Introduction
Hepatocellular carcinoma (HCC) is the seventh most
common cancer worldwide. Traditional therapy strategies currently
available for HCC include surgical procedures, radioactive particle
implantation, radiofrequency ablation, hepatic artery
chemoembolization (TACE), and chemotherapeutics. Recent studies
have indicated that these therapy strategies are still not fully
efficient and have multiple drawbacks, including post-treatment
relapse, chemotherapy drug resistance and metastasis (1–5). Thus,
discovering approaches to avoid recurrence and metastasis of liver
cancer and to provide novel therapeutic strategies is of outmost
importance in HCC. With the continuous progress in cancer stem cell
(CSC) research, many specific studies overexpressed on the surface
of CSCs have been discovered. These receptors are significantly
associated with growth and proliferation of tumor cells. To date,
scientists have isolated CSCs in various solid tumors, including
liver, breast, lung and brain cancer. Liver cancer stem cells
(LCSCs) represent a small fraction of cells in HCC cancer tissues
that possess the abilities of self-renewal, multi-directional
differentiation and indefinite proliferation, as well as high
tumorigenic ability (6–10). As specific markers of CSCs, the
CSC-specific overexpressed receptors may offer a new research
direction as therapeutic targets for the diagnosis and treatment of
tumors. Currently, potential clinical treatments targeting CSC
include: Blocking signal transduction pathways in CSCs; inducing
differentiation of CSCs; changing the microenvironment and
inhibiting telomerase activity in CSCs; specific gene therapy
targeting CSCs; specific compounds or drugs targeting CSCs; and
ligands targeting CSCs. In conclusion, the CSC theory may provide
an explanation as to the refractory nature of liver cancer and may
provide useful insights for scientists to design novel therapies
for HCC.
LCSCs and their origin
The liver has both exocrine and endocrine functions.
It is estimated that the normal liver can completely self-renew
within ~1 year (11), exhibiting a
strong regeneration capacity, which is also an important feature of
stem cells. While there is a large amount of endogenous stem cells
in the liver, the duration of their proliferative potential is
short. These cells are commonly derived from undifferentiated liver
oval cells, also known as hepatic precursor cells (HPC), and are
located in the terminal bile canaliculi and beside the interlobular
bile duct (12–14). Oval cells have both the ability to
differentiate into hepatocytes and bile duct cells, which, in human
HCC, display the properties of stem cells (15). Additionally, the majority of hepatic
stem cell surface markers are the same as hepatic oval cell markers
(OV)6, OV1, cytokeratin 7 (CK7) and CK19, α-fetoprotein (AFP), KIT
proto-oncogene receptor tyrosine kinase (c-kit), and Thy-1 cell
surface antigen (Thy-1). OV6 expression is a specific phenotype of
oval cells that was originally identified in the livers of
tumor-bearing rats, and is recognized as a surface marker of human
liver progenitor cells (16). Yang
et al (17) reported that
overexpression of OV6 enhances the invasiveness and metastasis
potential of HCC stem cells, and that increased numbers of
OV6+ CSCs in patients with liver cancer indicate worst
clinicopathological features and poorer prognosis. In addition,
Yang et al (17) demonstrated
that the stromal cell derived factor (SDF)-1/C-X-C motif chemokine
receptor (CXCR) 4 signaling pathway is significantly associated
with the migration ability of OV6+ HCC cells, suggesting
that OV6+ stem cells have an important role in HCC
metastasis. By contrast, exogenous liver stem cells, which are
derived from bone marrow or peripheral blood stem cells, are
usually fewer in number, but exhibit a longer duration of
proliferative potential (18).
Gene mutations, with the exception of mutations
affecting self-renewal capacity, are important events occurring in
the early stages of cancer. Previous studies have reported that
CSCs originate from normal stem/progenitor cells and exhibit
certain self-renewal ability (19).
However, whether this hypothesis applies to HCC is unknown.
Previous studies have demonstrated that there is indeed a small
subset of cells in HCC that display the characteristics of CSCs.
Side population (SP) cell sorting is widely used for the isolation
and identification of CSCs from other types of tumors. The subsets
of SP cells are identified by the ability of the ATP binding
cassette transporter to export the DNA dye, Hoechst 33342. In the
Huh7 and PLC/PRF/5 HCC cell lines, ~0.25–2.0% of the cells display
an SP phenotype (20).
LCSCs can self-replicate, differentiate, and present
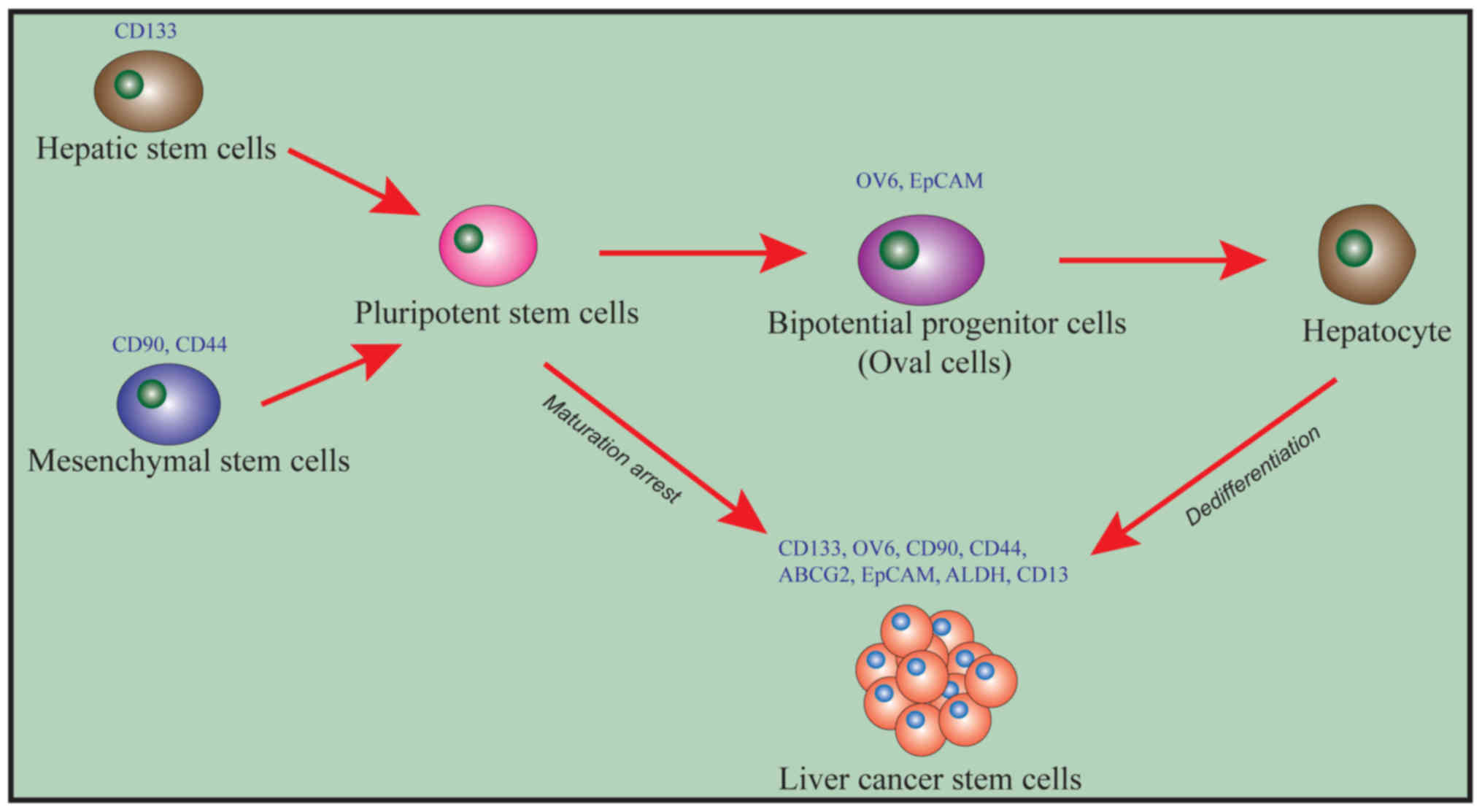
strong drug resistance. Liu et al (21) (Fig. 1)
have hypothesized that CSCs are not derived from a specific source
of cells in hepatitis-B (HBV)-associated HCC and may be derived
either from hematopoietic stem cells (HSC) or from mesenchymal stem
cells (MSC). The specific surface marker for HSCs is CD133, while
the specific surface markers for MSCs are CD90 and CD44. Both HSCs
and MSCs can differentiate into pluripotent stem cells (PSCs). PSCs
can then differentiate into liver precursor cells/oval cells that
express OV6 and epithelial cell adhesion molecule (EpCAM). PSCs and
liver precursor cells can be induced into CSCs by the mechanism of
‘maturation arrest’, thus leading to the occurrence of liver
cancer.
There are several theories regarding the origin of
HCC cells. One theory proposes that they are derived from
dedifferentiated mature liver cells. Gournay et al (22) have confirmed that dedifferentiation of
mature liver cells occurs during the formation of HCC in mice,
suggesting that proliferative liver cells may be one of the sources
of LCSCs. Other scholars argue that HCC cells are derived from the
abnormal differentiation of liver stem cells by ‘blocked
maturation’. For example, Sell et al (23) used chemical carcinogens and oncogenes
to intervene in the differentiation of liver oval cells and to
transform them into HCC pre-cancer cells. Dumble et al
(24) subcutaneously inoculated oval
cells into nude mice and reported the development of tumors similar
to HCC. Results from the detection of surface markers demonstrated
that the newly developed tumors were derived from differentiated
oval cells, suggesting that oval cells may be involved in the
occurrence of HCC (24). HCC tumors
have also been demonstrated to include intermediate cells between
HPC and mature hepatocytes. An increasing number of studies has
demonstrated that LCSCs can originate from the ‘blocked maturation’
LSCs (25–27), because most HCCs consist of mixtures
of mature cells and cells with a phenotype similar to HPCs.
Immunophenotyping analysis of HCCs has further indicated that
28–50% of HCC cells express HPC surface markers, such as CK7 and
CK19 (28). These tumors also include
intermediate cells between HPC and mature liver cells. Furthermore,
Yeh et al (29) reported that
the expression levels of CD133 were negatively correlated with the
expression levels of HBV surface antigen (HBsAg) in HBV-associated
liver cancer tissue samples, indicating that LCSCs more likely
originate from blocked liver stem cells, rather than differentiated
liver cells post-infection. Therefore, various LCSC markers can be
detected in HBV-associated clinical samples of HCC. There is also
evidence suggesting that LCSCs may be derived from bone marrow stem
cells (30) and SP cells (20,31).
Cancers exhibit immense tumor heterogeneity. If
cancers originate from few CSCs and these stem cells offer various
characteristics to the tissue, then the importance of cell abnormal
differentiation ability needs to be redefined to better explain the
heterogeneity of tumors. Dynamic analysis of the expression levels
of LCSC markers can help clarify the changes of biological
characteristics of LCSCs during hepatocarcinogenesis and explain
the clinical significance of the changes in marker expression
levels.
LCSCs and their characteristics in HCC
Drug resistance is associated with the recurrence
and metastasis of cancer (32). CSCs
resist chemotherapy-induced cell death through various mechanisms,
including intrinsic and external mechanisms. The intrinsic
mechanism consists of the self-renewal ability of CSCs, the
enhancement of DNA damage repair pathways, the high expression of
drug efflux-related proteins, the overactivation of growth pathways
and other stem-related pathways. The external mechanism refers to
the influence of tumor microenvironment factors on CSC resistance,
including hypoxia stimulation, epithelial-mesenchymal transition
(EMT) signals, and angiogenesis abnormalities (33). In HCC, SP cells or LCSCs expressing
other molecular markers (including EpCAM, CD133, CD90, CD44 and
CD13) exhibited resistance to radiotherapy and chemotherapy in
vitro and in vivo. The mechanisms involved include
increased expression of drug efflux-related proteins (31,34–36),
activation of anti-apoptotic pathways (37–39),
activation of stem cell-related pathways, and increased resistance
and maintenance of a certain number of LCSCs (16,40).
MicroRNAs (miRNAs) participate in the maintenance of the CSC
phenotype by regulating the expression of oncogenes and stem
cell-related genes (41,42). The half-life of mature tumor cells in
the circulation is very short and most of them die from natural
apoptosis, with a relatively small effect on tumor invasion and
metastasis. A previous study revealed that the viability, distant
metastasis, and homing ability of LCSCs in the circulatory system
were significantly higher than that of other tumor cells (43). This may be explained by the EMT status
of LCSCs, which enables them to serve a leading role in the
metastasis and invasion of HCC and to become the source of HCC
recurrence (43). Theoretically,
tumor recurrence may be effectively prevented if a method to
eliminate CSCs could be developed, making CSCs a desirable
diagnosis and treatment target for resistant tumors, including HCC
(44). This would be especially true
for cases with poor therapeutic effect by traditional methods.
LCSC-targeted therapy is thus hypothesized to achieve excellent
antitumor effects and to reduce the side effects of chemotherapy,
providing novel more efficient strategies for the treatment of
cancer.
Currently known LCSC surface markers
With the identification of specific surface markers,
LCSCs can be successfully separated and enriched through screening
for these markers by fluorescence-activated and magnetic-activated
cell sorting methods (45). If
LCSC-specific molecular markers are targeted and blocked, the
number of LCSCs may be reduced, potentially resulting to inhibited
tumor growth and recurrence.
To date, the commonly-reported LCSC surface markers
are EpCAM (also known as CD326), CD133, CD90 (also known as Thy-1),
CD44, and CD13 (40,46–49). In
addition, other surface markers have also been demonstrated to be
involved, including OV6, K19, c-kit (also known as CD117), ATP
binding cassette subfamily G member 2 (ABCG2), and aldehyde
dehydrogenase (ALDH).
Most of these markers normally exist on the surface
of HSCs. Cells expressing these markers have similar stem cell
properties, therefore these markers are hypothesized to be used as
molecular therapeutic targets to eliminate LCSCs and to overcome
cancer recurrence. Although certain other surface markers have been
reported on cancer stem cells, they are not specific to LCSCs
(50).
LCSC surface markers and targeted
therapies
Prominin-1 (CD133)
CD133 is one of the most studied stem cell surface
markers in recent years. In solid tumors, CD133 was first
discovered and isolated in brain tumors; Singh et al
(51) successfully isolated
CD133+ tumor cells from glioblastoma and demonstrated
that CD133+ glioblastoma tumor cells can form
neurosphere-like clones, with a strong self-renewal capacity,
differentiation potential and tumorigenicity in vivo. The
tumor cells and subtypes of tumors formed in mice were the same as
those obtained by orthotopic grafts, but could also be passaged
consecutively; therefore, CD133+ cells were identified
as tumor stem cells. Furthermore, CD133+ cells have an
important role in multiple other solid tumors, including gastric
(52), liver (53–55) and
colon cancer (56,57). CD133 is a transmembrane glycoprotein
with a unique structure of five transmembrane domains and two large
extracellular glycosylation chains, expressed in both hematopoietic
and neural stem cells (58). In
several studies of HCC, the HSC surface marker CD133 was used to
isolate LCSCs (47,54). CD133 is expressed on the surface of
stem cells in many solid tumors, including liver, colon, brain,
lung and prostate cancer, and in B16 melanoma (59). In human HCC cell lines, ~0–65% of
cells are CD133+ cells. CD133 is considered one of the
main LCSC markers, with self-renewal, multi-lineage differentiation
and chemoresistance abilities (37).
Methionine adenosyltransferase (MAT) is the only enzyme that can
catalyze the biosynthesis of S-adenosylmethionine (SAMe), which is
the principal biological methyl donor in cells. SAMe can regulate
hepatocyte growth and apoptosis. Exogenous SAMe inhibits the growth
of hepatoma cells and prevents HCC development. Similar results
were observed in a MAT deficiency-induced HCC mouse model (60). Xenografts of CD133+ cells
in nude mice formed tumors, while CD133− cells did not
(61); Yang et al (48) also reported that CD133+
liver cancer cells exhibited higher tumorigenic and proliferative
abilities, properties that are similar to the features of HPCs
(47). CD133 knockout may reduce the
tumorigenicity and change the cell cycle distribution in these
cells. Additionally, HCC patients with high CD133 expression in
their tumors have poor prognosis and increased recurrence,
indicating that CD133 expression may be associated with the
prognosis of liver cancer (47,54,62–64).
A previous study has demonstrated that the migration of
CD133+ and CD133− cells was not significantly
different, and that the CD133 expression pattern was inconsistent
with the clinical manifestations (65). A recent study reported that
CD133+ LCSCs were resistant to interferon-induced
autophagy (66). Therefore, the
identification of targeted molecular markers is of great
significance.
Monoclonal antibodies are commonly used as ligands
in CD133-targeted therapy. These antibodies can carry various drugs
or toxins to the target in order to enhance the immune response of
the human body towards the disease. Such methods have several
advantages that are absent in traditional anticancer drugs, namely,
relatively high target specificity, low molecular weight, less side
effects, and better patient compliance. Currently reported
antibodies against CD133 are AC133, 293C3 and AC141, among which
AC141 and 293C3 are antibodies targeting CD133/2. CD133/2 is a
variant of the CD133 antigen, which is predominantly expressed in
the fetal liver and kidney, but not in the adult pancreas and
placenta tissues. Prasad et al (67) prepared a compound antibody from CD133
and CD3 antibodies. This compound could specifically identify
glioma stem cells and recruit T cells to kill these stem cells,
demonstrating an excellent targeted therapeutic effect. Smith et
al (68) combined a mouse
anti-human CD133 antibody with the anti-microtubule cytotoxic drug
monomethyl auristatin E, and confirmed that this complex inhibited
the growth of CD133+ LCSC-like cells in vivo and
in vitro. Lang et al (69) prepared a 131I-CD133
monoclonal antibody (mAb) with high stability and specificity in
vitro. Additionally, in vivo experiments demonstrated
that the 131I-CD133 mAb had a high selectivity, and the
binding rates with CD133+ colon adenocarcinoma CSCs and
CD133− cells were 70.01±6.02 and 2.73±0.26%,
respectively (69). Imaging of the
transplanted tumor mice demonstrated that most of the
131I-CD133 mAb was deposited in the
CD133+-transplanted tumor sites in the mice, while this
was not observed in CD133− mice. The
131I-CD133 mAb may be therefore applied with high
selectivity and high stability in the clinical diagnosis of LCSCs,
as well as for immune imaging and radiation therapy of LCSCs, and
clinical trials are currently ongoing.
In the field of CD133-targeted polypeptides, Sun
et al (70) successfully
identified a short peptide LQNAPRS (LS-7) which can highly bind to
mouse CD133, by using phage display technology. Wang et al
(71) prepared a DSPEPEG micelle
system loaded with a 7-amino-acid peptide (TR short peptide), which
was used to investigate its targeting effect on brain stem cells.
Compared with the unmodified micelles, the uptake rate of the
TR-modified micelles was significantly increased in brain glial
stem cells. TR peptide-modified micelles exhibited specific binding
of the TR peptide to the CD1333 receptor, and improved anticancer
effects by targeting CD133+ glioma stem cells. In
conclusion, despite these studies demonstrating that CD133 can be
used for the isolation and identification of LCSCs in vitro
as well as for targeted therapy, the application of a single
surface marker remains limited.
CD13
CD13 is the earliest identified marker of normal and
malignant myeloid cells and has been used for many years to
characterize and classify leukemia or lymphoma cells (72). CD13, also known as aminopeptidase N
(73), is a zinc-binding protein. In
addition, CD13+ cells exhibit features similar to that
of stem cells, such as increased cell proliferation and tumor cell
formation, and increased resistance to chemotherapy.
CD13+ cells are resistant to adriamycin and fluorouracil
(5-FU) treatment, and expression of CD13 is enhanced by
chemotherapy. This is associated with the high resistance of
CD13+ cells; in the presence of chemotherapy drugs,
CD13− cells exhibit an increased response to oxygen
clusters, leading to DNA breakage and cell death. In
CD13+ cells, the expression of the glutamate-cysteine
ligase (GCLM) gene is significantly increased compared with other
cells. GCLM catalyzes intracellular antioxidant glutathione
synthesis, against reactive oxygen species induced by
chemotherapy/radiotherapy, thereby protecting DNA from DNA damage,
preventing apoptosis, and resulting in drug resistance (74). The classical cytotoxic antitumor
drugs, doxorubicin and 5-FU, kill CD90+ hepatoma cells,
but the proportion of the surviving CD13+ cells
increases. The percentage of CD13+/CD90− cell
subpopulation in clinical tissues from patients with HCC who
relapsed following arterial chemoembolization is significantly
higher compared with untreated HCC tissues. Following
administration of a low dose gradient of cyclophosphamide, the
remaining tumor cells exhibit a
hAFP+/CD13+/PCNA− phenotype, with
the CD13+ cells increased, indicating that
CD13+ cells are resistant to this chemotherapy. However,
combined treatment with Tegafur, a prodrug of 5-fluorouracil
(5-FU), and cyclophosphamide, using a low-dose rhythmic
administration, significantly reduced the number of tumor cells
(75,76). Studies have demonstrated that the
expression of CD13 may enhance the semi-static activity of CSCs.
Haraguchi et al (40) observed
that CD13+ cells are predominantly in the
G1/G0 phase of the cell cycle, suggesting
that CD13 may be a marker of the dormant or semi-stationary status
of LCSCs.
Downregulation of CD13, by use of a CD13
neutralizing antibody or inhibitors, can induce apoptosis in the
HCC cell lines Huh7 and PLC/PRF/5. When CD13+
hepatocytes were treated with 5-FU, which is directly targeted at
CD13 molecules, the number of cells with tumorigenic and
self-renewal abilities was significantly reduced (77). The coexpression of CD13 and CD90 has
an important role in the occurrence of liver cancer. The combined
application of CD13 and CD90 inhibitors significantly reduces tumor
volume, compared with the application of each individual inhibitor
alone. Reduction or inhibition of CD13 molecules on the surface of
HCC cells by interfering techniques also affect, to a certain
extent, the self-renewal and tumorigenic ability of LCSCs (40).
CD90 or Thy-1
In 1964, CD90 was first identified in the CH3 AKR
strain mice in an effort to develop an antileukemia xenoantibody,
and was named as θ antigen (78). In
1969, due to the fact that a study had demonstrated that the
precursors of T cells are mature in the thymus (79), the important surface marker of T
cells, θ antigen, was renamed as Thy-1. In the 1980s, CD90 was
isolated from the human T-cell leukemia cell line, MOLT-3,
indicating the presence of CD90 in humans (80). CD90 overexpression was demonstrated to
be associated with age in patients with HCC and HBV infection,
tissue staging and high CD90 expression were associated with poor
prognosis (81). The CD90+
cell population was selected from a HCC cell line, as well as
tissues and blood from patients with HCC, and it was demonstrated
that they presented increased tumorigenic abilities and indefinite
proliferation compared with the CD90− cell population,
suggesting that the CD90+ cells might be a
‘hepatocellular stem cell’ population (48,82). CD90
is a surface marker expressed in human HCC cell lines and
mesenchymal stem cells with a positive rate of ~0-2.5%, and often
serves as a surface marker for various stem cells. Yang et
al (48) noted that HCC tumor
samples and the majority of blood samples contain highly
tumorigenic CD90+/CD45− cells, while samples
from normal individuals or patients with chronic hepatitis do not.
Similarly, aspects of the aforementioned study, which focused on
the expression of CD90 in HCC cell lines, revealed that only
CD90+ cells exhibited tumorigenic ability. If the
surface marker glycoprotein CD44 was also expressed in the
CD90+ cells, the invasive phenotype was even stronger,
with increased metastatic and self-renewal capacities. When CD44
was blocked by an inhibiting antibody, the tumor formation and
metastasis abilities of CD90+ cells were decreased and
apoptosis was induced. In the same study, it was also noted that
CD45−/CD90+ cells were present in all tissue
samples and ~90% of blood samples from liver cancer patients, and
exhibited a more aggressive phenotype in immunodeficient mice;
while only a small population of CD90+ cells existed
among 6 different liver cancer cell lines, and exhibited a low
aggressive phenotype in immunodeficient mice. Transplant
experiments in nude mice demonstrated that CD90+ HCC
cells had a tumorigenic ability that was not present in the
CD90− cells. A further study has indicated that
CD45−/CD90+ cells also express other stem
cell markers, including CD133, epithelial specific antigen (ESA),
CXCR4, CD24, kinase insert domain receptor and CD44 (48). CD90, possibly one of the surface
markers of LCSCs, has been used in the identification of LCSCs in
recent years. CD45−/CD90+ cells may become a
new target for diagnosis and treatment of liver cancer. CD90 has
been shown to upregulate the expression of the molecular marker
CD133, and this abnormal expression can promote tumor progression.
The CD90/integrin/mechanistic target of rapamycin kinase
(mTOR)/AMP-activated protein kinase (AMPK)/CD133 signaling pathway
serves an important role in tumor formation, and inhibition of this
pathway by the energy-limited simulant, OSU-CG5, reduced the
proportion of CD90+ cells in fresh HCC specimens and
inhibited tumor growth (83).
CD44
CD44 is a glycoprotein encoded by a single gene, and
hyaluronic acid is its main receptor. As an important class of
adhesion molecules, CD44 is widely distributed on the cell surface
of various cell types, including lymphocytes, monocytes and
endothelial cells (84), and it is
involved in intercellular cell adhesion and cell migration. CD44
may be associated with tumor cell invasion and metastasis of liver
cancer (36). Under normal
circumstances, CD44 is in a relatively quiescent state on the cell
surface. However, CD44 is overexpressed in tumor cells and mainly
involved in heterotypic adhesion (the adhesion of tumor cells to
the host cells and the host matrix), thereby promoting tumor cell
invasion and metastasis. The relationship between CD44 and tumor
infiltration and metastasis has been investigated (85). CD44 is a stem cell marker of
pancreatic, gastric, and colorectal cancer. Subsequent studies
indicated that it is also one of the important markers of LCSCs,
and its coexpression with other markers can better identify LCSC
phenotypes. Mima et al (49)
observed in a nude mouse model that the tumor formation rate of
CD44+ cells was faster compared with CD44−
cells, and that only CD90+/CD44+ cells
appeared in the lung metastasis sites. Compared with
CD133+/CD44− cells,
CD133+/CD44+ HCC cells were more prone to
tumor formation and drug resistance, and expressed more
stem-associated genes (36).
CD133+/CD90+ cells were more aggressive than
CD44+ cells alone.
Blocking CD44 activity by use of a CD44-targeting
antibody can induce the apoptosis of CD90+ cells in
vitro and inhibit tumor formation of CD90+ cells in
immunodeficient mice in vivo (86). IM7 is a murine monoclonal antibody
specifically targeting CD44, which has a confirmed inhibitory
effect on tumor growth. Zhang et al (87) reported that RG7356, a humanized
antibody against CD44, could induce apoptosis of chronic
lymphocytic leukemia cells.
To date, given the characteristics of CD44, studies
on short peptides targeting CD44 are gradually increasing. Cho
et al (88) prepared a novel
short peptide complex PDPP targeting CD44 by combining the short
peptide with D-polylysine. The binding capacity of PDPP and CD44
was 4–10 times stronger than that of the CD44 antibody, suggesting
that PDPP may serve as a probe for the diagnosis and treatment of
cancer stem cells. Park et al (89) successfully identified a short peptide,
P7(FNLPLPSRPLLR), which can specifically bind to CD44 expressed on
the surface of breast cancer CSCs. Similar to the CD44 antibody,
the binding rate of P7 on MCF7 cells was high. Therefore, the
authors suggest that the short peptide P7 could be used for the
treatment of CSCs as a substitute for antibodies.
EpCAM
EpCAM is a transmembrane glycoprotein with a
relative molecular mass of 40,000 Da and currently used in research
for various tumor types (90–93). EpCAM is expressed during the early
liver development process, but not in normal mature liver cells.
EpCAM is expressed in human epithelial tissue and tumors, as well
as in precursor cells and stem cells. It is also present in liver
stem cells and hepatoblasts. Nevertheless, the high expression of
EpCAM is significantly associated with activation of cell
proliferation (94). EpCAM is also
expressed on the surface of LCSCs and pancreatic CSCs (95,96).
Recent studies have demonstrated that the tumor formation and
invasion abilities of EpCAM+ HCC cells were
significantly higher compared with EpCAM− HCC cells
(97). Liver stem cell surface
markers were expressed in EpCAM+ cells, while the
expression of mature hepatocyte markers was significantly increased
in EpCAM− cells (91).
Yamashita et al (98), EpCAM
expression was utilized to classify patients with HCC, and the
differential expression of AFP and EpCAM was verified in tumor
samples from two HCC cell lines. EpCAM+ cells exhibited
CSC-like characteristics, with high tumor formation ability in
vivo and in vitro. Compared with EpCAM−
cells, the increased CSC characteristics of EpCAM+ cells
in primary liver cancer samples were further confirmed. The
aforementioned study indicated that activation of the Wnt/β-catenin
signaling pathway may increase the proportion of EpCAM+
cells and block the reduction of EpCAM-induced tumorigenic ability
of these cells. Results of CD90 and EpCAM expression obtained from
HCC tumor cell lines were confirmed in human HCC samples. The
aforementioned studies offer direct evidence for the existence of
LCSCs in human HCC.
Targeted therapy towards the LCSC molecular marker
EpCAM can effectively eliminate the expression of EpCAM in LCSCs
(99,100). EpCAM antibodies currently available
in preclinical or clinical studies include edrecolomab,
adecatumumab, MT110 and catumaxomab, and they have been approved in
the EU for patients with EpCAM+ malignant ascites. Chen
et al (7) used an EpCAM
antibody (EpCAM-Ab) as the target to modify micelles loaded with
anticancer drugs or genes, and to develop a gene delivery system
targeting CSCs. This delivery system exhibited characteristics of
pH-responsive drug release, with the amount of drug released at pH
5.0 being 40% higher than that at pH 7.4. In vitro
experiments showed that the inhibitory effect of EpCAMAb-modified
adriamycin-loaded micelles on LCSCs was significantly enhanced,
with an IC50 of 0.051 mg/l, while the IC50 of
the EpCAMAb-unmodified adriamycin-loaded micelles was 0.24 mg/l,
which was 5 times that of the former. This targeted drug delivery
system offers a significant therapeutic effect, indicating the
feasibility of this antibody-mediated active CSC targeted therapy,
as well as its potential value for the clinical treatment of
cancer. Because RNA interference (RNAi) of EpCAM has been confirmed
to significantly reduce the number of stem cells and their
tumorigenic and invasive abilities (91,95), Bae
et al (101) reported that,
following EpCAM gene silencing by RNAi, HCC tumor grade,
proliferation, invasiveness and AFP levels were significantly
decreased.
Other LCSC targets and their applications in
tumor therapy
Delta-like 1 non-canonical Notch ligand 1 (DLK1) is
a progenitor marker in fetal liver and serves an important role in
the carcinogenesis of HCC. Tanimizu et al (102) in order to isolate and characterize
hepatobrobocytes, used the signal sequence trap method to search
for cell surface antigens expressed in mouse fetal hepatocytes.
They demonstrated that DLK1 (also known as Pref-1) was highly
expressed in fetal liver and reported that most of the
colony-forming DLK1+ cells could differentiate into
hepatocytes and bile duct epithelial cells. In addition, 7% of the
colony-forming DLK1+ cells exhibited a high degree of
proliferation, and were able to form a large colony containing
>100 cells following 5 days in culture. When they transplanted
donor DLK1+ cells into recipient spleens, they found
donor-derived hepatocytes in the recipient liver, indicating that
DLK1+ cells were able to differentiate into hepatocytes
in vivo. These results clearly suggest that DLK1 is a liver
hepatocyte marker (102). The
biological behavioral differences between DLK1+ and
DLK1− cells were assessed by growth curve, colony
formation assay, spherical colony formation, chemical resistance
and in vivo tumorigenicity. Knockdown of DLK1 reduced the
malignancy of HCC cells and may kill LCSCs directly (103), suggesting that DLK1 may be a
potential therapeutic target for LCSCs.
Assis et al (104) reported that CD24+ HCC
cells were highly important in the maintenance, self-renewal,
differentiation and metastasis of chemotherapy-tolerant HCC cell
xenografts, significantly affected the clinical prognosis, and
tumor recurrence following chemotherapy. The authors used
experiments based on lentivirus knockdowns and demonstrated that
CD24 is a functional LCSC marker that is regulated by signal
transducer and activator of transcription 3-mediated NANOG homeobox
regulation to generate CSCs. These results suggested that the CD24
cascade in LCSCs may provide an attractive therapeutic target for
HCC.
De Francesco et al (105) isolated the population of cells with
CSC properties and labeled the calcium channel α2δ1 in primary
hepatocellular carcinoma and their surgical margins using the
monoclonal antibody 1B50-1. It was demonstrated that α2δ1 serves an
important role in regulating the calcium oscillation amplitude,
which is important in maintaining the properties of CSCs. 1B50-1
can bind α2δ1 in CSCs and may have potential as a drug against HCC
by targeting α2δ1.
Recent studies have demonstrated that ICAM-1 is
expressed on a variety of stem cells, including bone marrow
mesenchymal stem cells, adipose-derived stem cells, periodontal
ligament stem cells and placental mesenchymal stem cells (103–106).
Based on the above findings, intercellular adhesion molecule-1
(ICAM-1) is also considered to be one of the surface markers of
LCSCs. Liu et al (107)
measured the sphere formation and tumor formation abilities of
ICAM-1+ cells in vivo and in vitro,
respectively. They also used a specific targeting system that
inhibited ICAM-1 expression in HBV transgenic mice (M-TgHBV) to
study whether inhibition of ICAM-1 expression reduced the incidence
and metastasis of tumors in vivo. ICAM-1 was demonstrated to
be significantly expressed in HCC tumor cell lines, tumor tissue
from patients or transgenic mice, and in circulating tumor cells
from patients. Compared with ICAM-1− tumor cells,
ICAM-1+ tumor cells exhibited greater tumorigenic
ability and increased expression of stem cell-related genes.
Specific inhibition of ICAM-1 reduced tumor formation and
metastasis in M-TgHBV mice. Increased numbers of
CD45−/ICAM-1+ cells in blood samples from
patients with HCC was an indicator of poor prognosis. Finally, this
review also reported that ICAM-1 expression was regulated by the
stem cell transcription factor Nanog.
Barclay and Brown (108) reported that CD47, a widely expressed
integrin-related protein, was upregulated in LCSCs. Since CD47 acts
as a ligand for signal-regulatory protein α (SIRPα), which is
mainly expressed on phagocytic cells (including macrophages and
dendritic cells), the activation of CD47 receptors can initiate a
signal transduction cascade and inhibit macrophage cell
phagocytosis (108–112). Majeti et al (113) demonstrated that CD47 expression in
leukemia stem cells was increased compared with normal controls,
and high expression of CD47 was associated with poor overall
survival in three independent adult AML patients. In addition, the
monoclonal antibody CD47 can cause leukemia stem cells to be
engulfed by macrophages. Anti-CD47 antibody was used to target AML
LSC in human AML LSC-transplanted mice, and the results
demonstrated that high expression of CD47 is an independent factor
indicating poor prognosis, and may be used as a target for the
treatment of AML. Chao et al (114) demonstrated that calreticulin is a
major pre-phagocytic signal in several human cancers. It provides
an explanation for the role of anti-CD47 antibody in selectively
targeting tumor cells and highlights the balance between
phagocytosis and anti-phagocytosis in hepatocellular carcinoma
immune escape. Willingham et al (115) blocked CD47 by use of targeting
monoclonal antibodies and demonstrated that inhibition of
macrophage phagocytosis by CD47 was relieved in in vitro
experiments. Then, they established a tumor model by transplanting
human tumor cells into immunodeficient mice, and treated them with
the anti-CD47 antibody. The results indicated that increased
treatment duration extended survival in mice. Treatment of larger
tumors with the anti-CD47 antibody inhibited tumor growth and
metastasis. Anti-CD47 antibodies have potential effects on the
treatment of smaller tumors, as well. The results demonstrated that
all human tumor cells require the expression of CD47 to inhibit the
innate immune monitoring and clearance of phagocytic cells, while
CD47 is a widely expressed marker in all cancers that help tumor
cells escape from phagocytosis and clearance. Thus, CD47 may be an
effective target for the treatment of cancer. Lee et al
(116) revealed that transplantation
of freshly isolated CD47+ cells in non-obese
diabetic/severe combined immunodeficiency (NOD/SCID) mice exhibit
strong tumorigenicity, self-renewal and distant metastasis. CD47
mRNA is preferentially expressed in
CD133+/CD24+ LCSCs. In addition, the
increased expression level of CD47 mRNA in HCC clinical samples is
positively correlated with patient survival. Knockout of CD47 using
lentivirus-based short hairpin RNA (shRNA) inhibited the
characteristics of stem cells, suggesting that CD47 has a key role
in regulating the stem cell characteristics of HCC. In addition,
cathepsin S (CTSS) is a downstream effector of CD47, which is
preferentially secreted by CD47+ HCC cells and could
regulate the function of hepatic CSCs by activating
protease-activated receptor 2 (PAR2) through autocrine pathways.
Clinically, the serum level of CTSS was significantly associated
with advanced tumor behavior in human HCC. HCC cell lines and
patient-derived xenograft models were established and the CTSS/PAR2
signaling pathway was blocked by the morpholino approach to achieve
chemosensitization effects. This review elucidates the signal
transduction function of CD47 and its role in the pathogenesis of
cancer through the CTSS/PAR2 pathway, suggesting a novel target in
HCC treatment.
Lei et al (117) used the lysine-specific demethylase
1A/prickle planar cell polarity protein 1/adenomatous polyposis
coli/β-catenin signal axis as a novel molecular circuit to regulate
the hepatocyte properties and chemoresistance of Lgr5+
LCSCs in liver, and the results confirmed that this signal axis may
be used as a target for chemotherapy sensitization of liver
cancer.
Conclusions and perspectives
Given the lack of sensitivity of liver cancer to
radiochemotherapy, current treatments of liver cancer include
surgical procedures, interventional therapy (including TACE,
microwave ablation, and particle implantation therapy), and
biological therapy (including immunotherapy and gene therapy).
However, even surgery-based comprehensive treatment cannot prevent
HCC recurrence and metastasis. Exploration of possible targeted
therapies towards LCSCs may offer the only way to break through the
bottleneck of HCC treatment. Current targeted therapy strategies
for HCC include the inhibition of LCSC proliferation and induction
of apoptosis; induction of LCSC differentiation to improve
sensitivity to radiochemotherapy; and destruction of the LCSC
microenvironment. Furthermore, direct targeting of LCSC surface
markers, including CD133, CD90 and EpCAM, may represent another
research direction. Although the molecular targeted drug,
sorafenib, alone or in combination may inhibit the progress of HCC,
drug resistance occurs fast and the numbers of CD90+
cells are not reduced. Therefore, radical treatment of HCC should
begin by eliminating the stem cells. Although various LCSC surface
markers have been identified, LCSCs of high purity cannot be
independently isolated using only one molecular marker, and some
regulation mechanisms of stem cells remain unclear. Findings on
LCSCs have been mainly obtained from in vitro experiments.
Without examining the role of LCSCs within their microenvironment
in the body, it is unclear whether the direct application of these
results to the human body will work effectively. Further studies
are therefore urgently required in order to develop efficient novel
treatments for liver cancer.
References
|
1
|
Fan ST, Lo Mau C, Poon RT, Yeung C, Liu
Leung C, Yuen WK, Ming LC, Ng KK and Chan Ching S: Continuous
improvement of survival outcomes of resection of hepatocellular
carcinoma: A 20-year experience. Ann Surg. 253:745–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woerns MA and Galle PR: Future
perspectives in hepatocellular carcinoma. Digest Liver Dis. 42
Suppl 3:S302–S309. 2010. View Article : Google Scholar
|
|
4
|
Rountree CB, Mishra L and Willenbring H:
Stem cells in liver diseases and cancer: Recent advances on the
path to new therapies. Hepatology. 55:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji J and Wang XW: Clinical implications of
cancer stem cell biology in hepatocellular carcinoma. Semin Oncol.
39:461–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skubitz AP, Taras EP, Boylan KL, Waldron
NN, Oh S, Panoskaltsis-Mortari A and Vallera DA: Targeting CD133 in
an in vivo ovarian cancer model reduces ovarian cancer progression.
Gynecol Oncol. 130:579–587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Liu Q, Xiao J and Du J:
EpCAM-antibody-labeled noncytotoxic polymer vesicles for cancer
stem cells-targeted delivery of anticancer drug and siRNA.
Biomacromolecules. 16:1695–1705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lawson DA, Bhakta NR, Kessenbrock K,
Prummel KD, Yu Y, Takai K, Zhou A, Eyob H, Balakrishnan S, Wang CY,
et al: Single-cell analysis reveals a stem-cell program in human
metastatic breast cancer cells. Nature. 526:131–135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y, Fan Y, Qi Y, Liu D, Wu K, Wen F
and Zhao S: Side population cells separated from A549 lung cancer
cell line possess cancer stem cell-like properties and inhibition
of autophagy potentiates the cytotoxic effect of cisplatin. Oncol
Rep. 34:929–935. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iacopino F, Angelucci C, Piacentini R,
Biamonte F, Mangiola A, Maira G, Grassi C and Sica G: Isolation of
cancer stem cells from three human glioblastoma cell lines:
Characterization of two selected clones. Plos One. 9:e1051662014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steiner JW, Perz ZM and Taichman LB: Cell
population dynamics in the liver. A review of quantitative
morphological techniques applied to the study of physiological and
pathological growth. Exp Mol Pathol. 5:146–181. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turner R, Lozoya O, Wang Y, Cardinale V,
Gaudio E, Alpini G, Mendel G, Wauthier E, Barbier C, Alvaro D and
Reid LM: Human hepatic stem cell and maturational liver lineage
biology. Hepatology. 53:1035–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Foster M, Al-Dhalimy M, Lagasse E,
Finegold M and Grompe M: The origin and liver repopulating capacity
of murine oval cells. Proc Natl Acad Sci USA. 100 Suppl
1:S11881–S11888. 2003. View Article : Google Scholar
|
|
14
|
Libbrecht L, De Vos R, Cassiman D, Desmet
V, Aerts R and Roskams T: Hepatic progenitor cells in
hepatocellular adenomas. Am J Surg Pathol. 25:1388–1396. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Crosby HA, Hubscher SG, Joplin RE, Kelly
DA and Strain AJ: Immunolocalization of OV-6, a putative progenitor
cell marker in human fetal and diseased pediatric liver.
Hepatology. 28:980–985. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu
LX, Zhang SH, Huang DD, Tang L, Kong XN, et al: Wnt/beta-catenin
signaling contributes to activation of normal and tumorigenic liver
progenitor cells. Cancer Res. 68:4287–4295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang W, Wang C, Lin Y, Liu Q, Yu LX, Tang
L, Yan HX, Fu J, Chen Y, Zhang HL, et al: OV6+
tumor-initiating cells contribute to tumor progression and invasion
in human hepatocellular carcinoma. J Hepatol. 57:613–620. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Navarro-Alvarez N, Soto-Gutierrez A and
Kobayashi N: Hepatic stem cells and liver development. Methods Mol
Biol. 640:181–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chiba T, Kita K, Zheng YW, Yokosuka O,
Saisho H, Iwama A, Nakauchi H and Taniguchi H: Side population
purified from hepatocellular carcinoma cells harbors cancer stem
cell-like properties. Hepatology. 44:240–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu LL, Fu D, Ma Y and Shen XZ: The power
and the promise of liver cancer stem cell markers. Stem Cells Dev.
20:2023–2030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gournay J, Auvigne I, Pichard V, Ligeza C,
Bralet MP and Ferry N: In vivo cell lineage analysis during
chemical hepatocarcinogenesis in rats using retroviral-mediated
gene transfer: Evidence for dedifferentiation of mature
hepatocytes. Lab Invest. 82:781–788. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sell S: Heterogeneity and plasticity of
hepatocyte lineage cells. Hepatology. 33:738–750. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dumble ML, Croager EJ, Yeoh GC and Quail
EA: Generation and characterization of p53 null transformed hepatic
progenitor cells: Oval cells give rise to hepatocellular carcinoma.
Carcinogenesis. 23:435–445. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Michalopoulos GK and DeFrances MC: Liver
regeneration. Science. 276:60–66. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shafritz DA, Oertel M, Menthena A,
Nierhoff D and Dabeva MD: Liver stem cells and prospects for liver
reconstitution by transplanted cells. Hepatology. 43:S89–S98. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao Z and Mishra L: Cancer stem cells and
hepatocellular carcinoma. Cancer Biol Ther. 8:1691–1698. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Durnez A, Verslype C, Nevens F, Fevery J,
Aerts R, Pirenne J, Lesaffre E, Libbrecht L, Desmet V and Roskams
T: The clinicopathological and prognostic relevance of cytokeratin
7 and 19 expression in hepatocellular carcinoma. A possible
progenitor cell origin. Histopathology. 49:138–151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeh CT, Kuo CJ, Lai MW, Chen TC, Lin CY,
Yeh TS and Lee WC: CD133-positive hepatocellular carcinoma in an
area endemic for hepatitis B virus infection. BMC Cancer.
9:3242009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okumoto K, Saito T, Haga H, Hattori E,
Ishii R, Karasawa T, Suzuki A, Misawa K, Sanjo M, Ito JI, et al:
Characteristics of rat bone marrow cells differentiated into a
liver cell lineage and dynamics of the transplanted cells in the
injured liver. J Gastroenterol. 41:62–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haraguchi N, Utsunomiya T, Inoue H, Tanaka
F, Mimori K, Barnard GF and Mori M: Characterization of a side
population of cancer cells from human gastrointestinal system. Stem
Cells. 24:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maugeri-Saccà M, Vigneri P and De Maria R:
Cancer stem cells and chemosensitivity. Clin Cancer Res.
17:4942–4947. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu C, Li H, Li J, Zhu Z, Yin S, Hao X, Yao
M, Zheng S and Gu J: Analysis of ABCG2 expression and side
population identifies intrinsic drug efflux in the HCC cell line
MHCC-97L and its modulation by Akt signaling. Carcinogenesis.
29:2289–2297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma S, Chan KW, Lee TK, Tang KH, Wo JY,
Zheng BJ and Guan XY: Aldehyde dehydrogenase discriminates the
CD133 liver cancer stem cell populations. Mol Cancer Res.
6:1146–1153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J and
Li J: Cancer stem/progenitor cells are highly enriched in
CD133+CD44+ population in hepatocellular
carcinoma. Int J Cancer. 126:2067–2078. 2010.PubMed/NCBI
|
|
37
|
Ma S, Lee TK, Zheng BJ, Chan KW and Guan
XY: CD133+ HCC cancer stem cells confer chemoresistance
by preferential expression of the Akt/PKB survival pathway.
Oncogene. 27:1749–1758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Piao LS, Hur W, Kim TK, Hong SW, Kim SW,
Choi JE, Sung PS, Song MJ, Lee BC, Hwang D and Yoon SK:
CD133+ liver cancer stem cells modulate radioresistance
in human hepatocellular carcinoma. Cancer Lett. 315:129–137. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xin HW, Ambe CM, Hari DM, Wiegand GW,
Miller TC, Chen JQ, Anderson AJ, Ray S, Mullinax JE, Koizumi T, et
al: Label-retaining liver cancer cells are relatively resistant to
sorafenib. Gut. 62:1777–1786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Haraguchi N, Ishii H, Mimori K, Tanaka F,
Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, et al:
CD13 is a therapeutic target in human liver cancer stem cells. J
Clin Invest. 120:3326–3339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma S, Tang KH, Chan YP, Lee TK, Kwan PS,
Castilho A, Ng I, Man K, Wong N, To KF, et al: miR-130b promotes
CD133(+) liver tumor-initiating cell growth and self-renewal via
tumor protein 53-induced nuclear protein 1. Cell Stem Cell.
7:694–707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang J, Luo N, Luo Y, Peng Z, Zhang T and
Li S: MicroRNA-150 inhibits human CD133-positive liver cancer stem
cells through negative regulation of the transcription factor
c-Myb. Int J Oncol. 40:747–756. 2012.PubMed/NCBI
|
|
43
|
Fan ST, Yang ZF, Ho DW, Ng MN, Yu WC and
Wong J: Prediction of posthepatectomy recurrence of hepatocellular
carcinoma by circulating cancer stem cells: A prospective study.
Ann Surg. 254:569–576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Philip PA, Mooney M, Jaffe D, Eckhardt G,
Moore M, Meropol N, Emens L, O'Reilly E, Korc M, Ellis L, et al:
Consensus report of the national cancer institute clinical trials
planning meeting on pancreas cancer treatment. J Clin Oncol.
27:5660–5669. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park CY, Tseng D and Weissman IL: Cancer
stem cell-directed therapies: Recent data from the laboratory and
clinic. Mol Ther. 17:219–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yamashita T, Honda M, Nakamoto Y, Baba M,
Nio K, Hara Y, Zeng SS, Hayashi T, Kondo M, Takatori H, et al:
Discrete nature of EpCAM+ and CD90+ cancer
stem cells in human hepatocellular carcinoma. Hepatology.
57:1484–1497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO,
Zheng BJ and Guan XY: Identification and characterization of
tumorigenic liver cancer stem/progenitor cells. Gastroenterology.
132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai
P, Chu PW, Lam CT, Poon RT and Fan ST: Significance of
CD90+ cancer stem cells in human liver cancer. Cancer
Cell. 13:153–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mima K, Okabe H, Ishimoto T, Hayashi H,
Nakagawa S, Kuroki H, Watanabe M, Beppu T, Tamada M, Nagano O, et
al: CD44s regulates the TGF-β-mediated mesenchymal phenotype and is
associated with poor prognosis in patients with hepatocellular
carcinoma. Cancer Res. 72:3414–3423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wilson GS, Hu Z, Duan W, Tian A, Wang XM,
McLeod D, Lam V, George J and Qiao L: Efficacy of using cancer stem
cell markers in isolating and characterizing liver cancer stem
cells. Stem Cells Dev. 22:2655–2664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
52
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fang D, Nguyen TK, Leishear K, Finko R,
Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE and Herlyn M: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Suetsugu A, Nagaki M, Aoki H, Motohashi T,
Kunisada T and Moriwaki H: Characterization of CD133+
hepatocellular carcinoma cells as cancer stem/progenitor cells.
Biochem Biophys Res Commun. 351:820–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tchoghandjian A, Baeza N, Colin C, Cayre
M, Metellus P, Beclin C, Ouafik L and Figarella-Branger D: A2B5
cells from human glioblastoma have cancer stem cell properties.
Brain Pathol. 20:211–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Miraglia S, Godfrey W, Yin AH, Atkins K,
Warnke R, Holden JT, Bray RA, Waller EK and Buck DW: A novel
five-transmembrane hematopoietic stem cell antigen: Isolation,
characterization and molecular cloning. Blood. 90:5013–5021.
1997.PubMed/NCBI
|
|
57
|
Shmelkov SV, Jun L, St Clair R, McGarrigle
D, Derderian CA, Usenko JK, Costa C, Zhang F, Guo X and Rafii S:
Alternative promoters regulate transcription of the gene that
encodes stem cell surface protein AC133. Blood. 103:2055–2061.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cioffi M, D'Alterio C, Camerlingo R,
Tirino V, Consales C, Riccio A, Ieranó C, Cecere SC, Losito NS,
Greggi S, et al: Identification of a distinct population of
CD133(+)CXCR4(+) cancer stem cells in ovarian cancer. Sci Rep.
5:103572015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Swaminathan SK, Roger E, Toti U, Niu L,
Ohlfest JR and Panyam J: CD133-targeted paclitaxel delivery
inhibits local tumor recurrence in a mouse model of breast cancer.
J Control Release. 171:280–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rountree CB, Ding W, He L and Stiles B:
Expansion of CD133-expressing liver cancer stem cells in
liver-specific phosphatase and tensin homolog deleted on chromosome
10-deleted mice. Stem Cells. 27:290–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yin S, Li J, Hu C, Chen X, Yao M, Yan M,
Jiang G, Ge C, Xie H, Wan D, et al: CD133 positive hepatocellular
carcinoma cells possess high capacity for tumorigenicity. Int J
Cancer. 120:1444–1450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kohga K, Tatsumi T, Takehara T, Tsunematsu
H, Shimizu S, Yamamoto M, Sasakawa A, Miyagi T and Hayashi N:
Expression of CD133 confers malignant potential by regulating
metalloproteinases in human hepatocellular carcinoma. J Hepatol.
52:872–879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yao J, Zhang T, Ren J, Yu M and Wu G:
Effect of CD133/prominin-1 antisense oligodeoxynucleotide on in
vitro growth characteristics of Huh-7 human hepatocarcinoma cells
and U251 human glioma cells. Oncol Rep. 22:781–787. 2009.PubMed/NCBI
|
|
64
|
Song W, Li H, Tao K, Li R, Song Z, Zhao Q,
Zhang F and Dou K: Expression and clinical significance of the stem
cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract.
62:1212–1218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Salnikov AV, Kusumawidjaja G, Rausch V,
Bruns H, Gross W, Khamidjanov A, Ryschich E, Gebhard MM,
Moldenhauer G, Büchler MW, et al: Cancer stem cell marker
expression in hepatocellular carcinoma and liver metastases is not
sufficient as single prognostic parameter. Cancer Lett.
275:185–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li J, Chen JN, Zeng TT, He F, Chen SP, Ma
S, Bi J, Zhu XF and Guan XY: CD133+ liver cancer stem
cells resist interferon-gamma-induced autophagy. BMC Cancer.
16:152016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Prasad S, Gaedicke S, Machein M, Mittler
G, Braun F, Hettich M, Firat E, Klingner K, Schuler J, Wider D, et
al: Effective eradication of glioblastoma stem cells by local
application of an AC133/CD133-specific T-cell-engaging antibody and
CD8 T cells. Cancer Res. 75:2166–2176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Smith LM, Nesterova A, Ryan MC, Duniho S,
Jonas M, Anderson M, Zabinski RF, Sutherland MK, Gerber HP, Van
Orden KL, et al: CD133/prominin-1 is a potential therapeutic target
for antibody-drug conjugates in hepatocellular and gastric cancers.
Br J Cancer. 99:100–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lang J, Lan X, Liu Y, Jin X, Wu T, Sun X,
Wen Q and An R: Targeting cancer stem cells with an 131I-labeled
anti-AC133 monoclonal antibody in human colorectal cancer
xenografts. Nucl Med Biol. 42:505–512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sun J, Zhang C, Liu G, Liu H, Zhou C, Lu
Y, Zhou C, Yuan L and Li X: A novel mouse CD133 binding-peptide
screened by phage display inhibits cancer cell motility in vitro.
Clin Exp Metastasis. 29:185–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang J and Zhang Q: Targeting glioblastoma
cancer stem cell marker CD133 by heptapeptide-modified DSPE-PEG
micelles. J Chin Pharm Sci. 24:2015. View Article : Google Scholar
|
|
72
|
Ashmun RA and Look AT: Metalloprotease
activity of CD13/aminopeptidase N on the surface of human myeloid
cells. Blood. 75:462–469. 1990.PubMed/NCBI
|
|
73
|
Look AT, Ashmun RA, Shapiro LH and Peiper
SC: Human myeloid plasma membrane glycoprotein CD13 (gp150) is
identical to aminopeptidase N. J Clin Invest. 83:1299–1307. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bralet MP, Pichard V and Ferry N:
Demonstration of direct lineage between hepatocytes and
hepatocellular carcinoma in diethylnitrosamine-treated rats.
Hepatology. 36:623–630. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Guzman-Rojas L, Rangel R, Salameh A,
Edwards JK, Dondossola E, Kim YG, Saghatelian A, Giordano RJ,
Kolonin MG, Staquicini FI, et al: Cooperative effects of
aminopeptidase N (CD13) expressed by nonmalignant and cancer cells
within the tumor microenvironment. Proc Natl Acad Sci USA.
109:1637–1642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Munoz R, Man S, Shaked Y, Lee CR, Wong J,
Francia G and Kerbel RS: Highly efficacious nontoxic preclinical
treatment for advanced metastatic breast cancer using combination
oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res.
66:3386–3391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Christ B, Stock P and Dollinger MM: CD13:
Waving the flag for a novel cancer stem cell target. Hepatology.
53:1388–1390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Reif AE and Allen JM: The AKR thymic
antigen and its distribution in leukemias and nervous tissues. J
Exp Med. 120:413–433. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Schlesinger M and Yron I: Antigenic
changes in lymph-node cells after administration of antiserum to
thymus cells. Science. 164:1412–1413. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ades EW, Zwerner RK, Acton RT and Balch
CM: Isolation and partial characterization of the human homologue
of Thy-1. J Exp Med. 151:400–406. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lu JW, Chang JG, Yeh KT, Chen RM, Tsai JJ
and Hu RM: Overexpression of Thy1/CD90 in human hepatocellular
carcinoma is associated with HBV infection and poor prognosis. Acta
Histochem. 113:833–838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yang ZF, Ngai P, Ho DW, Yu WC, Ng MN, Lau
CK, Li ML, Tam KH, Lam CT, Poon RT and Fan ST: Identification of
local and circulating cancer stem cells in human liver cancer.
Hepatology. 47:919–928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen WC, Chang YS, Hsu HP, Yen MC, Huang
HL, Cho CY, Wang CY, Weng TY, Lai PT, Chen CS, et al: Therapeutics
targeting CD90-integrin-AMPK-CD133 signal axis in liver cancer.
Oncotarget. 6:42923–42937. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Noto Z, Yoshida T, Okabe M, Koike C, Fathy
M, Tsuno H, Tomihara K, Arai N, Noguchi M and Nikaido T: CD44 and
SSEA-4 positive cells in an oral cancer cell line HSC-4 possess
cancer stem-like cell characteristics. Oral Oncol. 49:787–795.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Pesarrodona M, Ferrer-Miralles N, Unzueta
U, Gener P, Tatkiewicz W, Abasolo I, Ratera I, Veciana J, Schwartz
S Jr, Villaverde A and Vazquez E: Intracellular targeting of
CD44+ cells with self-assembling, protein only
nanoparticles. Int J Pharm. 473:286–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kimura O, Takahashi T, Ishii N, Inoue Y,
Ueno Y, Kogure T, Fukushima K, Shiina M, Yamagiwa Y, Kondo Y, et
al: Characterization of the epithelial cell adhesion molecule
(EpCAM)+ cell population in hepatocellular carcinoma
cell lines. Cancer Sci. 101:2145–2155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang S, Wu CC, Fecteau JF, Cui B, Chen L,
Zhang L, Wu R, Rassenti L, Lao F, Weigand S and Kipps TJ: Targeting
chronic lymphocytic leukemia cells with a humanized monoclonal
antibody specific for CD44. Proc Natl Acad Sci USA. 110:6127–6132.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cho JH, Lee SC, Ha NR, Lee SJ and Yoon MY:
A novel peptide-based recognition probe for the sensitive detection
of CD44 on breast cancer stem cells. Mol Cell Probes. 29:492–499.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Park HY, Lee KJ, Lee SJ and Yoon MY:
Screening of peptides bound to breast cancer stem cell specific
surface marker CD44 by phage display. Mol Biotechnol. 51:212–220.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Munz M, Baeuerle PA and Gires O: The
emerging role of EpCAM in cancer and stem cell signaling. Cancer
Res. 69:5627–5629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yamashita T, Ji J, Budhu A, Forgues M,
Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, et al:
EpCAM-positive hepatocellular carcinoma cells are tumor-initiating
cells with stem/progenitor cell features. Gastroenterology.
136:1012–1024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Schmelzer E and Reid LM: EpCAM expression
in normal, non-pathological tissues. Front Biosci. 13:3096–3100.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Terris B, Cavard C and Perret C: EpCAM, a
new marker for cancer stem cells in hepatocellular carcinoma. J
Hepatol. 52:280–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cioffi M, Dorado J, Baeuerle PA and
Heeschen C: EpCAM/CD3-bispecific T-cell engaging antibody MT110
eliminates primary human pancreatic cancer stem cells. Clin Cancer
Res. 18:465–474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Alibolandi M, Ramezani M, Sadeghi F,
Abnous K and Hadizadeh F: Epithelial cell adhesion molecule aptamer
conjugated PEG-PLGA nanopolymersomes for targeted delivery of
doxorubicin to human breast adenocarcinoma cell line in vitro. Int
J Pharm. 479:241–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yamashita T, Forgues M, Wang W, Kim JW, Ye
Q, Jia H, Budhu A, Zanetti KA, Chen Y, Qin LX, et al: EpCAM and
alpha-fetoprotein expression defines novel prognostic subtypes of
hepatocellular carcinoma. Cancer Res. 68:1451–1461. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gires O and Bauerle PA: EpCAM as a target
in cancer therapy. J Clin Oncol. 28:e239–e242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kurtz JE and Dufour P: Adecatumumab: An
anti-EpCAM monoclonal antibody, from the bench to the bedside.
Expert Opin Biol Ther. 10:951–958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Bae JS, Noh SJ, Jang KY, Park HS, Chung
MJ, Park CK and Moon WS: Expression and role of epithelial cell
adhesion molecule in dysplastic nodule and hepatocellular
carcinoma. Int J Oncol. 41:2150–2158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Tanimizu N, Nishikawa M, Saito H,
Tsujimura T and Miyajima A: Isolation of hepatoblasts based on the
expression of Dlk/Pref-1. J Cell Sci. 116:1775–1786. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sununliganon L and Singhatanadgit W:
Highly osteogenic PDL stem cell clones specifically express
elevated levels of ICAM1, ITGB1 and TERT. Cytotechnology. 64:53–63.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Assis AC, Carvalho JL, Jacoby BA, Ferreira
RL, Castanheira P, Diniz SO, Cardoso VN, Goes AM and Ferreira AJ:
Time-dependent migration of systemically delivered bone marrow
mesenchymal stem cells to the infarcted heart. Cell Transplant.
19:219–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
De Francesco F, Tirino V, Desiderio V,
Ferraro G, D'Andrea F, Giuliano M, Libondi G, Pirozzi G, De Rosa A
and Papaccio G: Human CD34/CD90 ASCs are capable of growing as
sphere clusters, producing high levels of VEGF and forming
capillaries. PLoS One. 4:e65372009. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Brooke G, Tong H, Levesque JP and Atkinson
K: Molecular trafficking mechanisms of multipotent mesenchymal stem
cells derived from human bone marrow and placenta. Stem Cells Dev.
17:929–940. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Liu S, Li N, Yu X, Xiao X, Cheng K, Hu J,
Wang J, Zhang D, Cheng S and Liu S: Expression of intercellular
adhesion molecule 1 by hepatocellular carcinoma stem cells and
circulating tumor cells. Gastroenterology. 144:1031–1041. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Barclay AN and Brown MH: The SIRP family
of receptors and immune regulation. Nat Rev Immunol. 6:457–464.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Oldenborg PA, Gresham HD and Lindberg FP:
CD47-signal regulatory protein alpha (SIRPalpha) regulates Fcgamma
and complement receptor-mediated phagocytosis. J Exp Med.
193:855–862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Okazawa H, Motegi S, Ohyama N, Ohnishi H,
Tomizawa T, Kaneko Y, Oldenborg PA, Ishikawa O and Matozaki T:
Negative regulation of phagocytosis in macrophages by the
CD47-SHPS-1 system. J Immunol. 174:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Blazar BR, Lindberg FP, Ingulli E,
Panoskaltsis-Mortari A, Oldenborg PA, Iizuka K, Yokoyama WM and
Taylor PA: CD47 (integrin-associated protein) engagement of
dendritic cell and macrophage counterreceptors is required to
prevent the clearance of donor lymphohematopoietic cells. J Exp
Med. 194:541–549. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Oldenborg PA, Zheleznyak A, Fang YF,
Lagenaur CF, Gresham HD and Lindberg FP: Role of CD47 as a marker
of self on red blood cells. Science. 288:2051–2054. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Majeti R, Chao MP, Alizadeh AA, Pang WW,
Jaiswal S, Gibbs KD Jr, van Rooijen N and Weissman IL: CD47 is an
adverse prognostic factor and therapeutic antibody target on human
acute myeloid leukemia stem cells. Cell. 138:286–299. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chao MP, Jaiswal S, Weissman-Tsukamoto R,
Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB,
Raveh T, Park CY, et al: Calreticulin is the dominant
pro-phagocytic signal on multiple human cancers and is
counterbalanced by CD47. Sci Transl Med. 2:63ra942010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Willingham SB, Volkmer JP, Gentles AJ,
Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin
R, Cohen JD, et al: The CD47-signal regulatory protein alpha
(SIRPa) interaction is a therapeutic target for human solid tumors.
Proc Natl Acad Sci USA. 109:6662–6667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lee TK, Cheung VC, Lu P, Lau EY, Ma S,
Tang KH, Tong M, Lo J and Ng IO: Blockade of CD47-mediated
cathepsin S/protease-activated receptor 2 signaling provides a
therapeutic target for hepatocellular carcinoma. Hepatology.
60:179–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lei ZJ, Wang J, Xiao HL, Guo Y, Wang T, Li
Q, Liu L, Luo X, Fan LL, Lin L, et al: Lysine-specific demethylase
1 promotes the stemness and chemoresistance of Lgr5+
liver cancer initiating cells by suppressing negative regulators of
β-catenin signaling. Oncogene. 34:32142015. View Article : Google Scholar : PubMed/NCBI
|