Introduction
Despite advances in cancer treatments, pancreatic
cancer remains one of the most lethal types of malignant tumor.
Pancreatic ductal adenocarcinoma (PDAC) is a major histological
subtype of pancreatic cancer, comprising 90% of all cases, and is
associated with a high mortality rate owing to its aggressive
growth and high metastatic rate (1).
Surgical treatment offers the only possible cure for PDAC; however,
80% of PDAC patients are inoperable at diagnosis, and the most
recently reported overall survival rate for patients with
pancreatic cancer is 8% (2). Notably,
by the year 2030, pancreatic cancer is expected to become the
second-leading cause of cancer-related deaths in the United States,
trailing only lung cancer (3).
A cancer stem cell (CSC) is defined as ‘a cell
within a tumor that possesses the capacity to self-renew and to
cause the heterogeneous lineages of cancer cells that comprise the
tumor’ (4). CSCs constitute a small
proportion of cancer cells and possess high tumorigenic potential
in vivo (5). CSCs, located at
the apex of the hierarchy, have the ability to undergo symmetric
and asymmetric cell division, enabling them to both self-renew and
give rise to the ‘differentiated’ tumor cell progeny that form the
bulk of a tumor. The ‘stem cell theory’ of cancer implies that CSCs
are responsible for tumor initiation, growth, and even metastasis
(6). Because CSCs chiefly remain in
the G0 phase of the cell cycle, they are less sensitive to
radiation and chemotherapy than proliferating cells (5). As such, they are believed to be
responsible for tumor recurrence after completion of adjuvant
therapy. Researchers therefore regard CSCs as an important
potential cancer therapeutic target (7,8).
Three major methods are employed to identify CSCs:
Detection of CSC-specific markers, detection of side population
(SP) cells, and the sphere formation assay. In the latter assay,
the CSCs of PDAC cells form floating colonies when cultivated in
ultra-low-attachment dishes (9–11).
Notably, after transplantation to immunodeficient mice,
sphere-forming cells show higher tumor formation rates than
adherent cells (12). In a previous
study, we reported that nestin, a pancreatic CSC marker, is more
highly expressed in the spheres of three PDAC cell lines than in
nonsphere cells (13). Moreover,
pancreatic cancer cells derived from metastatic foci of
immunodeficient mice form a greater number of spheres in
low-attachment plates than do their primary tumor counterparts
(14). These findings suggest that
CSCs are enriched in the spheres and play an important role in the
aggressiveness of PDAC. Nevertheless, a thorough study of CSCs and
the differentiation of cells from CSCs within spheres has yet to be
conducted at the ultramicroscopic level.
In the present study, we therefore analyzed the
surface and cross-sections of spheres isolated from a human PDAC
cell line, PANC-1, via scanning electron microscopy (SEM) and
transmission electron microscopy (TEM). To our knowledge, this is
the first report of the ultramicroscopic features of cancer spheres
consisting of cells that exhibit varying surface morphologies.
Materials and methods
Human pancreatic cancer cell line
The human pancreatic cancer cell line PANC-1 was
obtained from the Cell Resource Center for Biomedical Research
Center at the Institute of Development, Aging, and Cancer of Tohoku
University (Sendai, Japan). PANC-1 cells were grown in the RPMI
1640 medium supplemented with 10% fetal bovine serum (FBS) at 37°C
in a humidified atmosphere containing 5% CO2.
Sphere-forming assays
For sphere formation, PANC-1 cells
(103/well) were seeded in 24-well ultra-low-attachment
plates (Corning Inc., Kennebunk, ME, USA) in RPMI-1640 supplemented
with 10% FBS. After 7 days, the spheres were photographed using a
phase contrast microscope (Eclipse TS-100; Nikon Co., Ltd., Tokyo,
Japan). The spheres were then aspirated using micropipettes and
placed into microcentrifuge tubes.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells, using an RNeasy
Plus Mini kit (Qiagen, Venlo, The Netherlands), and subsequently
reverse-transcribed to cDNA, using a ReverTra Ace® qPCR
RT kit (Toyobo, Osaka, Japan) according to the manufacturer's
protocol. qPCR was performed using a Power SYBR® Green
kit (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and a StepOnePlus™ real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Expression of β-actin
was considered an internal control. The primer sets used for qPCR
analyses are listed in Table I. Gene
expression measurements were performed in triplicate.
 | Table I.List of primer sets for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
List of primer sets for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| Nestin |
TCCTGCTGTAGATGCAGAGATCAG |
ACCCTGTGTCTGGAGCAGAGA |
| Sox2 | TGCGAGCGCTGCACAT |
TCATGAGCGTCTTGGTTTTCC |
| CD44v9 |
AGCAGAGTAATTCTCAGAGCTT |
TGCTTGATGTCAGAGTAGAAGT |
| β-actin |
GGTCATCACCATTGGCAATGAG |
TACAGGTCTTTGCGGATGTCC |
TEM
Spheres from PANC-1 cells were fixed with 2.5%
glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), then postfixed
for 1 h with 2% OsO4 dissolved in distilled water,
dehydrated in a graded series of ethanol solutions, and embedded in
Epon. Ultrathin sections were generated using an ultramicrotome and
stained with uranyl acetate and lead citrate for examination under
a transmission electron microscope (H-7500; Hitachi
High-Technologies, Tokyo, Japan).
SEM
Spheres from PANC-1 cells were fixed for 1 h with
2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at room
temperature and then incubated at 4°C overnight. The following day,
the glutaraldehyde solution was removed and the spheres were washed
with PBS. After complete dehydration via a graded ethanol series,
sphere samples suspended in 100% ethanol were placed onto a
Nanopercolator (JEOL Ltd., Tokyo, Japan) and air-dried, then coated
with a platinum layer using an MSP-1S sputter coater (Shinku
Device, Ibaraki, Japan) and examined and photographed using a
Phenom Pro X desktop scanning electron microscope (Phenom-World BV,
Eindhoven, The Netherlands) (15,16).
Statistical analysis
Quantitative data are presented as means ± standard
deviations. Differences between two groups were analyzed by
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. Computations were performed
using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA,
USA).
Results
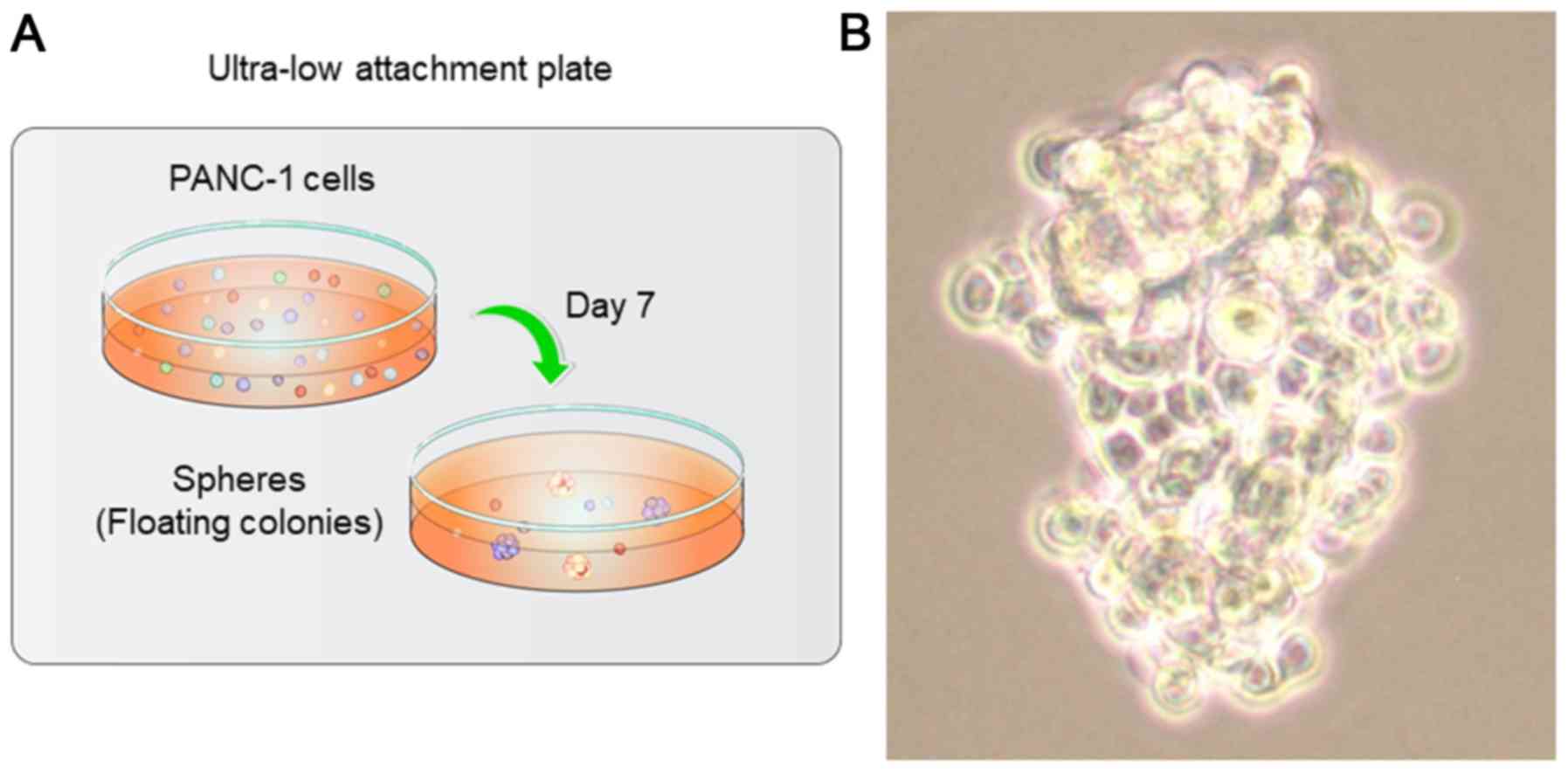
Spheres of PANC-1 cells in
ultra-low-attachment plates
After 7 days of incubation in ultra-low-attachment
dishes, we observed sphere formation in all 24-wells of each plate,
with each well containing approximately 10 spheres (Fig. 1A). Moreover, the PANC-1 cells
proliferated and formed irregular, oblong spheres. While certain
individual cells could be seen clearly (Fig. 1B, middle to lower parts), others
appeared grouped together, making their margins difficult to
distinguish under the phase contrast microscope (Fig. 1B, upper part).
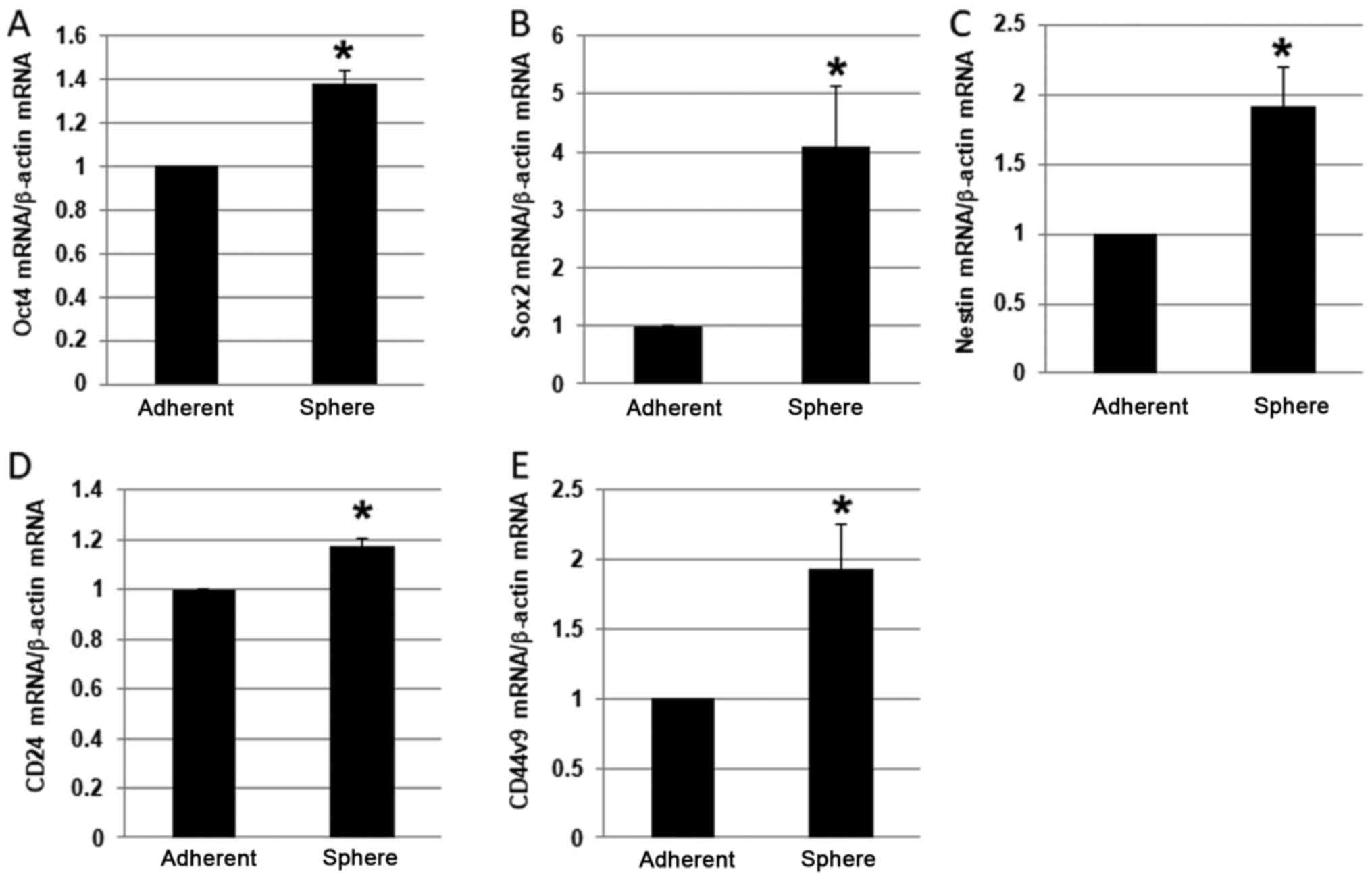
RT-qPCR
To confirm the stemness of the PANC-1 spheres, we
screened the spheres for expression of CSC markers, including Oct4,
sex determining region Y-box 2 (Sox2), nestin, CD24, and CD44v9
(7,17–19).
Notably, the mRNA expression level of each of these markers was
higher in PANC-1 spheres than in adherent cells (Fig. 2A-E). In particular, Sox2 was
expressed at 4-fold higher levels in spheres than in adherent
cells, which was the largest fold-difference observed.
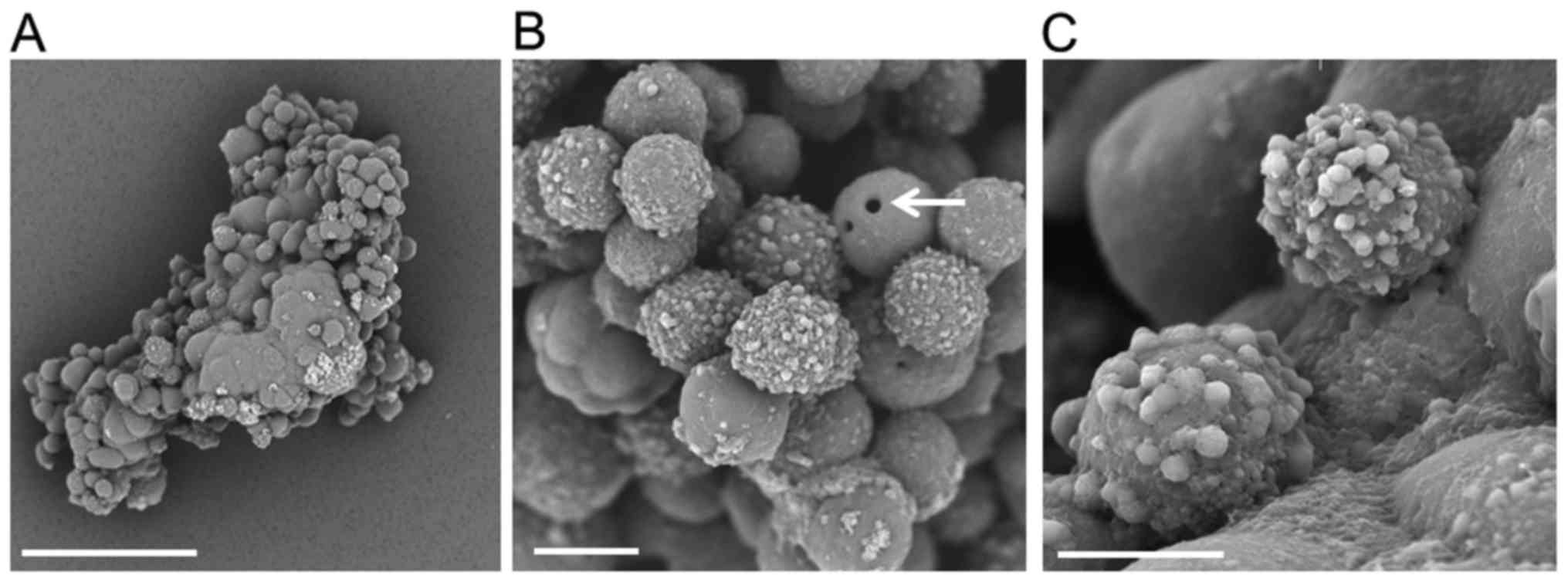
SEM analysis of spheres from PANC-1
cells
Low-magnification SEM imaging showed that the
spheres of PANC-1 cells exhibited an oval to club-like appearance.
The surface of the sphere was rugged, and some cells were fused.
The center was smooth, while the periphery contained both smooth
and protruding areas (Fig. 3A).
Additionally, at high-magnification, the spheres had a grape-like
appearance, with a mostly smooth surface and rough-surfaced PANC-1
cells (Fig. 3B). We observed a small
hole in the surface of certain PANC-1 cells (arrow), and some
sphere cells showed several irregular protrusions on their surfaces
(Fig. 3C), while others appeared to
be fused, with unclear margins.
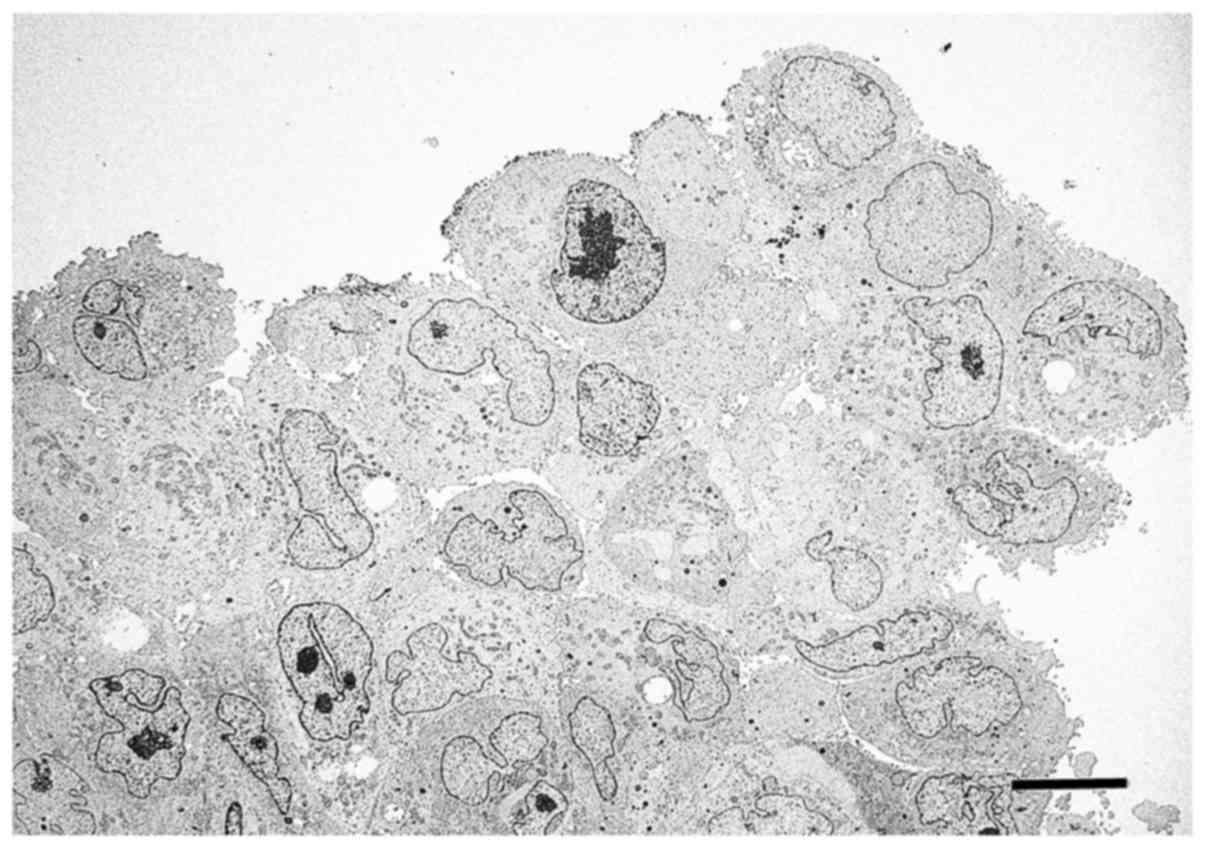
TEM analysis of spheres from PANC-1
cells
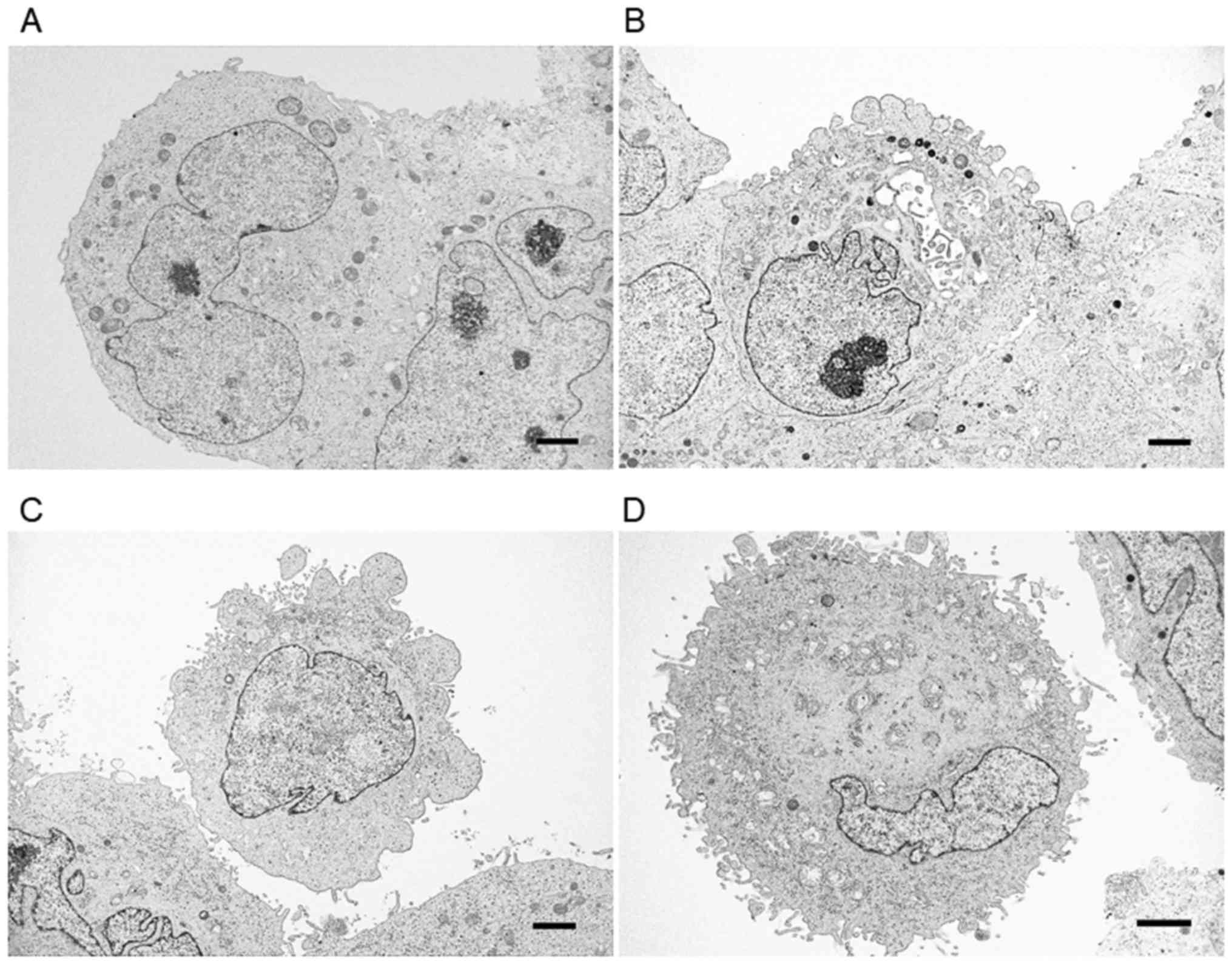
Low-magnification TEM indicated that PANC-1 cells
formed spheres with irregular-shaped nuclei and nucleoli, and that
the cell-to-cell border was not clear in some cells (Fig. 4). Additionally, there were small
clefts or cavities between PANC-1 cells in each sphere, and several
cells displayed irregular protrusions on their surface. The surface
of the PANC-1 cells in spheres was classified into four types:
Smooth surface (Fig. 5A), irregular
large protrusions (Fig. 5B),
protrusions and a small number of microvilli (Fig. 5C), and many microvilli located
throughout the entire cell surface (Fig.
5D). Most of the cells with smooth surfaces were located inside
the spheres and tightly attached to other cells. Conversely, those
with protrusions and microvilli, as well as those exhibiting large
numbers of microvilli throughout the surface (Fig. 5C and D), were located at the outside
of the spheres, with loose adhesion to other cancer cells.
Discussion
Spheres of PDAC cells represent round aggregates of
fused cells formed during the early stage of culture in
ultra-low-attachment plates, according to phase contrast
microscopic examination. Subsequently, these spheres grow and form
irregular, oblong to club-shaped floating colonies. There are
several reports of the electron microscopic analysis of cancer
spheres or spheroids, involving the hanging-drop method, culture on
a scaffold, or culture in low-attachment plates (20,21). SEM
images of PANC-1 cells in hanging-drop experiments show a ruffled
surface and lightly packed cells with deep pore-like structures
(22). The heterogeneous morphology
of PDAC spheres consisting of cancer cells is similar to that of
morphologically and functionally heterogeneous neurospheres
(consisting of neural stem cells and their progeny), which can
differentiate into glial cells, neurons, and oligodendrocytes
(23,24). The most immature clonogenic cells are
located in the core of the neurosphere, whereas progeny and
differentiated cells are located at the periphery (25,26). In
the present study, we found that the fused cell aggregates are
located in the core region of the PDAC spheres, while clearly
separated cancer cells exist around the spheres.
In this study, we used serum-containing medium
because PANC-1 cells most rapidly form large spheres under these
conditions. The spheres generated from these cells expressed high
mRNA levels of CSC markers, suggesting that they contain CSCs
(7,13,17–19). A
previous study showed that PANC-1 cells grown in a serum-containing
medium formed clusters that were heterogeneous for dye efflux,
while cells grown in the serum-free medium with supplements
commonly used to culture stem cells gave rise to colonies that
varied in their ability to efflux dye and respond to verapamil
(27). In neurospheres, mitotic cells
were found at the periphery but not in the inner part of the
spheres (23). Growth factors or
serum added to the medium might therefore play important roles in
the differentiation of the cancer cells on the outside of the
spheres, as well as the growth of cells in the spheres.
Our EM analyses detected four types of cancer cells
within PDAC spheres. Those with a smooth surface were located
inside the spheres and were fused to each other. In contrast, the
cells containing protrusions and/or microvilli on their surface
were localized at the periphery of the spheres. In a healthy human
pancreas, short microvilli are present on acinar cells and ductal
epithelial cells (28). Furthermore,
PDAC cells are comprised of epithelial cells that form lumens,
apical mucin granules, intermediate filaments, tight junctions, and
microvilli. In commonly used 2D culture, PANC-1 cells have
microvilli on their cell surface (29). SEM and TEM analyses in the present
study suggest that the cells with a smooth surface are CSCs of
PANC-1, while those with large protrusions, with protrusions and
some microvilli, and with large numbers of microvilli on their
surfaces represent distinct stages of the differentiation process
of pancreatic CSCs to non-CSCs. A recent study showed that lung
adenocarcinoma cells can divide into two cell types: Tumor cells
and support cells that form a part of a supporting microenvironment
known as the niche (30). Further
studies are needed to clarify the function of the different types
of pancreatic cancer cells in the spheres that were observed in our
study.
Here, we identified different types of cells present
in spheres formed by a human pancreatic cancer cell line, via EM
analysis. The cancer cells exhibited smooth surfaces, rounded
protrusions on the cell surface, surfaces containing protrusions
with a small number of microvilli, or the presence of large numbers
of microvilli across the entire cell surface. These findings
suggest that CSCs and differentiated non-CSCs have characteristic
morphologies. However, further studies are needed to clarify
correlations between cell surface features and cell types within
pancreatic cancer spheres.
Acknowledgements
The authors would like to thank Ms. Sanae Furusho,
Shoko Wada, Atsumi Ozaki and Mr. Hiroyuki Sugihara (Jasco
International Co., Ltd., Tokyo, Japan) for their technical
assistance with SEM. We thank Dr Seiichi Shinji (Nippon Medical
School), Dr Kimimasa Takahashi, M.A. Yuuki Shichi (Nippon
Veterinary and Life Science University), and Dr Naotaka
Izumiyama-Shimomura (Tokyo Metropolitan Institute of Gerontology)
for their helpful discussions. This study was supported in part by
JSPS KAKENHI (grant no. JP16K10613) to Dr Toshiyuki Ishiwata.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lonardo E, Hermann PC and Heeschen C:
Pancreatic cancer stem cells-update and future perspectives. Mol
Oncol. 4:431–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuda Y, Kure S and Ishiwata T: Nestin
and other putative cancer stem cell markers in pancreatic cancer.
Med Mol Morphol. 45:59–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishiwata T: Cancer stem cells and
epithelial-mesenchymal transition: Novel therapeutic targets for
cancer. Pathol Int. 66:601–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gou S, Liu T, Wang C, Yin T, Li K, Yang M
and Zhou J: Establishment of clonal colony-forming assay for
propagation of pancreatic cancer cells with stem cell properties.
Pancreas. 34:429–435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaviraghi M, Tunici P, Valensin S, Rossi
M, Giordano C, Magnoni L, Dandrea M, Montagna L, Ritelli R, Scarpa
A and Bakker A: Pancreatic cancer spheres are more than just
aggregates of stem marker-positive cells. Biosci Rep. 31:45–55.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin T, Wei H, Gou S, Shi P, Yang Z, Zhao G
and Wang C: Cancer stem-like cells enriched in Panc-1 spheres
possess increased migration ability and resistance to gemcitabine.
Int J Mol Sci. 12:1595–1604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Michishita M, Akiyoshi R, Yoshimura H,
Katsumoto T, Ichikawa H, Ohkusu-Tsukada K, Nakagawa T, Sasaki N and
Takahashi K: Characterization of spheres derived from canine
mammary gland adenocarcinoma cell lines. Res Vet Sci. 91:254–260.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuda Y, Ishiwata T, Yoshimura H,
Yamashita S, Ushijima T and Arai T: Systemic administration of
small interfering RNA targeting human nestin inhibits pancreatic
cancer cell proliferation and metastasis. Pancreas. 45:93–100.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuda Y, Yoshimura H, Ueda J, Naito Z,
Korc M and Ishiwata T: Nestin delineates pancreatic cancer stem
cells in metastatic foci of NOD/Shi-scid IL2Rγ (null) (NOG) mice.
Am J Pathol. 184:674–685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Konings J, Hoving LR, Ariens RS,
Hethershaw EL, Ninivaggi M, Hardy LJ, de Laat B, Ten Cate H,
Philippou H and Govers-Riemslag JW: The role of activated
coagulation factor XII in overall clot stability and fibrinolysis.
Thromb Res. 136:474–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schurgers E, Moorlag M, Hemker C, Lindhout
T, Kelchtermans H and de Laat B: Thrombin generation in zebrafish
blood. PLoS One. 11:e01491352016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma N, Nanta R, Sharma J, Gunewardena
S, Singh KP, Shankar S and Srivastava RK: PI3K/AKT/mTOR and sonic
hedgehog pathways cooperate together to inhibit human pancreatic
cancer stem cell characteristics and tumor growth. Oncotarget.
6:32039–32060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herreros-Villanueva M, Zhang JS, Koenig A,
Abel EV, Smyrk TC, Bamlet WR, de Narvajas AA, Gomez TS, Simeone DM,
Bujanda L and Billadeau DD: SOX2 promotes dedifferentiation and
imparts stem cell-like features to pancreatic cancer cells.
Oncogenesis. 2:e612013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kiuchi S, Ikeshita S, Miyatake Y and
Kasahara M: Pancreatic cancer cells express CD44 variant 9 and
multidrug resistance protein 1 during mitosis. Exp Mol Pathol.
98:41–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Breslin S and O'Driscoll L: The relevance
of using 3D cell cultures, in addition to 2D monolayer cultures,
when evaluating breast cancer drug sensitivity and resistance.
Oncotarget. 7:45745–45756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishiwata T, Teduka K, Yamamoto T, Kawahara
K, Matsuda Y and Naito Z: Neuroepithelial stem cell marker nestin
regulates the migration, invasion and growth of human gliomas.
Oncol Rep. 26:91–99. 2011.PubMed/NCBI
|
|
22
|
Ware MJ, Colbert K, Keshishian V, Ho J,
Corr SJ, Curley SA and Godin B: Generation of homogenous
three-dimensional pancreatic cancer cell spheroids using an
improved hanging drop technique. Tissue Eng Part C Methods.
22:312–321. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bez A, Corsini E, Curti D, Biggiogera M,
Colombo A, Nicosia RF, Pagano SF and Parati EA: Neurosphere and
neurosphere-forming cells: Morphological and ultrastructural
characterization. Brain Res. 993:18–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gage FH: Mammalian neural stem cells.
Science. 287:1433–1438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ilieva M and Dufva M: SOX2 and OCT4
mRNA-expressing cells, detected by molecular beacons, localize to
the center of neurospheres during differentiation. PLoS One.
8:e736692013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suslov ON, Kukekov VG, Ignatova TN and
Steindler DA: Neural stem cell heterogeneity demonstrated by
molecular phenotyping of clonal neurospheres. Proc Natl Acad Sci
USA. 99:pp. 14506–14511. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhagwandin VJ and Shay JW: Pancreatic
cancer stem cells: Fact or fiction? Biochim Biophys Acta.
1792:248–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hruban RH, Pitman MB and Klimstra DS:
Ductal adenocarcinoma(Tumors of the Pancreas. Atlas of Tumor
Pathology, 4th series). Silverberg SG and Sobin LH: American
Registry of Pathology. Washington, DC: pp. 111–164. 2007
|
|
29
|
Lu Y, Onda M, Uchida E, Yamamura S, Yanagi
K, Matsushita A, Kobayashi T, Fukuhara M, Aida K and Tajiri T: The
cytotoxic effects of bile acids in crude bile on human pancreatic
cancer cell lines. Surg Today. 30:903–909. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tammela T, Sanchez-Rivera FJ, Cetinbas NM,
Wu K, Joshi NS, Helenius K, Park Y, Azimi R, Kerper NR, Wesselhoeft
RA, et al: A Wnt-producing niche drives proliferative potential and
progression in lung adenocarcinoma. Nature. 545:355–359. 2017.
View Article : Google Scholar : PubMed/NCBI
|