Introduction
Glioma has one of the highest incidence rates of all
malignant tumor types and is the most difficult primary cerebral
tumor to treat (1). Cerebral
malignant glioma, also named high-grade glioma (HGG) (2), is the most common type of primary
intracranial malignant tumor and originates in cranial nerve glial
cells (3). The relapse rate of HGG is
high and results in the generation of metastases; thus, the median
survival time of patients with HGG is only 13 months (4). The typical biological characteristic of
this type of malignant tumor is invasive growth, including that
into the surrounding healthy brain tissue. Due to the location of
such tumors within the brain, it is difficult to remove them using
surgery alone, resulting in poor prognosis (5,6).
Therefore, the conventional treatment for glioma involves a
combination of surgery and chemotherapy. However, patients
receiving chemotherapy alone following surgery frequently relapse.
Further studies are required in order to develop novel and
effective treatments for patients with glioma.
Temozolomide (TMZ) is a small lipophilic molecule
that can efficiently pass through the blood-brain barrier (7). TMZ is an alkylating agent that can
induce base mismatch and DNA abruption, thus inhibiting tumor cell
growth and inducing cell death. Therefore, TMZ is regarded as an
important chemotherapeutic drug for the treatment of glioma
(8). However, Kalkan et al
(9) identified that the expression of
multidrug resistance-associated proteins, such as P-glycoprotein
and O6-methylguanine-DNA methyl transferase (MGMT), was upregulated
in malignant glioma cells following chemotherapy, which induced
resistance to chemotherapeutic drugs, such as TMZ. In addition,
this study identified an association between the expression of
these proteins and the relapse rate of malignant glioma.
There may be other mechanisms of drug resistance in
malignant glioma. Zhang et al (10) identified that the median survival time
of malignant glioma only increased from 14.6 to 21.7 months
following MGMT promoter methylation, which resulted in the down
regulation of MGMT. Previous studies have demonstrated that TMZ
induces and molecularly regulates the autophagy of glioma cells
(11). Based on these results, it has
been speculated that TMZ resistance could be associated with
autophagy. Therefore, in the present study, the combined effect of
TMZ and a specific inhibitor of autophagy was investigated in order
to elucidate the molecular mechanisms underlying autophagy in
cerebral malignant glioma.
Rapamycin (RAPA) is a macrolide antibiotic, which
was first widely used as an immunosuppressant, due to its
significant effects on multiple autoimmune diseases. In addition,
RAPA is used to prevent rejection following organ transplantation,
with few side effects. In previous studies, RAPA demonstrated
antitumor activity, in addition to specific autophagy inhibition
(12–14). The present study aimed to investigate
the combined biological effect of TMZ and RAPA on the
proliferation, survival, apoptosis, cell cycle distribution and
autophagy of cerebral glioma cells, and the underlying molecular
mechanisms involved, in order to develop more effective treatments
for patients with glioma.
Materials and methods
Reagents and apparatus
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Trypsin (0.25%) was purchased
from Hangzhou Evergreen (Hangzhou, China). Penicillin and
streptomycin were purchased from Wuhan Boster Biological
Technology, Ltd. (Wuhan, China). RAPA was purchased from the
Beyotime Institute of Biotechnology (Haimen, China). TMZ and
acridine orange (AO) were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany).
Cell culture
The human glioma cell line U251, purchased from the
Shanghai Institute of the Chinese Academy of Sciences (Shanghai,
China), was cultured in DMEM containing 100 U/ml penicillin and 100
µg/ml streptomycin, supplemented with 10% FBS and incubated at 37°C
with 5% CO2 in a humidified atmosphere. Medium was
changed every 1–2 days. When the cells covered 90% of the culture
flask, they were digested with trypsin and passaged.
Detection of U251 cell survival rate
using the cell counting kit-8 (CCK-8) assay
Glioma cells in the logarithmic phase of growth were
washed with PBS three times and then digested using 0.25% trypsin.
DMEM containing 10% FBS was used to achieve a solution of
~1×105 cells/ml. The resulting suspension was added to a
96-well plate (100 µl/well). The plate was incubated at 37°C with
5% CO2 for 24 h. The cells were subsequently divided
into the control groups (solvent and blank) and the experimental
groups (TMZ alone, RAPA alone, and TMZ and RAPA). Different
concentrations of TMZ (1–50 µM) and/or RAPA (0.625–20 nM) were
added to the experimental groups.
Dimethyl sulfoxide (1 µg/ml) was added to the
solvent control group and DMEM was added to the blank control
group. There were six wells per treatment. Forty-eight hour
following the addition of TMZ/RAPA, 10 µl CCK-8 reagent
(Sigma-Aldrich; Merck KGaA) was added to each well prior to
incubating the plate for a further 2–4 h. An enzyme-linked
spectrophotometer was used to detect cell proliferation via
measuring the absorbance at 450 nm. The following formula was used
to calculate cell viability: (D-B/C-B) ×100% (D,
absorbance of drug well at 450 nm; B, absorbance of blank
control well at 450 nm; C, absorbance of control well at 450
nm).
Detection of cell apoptosis using flow
cytometry
A total of 48 h following treatment with drugs as
described above, cells (~5×106 cells/ml) were collected)
by centrifugation at 200 × g for 5 min in room temperature. Cells
were subsequently washed in ice-cold PBS and centrifuged again.
Cells were resuspended in 100 µl 5X annexin binding buffer for flow
cytometry (Thermo Fisher Scientific Inc.). An additional 5 µl APC
annexin V and 5 µl propidium iodide (PI) (both 100 µg/ml) were
added to the suspension and incubated at room temperature for 15
min. PI fluorescence excitation was performed using an argon ion
laser at a wavelength of 488 nm. Annexin V staining was detected
using flow cytometry, as aforementioned. Flow cytometry (Attune NxT
Flow Cytometer Software, version 2.1, Thermo Fisher Scientific
Inc.) was used to detect the effect of the drugs on U251 cell
apoptosis.
Detection of cell cycle progression using flow
cytometry. A total of 48 h following treatment with drugs as
described above, the cell culture medium was collected into a
streaming dedicated pipe. Cells were washed once with 1 ml PBS and
cleaning fluid was added to the pipe. The cells were collected into
the pipe following digestion with trypsin. The remaining cells were
washed with 1 ml PBS and collected (~2×106 cells/ml)
through centrifugation at 200 × g for 5 min in room temperature.
This step was repeated to wash the cells. Subsequently, 300 µl PBS
was used to resuspend the cells and the cells were fixed with 700
µl of ice-cold 70% absolute ethanol, which was added dropwise. The
cells were immobilized at −20°C for ≥24 h without light. The
resultant mixture was centrifuged as described above to remove any
stationary liquid. PBS (500 µl) was used to resuspend the cells
prior to centrifugation as described above. PI (5 µl) was used for
staining at 4°C for 30 min without light. Annexin V staining was
detected using flow cytometry, as aforementioned.
Immunofluorescence detection
U251 cells were seeded into a 24-well plate with
sterile cover slips in place. The culture medium was discarded when
all cells had adhered. Cells were washed three times with PBS and
paraformaldehyde (4%) was applied at room temperature for 20 min to
immobilize cells. Cells growing on glass coverslips were
permeabilized with Triton X-100 solution (0.3%) for 15 min. The
cells were washed three times with PBS (5 min/wash). Normal goat
serum (10%; Thermo Fisher Scientific Inc.) was used to block
non-specific binding and the cells were subsequently incubated at
37°C for 60 min.
Cells were incubated with the primary antibody
(1:100, CD81 Monoclonal Antibody (1D6), cat. no. MA1-80820, Thermo
Fisher Scientific Inc.). Coverslips were then incubated at 4°C
overnight. The cells were subsequently placed at room temperature
for 30 min and rinsed three times with 0.01 M of PBS (5 min/wash).
Secondary antibody [dilution: 10 µg/ml, donkey anti-Goat IgG (H+L)
Cross-Adsorbed Secondary Antibody, Alexa Fluor 350, catalog no.
A-21081, Thermo Fisher Scientific Inc.] was added to the cells
cultured on glass coverslips and incubated at 37°C for 30 min.
Cells were counterstained with DAPI and incubated at 37°C for a
further 10 min. Cells were washed three times with PBS (5 min/wash)
and ProLong anti-fade solution (Thermo Fisher Scientific, Inc.) or
glycerinum (Thermo Fisher Scientific Inc.) was used for sealing.
Laser scanning confocal microscopy was used for observation and
image acquisition.
Detection of acidic vesicular
organelles (AVOs) in tumor cells using AO staining
The generation of AVOs is a specific process of
autophagy. AO is a fluorochrome that can freely cross the cell
membrane. AO accumulates in acidic cell components, generating red
fluorescence. This red fluorescence appears when acid phosphatase
activity increases during autolysosome formation. The present
experiment was performed as described in a previous study, which
used the FL1 pathway to detect green fluorescence with an emission
maximum at 532 nm and the FL3 pathway to detect red fluorescence by
using 6HFFRPWL Shower Lens, in order to analyze AVO generation in
U251 cells (15). U251 cells in the
logarithmic phase of growth (1×104 cells/ml), were
inoculated into a 24-well plate (1 ml/well). Drugs were added the
next day as described above and the cells were cultured for a
further 72 h. AO (1 µg/ml) was used at 37°C to stain the cells for
15 min. The distribution of AVOs was subsequently observed under a
fluorescence microscope. Flow cytometry (Attune NxT Flow Cytometer
Software, version 2.1, Thermo Fisher Scientific Inc.) was used to
quantitatively analyze the rate of AVO generation.
Detection of autophagy marker
proteins, apoptosis-associated proteins and protein kinase B
(Akt)/mammalian target of rapamycin (mTOR) signaling protein
pathway expression using western blotting
After 48 h, cells treated with drugs as described
above were collected and left on ice for 30 min, and then sonicated
for 10 min for lysation. The cells were centrifuged at 2,400 × g,
12,000 rpm at 4°C for 15 min. Protein concentration was detected
using the protein assay kit (Quant-iT™ Protein Assay Kit, Thermo
Fisher Scientific Inc.). Loading buffer (2.5 µg/µl, 20 µl) was
added and the samples were boiled for 5 min to prepare protein
specimens. SDS-PAGE was performed using 20 µg protein/lane.
Proteins were then transferred to a nitrocellulose membrane.
Skimmed milk powder (5%) was used at room temperature for 1 h to
block the membrane. The membrane was treated with anti-LC3-I
(1:1,000; cat. no. PA1-16931), LC3-II (1:1,000; cat. no.
PA1-16931), mammalian target of rapamycin (mTOR; 1:1,000; cat. no.
PA1-518), phosphorylated (p)-mTOR (1:1,000; cat. no. 25-9718-42),
4E-binding protein 1 (4E-BP1; 1:250; cat. no. AHO1382), P70S6K
(1:500; cat. no. 710095), p-ribosomal protein S6 kinase (P70S6K;
1:500) and GAPDH (1:1,000; cat. no. MA5-15738) antibodies at 4°C
overnight. All the above antibodies were purchased from Thermo
Fisher Scientific Inc. Horseradish peroxidase-labeled secondary
antibody (1:3,000, cat. no. ab6721, Abcam. Cambridge, UK) was
applied at room temperature for 1 h. Enhanced chemiluminescence
reagent (Pierce ECL Western Blotting Substrate kit, cat. no. 32106,
Thermo Fisher Scientific Inc.) was added to analyze specimens using
a chemiluminescence imaging system (Alliance MINI HD9 AUTO Western
Blot Imaging system, Biocompare, San Francisco, CA, USA). Western
blots were semi-quantified using Quantity One (version 4.6.2;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) using three
different experimental results.
Statistical analysis
Results are presented as the mean ± standard
deviation. SPSS software (version 13.0; SPSS, Inc., Chicago, IL,
USA) was used for statistical analyses. An unpaired t-test was used
to compare differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of TMZ and RAPA on the
proliferation and survival of U251 glioma cells
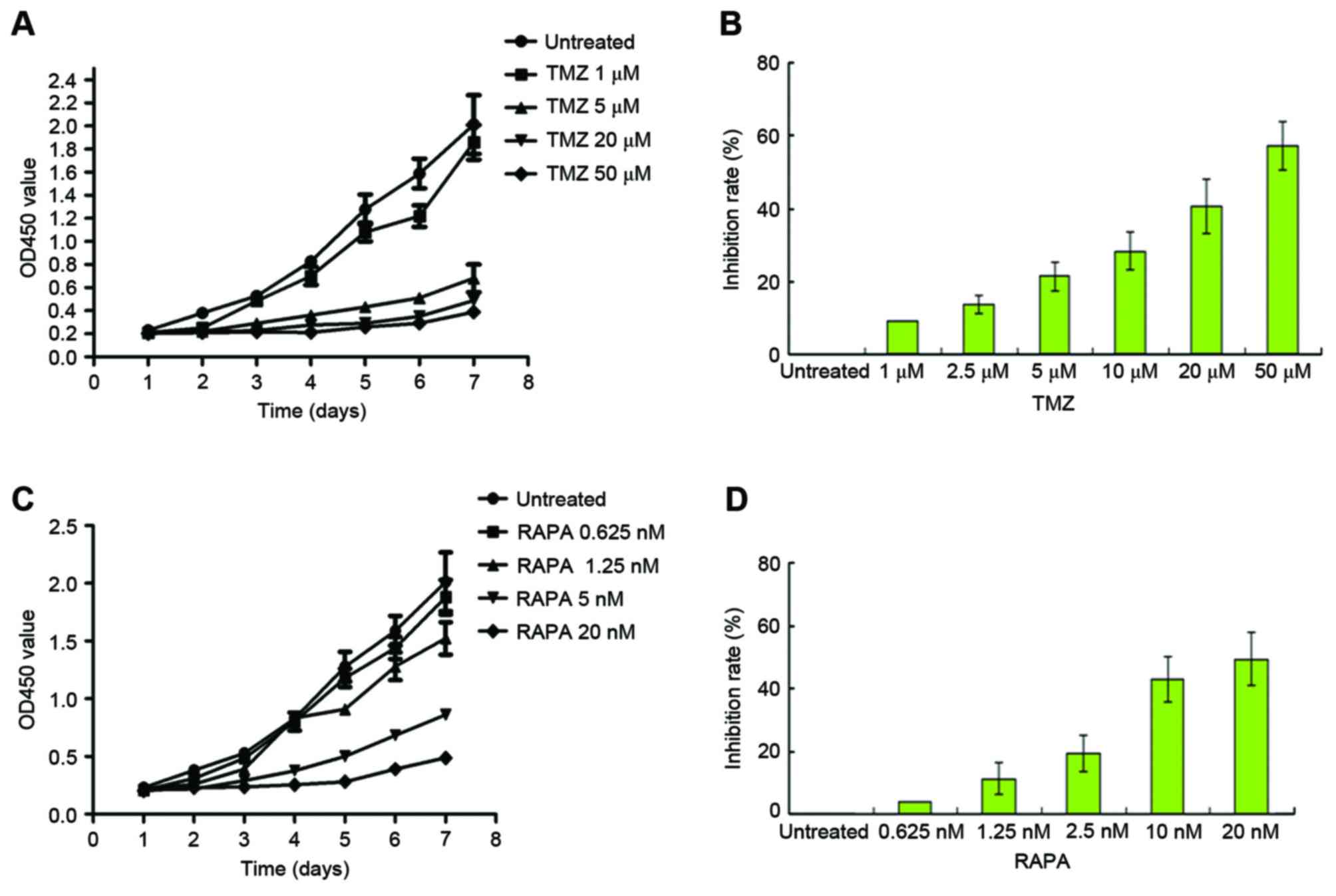
Following treatment with TMZ, U251 cell growth was
increased in a dose-dependent manner (Fig. 1A). Growth of the 5 µM TMZ-treated
group was markedly inhibited by the fourth day (P<0.05, vs. the
untreated cells). A total of 48 h following treatment with TMZ, the
half maximal inhibitory rate (IC50) of TMZ was
calculated. The IC50 of TMZ was 22.5±3.23 M, as
illustrated in Fig. 1B. Following
treatment with mTOR inhibitor RAPA, the growth of U251 cells was
inhibited in a dose-dependent manner (Fig. 1C, P<0.05, vs. the untreated cells).
The IC50 of RAPA was 12.93±1.36 nM, as illustrated in
Fig. 1D.
Effect of TMZ combined with RAPA on
the survival of U251 glioma cells
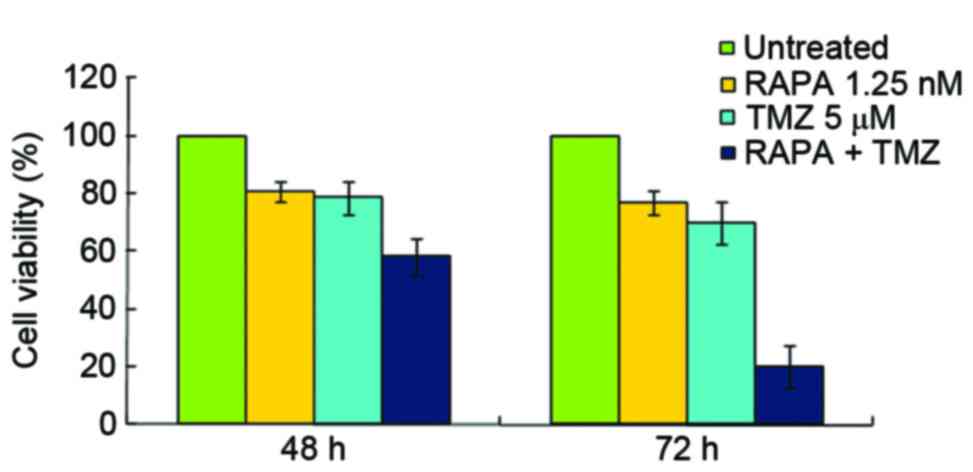
The present study demonstrated that the inhibitory
rates of 5 µM TMZ and 1.25 nM RAPA were 21.36 and 19.23%,
respectively (Fig. 2). The inhibitory
rate of 50 µM TMZ on U251 cell growth was 57.27%, but it showed
strong cytotoxicity (data not shown). This is consistent with the
results of a previous report (16).
In the present study, 5 µM TMZ combined with 1.25 nM RAPA was used
in order to reduce the cytotoxic effects of TMZ. The results
obtained from the CCK-8 assay demonstrated that the inhibitory
effect of TMZ combined with RAPA on cell growth was stronger
compared with TMZ alone (P<0.05, in 72 h). The IC50
of TMZ and RAPA combined was notably lower compared with TMZ alone
(10.35±2.81 µM vs. 22.5±3.23 µM, respectively; Fig. 2).
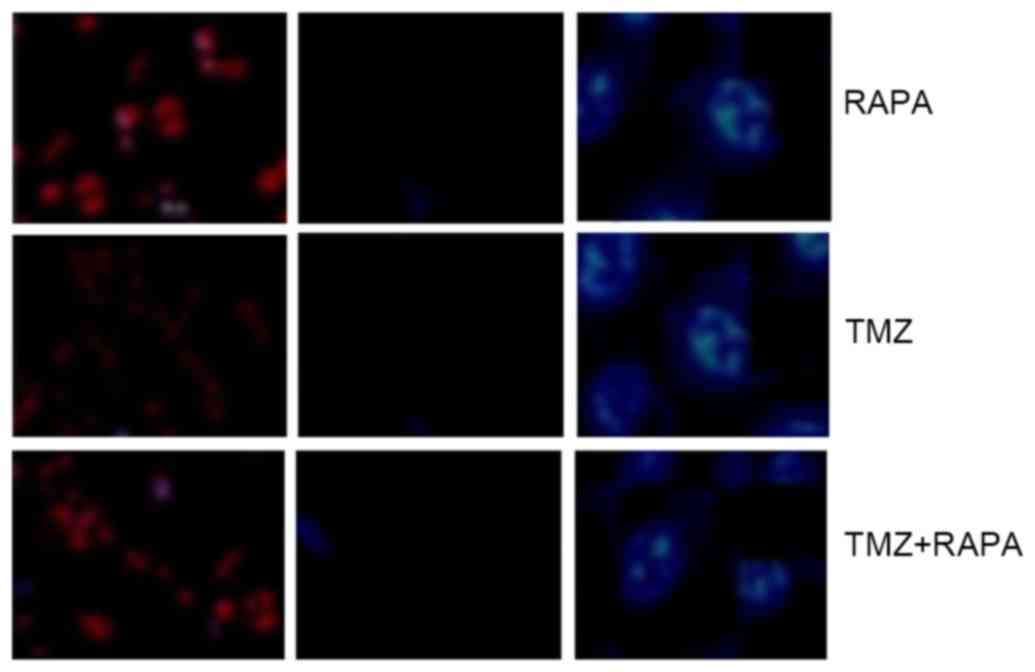
Effect of TMZ combined with RAPA on
the apoptosis of U251 glioma cells
The high blue: low red apoptotic cells shows no
significant difference, which indicates that the
apoptosis-inhibiting effect of TMZ combined with RAPA was not
notable compared with TMZ alone (Fig.
3).
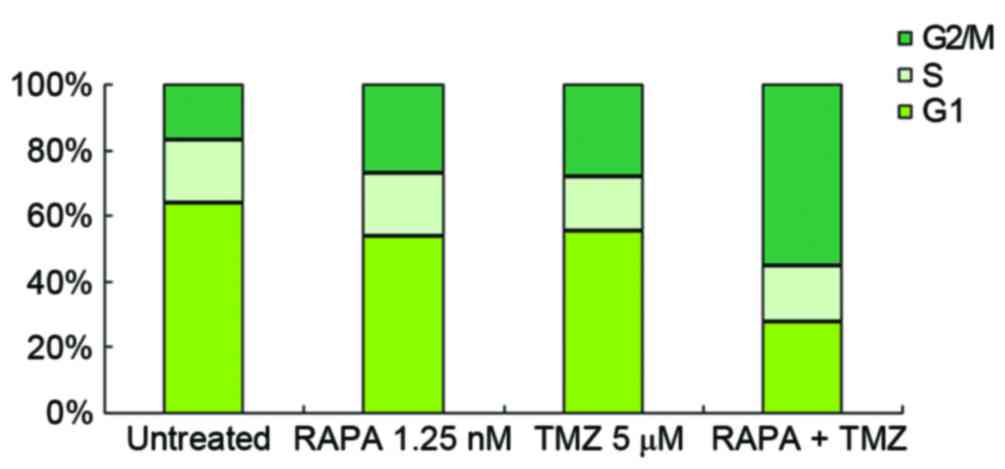
Effect of TMZ combined with RAPA on
the cell cycle distribution of U251 glioma cells
U251 cells were treated with 5 µM TMZ and/or 1.25 nM
RAPA. Compared with the blank control group, the number of cells in
G1 decreased and the number of cells in G2/M
increased in the groups treated with 5 µM TMZ or 1.25 nM RAPA
alone. There was no change in S stage cell number (Fig. 4). Following treatment with 5 µM TMZ
combined with 1.25 nM RAPA, G2/M stage cell number was
notably higher (61.07±2.37) compared with the groups treated with
TMZ (31.07±1.39) or RAPA (27.07±1.82) alone (P<0.05, vs. the
other groups; Fig. 4).
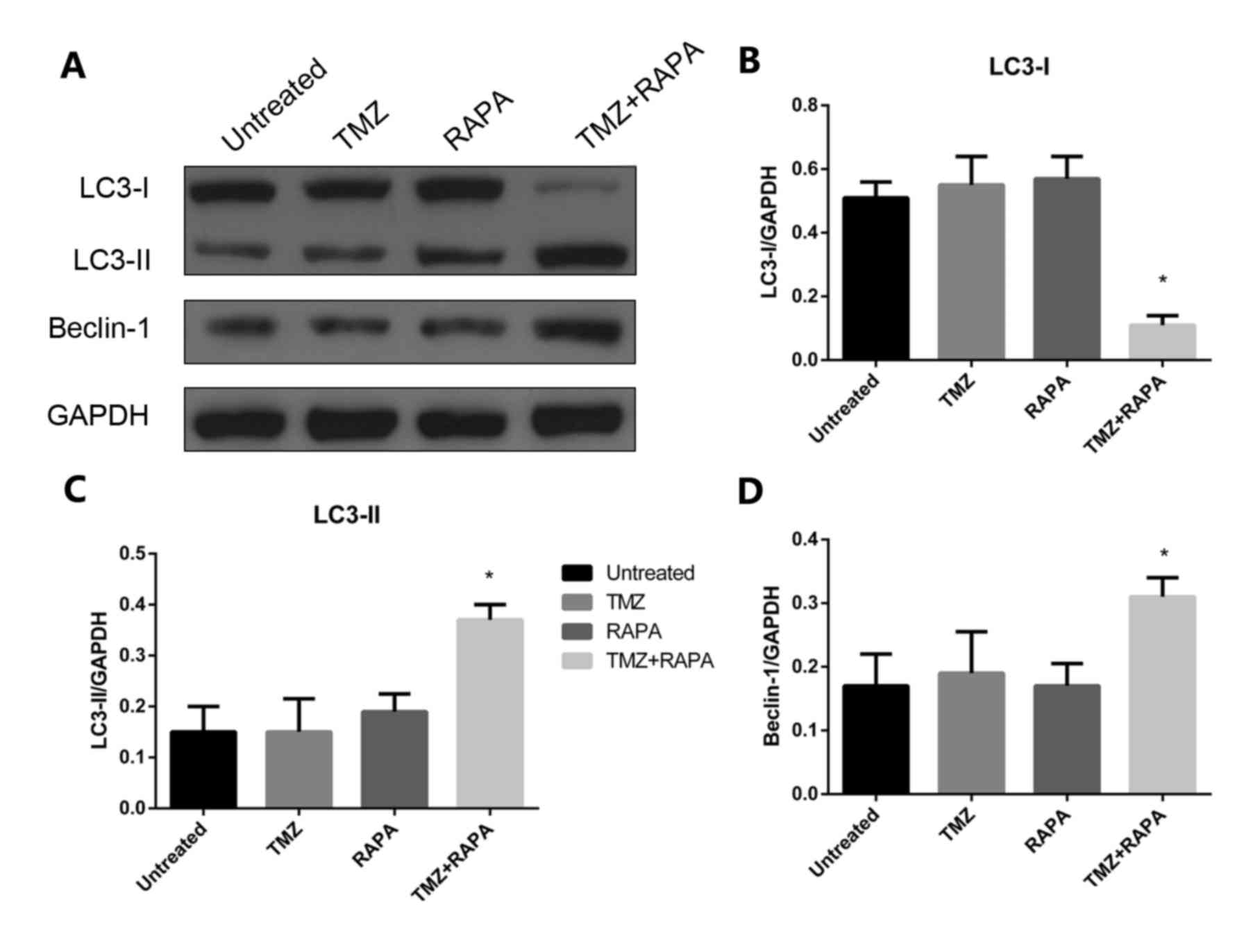
Effect of TMZ combined with RAPA on
the autophagy of U251 glioma cells
In the present study, 5 µM TMZ combined with 1.25 nM
RAPA was used to reduce the cytotoxic effects of TMZ. Following a
24 h treatment, the expression of autophagy-related protein LC3-II
was notably increased compared with the control group (P<0.05;
Fig. 5). There was no significant
change in LC3-II expression. The Beclin-1 gene is a congener of
yeast autophagy-related genes in mammals and an autophagy regulator
gene. Expression of Beclin-1 protein was notably increased
following treatment with TMZ combined with RAPA (P<0.05;
Fig. 5A and D).
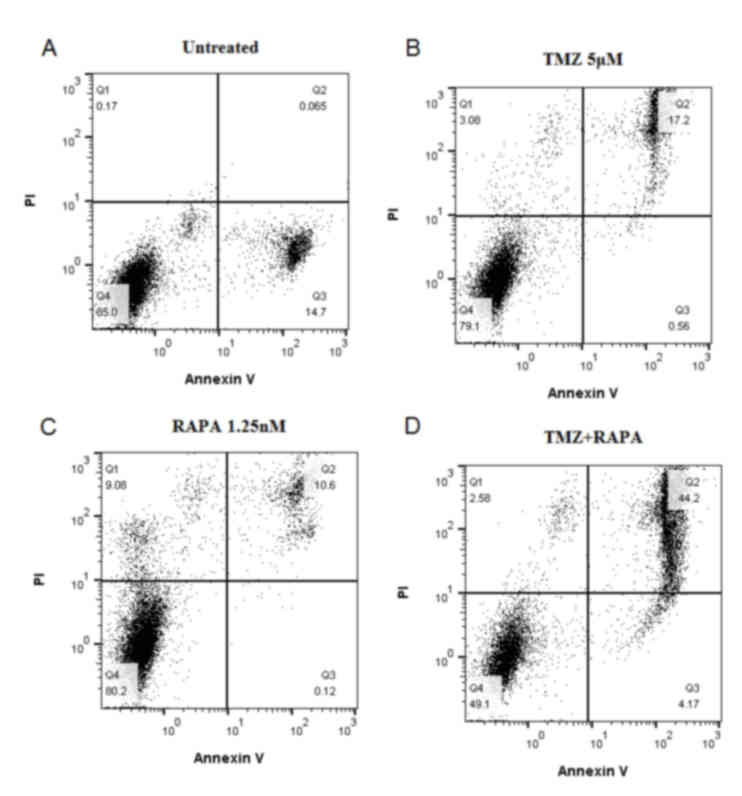
Detection of U251 AVO generation by AO
staining
AO staining is used to analyze
autophagy mechanisms at a molecular level
The results from AO staining (Fig. 6) identified no AVO accumulation in the
cytoplasm of the control group. The rate of AVO accumulation was
17.2% following treatment with 5 µM TMZ alone, which was
significantly increased compared with the control group (0.065%;
P<0.05). A similar result was seen following treatment with RAPA
alone (AVO accumulation rate, 10.6%). This indicates that treatment
with TMZ or RAPA alone at low concentrations does not effectively
induce AVO production in U251 cells. However, the rate of AVO
accumulation was notably increased in the cytoplasm of the group
treated with TMZ combined with RAPA (44.2%) compared with the
groups treated with TMZ (17.2%) or RAPA (10.6%) alone (P<0.05;
Fig. 6).
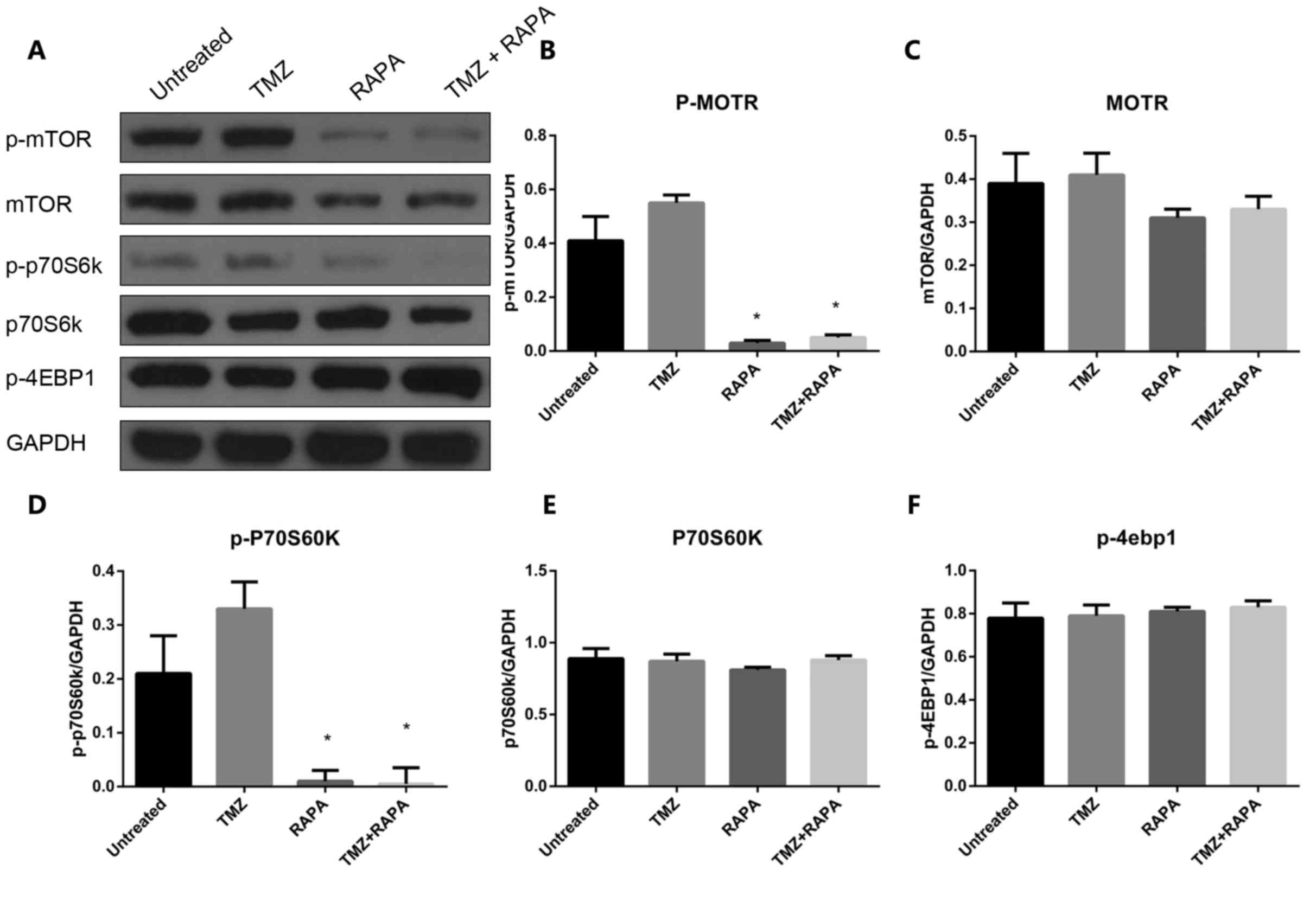
Effect of TMZ combined with RAPA on
the autophagy of U251 cells through the mTOR signaling pathway
In order to investigate the molecular mechanisms
underlying the induction of autophagy in U251 cells treated with
TMZ combined with RAPA, the expression and activation of important
components of the mTOR signaling pathway, such as p-mTOR, p-p70S6K
and P-4EBP1, was measured following treatment with TMZ alone, RAPA
alone or TMZ combined with RAPA. The results demonstrated that the
expression of p-mTOR and p-p70S6 significantly decreased in the
combination treatment group compared with the groups treated with
TMZ or RAPA alone (P<0.05, Fig.
7). A similar trend was seen in the expression of mTOR
(P<0.05, Fig. 7A and C). There was
no notable change in the expression of p70S6K and P-4EBP1 in the
combination treatment group compared with the single treatment
groups (P>0.05, Fig. 7E and F).
These results indicate that TMZ and RAPA-induced autophagy of U251
cells is associated with the mTOR signaling pathway.
Discussion
The three types of cell death include apoptosis,
autophagy and necrosis. The majority of chemotherapeutic drugs
induce the apoptosis of tumor cells. Autophagy is a type of
programmed cell death (type II). In autophagy, an autophagosome is
a monolayer or bilayer membrane that accumulates products to be
degraded in the cytoplasm and delivers them to the lysosomes, thus
forming autolysosomes. Autolysosomes carry out the digestion and
degradation of these products using multiple enzymes, in order to
achieve the metabolic needs of the cell and the renewal of specific
organelles (17–19). Autophagy serves an important role in
maintaining cell homeostasis. In certain tumors, inducing autophagy
can significantly inhibit growth and proliferation (20,21). Using
AO staining, the present study demonstrated that treatment with TMZ
alone induces the generation of AVOs in U251 cells in a
dose-dependent manner. The IC50 of TMZ in U251 cells was
22.5±3.23 µM; however, this concentration is highly toxic and known
to induce side effects in patients (20). Low concentrations of RAPA (≤2 nM)
strengthened TMZ-induced autophagic cell death; thus, the dosage of
TMZ was able to be decreased (20).
The combined use of RAPA and TMZ increased the generation of AVOs
in U251 glioma cells. As the primary indicator of autophagic cell
death is the generation of AVOs, these results indicate that the
combination of these two drugs may be used to synergistically
inhibit glioma cell growth.
The induction of autophagy has been associated with
a series of evolutionarily conserved gene products that were first
discovered in yeast and named autophagy-related gene (Atg)
proteins. In mammals, LC3-I is an autophagic vacuolar protein that
is isogenous with Atg 8 (22). During
cell autophagy, LC3-I is cleaved to produce LC3-II, which serves an
important role in AVO generation and is regarded as a molecular
marker of autophagic cell death (23,24). TMZ
induces autophagy in other ways (25). Zou et al (26) identified that TMZ inhibited the growth
of glioma cells and decreased the number of cells in S and
G2/M, leading to atypical cell apoptosis. The present
study identified that the use of TMZ alone to treat U251 cells
induces a slight increase in autophagy-related proteins and may
induce the apoptosis of glioma cells to a certain extent, which is
consistent with a previous study (15).
mTOR is a central regulator of cell growth,
proliferation, survival, migration, self-renewal and cell cycle
progression (27–29). The downstream effectors of mTOR
include eukaryotic translation initiation factor 4E-binding protein
1 and P70S6K. These two downstream effectors control expression of
cyclin D1 and cyclin-dependent kinase in eukaryotes (30–32).
Thoury et al (33) combined
RAPA derivative RAD001 with AEE788 (an epidermal growth factor
receptor/vascular endothelial growth factor receptor double
tyrosine kinase inhibitor) to treat D54MG glioma in a murine model.
This combination was demonstrated to significantly inhibit tumor
cell growth and proliferation, and the median survival time of the
mice was significantly longer in the combined treatment group
compared with the groups treated with AEE788 or RAD001 alone. Wang
et al (34) demonstrated that
combining RAPA with an inhibitor of the mTOR upstream regulator
molecules phosphoinositide 3-kinase and Akt significantly
sensitized cells to RAPA-mediated autophagy and resulted in
radiosensitization.
The present study demonstrated that
autophagy-associated protein concentrations in U251 cells markedly
increased following treatment with TMZ combined with RAPA.
Furthermore, the number of G2/M stage cells
significantly increased in the combined treatment group compared
with the single treatment groups. AO staining identified that the
amount of AVOs increased in cells treated with TMZ combined with
RAPA, indicating that the combination promotes autophagic cell
death. These results suggest that RAPA may be combined with other
drugs or therapies to effectively inhibit the growth and
proliferation of glioma cells.
The results of the current study demonstrated that
RAPA combined with TMZ induces the expression of Beclin-1 and
LC3-II in U251 cells. These proteins serve important roles in
autophagy. Beclin-1 is upregulated by certain autophagy-inducing
agents, such as the histone deacetylase inhibitor suberoylanilide
hydroxamic acid-d5. The findings of the present study are
consistent with those reported by Ye et al (35), in which small interfering RNA
knockdown of Beclin-1 expression resulted in the inhibition of
autophagic cell death.
To conclude, the results of the present study
indicate that a low concentration of RAPA significantly strengthens
TMZ-induced autophagic death of U251 cells, thus providing a novel
therapeutic approach for the treatment of patients with malignant
glioma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81270039 and
30901538), the Chinese Postdoctoral Science Foundation (grant no.
2013M530388) and the Chongqing Postdoctoral Science Foundation
(grant no. Xm201341).
References
|
1
|
Strowd RE III, Holdhoff M and Grossman SA:
Chemotherapy for treatment of grade II gliomas. Oncology (Williston
Park). 28:1036–1043. 2014.PubMed/NCBI
|
|
2
|
Pedeutour-Braccini Z, Burel-Vandenbos F,
Gozé C, Roger C, Bazin A, Costes-Martineau V, Duffau H and Rigau V:
Microfoci of malignant progression in diffuse low-grade gliomas:
Towards the creation of an intermediate grade in glioma
classification? Virchows Arch. 466:433–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chamberlain MC: Glioblastoma in the
Elderly. Current Understanding and Treatment of Gliomas. 1–170.
2015.
|
|
4
|
Friedmann-Morvinski D: Glioblastoma
heterogeneity and cancer cell plasticity. Crit Rev Oncog.
19:327–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Zhao HY, Zhang FC, Sun Y, Xiong ZY
and Jiang XB: Dendritic cell-based vaccine for the treatment of
malignant glioma: A systematic review. Cancer Invest. 32:451–457.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Altieri R, Agnoletti A, Quattrucci F,
Garbossa D, Specchia FM Calamo, Bozzaro M, Fornaro R, Mencarani C,
Lanotte M, Spaziante R and Ducati A: Molecular biology of gliomas:
Present and future challenges. Transl Med UniSa. 10:29–37.
2014.PubMed/NCBI
|
|
7
|
Simonetti G, Gaviani P, Innocenti A,
Botturi A, Lamperti E and Silvani A: Update on treatment strategies
for anaplastic glioma: A review of literature. Neurol Sci.
35:977–981. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okita Y, Nonaka M, Umehara T, Kanemura Y,
Kodama Y, Mano M and Nakajima S: Efficacy of temozolomide and
bevacizumab for the treatment of leptomeningeal dissemination of
recurrent glioblastoma: A case report. Oncol Lett. 9:1885–1888.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalkan R, Atli Eİ, Özdemir M, Çiftçi E,
Aydin HE, Artan S and Arslantaş A: IDH1 mutations is prognostic
marker for primary glioblastoma multiforme but MGMT
hypermethylation is not prognostic for primary glioblastoma
multiforme. Gene. 554:81–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Wang SX, Ma JW, Li HY, Ye JC, Xie
SM, Du B and Zhong XY: EGCG inhibits properties of glioma stem-like
cells and synergizes with temozolomide through downregulation of
P-glycoprotein inhibition. J Neurooncol. 121:41–52. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Turner KM, Sun Y, Ji P, Granberg KJ,
Bernard B, Hu L, Cogdell DE, Zhou X, Yli-Harja O and Nykter M:
Genomically amplified Akt3 activates DNA repair pathway and
promotes glioma progression. Proc Natl Acad Sci USA. 112:pp.
3421–3426. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barber NA and Ganti AK: Pulmonary
toxicities from targeted therapies: A review. Target Oncol.
6:235–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuo PL, Hsu YL and Cho CY: Plumbagin
induces G2-M arrest and autophagy by inhibiting the AKT/mammalian
target of rapamycin pathway in breast cancer cells. Mol Cancer
Ther. 5:3209–3221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zangari M, Cavallo F and Tricot G:
Farnesyltransferase inhibitors and rapamycin in the treatment of
multiple myeloma. Curr Pharm Biotechnol. 7:449–453. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang JH, Ma ZX, Huang GH, Xu QF, Xiang Y,
Li N, Sidlauskas K, Zhang EE and Lv SQ: Downregulation of HIF-1a
sensitizes U251 glioma cells to the temozolomide (TMZ) treatment.
Exp Cell Res. 343:148–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kouroussis C, Vamvakas L, Vardakis N,
Kotsakis A, Kalykaki A, Kalbakis K, Saridaki Z, Kentepozidis N,
Giassas S and Georgoulias V: Continuous administration of daily
low-dose temozolomide in pretreated patients with advanced
non-small cell lung cancer: A phase II study. Oncology. 76:112–117.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu H, Cao Y, Tong T, Shi J, Zhang Y, Yang
Y and Liu C: Autophagy in atherosclerosis: A phenomenon found in
human carotid atherosclerotic plaques. Chin Med J (Engl).
128:69–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sheng R and Qin ZH: The divergent roles of
autophagy in ischemia and preconditioning. Acta Pharmacol Sin.
36:411–420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gewirtz DA: Autophagy and senescence in
cancer therapy. J Cell Physiol. 229:6–9. 2014.PubMed/NCBI
|
|
20
|
Morselli E, Galluzzi L, Kepp O, Vicencio
JM, Criollo A, Maiuri MC and Kroemer G: Anti- and pro-tumor
functions of autophagy. Biochim Biophys Acta. 1793:1524–1532. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kondo Y and Kondo S: Autophagy and cancer
therapy. Autophagy. 2:85–90. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mareninova O, Jail W, Elperin J, Lotshaw
E, Pimiental M, Reicher B, Gukovsky I and Gukovskaya A: LC3
overexpression perturbs pancreatic acinar cell homeostasis and
alters pancreatitis responses. FASEB J. 30 1 Suppl 920:S122016.
|
|
23
|
Xiong Y, Yepuri G, Forbiteh M, Yu Y,
Montani JP, Yang Z and Ming XF: ARG2 impairs endothelial autophagy
through regulation of mTOR and PRKAA/AMPK signaling in advanced
atherosclerosis. Autophagy. 10:2223–2238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo L, Lu AM, Wang Y, Hong A, Chen Y, Hu
J, Li X and Qin ZH: Chronic resistance training activates autophagy
and reduces apoptosis of muscle cells by modulating IGF-1 and its
receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp
Gerontol. 48:427–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang MC, Loh JK, Li YY, Huang WS, Chou CH,
Cheng JT, Wang YT, Lieu AS, Howng SL, Hong YR and Chou AK: Bcl2L12
with a BH3-like domain in regulating apoptosis and TMZ-induced
autophagy: A prospective combination of ABT-737 and TMZ for
treating glioma. Int J Oncol. 46:1304–1316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou Y, Wang Q, Li B, Xie B and Wang W:
Temozolomide induces autophagy via ATM-AMPK-ULK1 pathways in
glioma. Mol Med Rep. 10:411–486. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng DM, Bian Z, Furuya N, Trejo JA
Oliva, Takeda-Ezaki M, Takahashi K, Hiraoka Y, Mineki R, Taka H and
Ikeda S: A treadmill exercise reactivates the signaling of the
mammalian target of rapamycin (mTOR) in the skeletal muscles of
starved mice. Biochem Biophys Res Commun. 456:519–526. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sarkar S: Regulation of autophagy by
mTOR-dependent and mTOR-independent pathways: Autophagy dysfunction
in neurodegenerative diseases and therapeutic application of
autophagy enhancers. Biochem Soc Trans. 41:1103–1130. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kubrusly MS, Corrêa-Giannella ML,
Bellodi-Privato M, de Sá SV, de Oliveira CP, Soares IC, Wakamatsu
A, Alves VA, Giannella-Neto D, Bacchella T, et al: A role for
mammalian target of rapamycin (mTOR) pathway in non-alcoholic
steatohepatitis related-cirrhosis. Histol Histopathol.
25:1123–1131. 2010.PubMed/NCBI
|
|
30
|
Zhang J, Cao J, Weng Q, Wu R, Yan Y, Jing
H, Zhu H, He Q and Yang B: Suppression of hypoxia-inducible factor
1α (HIF-1α) by tirapazamine is dependent on eIF2α phosphorylation
rather than the mTORC1/4E-BP1 pathway. PLoS One. 5:e139102010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guan L, Song K, Pysz MA, Curry KJ, Hizli
AA, Danielpour D, Black AR and Black JD: Protein kinase C-mediated
downregulation of cyclin D1 involves activation of the
translational repressor 4E-BP1 via a phosphoinositide
3-kinase/Akt-independent, protein phosphatase 2A-dependent
mechanism in intestinal epithelial cells. J Biol Chem.
282:14213–14225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fan QW and Weiss WA: Targeting the
RTK-PI3K-mTOR axis in malignant glioma: Overcoming resistance. Curr
Top Microbiol Immunol. 347:279–96. 2010.PubMed/NCBI
|
|
33
|
Thoury A, Descatoire V, Kotelevets L,
Kannengiesser C, Bertrand G, Theou-Anton N, Frey C, Genestie C,
Raymond E, Chastre E, et al: Evidence for different expression
profiles for c-Met, EGFR, PTEN and the mTOR pathway in low and high
grade endometrial carcinomas in a cohort of consecutive women.
Occurrence of PIK3CA and K-Ras mutations and microsatellite
instability. Histol Histopathol. 29:1455–1466. 2014.PubMed/NCBI
|
|
34
|
Wang S, Wu M, Yao G, Zhang J and Zhou J:
The cytoplasmic tail of FPC antagonizes the full-length protein in
the regulation of mTOR pathway. PLoS One. 9:e956302014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye LX, Yu J, Liang YX, Zeng JS, Huang RX
and Liao SJ: Beclin 1 knockdown retards re-endothelialization and
exacerbates neointimal formation via a crosstalk between autophagy
and apoptosis. Atherosclerosis. 237:146–154. 2014. View Article : Google Scholar : PubMed/NCBI
|