL-4+ and
iNKT+IFN-γ+ or the
iNKT+IFN-γ+: iNKT+IL-4+
ratio between patients requiring therapy, as compared with patients
without treatment, during the observation period (Table III).
 | Table III.Percentage of iNKT cells with
intracellular expression of IL-4 or IFN-γ in untreated and
requiring therapy patients with CLL. |
Table III.
Percentage of iNKT cells with
intracellular expression of IL-4 or IFN-γ in untreated and
requiring therapy patients with CLL.
|
| NKT/IL-4% | NKT/IFN % | IFN:IL-4 |
|---|
|
|
|
|
|
|---|
|
| Untreated
patients | Requiring
therapy | Untreated
patients | Requiring
therapy | Untreated
patients | Requiring
therapy |
|---|
| Median | 34.36 | 35.20 | 24.50 | 23.61 | 0.71 | 0.53 |
| Minimum | 2.36 | 12.07 | 0.60 | 11.11 | 0.04 | 0.32 |
| Maximum | 92.86 | 58.82 | 83.33 | 60.00 | 3.73 | 2.40 |
Expression of IL-4 and IFN-γ mRNAs in
iNKT cells
Purified iNKT cells were analyzed for IL-4 and IFN-γ
mRNA expression using RT-qPCR. For the analysis of IL-4 and IFN-γ
mRNA expression, each sample was normalized to β-actin. Generally,
after 24 h of culture without stimulation, no IL-4 or IFN-γ mRNA
was identified in the iNKT cells from patients with CLL or from the
HVs. Only in one patient (p#5) was the presence of IL-4 and IFN-γ
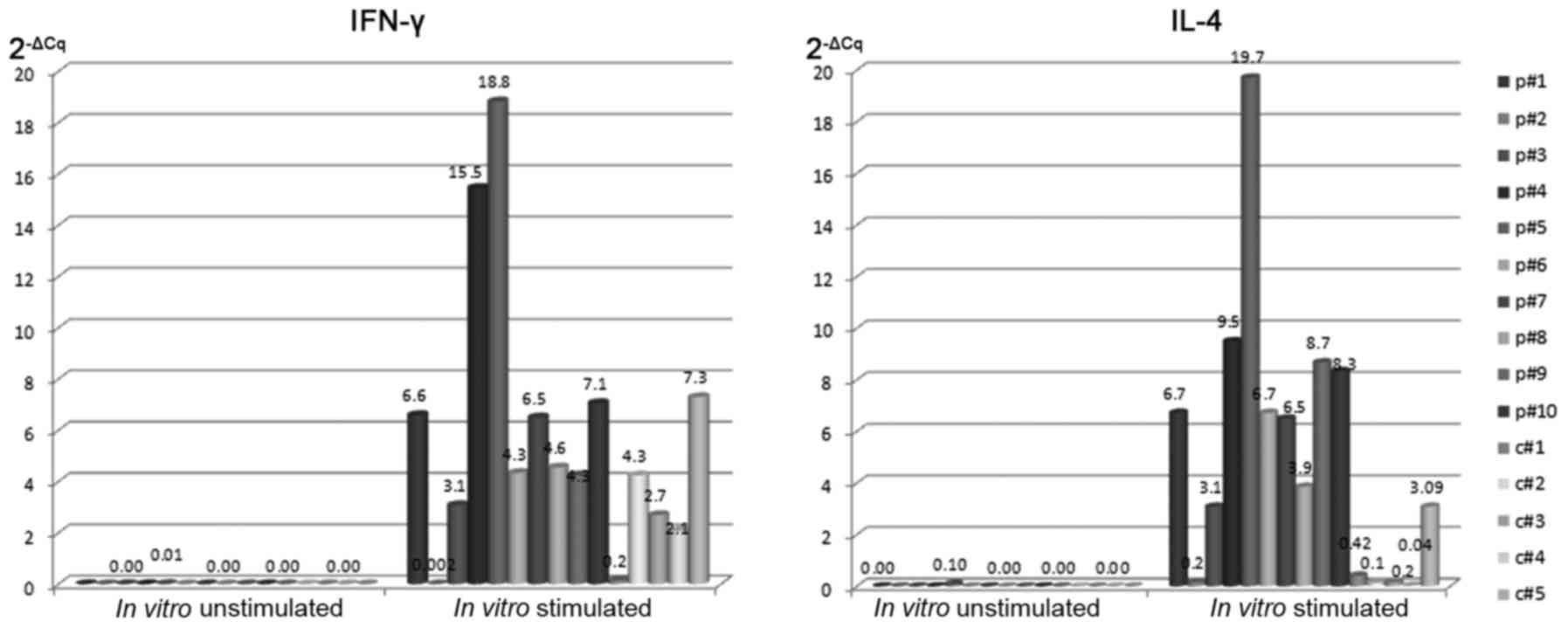
mRNA in an unstimulated culture observed (Fig. 6). iNKT cells from patients with CLL
and HVs were identified to express IL-4 (median 2−ΔCq:
6.70 vs. 0.20) and IFN-γ (median 2−ΔCq: 5.50 vs. 2.70)
mRNAs after α-GalCer stimulation (Fig.
6). After 24-h α-GalCer stimulation the presence of IL-4 and
IFN-γ mRNAs was determined in iNKT cells isolated from patients
with CLL as well as HVs. However, IL-4 or IFN-γ mRNAs were
identified at higher levels in iNKT cells from patients with CLL
compared with iNKT cells of HVs (P<0.05). In the present study,
IL-4 mRNA levels directly correlated with the percentage of
IL-4-positive iNKT cells (R=0.481; P<0.01). Similarly, IFN-γ
mRNA levels directly correlated with the percentage of iNKT cells
with intracellular IFN-γ expression (R=0.473; P<0.01).
Membrane CD1d expression on B cells
and monocytes from patients with CLL and HVs
Our previous data (25) indicated that the median percentage of
CD1d-positive B cells in patients with CLL was significantly lower
than in HVs. Similarly, when the level of membrane CD1d expression
determined by MFI on B cells was compared between patients with CLL
and HVs, we identified a significant difference between the groups.
In the present study, our previous results (25) that the percentage of B cells CD1d+ in
CLL patients was significantly lower than in HVs were confirmed.
Furthermore, in the current study, CD1d expression was detected on
monocytes. Fig. 2 presents two
representative types of monocytes with CD1d expression for patients
with CLL (CLL1-CLL2), and two representative types of CD1d-positive
monocytes for HVs (HV1-HV2). The percentage of monocytes with CD1d
expression was significantly lower in patients with CLL in
comparison with the HVs (median, 85.73%; range, 65.78–99.15% vs.
median, 92.86%; range, 88.55–99.89%; P<0.05). Similarly, the
level of membrane CD1d expression determined by MFI on monocytes
was reduced in patients with CLL (median 182.60 MFI in HVs; 161.40
MFI in the CLL group). However, this difference was not
statistically significant. Furthermore, no significant association
between the expression levels of IL-4 or IFN-γ in iNKT cells and
CD1d expression on leukemic B lymphocytes or monocytes from
patients with CLL was identified.
Apoptosis
The ex vivo percentage of apoptotic B
lymphocytes (∆Ψmlow/CD19+) was significantly
lower than the percentage of ∆Ψmlow/CD19+
lymphocytes in 24 h in vitro culture (P<0.05). However,
there was no significant difference in the percentage of apoptotic
CD19+ lymphocytes between the cultures with and without
α-GalCer (Table IV). No correlation
between the percentage of iNKT+IL-4+ or
iNKT+IFN-γ+ cells and the percentage of
∆Ψmlow/CD19+ lymphocytes was noted. iNKT
cells with various cytokine profiles did not affect B cell
apoptosis. Additionally, no correlation was identified between the
percentage of iNKT cells in PB from patients with CLL and the
percentage of ∆Ψmlow/CD19+ lymphocytes
determined directly ex vivo.
 | Table IV.Ex vivo and in vitro
(with and without α-GalCer stimulation) percentage of apoptotic
CD19+ cells (∆Ψmlow/CD19+) evaluated by
CMXRos. |
Table IV.
Ex vivo and in vitro
(with and without α-GalCer stimulation) percentage of apoptotic
CD19+ cells (∆Ψmlow/CD19+) evaluated by
CMXRos.
| Conditions |
∆Ψmlow/CD19+ (%)
Median (range) |
|---|
| ex vivo | 3.60
(0.40–26.70) |
| 24-h in
vitro culture without stimulation | 19.20
(5.30–30.60) |
| 24-h in
vitro culture with α-GalCer | 22.50
(7.60–28.70) |
Discussion
Deficiencies in cytokine production by the
T-lymphocytes of patients with CLL have previously been noted in
certain studies (29,30), but few concentrated on the small but
essential T cell subpopulation of iNKT (31). iNKT cells recognize lipid antigens,
such as αGalCer, when presented in a complex with CD1d (32). To evaluate the functionality of iNKT
lymphocytes, they were cultured in vitro and stimulated with
the iNKT-specific ligand α-GalCer. It is challenging to select
suitable stimulation methods for the analysis of cytokine
production, especially for rare cell populations (e.g., iNKT
cells). In the present study, whole blood samples were stimulated
in vitro. Analysis of cytokine synthesis in whole blood has
been utilized in previous studies (33–35). Such
a method may imitate the natural in vivo environment
(34). It was identified that
α-GalCer stimulation induced a stronger intracellular cytokine
response in patients with CLL, when compared with HVs. Following
culture with α-GalCer, iNKT cells exhibited upregulated expression
of CD69, an early activation marker, indicating that the examined
cells retained the ability to respond to stimulation. In patients
with CLL, the percentage of iNKT+IFN-γ+
cells, and of iNKT+IL-4+, was increased with
the noticeable dominance of a iNKT subset with intracellular
expression of IL-4, while in HVs, the percentage of iNKT cells with
IL-4 and IFN-γ expression was similar.
Changes in T cell cytokine secretion profile,
associated with a Th2 shift, were described for advanced CLL cases
(30). Hill et al (36) reported a reduction in IFN-γ and IL-4
expression by CD4+ T cells from patients with CLL.
However, little data concerning cytokine production by iNKT cells
have previously been presented. Weinkove et al (31) analyzed the cytokine profile and the
proliferative capacity of circulating iNKT cells from patients with
CLL. These authors observed an increased tendency towards an iNKT
CD4+ subset (characterized by a production of Th2
cytokines) and a reduced tendency towards an iNKT CD8+
subset, although the results were not statistically significant. A
comparison of patients with CLL and HVs revealed a lack of
functional differences in, and no difference in the numbers of,
iNKT cells; the cytokine production and in vitro
proliferation of iNKT were similar. In the study, the authors
evaluated a population of iNKT cells obtained from patients
primarily in the early stages of the disease (31). It was suggested that the analysis of
iNKT cells from patients with advanced-stage CLL could provide more
diverse results. In the present study, no significant differences
were observed in the percentage of iNKT+IL-4+
cells between the CLL risk groups. However, patients with CLL at
Rai stage III–IV had a lower percentage of
iNKT+IFN-γ+ cells than those at Rai stage 0.
Concordant with our results, Tahir et al (21) identified a strong Th2 bias;
α-GalCer-stimulated iNKT cells from patients with prostate cancer
predominantly produced IL-4, while the production of IFN-γ was
decreased. It must be emphasized that, in the present study, the
iNKT+IFN-γ+: iNKT+IL-4+
ratio was decreased in the CLL group as compared with in the HVs.
An inverse correlation was identified between the
iNKT+IFN-γ+: iNKT+IL-4+
ratio and the WBC count, as well as the β2-microglobulin
levels. Furthermore, the ratio was lower in advanced-stages
compared with the early stages of the disease, and was lower in
ZAP-70-positive patients. Similarly, a tendency towards the
reduction in the ratio of iNKT+IFN-γ+ to
iNKT+IL-4+ was observed in patients with CLL
who also had del (11q22.3) and/or del (17p13.1). In a study of
Dhodapkar et al (37), the
loss of IFN-γ production by freshly isolated iNKT cells in the
course of progressive myeloma was observed. An increase in IL-4
production, in comparison to IFN-γ expression, by iNKT cells was
identified in mice following immunization with α-GalCer (38,39). Tahir
et al (21) reported decreased
IFN-γ:IL-4 ratio in prostate cancer patients. IL-4 inhibits the
programmed death of CLL B cells and prolongs the cell lifespan
(40). This effect was not detected
for the B cells obtained from healthy subjects (4). Smyth et al (41) suggested that IL-4 is not required for
mediating α-GalCer activity against cancer. The results suggest
that the frequency of iNKT cells and the profile of cytokines
expressed by iNKT cells does not affect B cell apoptosis in
patients with CLL. The results obtained by Palmer et al
(42) indicate that iNKT cells are
dispensable in the development or accumulation of CD5+ B
cells in mice prone to benign or leukemic CLL-like B cell
expansion.
According to the literature, numerous studies have
noted not only qualitative, but also quantitative changes
concerning iNKT cells in the course of neoplastic diseases
(21,43–47). The
number of analyzed iNKT cells decreased in patients with solid
tumors (21,43,47) and
hematological malignancies, in comparison with healthy subjects
(45,46,48). Fais
et al (49) observed a
significantly lower number of iNKT cells in patients with CLL
compared with HVs. The results of the present study, and of our
previous study (46), indicated a
significantly lower percentage of iNKT cells in the PB of patients
with CLL when compared with that of HVs. Certain prior studies have
associated a reduced number of iNKT cells not with a tumor, but
with a risk of tumor growth (43,50).
Conversely, Weinkove et al (31) suggested that the absolute number of
circulating iNKT cells in patients with untreated CLL is normal,
and the reduction occurred in a group of patients undergoing
chemotherapy. The data obtained in the current study are concordant
with those recently reported by Weinkove et al (31), who determined there was no significant
association between the iNKT cell frequency and the clinical
disease stage or expression of adverse prognostic markers (i.e.,
ZAP-70).
In the present study, flow cytometry and RT-qPCR
were used to detect the levels of IL-4 and IFN-γ mRNA in iNKT
cells. The level of transcription for IL-4 and IFN-γ was higher in
leukemic B cells compared with HVs. Similarly, the percentage of
iNKT cells expressing these molecules was higher in patients with
CLL. However, the methods used in the present study were not able
to indicate whether iNKT cells with IL-4 and IFN-γ expression could
also secrete IL-4 and IFN-γ. Nevertheless, the pattern of IL-4 and
IFN-γ expression in the cytoplasm of iNKT cells together with the
expression of IL-4 and IFN-γ mRNA may suggest that iNKT cells are
able to produce these cytokines. Further study is required in order
to determine whether a change in the pattern of released cytokines
may have an important role in the pathogenesis of CLL. It must be
noted that no significant association was identified between the
percentage of iNKT cells with intracellular IL-4 or IFN-γ
expression and the TTT. Furthermore, no statistically significant
difference was observed in the iNKT+IFN-γ+:
iNKT+IL-4+ ratio between the patients
requiring therapy and the patients without treatment.
CD1d expression is crucial for the presentation of
glycolipids to iNKT cells (31). It
was previously demonstrated that there is lower CD1d molecule
expression in the B cells of patients with CLL, as compared with
HVs (25). Similarly, Weinkove et
al (31) identified that leukemic
B cells express CD1d at lower levels. The results of the current
study are consistent with those of Weinkove et al (31), who reported reduced CD1d expression on
the monocytes of patients with CLL. Nevertheless, the role of CD1d
in antitumor immunity is not well understood. It has been reported
that iNKT cells fail to develop in CD1d−/− mice
(51). Wang et al (52) identified that resting iNKT cells that
had not been exposed to APC with CD1d expression did not contain
detectable levels of IFN-γ mRNA. Fais et al (49) reported that CD1d on leukemic B cells
was able to present α-GalCer to NKT cells, as revealed by cytokine
production, cytotoxicity and proliferation assays. In the present
study, no association between the expression levels of IL-4 or
IFN-γ in iNKT cells and CD1d molecule expression on leukemic B
lymphocytes or monocytes of patients with CLL was identified.
Numerous issues concerning the development and
progression of CLL are still unclear. Over the years, numerous
abnormalities concerning leukemic B lymphocytes and non-leukemic
cells of the immune system that occur during the course of CLL have
been characterized (53). The
functional dysfunction of NKT-like cells in terms of cytokine
production have previously been demonstrated (54). However, there are currently few
publications concerning the cytokine expression profiles of iNKT
cells obtained from patients with CLL (31,49). By
producing a variety of cytokines, the iNKT lymphocytes modify the
microenvironment and, therefore, may influence tumor growth
(21,55,56). As an
important regulator of the Th1/Th2 balance (21,56), iNKT
cells may have a significant role in the pathogenesis of CLL. An
attempt was made to evaluate the ratio of
iNKT+IFN-γ+: iNKT+IL4+
in patients with CLL. It was expected that its decrease may result
in the promotion of leukemic B lymphocyte survival. However, in the
present study iNKT cells with multiple cytokines profiles did not
affect B cell apoptosis. Although iNKT cells are considered to
enhance the antitumor response (57,58) in
certain tumors, such as CLL, the cells may gain specific
immunosuppressive properties (25,54).
However, further studies are required. In the present study,
CD4+CD25+Foxp3+ regulatory T cells
(Tregs) were not analyzed. Nevertheless, an inverse
correlation was identified between the percentages of iNKT cells
and CD4+CD25high T cells (data not
presented). Tregs can suppress the proliferation,
cytokine secretion and cytotoxic activity of NKT cells (59). Monitoring the number and function of
iNKT cells may be important for assessing immunological dysfunction
in patients with CLL. However, currently it cannot unequivocally be
said that monitoring the percentage of iNKT cells or their function
can provide useful information concerning the activity or
progression of the disease. In the present study, only two
cytokines were analyzed. They were chosen since they represent
cytokines typical for Th1- and Th2-type lymphocytes with a
well-known antagonizing action. Further analysis of other cytokines
produced by iNKT cells, including IL-17 and TGF-β, may be important
for understanding the pathogenesis of CLL.
Acknowledgements
The present study was supported in part by a
research grant (grant no. N N402 439139) from the State Funds for
Scientific Research National Science Centre (NCN) and by a grant
from the Medical University of Lublin (grant no. DS 458).
References
|
1
|
Joyce JA: Therapeutic targeting of the
tumor microenvironment. Cancer Cell. 7:513–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burger JA and Gribben JG: The
microenvironment in chronic lymphocytic leukemia (CLL) and other B
cell malignancies: Insight into disease biology and new targeted
therapies. Semin Cancer Biol. 24:71–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tinhofer I, Rubenzer G, Holler C,
Hofstaetter E, Stoecher M, Egle A, Steurer M and Greil R:
Expression levels of CD38 in T cells predict course of disease in
male patients with B-chronic lymphocytic leukemia. Blood.
108:2950–2956. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mainou-Fowler T and Prentice AG:
Modulation of apoptosis with cytokines in B-cell chronic
lymphocytic leukaemia. Leuk Lymphoma. 21:369–377. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Terabe M, Swann J, Ambrosino E, Sinha P,
Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ and
Berzofsky JA: A nonclassical non-Valpha14Jalpha18 CD1d-restricted
(type II) NKT cell is sufficient for down-regulation of tumor
immunosurveillance. J Exp Med. 202:1627–1633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gapin L: Development of invariant natural
killer T cells. Curr Opin Immunol. 39:68–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dellabona P, Abrignani S and Casorati G:
iNKT-cell help to B cells: A cooperative job between innate and
adaptive immune responses. Eur J Immunol. 44:2230–2237. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Exley M, Porcelli S, Furman M, Garcia J
and Balk S: CD161 (NKR-P1A) costimulation of CD1d-dependent
activation of human T cells expressing invariant V alpha 24 J alpha
Q T cell receptor alpha chains. J Exp Med. 188:867–876. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seino K and Taniguchi M: Functionally
distinct NKT cell subsets and subtypes. J Exp Med. 202:1623–1626.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Godfrey DI, MacDonald HR, Kronenberg M,
Smyth MJ and Van Kaer L: NKT cells: What's in a name? Nat Rev
Immunol. 4:231–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawano T, Cui J, Koezuka Y, Toura I,
Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al:
CD1d-restricted and TCR-mediated activation of valpha14 NKT cells
by glycosylceramides. Science. 278:1626–1629. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spada FM, Koezuka Y and Porcelli SA:
CD1d-restricted recognition of synthetic glycolipid antigens by
human natural killer T cells. J Exp Med. 188:1529–1534. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hayakawa Y, Godfrey DI and Smyth MJ:
Alpha-galactosylceramide: Potential immunomodulatory activity and
future application. Curr Med Chem. 11:241–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuda JL, Naidenko OV, Gapin L, Nakayama
T, Taniguchi M, Wang CR, Koezuka Y and Kronenberg M: Tracking the
response of natural killer T cells to a glycolipid antigen using
CD1d tetramers. J Exp Med. 192:741–754. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsuda JL, Mallevaey T, Scott-Browne J
and Gapin L: CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of
the immune system. Curr Opin Immunol. 20:358–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gumperz JE, Miyake S, Yamamura T and
Brenner MB: Functionally distinct subsets of CD1d-restricted
natural killer T cells revealed by CD1d tetramer staining. J Exp
Med. 195:625–636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brennan PJ, Brigl M and Brenner MB:
Invariant natural killer T cells: An innate activation scheme
linked to diverse effector functions. Nat Rev Immunol. 13:101–117.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Godfrey DI and Kronenberg M: Going both
ways: Immune regulation via CD1d-dependent NKT cells. J Clin Inves.
114:1379–1388. 2004. View Article : Google Scholar
|
|
19
|
Fujii S, Shimizu K, Smith C, Bonifaz L and
Steinman RM: Activation of natural killer T cells by
alpha-galactosylceramide rapidly induces the full maturation of
dendritic cells in vivo and thereby acts as an adjuvant for
combined CD4 and CD8 T cell immunity to a coadministered protein. J
Exp Med. 198:267–279. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smyth MJ and Godfrey DI: NKT cells and
tumor immunity-a double-edged sword. Nat Immunol. 1:459–460. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tahir SM, Cheng O, Shaulov A, Koezuka Y,
Bubley GJ, Wilson SB, Balk SP and Exley MA: Loss of IFN-gamma
production by invariant NK T cells in advanced cancer. J Immunol.
167:4046–4050. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hallek M, Cheson BD, Catovsky D,
Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ,
Montserrat E, Rai KR, et al: Guidelines for the diagnosis and
treatment of chronic lymphocytic leukemia: A report from the
international workshop on chronic lymphocytic leukemia updating the
national cancer institute-working group 1996 guidelines. Blood.
111:5446–5456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rai KR, Sawitsky A, Cronkite EP, Chanana
AD, Levy RN and Pasternack BS: Clinical staging of chronic
lymphocytic leukemia. Blood. 46:219–234. 1975.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bojarska-Junak A, Hus I, Chocholska S,
Tomczak W, Woś J, Czubak P, Putowski L and Roliński J: CD1d
expression is higher in chronic lymphocytic leukemia patients with
unfavorable prognosis. Leuk Res. 38:435–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bojarska-Junak A, Hus I, Chocholska S,
Wasik-Szczepanek E, Sieklucka M, Dmoszyńska A and Roliński J: BAFF
and APRIL expression in B-cell chronic lymphocytic leukemia:
Correlation with biological and clinical features. Leuk Res.
33:1319–1327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sieklucka M, Pozarowski P, Bojarska-Junak
A, Hus I, Dmoszynska A and Rolinski J: Apoptosis in B-CLL: The
relationship between higher ex vivo spontaneous apoptosis
before treatment in III–IV Rai stage patients and poor outcome.
Oncol Rep. 19:1611–1620. 2008.PubMed/NCBI
|
|
28
|
Bojarska-Junak A, Hus I, Olszewska-Bożek
K, Chocholska S, Wąsik-Szczepanek E, Tomczak W, Miłczak J,
Dmoszyńska A and Roliński J: Analysis of ex vivo apoptosis of B and
T cells from peripheral blood and bone marrow of patients with
chronic lymphocytic leukemia. Acta Haematol Pol. 43:336–341. 2012.
View Article : Google Scholar
|
|
29
|
Christopoulos P, Pfeifer D, Bartholomé K,
Follo M, Timmer J, Fisch P and Veelken H: Definition and
characterization of the systemic T-cell dysregulation in untreated
indolent B-cell lymphoma and very early CLL. Blood. 117:3836–3846.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rossmann ED, Lewin N, Jeddi-Tehrani M,
Osterborg A and Mellstedt H: Intracellular T cell cytokines in
patients with B cell chronic lymphocytic leukaemia (B-CLL). Eur J
Haematol. 68:299–306. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weinkove R, Brooks CR, Carter JM, Hermans
IF and Ronchese F: Functional invariant natural killer T-cell and
CD1d axis in chronic lymphocytic leukemia: Implications for
immunotherapy. Haematologica. 98:376–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC,
Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J
and Rossjohn J: CD1d-lipid-antigen recognition by the
semi-invariant NKT T-cell receptor. Nature. 448:44–49. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fernandez CS, Cameron G, Godfrey DI and
Kent SJ: Ex-vivo α-galactosylceramide activation of NKT
cells in humans and macaques. J Immunol Methods. 382:150–159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ai W, Li H, Song N, Li L and Chen H:
Optimal method to stimulate cytokine production and its use in
immunotoxicity assessment. Int J Environ Res Public Health.
10:3834–3842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Z, Yuan X, Luo Y, He Y, Jiang Y, Chen
ZK and Sun E: Evaluating the effects of immunosuppressants on human
immunity using cytokine profiles of whole blood. Cytokine.
45:141–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hill SJ, Peters SH, Ayliffe MJ, Merceica J
and Bansal AS: Reduced IL-4 and interferon-gamma (IFN-gamma)
expression by CD4 T cells in patients with chronic lymphocytic
leukaemia. Clin Exp Immunol. 117:8–117. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dhodapkar MV, Geller MD, Chang DH, Shimizu
K, Fujii S, Dhodapkar KM and Krasovsky J: A reversible defect in
natural killer T cell function characterizes the progression of
premalignant to malignantmultiple myeloma. J Exp Med.
197:1667–1676. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh N, Hong S, Scherer DC, Serizawa I,
Burdin N, Kronenberg M, Koezuka Y and Van Kaer L: Cutting edge:
Activation of NK T cells by CD1d and alpha-galactosylceramide
directs conventional T cells to the acquisition of a Th2 phenotype.
J Immunol. 163:2373–2377. 1999.PubMed/NCBI
|
|
39
|
Burdin N, Brossay L and Kronenberg M:
Immunization with alpha-galactosylceramide polarizes CD1-reactive
NK T cells towards Th2 cytokine synthesis. Eur J Immunol.
29:2014–2025. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dancescu M, Rubio-Trujillo M, Biron G,
Bron D, Delespesse G and Sarfati M: Interleukin 4 protects chronic
lymphocytic leukemic B cells from death by apoptosis and
upregulates Bcl-2 expression. J Exp Med. 176:1319–1326. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Smyth MJ, Crowe NY, Pellicci DG,
Kyparissoudis K, Kelly JM, Takeda K, Yagita H and Godfrey DI:
Sequential production of interferon-gamma by NK1.1(+) T cells and
natural killer cells is essential for the antimetastatic effect of
alpha-galactosylceramide. Blood. 99:1259–1266. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Palmer VL, Nganga VK, Rothermund ME, Perry
GA and Swanson PC: Cd1d regulates B cell development but not B cell
accumulation and IL10 production in mice with pathologic CD5(+) B
cell expansion. BMC Immunol. 16:662015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Molling JW, Kölgen W, van der Vliet HJ,
Boomsma MF, Kruizenga H, Smorenburg CH, Molenkamp BG, Langendijk
JA, Leemans CR, von Blomberg BM, et al: Peripheral blood
IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are
decreased in cancer patients independent of tumor type or tumor
load. Int J Cancer. 116:87–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Giaccone G, Punt CJ, Ando Y, Ruijter R,
Nishi N, Peters M, von Blomberg BM, Scheper RJ, van der Vliet HJ,
van den Eertwegh AJ, et al: A phase I study of the natural killer
T-cell ligand alpha-galactosylceramide (KRN7000) in patients with
solid tumors. Clin Cancer Res. 8:3702–3709. 2002.PubMed/NCBI
|
|
45
|
Yoneda K, Morii T, Nieda M, Tsukaguchi N,
Amano I, Tanaka H, Yagi H, Narita N and Kimura H: The peripheral
blood Valpha24+ NKT cell numbers decrease in patients with
haematopoietic malignancy. Leuk Res. 29:147–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hus I, Bojarska-Junak A, Gonet-Sebastianka
J, Glazer M, Drab E, Woś J and Roliński J: iNKT cell percentage is
decreased in patients with chronic lymphocytic leukemia and
correlates inversely with the clinical stage and negative
prognostic factors. Centr Eur J Immunol. 36:79–84. 2011.
|
|
47
|
Singh AK, Shukla NK and Das SN: Altered
invariant natural killer T cell subsets and its functions in
patients with oral squamous cell carcinoma. Scand J Immunol.
78:468–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fujii S, Shimizu K, Klimek V, Geller MD,
Nimer SD and Dhodapkar MV: Severe and selective deficiency of
interferon-gamma-producing invariant natural killer T cells in
patients with myelodysplastic syndromes. Br J Haematol.
122:617–622. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fais F, Morabito F, Stelitano C, Callea V,
Zanardi S, Scudeletti M, Varese P, Ciccone E and Grossi CE: CD1d is
expressed on B-chronic lymphocytic leukemia cells and mediates
alpha-galactosylceramide presentation to natural killer T
lymphocytes. Int J Cancer. 109:402–411. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fujii S, Shimizu K, Okamoto Y, Kunii N,
Nakayama T, Motohashi S and Taniguchi M: NKT cells as an ideal
anti-tumor immunotherapeutic. Front Immunol. 4:4092013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mendiratta SK, Martin WD, Hong S,
Boesteanu A, Joyce S and Van Kaer L: CD1d1 mutant mice are
deficient in natural T cells that promptly produce IL-4. Immunity.
6:469–477. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang X, Bishop KA, Hegde S, Rodenkirch LA,
Pike JW and Gumperz JE: Human invariant natural killer T cells
acquire transient innate responsiveness via histone H4 acetylation
induced by weak TCR stimulation. J Exp Med. 209:987–1000. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lanasa MC: Novel insights into the biology
of CLL. Hematology Am Soc Hematol Educ Program. 2010:70–76.
2010.PubMed/NCBI
|
|
54
|
Bojarska-Junak A, Hus I, Sieklucka M,
Wasik-Szczepanek E, Mazurkiewicz T, Polak P, Dmoszynska A and
Rolinski J: Natural killer-like T CD3+/CD16+CD56+ cells in chronic
lymphocytic leukemia: Intracellular cytokine expression and
relationship with clinical outcome. Oncol Rep. 24:803–810. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Robertson FC, Berzofsky JA and Terabe M:
NKT cell networks in the regulation of tumor immunity. Front
Immunol. 5:5432014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang Y, Hu X, Guo C, Zhang Q, Peng J,
Zhang J, Li L, Zhang T and Xu C: Polarization of natural killer T
cells towards an NKT2 subpopulation occurs after stimulation with
alpha-galactosylceramide and rhG-CSF in aplastic anemia. Acta
Haematol. 119:178–186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mattarollo SR and Smyth MJ: NKT cell
adjuvants in therapeutic vaccines against hematological cancers.
Oncoimmunology. 2:e226152013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
McEwen-Smith RM, Salio M and Cerundolo V:
The regulatory role of invariant NKT cells in tumor immunity.
Cancer Immunol Res. 3:425–435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
La Cava A, Van Kaer L and Fu-Dong-Shi:
CD4+CD25+ Tregs and NKT cells: Regulators regulating regulators.
Trends Immunol. 27:322–327. 2006. View Article : Google Scholar : PubMed/NCBI
|