Introduction
Cervical cancer has become a malignant tumor of
importance. It threatens women's health, as its morbidity and
mortality rates are second only to lung cancer, with a trend
towards younger patients and increasing rates (1). It is generally believed that the
occurrence of cervical cancer is related to high-risk human
papilloma virus (HPV) infection and abnormal expressions of a
variety of pathogenic genes (2).
Galectin-3 is a member of lectin family and regulates intercellular
adhesion, metastasis, angiogenesis, apoptosis, immune tolerance and
other behaviors (3). Angiogenesis is
a necessary condition for the in situ and metastatic growth
of tumor cells, as well as an important factor for target organ
localization and circulating blood metastasis (4). Markowska et al (5) confirmed that galectin-3 can activate
N-acetylglucosamine transferase V, leading to the phosphorylation
of vascular endothelial growth factor (VEGF)-2 and promoting
angiogenesis. Many scholars (6)
believe that elevated expression of galectin-3 plays an important
role in the occurrence and development of cervical cancer. However,
some scholars hold the opposite opinion (7), pointing out that galectin-3 shows
strongly positive expression in normal cervix and chronic
cervicitis tissues, but lower levels in cervical precancerous
lesions and invasive carcinomas. Therefore, the correlation between
galectin-3 expression and cervical cancer occurrence needs in-depth
analysis.
In addition, changes in the tumor cell cycle also
play a key role in the occurrence of disease. The
p27kip1 protein is a multifunctional cyclin-dependent
kinase inhibitor, which can stagnate cells in the G1 phase and
reduce G1/S phase transition via the inhibition of cyclin D-CDK 4,
cyclin E-CDK 2, and other complexes (8). p27kip1 can also affect cell
differentiation and apoptosis, thereby inhibiting cell
proliferation (9). A number of
studies (10) suggest that the
downregulation of p27kip1 may be a sensitive index for
the early diagnosis and prognosis evaluation of cervical
cancer.
This study therefore analyzed the relationship
between galectin-3 and p27kip1 protein expression in
cervical precancerous lesion tissues and clinical prognosis, in
order to provide a reference for the early diagnosis of
disease.
Materials and methods
Patient data
A total of 74 patients diagnosed with cervical
intraepithelial neoplasia (CIN) via hysteroscopic biopsy and
pathology at Yuyao People's Hospital between January 2015 and June
2016 were continuously selected. There were 20 cases classified as
CIN I, 24 cases as CIN II and 30 cases as CIN III. Patients in the
CIN I group were aged 45–66 years (average, 51.2±8.6 years), with
12 cases of high-risk HPV infection. Patients in the CIN II group
were aged 44–68 years (average, 53.2±7.9 years), with 16 cases of
high-risk HPV infection. Patients in the CIN III group were aged
46–52 years (average, 52.6±6.8 years), with 20 cases of high-risk
HPV infection. No differences in age and high-risk HPV infection
rate were observed among the three groups (p>0.05). Informed
consent was obtained from all patients in this study. Conization of
cervix or radical operation was performed and radiotherapy and
chemotherapy were not adopted.
Research methods
Galectin-3, p27kip1, VEGF-2 and cyclin D
expression was detected via immunohistochemical staining and
reverse transcription polymerase chain reaction (RT-PCR). Tissue
sections (5 µm-thick) were made conventionally and stored at −70°C.
Follow-up lasted for 6–22 months (median, 13.5 months) and
recurrence rate was compared.
Immunohistochemical staining
Tissues were dewaxed using dimethylbenzene, hydrated
using an ethanol gradient, and then were incubated in 3%
H2O2 for 20 min and then in normal goat serum
working solution for 30 min. Rat anti-human galectin-3,
p27kip1, VEGF-2 and cyclin D monoclonal antibodies and
β-actin (internal reference) primary antibody (working
concentration of 1:2,000; Jiangsu Beyotime Institute of
Biotechnology Co., Ltd., Jiangsu, China) were used to incubate
tissues using a wet box at 4°C overnight. Following which, a rabbit
anti-mouse IgG polyclonal secondary antibody (working concentration
of 1:500; Jiangsu Beyotime Institute of Biotechnology) was used to
incubate tissues using a wet box at 27°C for 20 min. Finally,
horseradish peroxidase-labeled streptavidin working solution
(Jiangsu Beyotime Institute of Biotechnology) was used to incubate
tissues using a wet box at 27°C for 20 min, washed with
phosphate-buffered saline (PBS), subjected to DAB staining and
hematoxylin counter-staining, sealed with neutral gum, dried at
room temperature and observed under an optical microscope. Staining
results were evaluated using a semi-quantitative method according
to stain intensity and positive cell rate. Dark brown-stained
cytoplasm or nucleus was regarded as a positive indicator; no
positive staining was scored as 0 points, weakly positive staining
as 1 point, moderately positive staining as 2 points and strongly
positive staining as 3 points. A positive cell rate ≤5% was scored
as 0 points, 6–25% as 1 point, 26–50% as 2 points, 51–75% as 3
points and >75% as 4 points. A sum between 0–3 points was
interpreted as negative and a sum between 4–12 points as
positive.
RT-PCR
Total cellular RNA was extracted using TRIzol
reagent and RNA concentration and purity were determined via
ultraviolet spectrophotometer. cDNA was synthesized using a reverse
transcription kit. Primers were produced by Shanghai Sangon Biotech
Company according to Gene Bank sequences: Galectin-3 forward,
5′-GGTTTCATCCAGGATCGAGCAGG-3′ and reverse,
5′-ACAAAGATGGTCACGGTCTGCC-3′, 445 bp; p27kip1 forward,
5′-ACTACTTCTCCCGCCGCTAC-3′ and reverse,
5′-GAAATCAAACAGAGGCCGCATG-3′, 332 bp; VEGF-2 forward,
5′-TACCAGTGGAGGCCGACTTC-3′ and reverse,
5′-GCACAAAGCGACTGGATGAAC-3′, 103 bp; cyclin D forward,
5′-TTCCTCTTCCTACAGTACTC-3′ and reverse, 5′-GCAACCAGCCCTGTCGTCTC-3′,
399 bp; GAPDH forward, 5′-CGCGAGAAGATGACCCAGAT-3′ and reverse,
5′-GCACTGTGTTGGCGTACAGG-3′, 225 bp. The following reaction system
mixture was employed: 2 µl cDNA, 3 µl forward primer, 3 µl lower
primer, 0.5 µl Taq polymerase, 1 µl dNTPs, 3 µl MgCl2, 5
µl 10X buffer and water up to a final volume of 25 µl. Reaction
conditions were as follows: 95°C for 5 min, 95°C for 30 sec, 58°C
for 30 sec and 72°C for 60 sec for 30 cycles and concluding with a
termination cycle at 72°C for 10 min. PCR products were identified
via 2% agarose gel electrophoresis followed by UV imaging using a
gel imaging analysis system and gray value analysis via digital
photography.
Statistical analysis
SPSS 20.0 (SPSS, Inc., Chicago, IL, USA) software
was used for statistical analysis. Measurement data were presented
as mean ± standard deviation (SD). One-way analysis of variance
(ANOVA) was used for intergroup comparisons and LSD t-test was used
for pairwise comparisons. Enumeration data were presented as cases
or percentages (%) and Chi-square test was used for intergroup
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Ethics statement
The study was approved by the Ethics Committee of
Yuyao People's Hospital and written informed consents were signed
by the patients and/or guardians.
Results
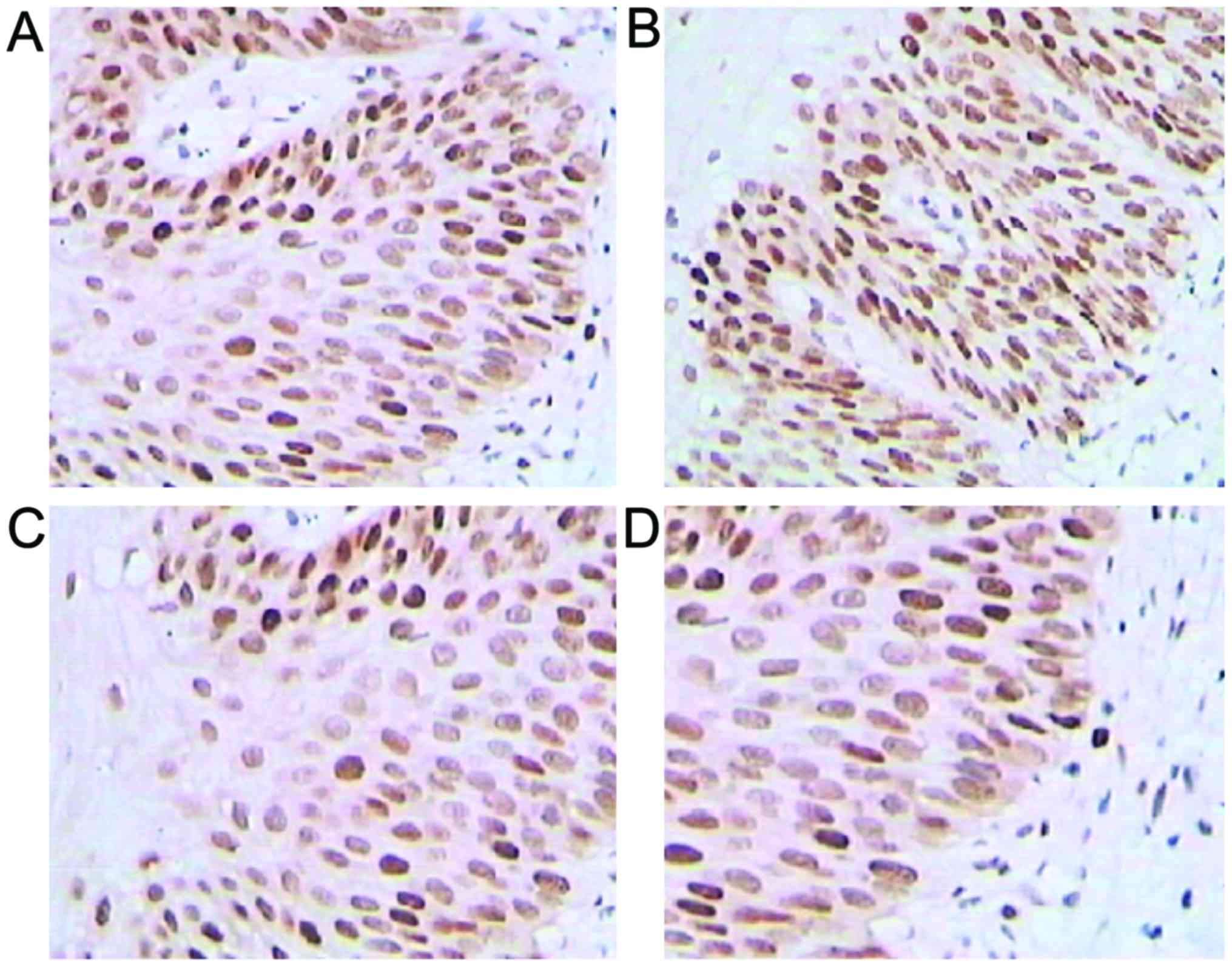
Immunohistochemical staining
Positive expression rates for galectin-3, VEGF-2 and
cyclin D increased in higher CIN-stage patients, but
p27kip1 expression presented the opposite result
(p<0.05; Fig. 1 and Table I).
 | Table I.Positive staining rate for each
protein. |
Table I.
Positive staining rate for each
protein.
| Group | Cases | Galectin-3 n (%) | p27kip1 n
(%) | VEGF-2 n (%) | Cyclin D n (%) |
|---|
| CIN I | 20 | 6 (30.0) | 14 (70.0) | 5 (25.0) | 7 (35.0) |
| CIN II | 24 | 9 (37.5) | 11 (45.8) | 10 (41.7) | 12 (50.0) |
| CIN III | 30 | 19 (63.3) | 10 (33.3) | 21 (70.0) | 23 (76.7) |
| χ2 |
| 6.389 | 6.503 | 10.420 | 9.149 |
| P-value |
| 0.041 | 0.039 | 0.005 | 0.010 |
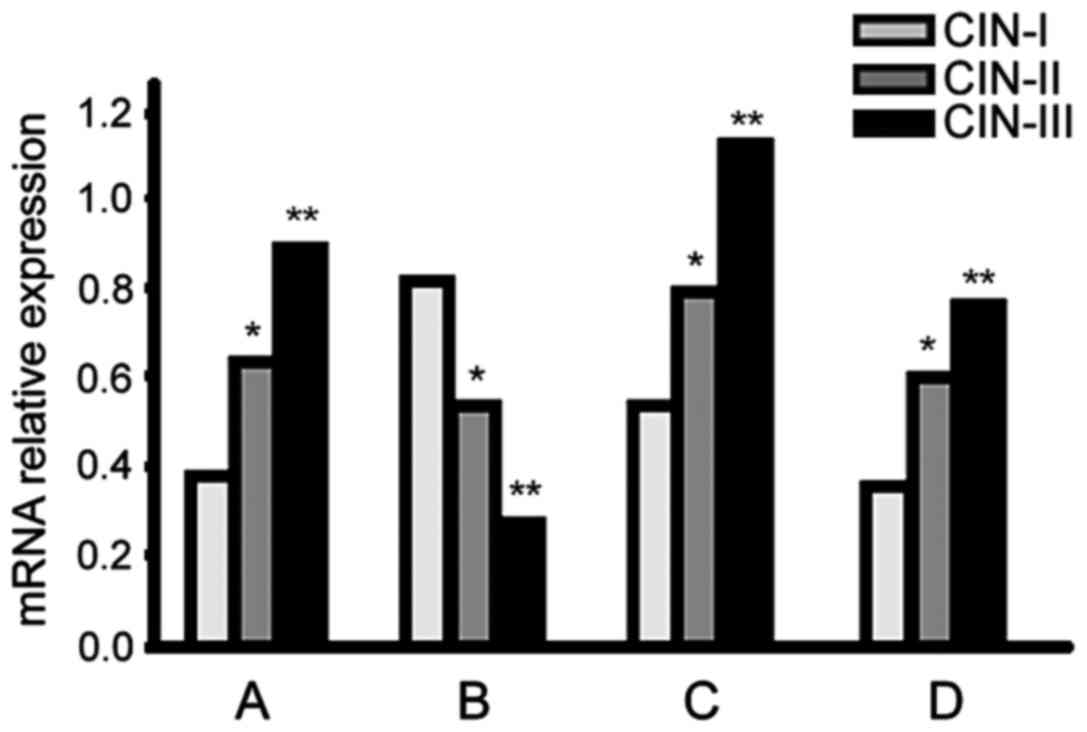
RT-PCR
Galectin-3, VEGF-2 and cyclin D mRNA expression
levels increased in higher CIN-stage patients, but
p27kip1 expression presented the opposite result
(p<0.05; Fig. 2).
Recurrence rate in the follow-up
During follow-up, 3 cases (15.0%) of recurrence were
noted in the CIN I group, 5 cases (20.8%) in the CIN II group and 9
cases (30.0%) in the CIN III group. There was no significant
differences in recurrence rate among the groups (p>0.05).
Discussion
This study showed galectin-3, VEGF-2 and cyclin D
expression increased in patients with higher CIN stage
classifications, but p27kip1 showed the opposite trend.
More than 80% of cervical cancer originates from CIN, so
investigations regarding CIN tissue characteristics is extremely
important for the study of cervical cancer occurrence. The
galectin-3 gene is located on the human chromosome 14q21-22 and is
comprised of a special chimeric structure composed of three parts:
An N-terminal domain (with a serine phosphorylation site that
regulates cell targeting), a collagen-like structure rich in
repeating sequences of proline, glycine and tyrosine (as a matrix
metalloproteinase substrate) and a C-terminal
carbohydrate-recognition domain (with a specific affinity for
β-galactose residues) (11). Zhao
et al (12) pointed out that
galectin-3 can lead to conformational changes in mucoprotein 1 on
the tumor surface, exposing the binding pockets of the adhesion
molecule CD44 and its ligand and promote tumor cell adhesion and
metastasis. Oka et al (13)
confirmed that galectin-3 can inhibit tumor cell apoptosis induced
by tumor necrosis factors such as CD95, resulting in tolerance to
radiotherapy and chemotherapy. Peng et al (14) found that galectin-3 can induce T cell
apoptosis and immune escape in vitro and in vivo and
promote tumor proliferation. A number of studies (15) have argued that the positive expression
of galectin-3 is related to pelvic lymph node metastasis and the
poor prognosis of patients with cervical cancer. After VEGF binds
to its receptor, signal transduction pathway activation results in
the promotion of angiogenesis and lymphangiogenesis, providing a
material basis for tumor growth and metastasis. Mitsuhashi et
al (16) noted that the levels of
serum VEGF and its receptors in patients with cervical squamous
carcinoma were significantly higher than those in healthy controls,
but no such differences were found in adenocarcinoma patients.
p27kip1 is usually expressed in the
nucleus of the middle-layer and superficial epithelial cells, but
levels are lower or absent in basal-layer cells with active
hyperplasia, suggesting that the p27kip1 protein
functions by inhibiting cell division and promoting differentiation
and maturation (17). HPV gene
products release a large number of transcription factors in the G1
phase, activating gene expression required in the S phase and
producing a variety of proteins required for the cell cycle
including cyclin E. Cyclin E and p27kip1 can bind to
each other to induce the degradation of HPV gene products (18). Therefore, the downregulation of
p27kip1 protein expression may be associated with HPV
infection (19) or the result of cell
cycle regulation disorders (20).
There was no difference in recurrence rate between
groups during follow-up, but an increasing trend was noted, which
may be related to the small sample size, shorter follow-up time and
radical cure of lesion. In conclusion, the upregulation of
galectin-3 expression and the downregulation of p27kip1
expression in CIN tissues may be related to tumor progression and
involving multiple mechanisms, such as angiogenesis, cell adhesion,
apoptosis inhibition, immune escape, HPV infection and cell cycle
regulation. This provides an important reference index for the
early diagnosis and prognosis evaluation of cervical cancer, but
its clinical application value needs to be further verified.
References
|
1
|
Xu XX, Zhou JS, Yuan SH, Yu H and Lou HM:
Distribution of HPV genotype in invasive cervical carcinoma and
cervical intraepithelial neoplasia in Zhejiang province, Southeast
China: Establishing the baseline for surveillance. Int J Environ
Res Public Health. 12:10794–10805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Magaldi TG, Almstead LL, Bellone S,
Prevatt EG, Santin AD and DiMaio D: Primary human cervical
carcinoma cells require human papillomavirus E6 and E7 expression
for ongoing proliferation. Virology. 422:114–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Than NG, Romero R, Balogh A, Karpati E,
Mastrolia SA, Staretz-Chacham O, Hahn S, Erez O, Papp Z and Kim CJ:
Galectins: Double-edged swords in the cross-roads of pregnancy
complications and female reproductive tract inflammation and
neoplasia. J Pathol Transl Med. 49:181–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu H, Zhang S, Zhang R and Zhang L: The
role of VEGF-C/D and Flt-4 in the lymphatic metastasis of
early-stage invasive cervical carcinoma. J Exp Clin Cancer Res.
28:982009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Markowska AI, Jefferies KC and Panjwani N:
Galectin-3 protein modulates cell surface expression and activation
of vascular endothelial growth factor receptor 2 in human
endothelial cells. J Biol Chem. 286:29913–29921. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Punt S, Thijssen VL, Vrolijk J, de Kroon
CD, Gorter A and Jordanova ES: Galectin-1, −3 and −9 expression and
clinical significance in squamous cervical cancer. PLoS One.
10:e01291192015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ebrahim AH, Alalawi Z, Mirandola L,
Rakhshanda R, Dahlbeck S, Nguyen D, Jenkins M, Grizzi F, Cobos E,
Figueroa JA, et al: Galectins in cancer: Carcinogenesis, diagnosis
and therapy. Ann Transl Med. 2:882014.PubMed/NCBI
|
|
8
|
Liu X, Yang WT and Zheng PS: Msi1 promotes
tumor growth and cell proliferation by targeting cell cycle
checkpoint proteins p21, p27 and p53 in cervical carcinomas.
Oncotarget. 5:10870–10885. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pavlides SC, Huang KT, Reid DA, Wu L,
Blank SV, Mittal K, Guo L, Rothenberg E, Rueda B, Cardozo T, et al:
Inhibitors of SCF-Skp2/Cks1 E3 ligase block estrogen-induced growth
stimulation and degradation of nuclear p27kip1: Therapeutic
potential for endometrial cancer. Endocrinology. 154:4030–4045.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu XL and Zheng PS: Undifferentiated
embryonic cell transcription factor-1 (UTF1) inhibits the growth of
cervical cancer cells by transactivating p27Kip1. Carcinogenesis.
34:1660–1668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kovak MR, Saraswati S, Schoen DJ and
Diekman AB: Investigation of galectin-3 function in the
reproductive tract by identification of binding ligands in human
seminal plasma. Am J Reprod Immunol. 72:403–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Q, Guo X, Nash GB, Stone PC, Hilkens
J, Rhodes JM and Yu LG: Circulating galectin-3 promotes metastasis
by modifying MUC1 localization on cancer cell surface. Cancer Res.
69:6799–6806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oka N, Nakahara S, Takenaka Y, Fukumori T,
Hogan V, Kanayama HO, Yanagawa T and Raz A: Galectin-3 inhibits
tumor necrosis factor-related apoptosis-inducing ligand-induced
apoptosis by activating Akt in human bladder carcinoma cells.
Cancer Res. 65:7546–7553. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng W, Wang HY, Miyahara Y, Peng G and
Wang RF: Tumor-associated galectin-3 modulates the function of
tumor-reactive T cells. Cancer Res. 68:7228–7236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W, Guo H, Geng J, Zheng X, Wei H, Sun
R and Tian Z: Tumor-released Galectin-3, a soluble inhibitory
ligand of human NKp30, plays an important role in tumor escape from
NK cell attack. J Biol Chem. 289:33311–33319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitsuhashi A, Suzuka K, Yamazawa K, Matsui
H, Seki K and Sekiya S: Serum vascular endothelial growth factor
(VEGF) and VEGF-C levels as tumor markers in patients with cervical
carcinoma. Cancer. 103:724–730. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui N, Yang WT and Zheng PS: Slug inhibits
the proliferation and tumor formation of human cervical cancer
cells by up-regulating the p21/p27 proteins and down-regulating the
activity of the Wnt/β-catenin signaling pathway via the
trans-suppression Akt1/p-Akt1 expression. Oncotarget.
7:26152–26167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang K-T, Pavlides SC, Lecanda J, Blank
SV, Mittal KR and Gold LI: Estrogen and progesterone regulate
p27kip1 levels via the ubiquitin-proteasome system: Pathogenic and
therapeutic implications for endometrial cancer. PLoS One.
7:e460722012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou N, Yuan S, Wang R, Zhang W and Chen
JJ: Role of dual specificity tyrosine-phosphorylation-regulated
kinase 1B (Dyrk1B) in S-phase entry of HPV E7 expressing cells from
quiescence. Oncotarget. 6:30745–30761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rath SL and Senapati S: Mechanism of p27
unfolding for CDK2 reactivation. Sci Rep. 6:264502016. View Article : Google Scholar : PubMed/NCBI
|