Introduction
Head and neck squamous cell carcinoma (HNSCC), the
sixth most common type of cancer in the world, occurs at various
sites, including the oral cavity, oropharynx, hypopharynx and
larynx (1). The most common risk
factors for HNSCC are tobacco use, betel quid chewing, alcohol
consumption and human papillomavirus (HPV) infection (2). Previous studies have identified the
distinct etiologies of HNSCC arising from different anatomical
locations (3,4). In cancer arising from the oropharynx,
such as oropharyngeal squamous cell carcinoma (OPSCC), HPV is the
major causative factor and it has been reported that the expression
of p16INK4A, an important tumor suppressor protein
encoded by the cyclin dependent kinase inhibitor 2A (CDKN2A)
gene, is a biomarker for HPV infection and indicates good patient
prognosis (5). By contrast, in cancer
arising from the non-oropharyngeal head and neck region, such as
non-oropharyngeal head and neck squamous cell carcinoma
(non-OPHNSCC), the roles of HPV infection and p16INK4A
expression have not been clearly defined. The causes of non-OPHNSCC
may be complex as environmental carcinogens, including alcohol,
tobacco and betel quid serve a role in tumor initiation and
progression (6). It has been
demonstrated that p16INK4A expression is a poor
surrogate biomarker of HPV infection (7) and is controversial for its prognostic
value in non-OPHNSCC (8). In Taiwan,
a country with a high prevalence of betel quid chewing, the
predictive value of p16INK4A expression for HPV
infection in non-OPHNSCC is low (9).
Inflammatory tumor microenvironments contribute to
the carcinogenesis and progression of HNSCC (10); however, few studies have investigated
the association between p16INK4A expression and tumor
inflammation or immunity. An association between
p16INK4A and inflammatory factors has been identified. A
previous study demonstrated that the expression of
p16INK4A may be inhibited by Toll-like receptors
(11). Furthermore, the expression of
alternate reading frame protein, which is associated with
macrophages surrounding the tumor, is correlated with
p16INK4A expression in pancreatic cancer (12). In addition, environmental carcinogens
damage normal mucosal cells in the upper aerodigestive tract due to
repeated inflammation and are correlated with gene polymorphisms
including CTLA4 or TNFα that are important in
determining the prognosis of patients with HNSCC (13,14).
However, the role of p16INK4A in non-OPHNSCC remains
unclear.
Programmed cell death 1-ligand 1 (PD-L1) is an
immune modulatory molecule in cancer cells that inhibits cytotoxic
T cell activity (15). The expression
of PD-L1, which belongs to the B7 superfamily of proteins, can be
induced in certain types of solid and hematological cancer. PD-L1
binds to programmed cell death protein 1 (PD-1) and cluster of
differentiation 80 in T cells in the tumor microenvironment to
modulate immunity. This is one of the mechanisms by which cancer
cells evade the immune system (16).
In non-OPHNSCC, interferon (INF)-α induces PD-L1 expression in
cancer cells via the protein kinase D isoform 2 (PKD2) pathway to
evade recognition by tumor antigen specific T cells (17). Studies have identified varying levels
of PD-L1 expression in human HNSCC tissues, ranging from 40–100%;
however, most of the data available pertain to OPSCC (18–20). PD-L1
expression may cause immune evasion of HPV, which in turn leads to
malignant transformation. Furthermore, it has been reported that
HPV-positive patients exhibit a higher expression of PD-L1 than
HPV-negative patients with OPSCC (19). However, in patients with non-OPHNSCC,
the expression of PD-L1 and p16INK4A, as well as their
association, remains unclear. Furthermore, the prognostic value of
PD-L1 in HNSCC has not been clearly established, as its expression
may not reflect the fluid interactions of PD-L1 to the dynamic
immune response in the tumor microenvironment (21). To the best of our knowledge, the
current study is the first to evaluate the expression of PD-L1 in
non-OPHNSCC and its association with p16INK4A
expression, as well as other clinicopathological characteristics.
The prognostic role of PD-L1 was also evaluated.
Patients and methods
Patients
Between January 2007 and August 2014, 106 patients
with non-OPHNSCC that was pathologically proven, at the Taipei
Veterans General Hospital (Taipei, Taiwan) were retrospectively
reviewed. Information regarding patient characteristics, including
patient age, sex, history of betel quid chewing, tobacco use,
alcohol consumption and treatment history was collected.
Information about the pathological characteristics of perineural
invasion, lymphovascular invasion, tumor emboli and extra-capsular
spread was also collected. Cancer staging was established according
to the 7th American Joint Committee on Cancer Staging Manual
(22). The current study was approved
by The Institutional Review Board of Taipei Veterans General
Hospital (TVGHTPE-2017-08-002BC). Since the current study was
retrospective, patient consent was waived.
Immunohistochemical (IHC) staining of
PD-L1 and p16INK4A
Tissue arrays (depth of 1.5 mm) were constructed as
described previously (23). Xylene
was used to deparaffinize the samples and serial dilutions of
alcohol (100, 95, 75 and 50%) were used to rehydrate the array
samples. Antigen retrieval was performed by placing samples in a
citrate buffer (pH 6.0) and heating to 121°C in an autoclave for 10
min. Following this, samples were bathed in the blocking agent, 3%
bovine serum albumin (BSA), for 30 min at room temperature. Samples
were then incubated overnight at 4°C with primary antibodies,
anti-PD-L1 (cat. no. 13684S; dilution, 1:200; Cell Signaling
Technology, Inc., Danvers, MA, USA) and a monoclonal anti-mouse
p16INK4A (cat. no. sc-81157; dilution, 1:100; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). By using MultiLink + HRP
label kits (Super Sensitive™ IHC Detection Systems; BioGenex
Laboratories, Inc., Fremont, CA, USA), samples were incubated with
secondary antibody (a mix of anti-mouse and anti-rabbit IgGs
conjugated to multiple biotin molecules) for 20 min at room
temperature. Subsequently, a horseradish peroxidase
(HRP)-conjugated streptavidin solution (Streptavidin/HRP complex;
Multi-Link Biogenex, BioGenex Laboratories) was used for incubation
for 20 min at room temperature. AEC substrates (cat. no. HK139-50K;
ready to use; BioGenex Laboratories, CA, USA) was used for staining
for 2 min at room temperature and the tissues were counterstained
with hematoxylin for 1 min at room temperature. The sections were
then examined by a light microscope (Eclipse 80i; Nikon
Corporation, Tokyo, Japan).
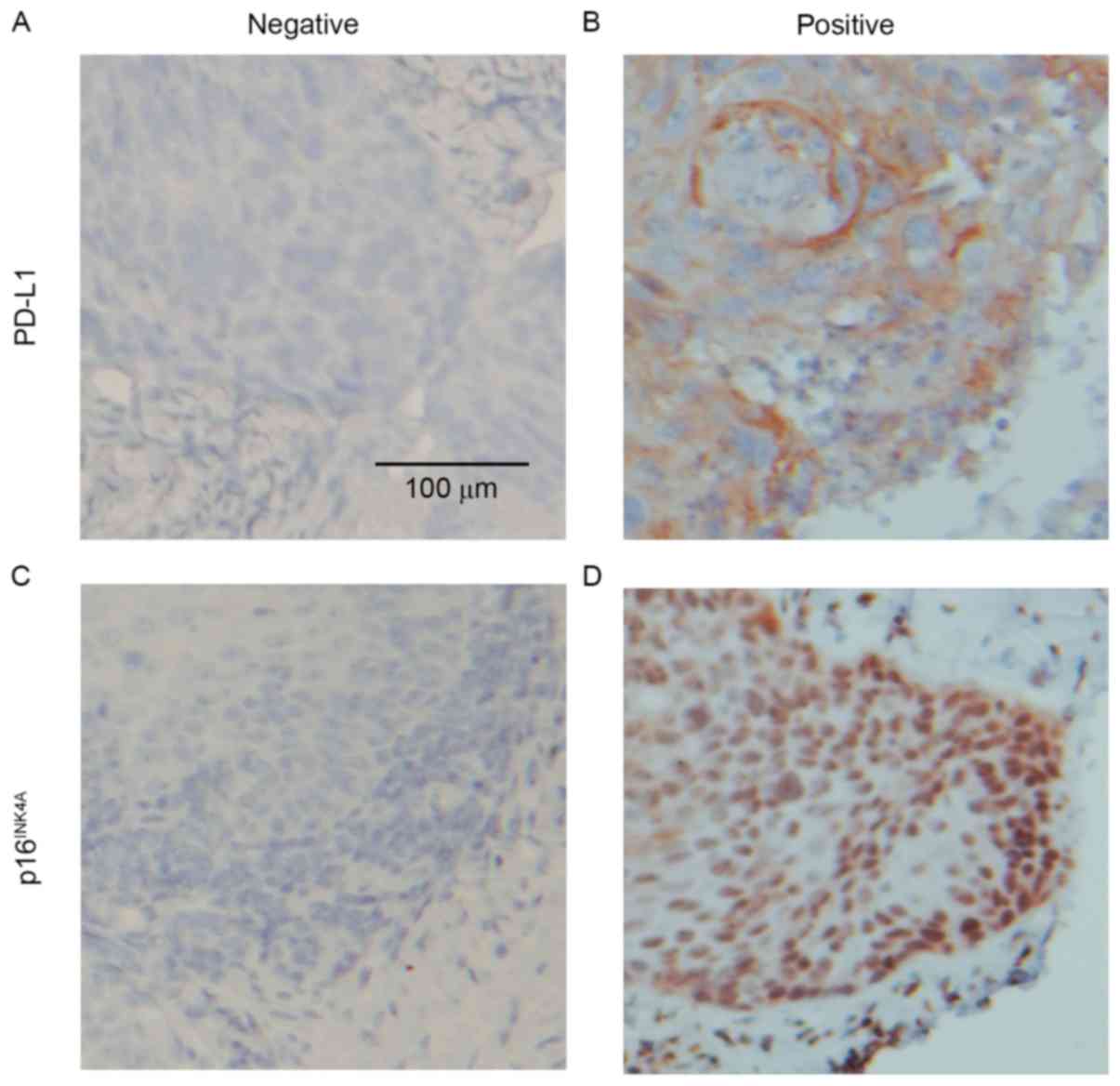
Tumor cells exhibiting membranous and cytoplasmic
staining were defined as positive for PD-L1 and those exhibiting
nuclear and cytoplasmic staining were defined as positive for
p16INK4A. The distribution of staining was categorized
as follows: 0, 0–5% staining; 1+, 5–20% staining; 2+, 20–50%; 3+,
≥51%. Cases were classified binarily as positive for PD-L1 when
there was staining >5% (1+, 2+ and 3+) of cancer cells (20,24) and
positive for p16INK4A when staining was >20% (2+ and
3+) (25). Staining was analyzed by
two independent investigators (five random fields at magnification,
×200).
Statistical analysis
The Mann-Whitney test was used to compare continuous
variables and the χ2 or Fisher's exact test was used to
compare categorical variables between groups. Progression-free
survival (PFS) was defined as the time period from diagnosis until
disease progression. Overall survival (OS) was calculated from the
time of diagnosis to mortality. Cox proportional analysis was also
used to determine risk factors for disease progression and
mortality. The log-rank test to compare Kaplan-Meier curves.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient clinicopathological
characteristics
Of the 106 patients with non-OPHNSCC, there were 99
(93.4%) males and 7 (6.6%) females, with a mean age of 58.8±11.5
years. The tumor sites included the oral cavity (63.2%),
hypopharynx (27.4%) and larynx (9.4%). A total of 33 patients
(31.1%) were diagnosed as having stage I/II disease and 73 (68.9%)
had stage III/IV disease. With respect to risk factors for HNSCC,
55 (51.9%) patients partook in chewing betel quid, 84 (79.2%) had
used tobacco and 66 (62.3%) consumed alcohol. Regarding treatment,
40 (37.7%) patients received radical surgery alone and 50 (47.2%)
patients received surgery followed by adjuvant therapy, consisting
of chemotherapy (cisplatin 25 mg/m2 IV weekly plus
tegafur-uracil 400 mg daily for up to 7 weeks), radiotherapy (60–66
Gy) and concurrent chemoradiotherapy. A total of 16 (15.1%)
patients received definitive chemoradiotherapy (cisplatin 80
mg/m2 on day 1 plus 5-fluorouracil 400
mg/m2/day by continuous infusion on days 1–4, every 28
days for 2 cycles plus radiation 66–72 Gy); whereas 10 (9.4%) were
administered induction chemotherapy (cisplatin 80 mg/m2
on day 1 plus 5-fluorouracil 600 mg/m2/day by continuous
infusion on days 1–4 every 28 days for 2 cycles; or docetaxel 60
mg/m2 plus cisplatin 75 mg/m2 on day 1 plus
5-fluorouracil 850 mg/m2/day by continuous infusion on
days 1–4 every 28 days for 2 cycles; Table I). A total of 34 patients (32.1%)
exhibited PD-L1 expression (Fig. 1A and
B) and 22 (20.8%) exhibited p16INK4A expression
(Fig. 1C and D).
 | Table I.Demographic and clinical
characteristics of the study population. |
Table I.
Demographic and clinical
characteristics of the study population.
|
| Case number
(n=106) |
|---|
|
|
|
|---|
| Characteristic | Number | % |
|---|
| Age (mean ±
standard deviation) | 58.8±11.5 |
| Male | 99 | 93.4 |
| Sites |
|
|
| Oral
cavity | 67 | 63.2 |
|
Hypopharynx | 29 | 27.4 |
|
Larynx | 10 | 9.4 |
| Stage |
|
|
|
I/II | 33 | 31.1 |
|
III/IV | 73 | 68.9 |
| Betel quid chewing
user |
|
|
|
Yes | 55 | 51.9 |
| No | 51 | 48.1 |
| Tobacco user |
|
|
|
Yes | 84 | 79.2 |
| No | 22 | 20.8 |
| Alcohol
consumption |
|
|
|
Yes | 66 | 62.3 |
| No | 40 | 37.7 |
| Pathological
characteristics |
|
|
| PD-L1
expression | 34 | 32.1 |
|
p16INK4A
expression | 22 | 20.8 |
| Definite
treatment |
|
|
| Surgery | 90 | 84.9 |
| Surgery
alone | 40 | 37.7 |
|
Adjuvant therapy | 50 | 47.2 |
| CCRT | 16 | 15.1 |
| CCRT
alone | 6 | 5.7 |
| IC
followed by CCRT | 10 | 9.4 |
Association between PD-L1 expression
and clinicopathological characteristics
Positive p16INK4A expression was
significantly higher in the group exhibiting positive expression of
PD-L1 compared with the group exhibiting negative expression of
PD-L1 (38.2 vs. 12.5%; P<0.01; Table
II). Furthermore, the mean age of patients exhibiting positive
PD-L1 expression was significantly higher than those exhibiting
negative PD-L1 expression (62.5±10.4 vs. 57.0±11.7; P<0.01;
Table II). However, positive PD-L1
expression was not associated with clinical stage, oral habits or
primary cancer sites (Table II).
Since it has been demonstrated that PD-L1 is associated with the
inflammatory tumor microenvironment (26), the association between PD-L1 and
systemic inflammatory factors at diagnosis, including total white
blood cell count, absolute neutrophil count, absolute lymphocyte
count, absolute monocyte count, neutrophils/lymphocyte ratio and
C-reactive protein levels, were investigated. However, there was no
significant association between PD-L1 expression and any of the
aforementioned inflammatory factors (Table II).
 | Table II.Association between PD-L1 expression
and patient clinicopathological characteristics. |
Table II.
Association between PD-L1 expression
and patient clinicopathological characteristics.
|
| PD-L1 negative,
n=72 | PD-L1 expression,
n=34 | P-value |
|---|
| Age | 57.0±11.7 | 62.5±10.4 | 0.01a |
| Stage |
|
|
|
| I/II
(%) | 22 (30.6%) | 11 (32.4%) | 0.85 |
| III/IV
(%) | 50 (69.4%) | 23 (67.6%) |
|
| Habits |
|
|
|
| Betel
quid chewing (%) | 41 (59.4) | 16 (48.5) | 0.30 |
| Tobacco
use (%) | 60 (87.0) | 26 (78.8) | 0.28 |
| Alcohol
consumption (%) | 45 (67.2) | 22 (66.7) | 0.96 |
| Sites |
|
|
|
| Oral
(%) | 47 (65.3) | 20 (58.8) | 0.44 |
|
Hypopharynx (%) | 20 (27.8) | 9 (26.5) |
|
| Larynx
(%) | 5 (6.9) | 5 (14.7) |
|
| Pathological
characteristics |
|
|
|
| p16
INK4A expression (%) | 9 (12.5) | 13 (38.2) |
<0.01a |
| PNI
(%) | 21 (41.2) | 18 (58.1) | 0.14 |
| LVI
(%) | 29 (58.0) | 19 (61.3) | 0.77 |
| Tumor
emboli (%) | 15 (31.9) | 15 (48.4) | 0.14 |
| ECS
(%) | 11 (59.4) | 8 (61.5) | 0.83 |
| Systemic
inflammatory factors |
|
|
|
| WBC
count (/cumm) | 7,969±2,378 | 7,494±3,603 | 0.42 |
| ANC
(/cumm) | 5,274±2,086 | 5,035±3,358 | 0.65 |
| ALC
(/cumm) | 1,953±1,316 | 1,663±676 | 0.23 |
| AMC
(/cumm) | 622±248 | 554±232 | 0.18 |
|
N/L | 3.3±1.8 | 3.7±4.1 | 0.42 |
| CRP
(mg/dl) | 6.8±5.5 | 8.7±6.8 | 0.25 |
Risk factors for PFS and OS
Univariate Cox proportional hazards analysis
demonstrated that only advanced cancer stage (III, IV) was a
prognostic factor of OS (HR, 7.53; P=0.05). Neither oral habits,
nor pathological characteristics, including PD-L1 and
p16INK4A expression, were risk factors for disease
progression and survival (Table
III). Following adjustment for cancer stage, PD-L1 and
p16INK4A expression did not qualify as independent risk
factors.
 | Table III.Univariate analysis of progression
and survival. |
Table III.
Univariate analysis of progression
and survival.
|
| PFS | OS |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age ≥60 years | 1.10
(0.54–2.23) | 0.79 | 1.24
(0.44–3.51) | 0.68 |
| Stage (III,
IV) | 1.31
(0.61–2.83) | 0.50 | 7.53
(0.99–57.35) | 0.05 |
| Betel quid
chewing | 1.39
(0.68–2.84) | 0.37 | 1.99
(0.63–6.36) | 0.24 |
| Tobacco use | 1.85
(0.56–6.07) | 0.31 | 2.41
(0.32–18.46) | 0.40 |
| Alcohol
consumption | 2.42
(1.00–5.92) | 0.05 | 1.38
(0.43–4.40) | 0.59 |
| Pathological
characteristics |
|
|
|
|
| PD-L1
expression | 1.29
(0.62–2.69) | 0.49 | 1.24
(0.42–3.63) | 0.70 |
|
p16INK4A
expression | 1.62
(0.67–3.80) | 0.26 | 1.14
(0.39–3.37) | 0.81 |
| Close
margin | 1.35
(0.66–2.76) | 0.42 | 0.57
(0.16–2.02) | 0.38 |
|
PNI | 1.87
(0.84–4.16) | 0.13 | 2.94
(0.76–11.37) | 0.12 |
|
LVI | 1.22
(0.52–2.77) | 0.63 | 1.54
(0.40–5.98) | 0.53 |
| Tumor
emboli | 1.42
(0.63–3.21) | 0.39 | 1.98
(0.57–6.84) | 0.28 |
|
ECS | 2.52
(0.66–9.65) | 0.18 | 2.21
(0.43–11.46) | 0.34 |
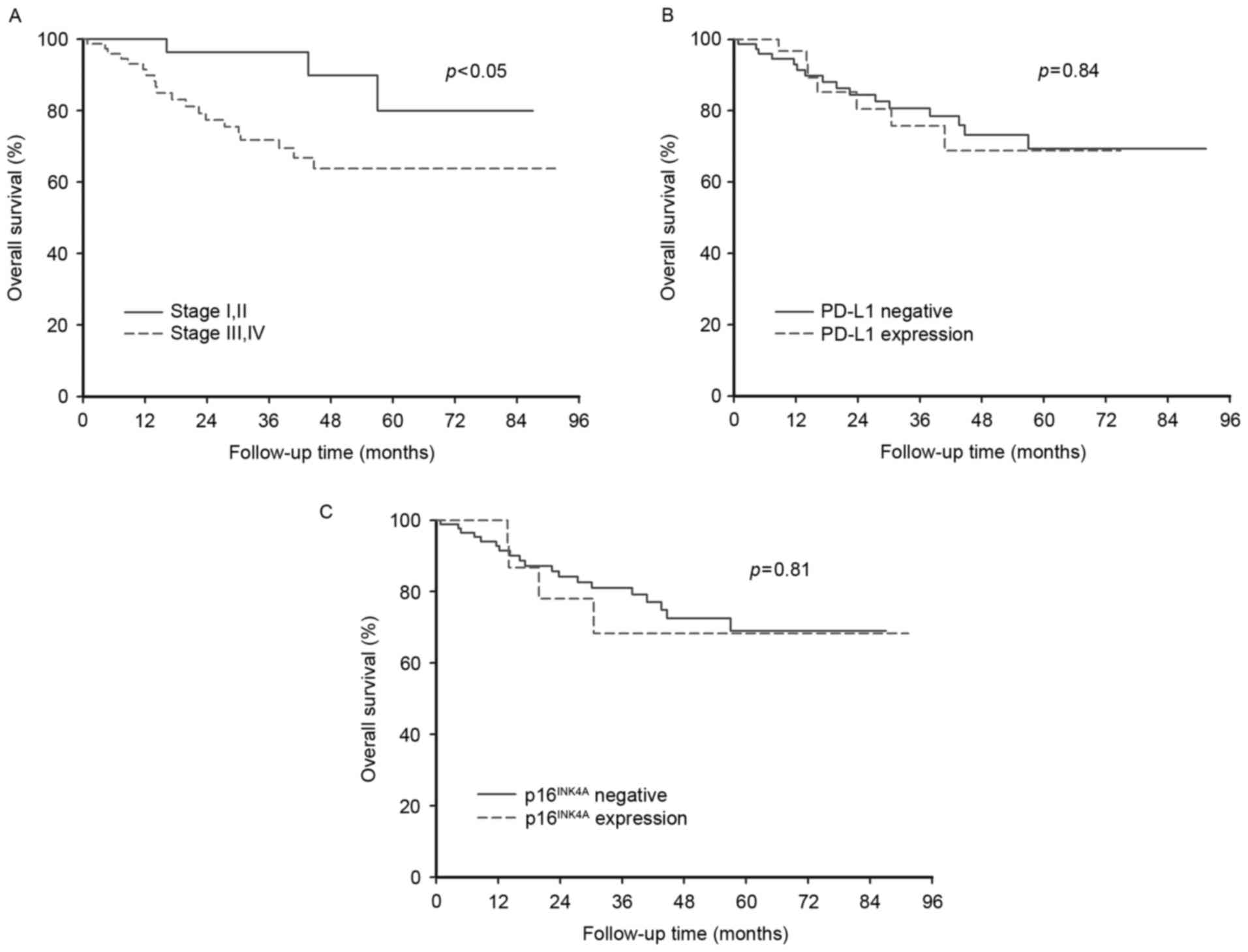
Patients with early stage cancer (I or II) had a
significantly better survival rate (P<0.05) than those with
advanced stage cancer (III or IV; Fig.
2A). However, the differing status of PD-L1 and
p16INK4A expression did not significantly affect the OS
of patients (Fig. 2B and C).
Discussion
The results of the current study demonstrate that
PD-L1 is expressed in a proportion of patients with non-OPHNSCC and
that PD-L1 expression is significantly associated with
p16INK4A expression. However, PD-L1 expression is not a
prognostic factor for non-OPHNSCC. In the current study, 32.1% of
subjects exhibited positive PD-L1 expression, comparable to the
results of previous studies, which demonstrated that positive PD-L1
expression occurred in 19–66% of HNSCC cases (18,24,27) and
46–59% in OPSCC cases (19,20). Positive expression of PD-L1 was
observed in 50% of larynx squamous cell carcinoma cases, a
relatively high proportion, however the number of cases included in
this study was relatively small (28). The variation in the level of PD-L1
expression may be attributed to the heterogeneity of subjects, a
small sample size and the inclusion of different ethnic groups. In
the current study, analysis of the levels of systemic inflammation
factors demonstrated that they were not associated with PD-L1
expression, suggesting that the tumor microenvironment, not
systemic inflammation, is an important factor influencing tumor
immune evasion. The identification of PD-L1 has led to the
development of PD-L1 antibodies to treat types of cancer that were
previously considered to be immune-responsive, including non-small
cell lung cancer and HNSCC (24). The
results of the current study may provide information that may be
important in the investigation of immune checkpoint blockage in
non-OPHNSCC.
In the present study, it was demonstrated that there
was an association between PD-L1 and p16INK4A expression
in cancer cells, which may be explained by the response of cancer
cells to immune attack. It has been demonstrated that IFN-γ
produced by inflammatory cells in the tumor microenvironment
directly induces p16INK4A expression and downstream
retinoblastoma (Rb) protein hypophosphorylation in cancer cells,
which leads to permanent growth arrest in tumors (29). This may be a general mechanism for
arresting tumor progression. By contrast, in OPSCC, it has been
suggested that p16INK4A expression is caused by HPV
infection that results in the inactivation by Rb by E7 oncoprotein
(30). Furthermore, in non-OPHNSCC,
IFN-γ induces cancer cells to express PD-L1 via the PKD2 pathway
(17). Similar results have been
reported in ovarian cancer, where IFN-γ stimulated PD-L1
expression, thus promoting tumor progression (31). The results of the current study
identified the co-occurrence of senescence and immune evasion of
cancer cells, which may be used to develop novel agents targeting
non-OPHNSCC in the future.
It remains unknown whether PD-L1 expression is
associated with cancer stage and patient prognosis. The present
study demonstrated that PD-L1 expression is not associated with
non-OPHNSCC stage or sites of occurrence, which is in accordance
with the results of previous studies. Ukpo et al (20) reported that PD-L1 expression is not
associated with nodal disease and tumor-node-metastasis stage. With
regards to prognosis, previous studies have indicated that there is
no correlation of survival rate with PD-L1 expression in oral
squamous cell carcinoma (20,28), which is consistent with the results of
the present study. The association between PD-L1 expression and
patient outcomes is controversial; it has been demonstrated in lung
cancer that PD-L1 expression is correlated with an improved outcome
(32), however, this has not been the
case in the other study (33). Such
discrepancies may be due to the complex interactions that occur
between tumor and immune cells in the tumor microenvironment. It
has previously been established that PD-L1 expression helps cancer
cells to evade immune attack, which may lead to tumor progression
and poorer patient outcomes. However, the co-expression of PD-L1
and p16INK4A may attenuate tumor growth and turn tumor
cells into senescent cells, offsetting tumor aggression.
Furthermore, immune evasion is not only determined by upregulation
of PD-L1 but also by PD-1 expression in tumor-infiltrating T cells
(18). Due to these factors, PD-L1
expression cannot be used as a prognostic factor in
non-OPHNSCC.
There were several limitations of the present study.
Although a significant association between PD-L1 and
p16INK4A expression was identified, the mechanism
between immune checkpoint and senescence remains unclear. As well
as the immune response, the expression of other genes or proteins
may affect the expression of PD-L1 (34) and p16INK4A (35). In addition, the patients included in
the current study underwent different treatment strategies due to
differences in cancer stage, which is a common selection bias of
retrospective studies. Although adjustments for cancer stage were
made, this bias may not have been fully corrected. Finally, there
is no standard cutoff value of IHC expression to define PD-L1 and
p16 positive. Having a different cutoff value may generate
inconsistent results and further studies are required to establish
standard values.
In conclusion, the present study identified an
association between PD-L1 and p16INK4A expression in
non-OPHNSCC. The poor association between PD-L1 expression and
clinical and prognostic status highlight the complex interactions
between the tumor and its microenvironment. Further investigations
into cancer cell senescence and immune evasion in microenvironment
are required.
Acknowledgements
The current study was supported by the Ministry of
Science and Technology (103-2314-B-010-034-MY3 to M.-H.Y.), Taipei
Veterans General Hospital (V104-E8-001 to M,-H.Y.) and a grant from
Ministry of Health and Welfare, Center of Excellence for Cancer
Research (MOHW104-TDU-B-211-124-001 to P.-Y.C.). The current study
was partly assisted by the Division of Experimental Surgery,
Department of Surgery of Taipei Veterans General Hospital. The
authors would like to acknowledge the support by the Biobank of
Taipei Veterans General Hospital. The abstract of the current study
was presented at the 2017 ASCO-SITC Clinical Immuno-Oncology
Symposium in Orlando, US on Feb 24, 2017.
References
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kreimer AR, Clifford GM, Boyle P and
Franceschi S: Human papillomavirus types in head and neck squamous
cell carcinomas worldwide: A systematic review. Cancer Epidemiol
Biomarkers Prev. 14:467–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rischin D, Young RJ, Fisher R, Fox SB, Le
QT, Peters LJ, Solomon B, Choi J, O'Sullivan B, Kenny LM and
McArthur GA: Prognostic significance of p16INK4A and human
papillomavirus in patients with oropharyngeal cancer treated on
TROG 02.02 phase III trial. J Clin Oncol. 28:4142–4148. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hashibe M, Brennan P, Benhamou S,
Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova
E, Fernandez L, et al: Alcohol drinking in never users of tobacco,
cigarette smoking in never drinkers, and the risk of head and neck
cancer: Pooled analysis in the international head and neck cancer
epidemiology consortium. J Natl Cancer Inst. 99:777–489. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith EM, Wang D, Kim Y, Rubenstein LM,
Lee JH, Haugen TH and Turek LP: P16INK4a expression, human
papillomavirus, and survival in head and neck cancer. Oral Oncol.
44:133–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salazar CR, Anayannis N, Smith RV, Wang Y,
Haigentz M Jr, Garg M, Schiff BA, Kawachi N, Elman J, Belbin TJ, et
al: Combined P16 and human papillomavirus testing predicts head and
neck cancer survival. Int J Cancer. 135:2404–2412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen SF, Yu FS, Chang YC, Fu E, Nieh S and
Lin YS: Role of human papillomavirus infection in carcinogenesis of
oral squamous cell carcinoma with evidences of prognostic
association. J Oral Pathol Med. 41:9–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ochi A, Graffeo CS, Zambirinis CP, Rehman
A, Hackman M, Fallon N, Barilla RM, Henning JR, Jamal M, Rao R, et
al: Toll-like receptor 7 regulates pancreatic carcinogenesis in
mice and humans. J Clin Invest. 122:4118–4129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Través PG, Luque A and Hortelano S:
Macrophages, inflammation, and tumor suppressors: ARF, a new player
in the game. Mediators Inflamm. 2012:5687832012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wong YK, Chang KW, Cheng CY and Liu CJ:
Association of CTLA-4 gene polymorphism with oral squamous cell
carcinoma. J Oral Pathol Med. 35:51–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu CJ, Wong YK, Chang KW, Chang HC, Liu
HF and Lee YJ: Tumor necrosis factor-alpha promoter polymorphism is
associated with susceptibility to oral squamous cell carcinoma. J
Oral Pathol Med. 34:608–612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y
and Zhang P: Interferon-γ-induced PD-L1 surface expression on human
oral squamous carcinoma via PKD2 signal pathway. Immunobiology.
217:385–393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Badoual C, Hans S, Merillon N, Van Ryswick
C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A,
Besnier N, et al: PD-1-expressing tumor-infiltrating T cells are a
favorable prognostic biomarker in HPV-associated head and neck
cancer. Cancer Res. 73:128–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lyford-Pike S, Peng S, Young GD, Taube JM,
Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, et
al: Evidence for a role of the PD-1:PD-L1 pathway in immune
resistance of HPV-associated head and neck squamous cell carcinoma.
Cancer Res. 73:1733–1741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ukpo OC, Thorstad WL and Lewis JS Jr:
B7-H1 expression model for immune evasion in human
papillomavirus-related oropharyngeal squamous cell carcinoma. Head
Neck Pathol. 7:113–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zandberg DP and Strome SE: The role of the
PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck.
Oral Oncol. 50:627–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen YW, Kao SY and Yang MH: Analysis of
p16(INK4A) expression of oral squamous cell carcinomas in Taiwan:
Prognostic correlation without relevance to betel quid consumption.
J Surg Oncol. 106:149–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen YW, Kao SY, Wang HJ and Yang MH:
Histone modification patterns correlate with patient outcome in
oral squamous cell carcinoma. Cancer. 119:4259–4267. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:pp. 12293–12297. 2002;
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Strome SE, Dong H, Tamura H, Voss SG,
Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, et al:
B7-H1 blockade augments adoptive T-cell immunotherapy for squamous
cell carcinoma. Cancer Res. 63:6501–6505. 2003.PubMed/NCBI
|
|
28
|
Cho YA, Yoon HJ, Lee JI, Hong SP and Hong
SD: Relationship between the expressions of PD-L1 and
tumor-infiltrating lymphocytes in oral squamous cell carcinoma.
Oral Oncol. 47:1148–4253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Braumüller H, Wieder T, Brenner E, Aßmann
S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M,
Griessinger C, et al: T-helper-1-cell cytokines drive cancer into
senescence. Nature. 494:361–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parry D, Bates S, Mann DJ and Peters G:
Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with
high levels of p16INK4/MTS1 tumour suppressor gene product. Embo J.
14:503–511. 1995.PubMed/NCBI
|
|
31
|
Abiko K, Matsumura N, Hamanishi J,
Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I
and Mandai M: IFN-γ from lymphocytes induces PD-L1 expression and
promotes progression of ovarian cancer. Br J Cancer. 112:1501–1509.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Velcheti V, Schalper KA, Carvajal DE,
Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L
and Rimm DL: Programmed death ligand-1 expression in non-small cell
lung cancer. Lab Invest. 94:107–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu J, Chen L, Zou L, Yang P, Wu R, Mao Y,
Zhou H, Li R, Wang K, Wang W, et al: MiR-20b, −21, and −130b
inhibit PTEN expression resulting in B7-H1 over-expression in
advanced colorectal cancer. Hum Immunol. 75:348–353. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sage J, Miller AL, Pérez-Mancera PA,
Wysocki JM and Jacks T: Acute mutation of retinoblastoma gene
function is sufficient for cell cycle re-entry. Nature.
424:223–228. 2003. View Article : Google Scholar : PubMed/NCBI
|