Introduction
Lung cancer is the most common malignant tumor and
the leading cause of death from malignancies. Five-year survival of
the lung cancer is around 15% (1). In
patients with no distant metastases (M0), the status of mediastinal
lymph nodes is crucial for the prognosis of the disease. Two-year
and five-year survival of a patient with metastasis spreading to
the mediastinal lymph nodes (stage IIIA-N2) treated with surgery
and adjuvant chemotherapy is 50 and 22%, respectively (2). TNM classification of malignant tumors,
including lung cancer, is still the most accurate way of estimating
the survival of patients with malignant tumors.
Angiogenesis is defined as the formation of new
blood vessels from preexisting vessels. It is determined by complex
interaction of multiple pro-angiogenic and anti-angiogenic factors.
Angiogenesis is a prerequisite for tumor growth beyond 1–2 mm in
diameter, when tumor cells can no longer be supplied with oxygen
and nutrients by simple diffusion (3,4). In order
to increase in size, tumors undergo an angiogenic switch, where the
action of pro-angiogenic factors leads to angiogenesis and tumor
growth (5). Tumor blood vessels are
irregular in shape and branching, disordered and have fragmented
basement membrane, which makes them permeable to tumor cells and
thus enable metastasis (6).
Angiogenesis is estimated by determining the average density of
tumor blood vessels (MVD).
VEGF-A is the most potent and specific
pro-angiogenic factor. It plays a critical role in tumor growth and
metastasis of epithelial tumors including colorectal cancer, breast
cancer, renal cell cancer, cervical cancer as well as head and neck
tumors and lung cancer (7–11). The binding of VEGF-A to its receptors
triggers multiple signaling cascades resulting in endothelial cell
proliferation, migration and differentiation. VEGF-A mediates
increased vascular permeability promoting tumor dissemination via
circulation. By induction of anti-apoptotic signals, such as bcl-2
and surviving, VEGF-A protects new vasculature from destruction
(12,13).
Although numerous studies point out that MVD is
associated with the outcome of malignant tumors, the significance
of MVD as a prognostic factor in patients with advanced NSCLC, who
were initially surgically treated, was never investigated before
(14). Furthermore, results of
studies evaluating the prognostic significance of VEGF-A in NSCLC
are controversial. High expression of VEGF-A, as a powerful
pro-angiogenic factor, was correlated with high MVD in most studies
(15–17).
In this retrospective study, we evaluated the
prognostic significance of MVD and VEGF-A and their correlation
with clinical parameters in advanced NSCLC (stage IIIA). MVD was
assessed using CD31 and platelet endothelial cell adhesion molecule
(PECAM-1), a protein found on the surface of endothelial cells.
Immunohistochemical analysis was performed to evaluate the
expression of CD 31 using a monoclonal anti-CD31 antibody and
VEGF-A expression using polyclonal anti-VEGF-A antibody.
Materials and methods
Tissues
From the archives of the Department of Pathology in
Zadar General Hospital, 50 formalin fixed, paraffin embedded cancer
specimens were obtained from patients with histologically confirmed
metastases to mediastinal lymph nodes, without distant metastases.
The surgery on these patients was performed at the Department of
Thoracic Surgery, Zadar General Hospital, Croatia between March1,
2007 and March 1, 2009. Samples from patients who died within 2
months of surgery were excluded from the study to eliminate
perioperative complications as the cause of death. No patient
underwent neoadjuvant chemotherapy or radiation therapy.
Histo-pathological properties of tumors were
classified according to the World Health Organization. All patients
received the same chemotherapy protocols postoperatively. The
organization and stage of tumors were determined according to the
guidelines of the International Association for the Study of Lung
Cancer (IASLC) (18).
Immunohistochemical analysis of tumor tissue samples was performed
at the Department of Pathology, University of Rijeka School of
Medicine. Patient survival was followed for a period of two years.
Data on patients' survival were obtained from the Croatian National
Cancer Registry. This study was approved by the Ethics Committee of
Zadar General Hospital.
Immunohistochemical analysis
In each paraffin block, new sections were obtained
and stained with hematoxylin eosin (HE) to determine a
representative tumor area. The selected area of tumor tissue was
transferred to the donor paraffin block and samples were taken from
three different areas, to create tissue microarrays (TMA). With MTA
Booster OI (Alphelys, Plaisir, France), 3 tissue cores, each
measuring 1 mm in diameter, were taken from the marked spot on
donor block and transferred into a new recipient paraffin block
(recipient block microarrays) at given coordinates. The recipient
paraffin block was left overnight at 45°C for tissue cores from the
donor block to interconnect with the recipient block. Four µm thick
sections were cut from the TMA block for immunohistochemical
analysis. After drying, samples were deparaffinized in xylene and
rehydrated in ethanol.
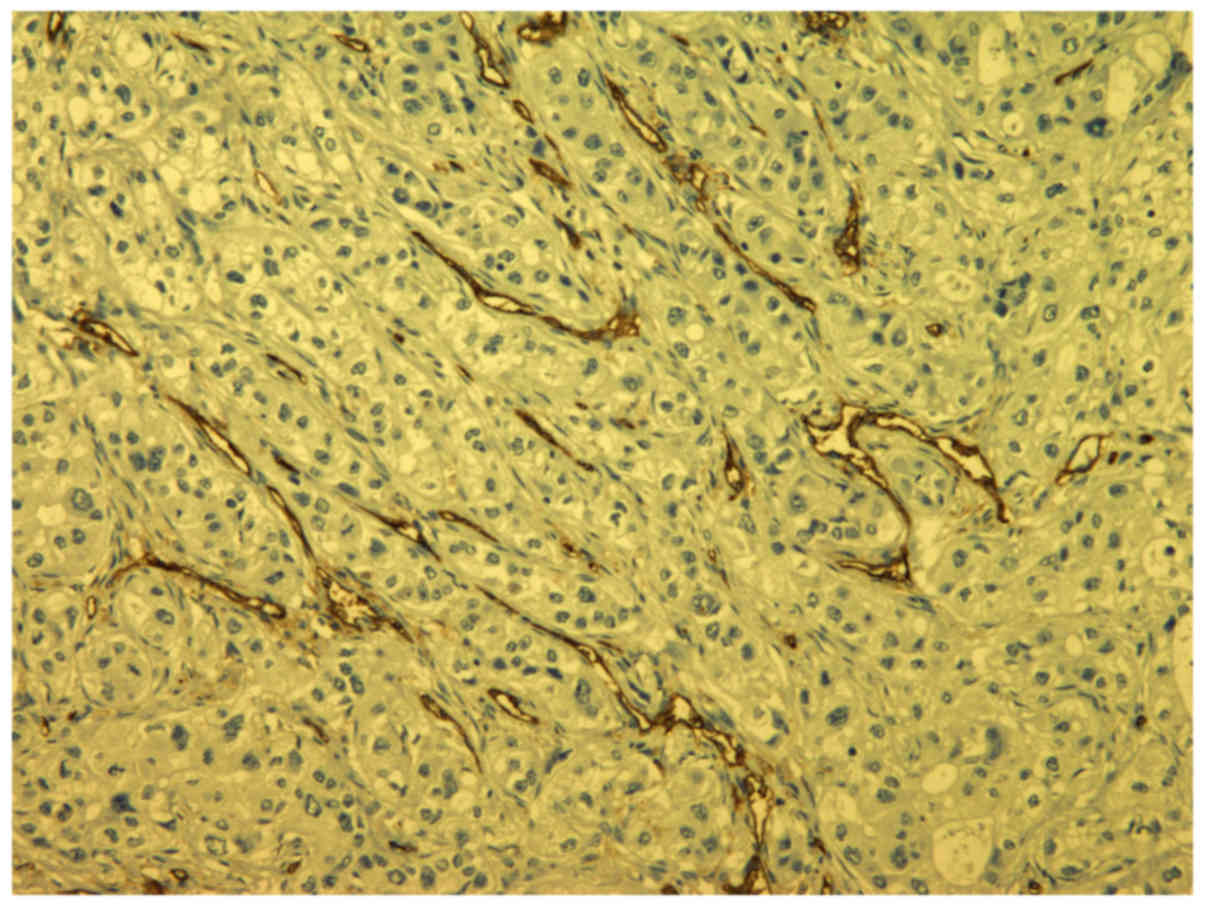
Sections were incubated with anti-CD31 monoclonal
antibodies (1/50 dilution, JC70; NeoMarkers, Freemont, California,
USA) at room temperature for 30 min in order to visualize the blood
vessels. Positive controls were endothelial cells in normal blood
vessels of the lungs.
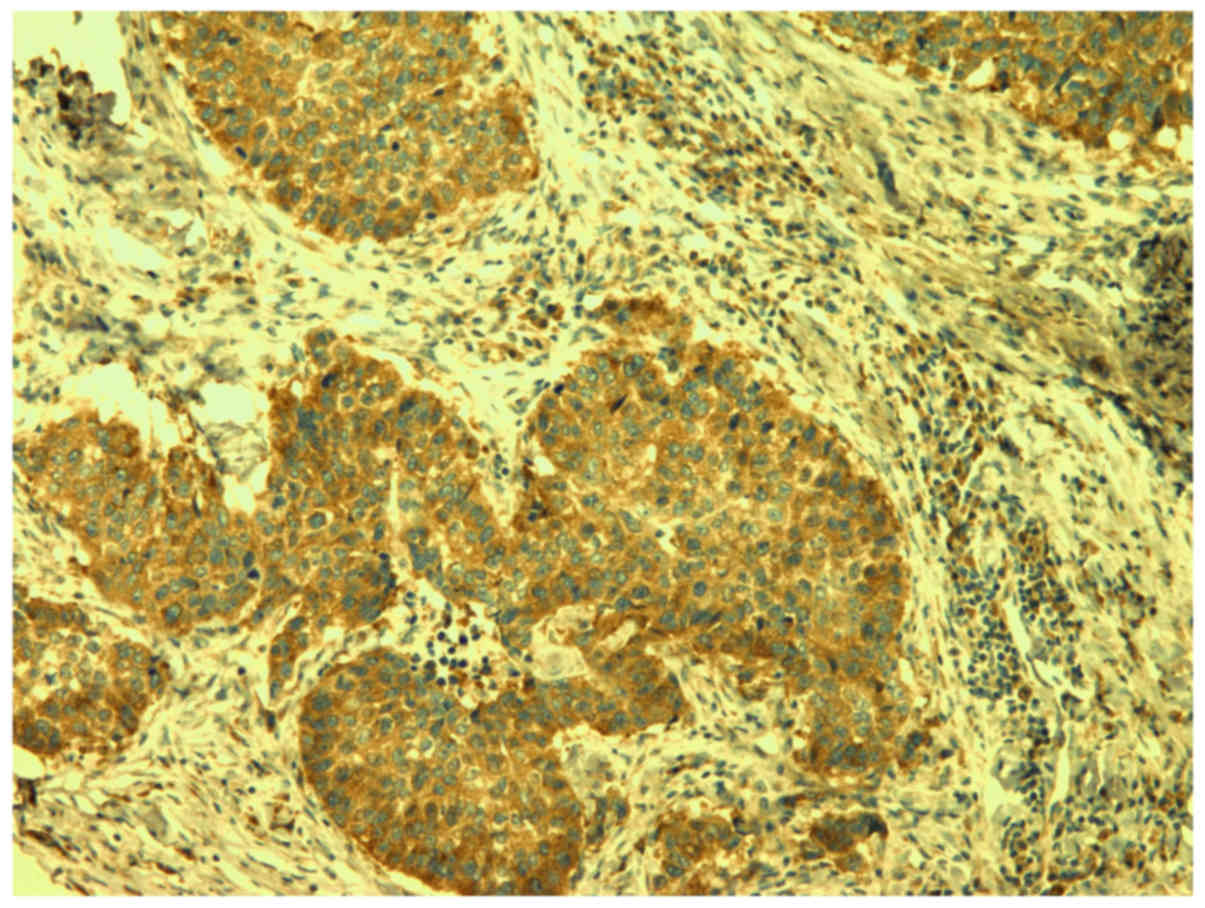
Sections were incubated with the anti-VEGF-A
polyclonal antibodies (A-20, 1/200 dilution; Santa Cruz
Biotechnology, Santa Cruz, California, USA) at room temperature for
two h to determine VEGF-A (Fig.
1).
Microvessel counting was used to evaluate
angiogenesis. Tumor sections stained with CD 31 were examined at
low magnification (×40) to determine areas with the greatest vessel
density (hot spots). Counting of blood vessels to determine MVD was
performed at high magnification (×400) in selected areas (Fig. 2).
Individual endothelial cells or clusters of
endothelial cells with barely visible lumen or no lumen were
considered individual vessels and therefore included in the
analysis.
VEGF-A expression was assessed semi quantitatively
by estimating the percentage of tumor cells stained on tumors
slides. Four groups were formed as follows: 1) Positive staining in
less than 25% of tumor cells; 2) positive staining in 25 to 50% of
tumor cells; 3) positive staining in 50 to 75% of e tumor cells and
4) positive staining in more than 75% of tumor cells. The intensity
of smooth muscle cells' staining in the blood vessels walls was
positive internal control.
‘Statistica 10’ software program (StatSoft, Inc,
Tulsa, Oklahoma, USA) was used for statistical analysis. Chi-square
test with Yeates's correction was used to assess the correlations
between MVD and VEGF-A with clinical parameters and with survival.
Values of P<0.05 were considered statistically significant.
Survival curves were determined by Kaplan-Meier's method.
Results
The average age of patients was 63 years (ranging
between 41 and 80), and there were 9 women and 41 men. There were
28 adenocarcinomas (56%) and 22 squamous cell carcinomas (44%) in
our tissue sample. Macroscopic features of the primary tumors were
estimated as follows: T1 tumors were found in 8 (16%) patients, T2
in 19 patients (38%) and T3 tumors in 23 patients (46%).
Noninvasive and invasive ‘staging’ was conducted with all patients
whereby tumor dissemination in mediastinal lymphatic nodes was not
proved and neither in distant metastases.
The median MVD was 16.5 (range 8–30) and the mean of
MVD values was 16.7, so 17 is taken as cut-off value. Patients were
classified into two groups: With low MVD (<17) and high MVD
(≥17). Low MVD was present in 26 (52%) patients and high MVD in 24
(48%) patients.
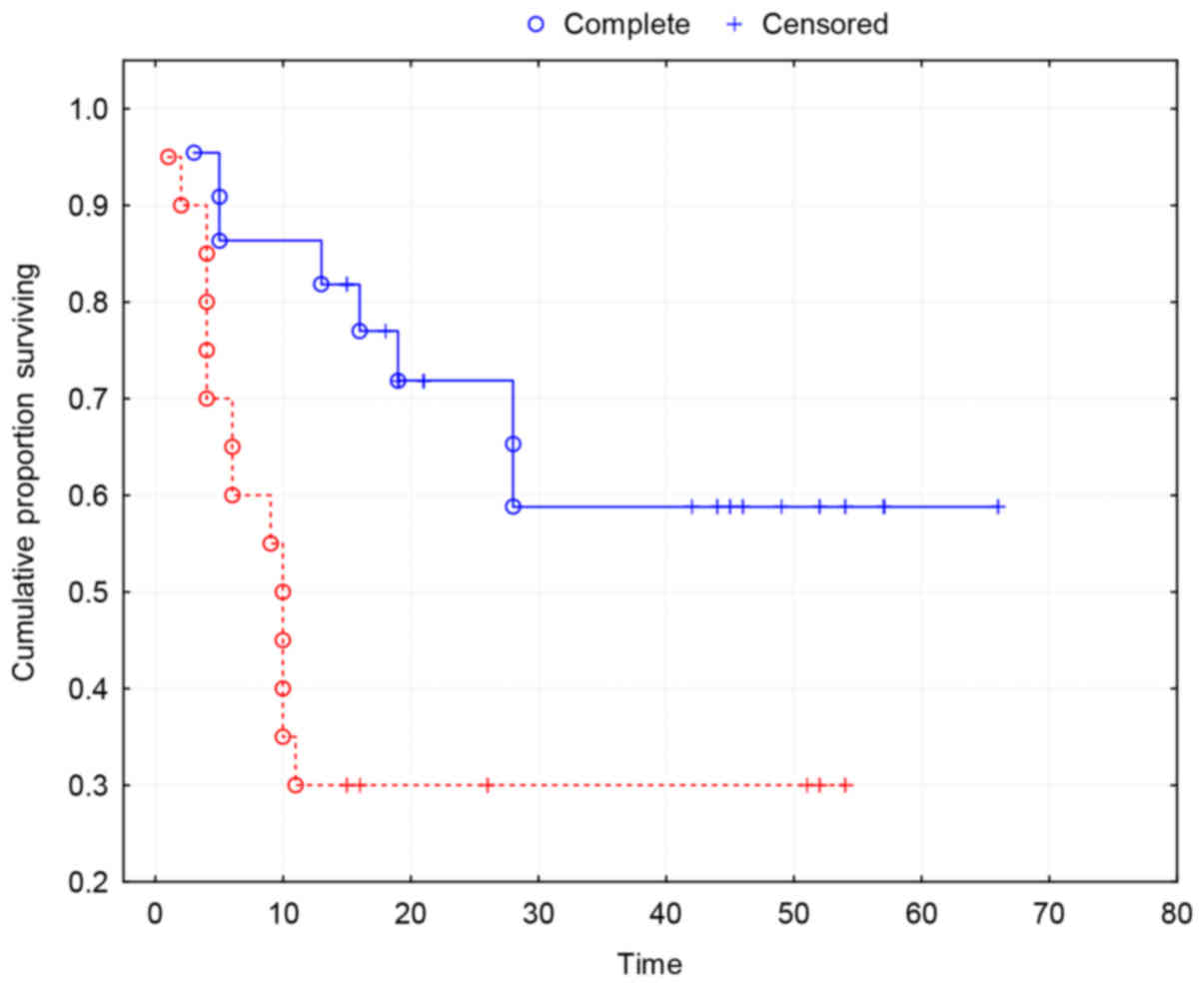
The overall two-year survival of patients in the
study group was 52%. The two-year survival of patients with high
MVD was 21% and of patients with low MVD was 77%. The results
indicate a significantly increased survival of patients with low
MVD (χ2=11.48; P=0.0007) (Table I).
 | Table I.Associations between micro vascular
density, and clinicopathological parameters and survival. |
Table I.
Associations between micro vascular
density, and clinicopathological parameters and survival.
|
| MVD |
|
|---|
|
|
|
|
|---|
| Clinicopathological
parameters | ≥17, n (%) | <17, n (%) | P-value |
|---|
| Age, years |
|
|
|
|
>63 | 9 (18) | 14 (28) | 0.2400 |
|
<63 | 15 (30) | 12 (24) |
|
| Gender |
|
|
|
| Male | 17 (34) | 24 (48) | 0.5900 |
|
Female | 5 (10) | 4 (8) |
|
| pT |
|
|
|
| 1,2 | 12 (24) | 15 (30) | 0.3900 |
| 3 | 13 (26) | 10 (20) |
|
| Histological type of
NSCLC |
|
|
|
|
Adenocarcinoma | 12 (24) | 13 (26) | 1.0000 |
| Squamous
cell carcinoma | 12 (24) | 13 (26) |
|
| Differentiation |
|
|
|
| Good (G1,
2) | 11 (22) | 16 (32) | 0.2600 |
| Poor (G3,
4) | 13 (26) | 10 (20) |
|
| Survival |
|
|
|
| <24
months | 18 (36) | 6 (12) | 0.0007 |
| >24
months | 6 (12) | 20 (40) |
|
| Expression of
VEGF-A |
|
|
|
| 1
(<25%) | 1 (2) | 1 (2) | 0.5900 |
| 2
(25–50%) | 4 (8) | 3 (6) |
|
| 3
(50–75%) | 11 (22) | 13 (26) |
|
| 4
(>75%) | 11 (22) | 6 (12) |
|
None of the standard clinically-relevant parameters
(age, gender, histological type, pT and tumor differentiation) was
significantly associated with MVD (Table
I).
Kaplan-Meier survival curves show a strong
association between MVD and two-year survival (P=0.0035) (Fig. 3).
VEGF-A was highly expressed (positive staining in
more than 50% of tumor cells) in the majority of tumors (68%), but
no association was found between VEGF-A expression and high MVD
(P=0.59) (Table I).
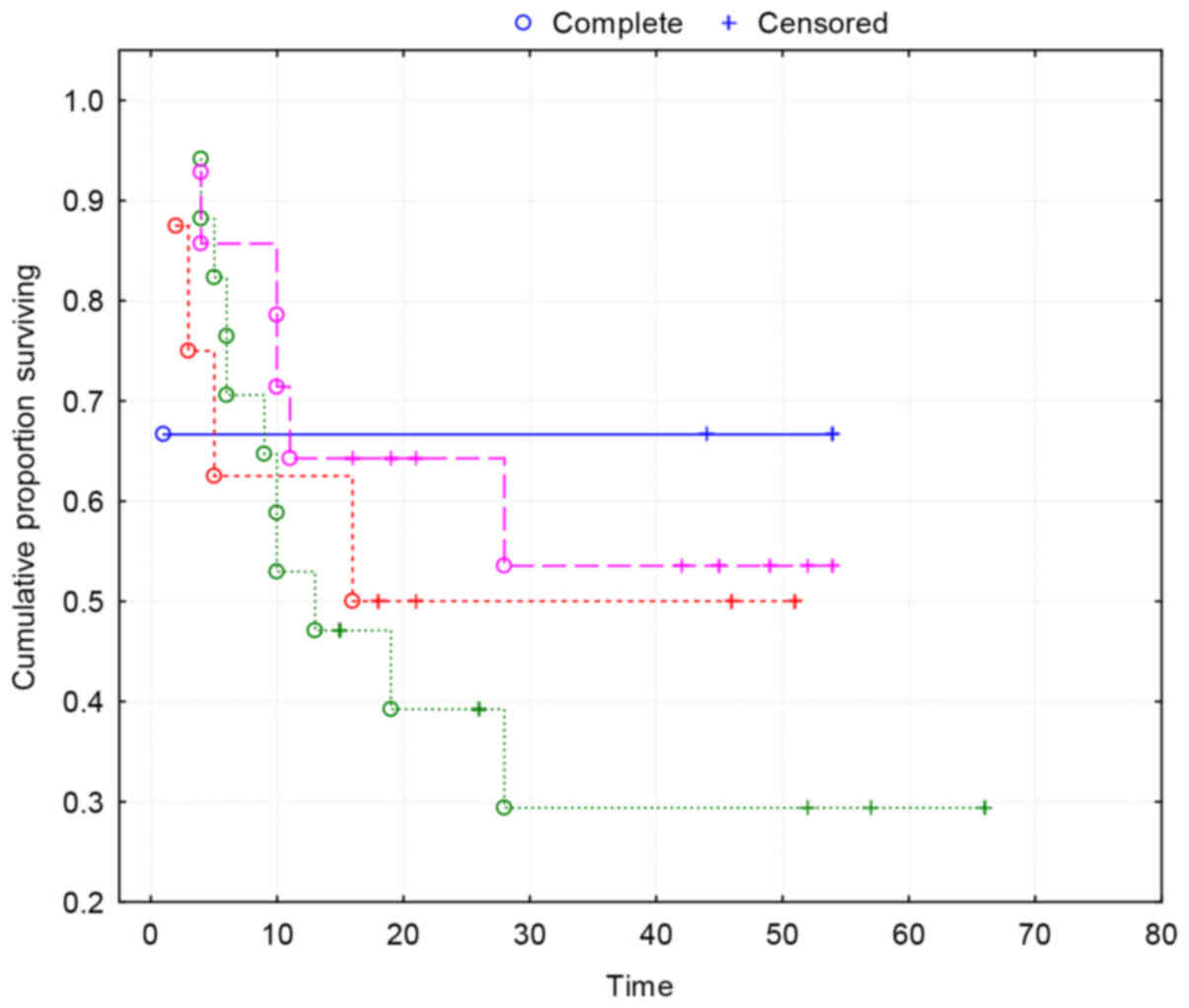
VEGF-A expression was not associated with standard
clinical parameters nor with survival (P>0.05) (Table II).
 | Table II.Associations between vascular
endothelial growth factor-A expression, and clinicopathological
parameters and survival. |
Table II.
Associations between vascular
endothelial growth factor-A expression, and clinicopathological
parameters and survival.
|
| Expression of VEGF-A
(%) |
|
|---|
|
|
|
|
|---|
| Clinicopathological
parameters | Group 1
(<25%) | Group 2 (25–50%) | Group 3 (50–75%) | Group 4
(>75%) | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
>63 | 1 | 7 | 12 | 6 | 0.77 |
|
<63 | 1 | 4 | 10 | 9 |
|
| Gender |
|
|
|
|
|
|
Male | 1 | 8 | 19 | 13 | 0.95 |
|
Female | 1 | 3 | 3 | 2 |
|
| pT |
|
|
|
|
|
|
1,2 | 0 | 6 | 11 | 7 | 0.92 |
| 3 | 2 | 5 | 11 | 8 |
|
| Histological type
of NSCLC |
|
|
|
|
|
|
Adenocarcinoma | 1 | 5 | 14 | 6 | 0.82 |
|
Squamous cell carcinoma | 1 | 6 | 8 | 9 |
|
|
Differentiation |
|
|
|
|
|
| Good
(G1, 2) | 1 | 7 | 17 | 9 | 0.87 |
| Poor
(G3, 4) | 1 | 4 | 5 | 6 |
|
| Survival
(months) |
|
|
|
|
|
|
<24 | 1 | 3 | 12 | 8 | 0.62 |
|
>24 | 1 | 8 | 10 | 7 |
|
Kaplan-Maier survival curves show no correlation
between VEGF-A and survival (Fig.
4).
Discussion
Lung cancer is the most common malignant tumor and
the leading cause of death from malignancies. Five-year survival of
lung cancer is around 15% (1).
Despite the overall decreasing trend, Croatia is still among the
European countries with the highest male lung cancer incidence and
mortality (19). TNM stage is the
most important predictor of outcome in patients with NSCLC.
However, patients within the same stage or tumor usually have a
very variable prognosis, which depends not only on the stage, but
also on the biological characteristics of the tumor. Angiogenesis
was validated as an independent prognostic factor in a variety of
solid malignancies. Several studies demonstrated that angiogenesis,
assessed by MVD, is a significant prognostic factor for patients
with NSCLC (3,7,20–23). However, most studies have been
conducted on patients with NSCLC in early stages and studies
dealing specifically with the prognostic impact of angiogenesis in
NSCLC with metastasis in mediastinal lymph nodes are lacking. The
only study, to our knowledge, which examines the impact of
angiogenesis on prognosis of patients in stage IIIA (T1-3N2), who
were initially surgically treated, was performed by Angeletti and
al. in 1996 and in this study MVD was identified as a significant
prognostic factor (23).
Several studies have not found MVD to be a predictor
of survival (24–26).
Since those papers did not prove the correlation MVD
and survival it is obvious that the role of tumor angiogenesis
prognosis in the disease's survival is still controversial, we
decided to examine the correlation MVD and survival with our
patients by using CD 31 monoclonal antibodies to visualize the
blood vessels instead of anti-factor VII polyclonal antibodies
which were used in the mentioned researches.
The results of studies which examined the
correlation of VEGF-A and survival of patients with NSCLC are
controversial. Although many data support the statement that the
VEGF-A is correlated with MVD and survival (16,25–27),
others did not confirm VEGF-A as an independent factor of survival
(28–30). The prognostic significance of VEGF-A
in patients with advanced NSCLC, who were primary surgically
treated, has never been investigated. In our study, we observed the
high expression of VEGF-A in most patients, but there was no
correlation with MVD and survival. This lack of correlation
indicates that other angiogenic factors may have an important role
in angiogenesis in advanced NSCLC.
Our study is retrograded and includes patients with
metastases to mediastinal lymph nodes that were discovered during
thoracotomy and subsequent histopathological examination, and for
these reasons were not subjected to neoadjuvant chemotherapy.
All patients included in the study received adjuvant
chemotherapy according to the appropriate protocol and in full
dose. Not one of the patients was subjected to anti-VEGF antibodies
(bevacizumab) therapy. Our results show that patients who were
classified by TNM classification in the same prognostic stage have
a highly variable prognosis. We found that high MVD has a strong
negative prognostic value for survival of patients with lung
cancer.
The introduction of anti-angiogenic therapy in
cancer treatment makes methods of angiogenesis evaluation even more
important. Patients with highly vascularized tumors, due to their
worse prognosis, may be candidates for more aggressive therapy
especially neoadjuvant multimodal chemotherapy. Assessment of
angiogenesis can become an integral part of the tumor staging and
ultimately helping to choose the most efficient therapeutic
approach.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu Y,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pisters KM and Darling G: The IASLC lung
cancer staging project: ‘The Nodal Zone’. J Thorac Oncol.
2:583–584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
4
|
Folkman J: The role of angiogenesis in
tumor growth. Semin Cancer Biol. 3:65–71. 1992.PubMed/NCBI
|
|
5
|
Cox G, Jones JL, Walker RA, Steward WP and
O'Byrne KJ: Angiogenesis and non-small cell lung cancer. Lung
Cancer. 27:81–100. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fox SB, Gatter KC and Harris AL: Tumour
angiogenesis. J Pathol. 179:232–237. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mineo TC, Ambrogi V, Baldi A, Rabitti C,
Bollero P, Vincenzi B and Tonin G: Prognostic impact of VEGF, CD31,
CD34, and CD105 expression and tumour vessel invasion after radical
surgery for IB-IIA non-small cell lung cancer. J Clin Pathol.
57:591–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abu-Jawdeh GM, Faix JD, Niloff J, Tognazzi
K, Manseau E, Dvorak HF and Brown LF: Strong expression of vascular
permeability factor (vascular endothelial growth factor) and its
receptors in ovarian borderline and malignant neoplasms. Lab
Invest. 74:1105–1115. 1996.PubMed/NCBI
|
|
9
|
Claffey KP, Brown LF, del Aguila LF,
Tognazzi K, Yeo KT, Manseau EJ and Dvorak HF: Expression of
vascular permeability factor/vascular endothelial growth factor by
melanoma cells increases tumor growth, angiogenesis, and
experimental metastasis. Cancer Res. 56:172–181. 1996.PubMed/NCBI
|
|
10
|
Maeda K, Chung YS, Ogawa Y, Takatsuka S,
Kang SM, Ogawa M, Sawada T and Sowa M: Prognostic value of vascular
endothelial growth factor expression in gastric carcinoma. Cancer.
77:858–863. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi Y, Kitadai Y, Bucana CD, Cleary
KR and Ellis LM: Expression of vascular endothelial growth factor
and its receptor, KDR, correlates with vascularity, metastasis, and
proliferation of human colon cancer. Cancer Res. 55:3964–3968.
1995.PubMed/NCBI
|
|
12
|
Maharaj AS, Saint-Geniez M, Maldonado AE
and D'Amore PA: Vascular endothelial growth factor localization in
the adult. Am J Pathol. 168:639–648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrera N: Vascular endothelial growth
factor. Trends Cardiovasc Med. 3:244–250. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meert AP, Paesmans M, Martin B, Delmotte
P, Berghmans T, Verdebout JM, Lafitte JJ, Mascaux C and Sculier JP:
The role of microvessel density on the survival of patients with
lung cancer: A systematic review of the literature with
meta-analysis. Br J Cancer. 87:694–701. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kadota K, Huang CL, Liu D, Ueno M, Kushida
Y, Haba R and Yokomise H: The clinical significance of
lymphangiogenesis and angiogenesis in non-small cell lung cancer
patients. Eur J Cancer. 44:1057–1067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fontanini G, Vignati S, Boldrini L, Chinè
S, Silvestri V, Lucchi M, Mussi A, Angeletti CA and Bevilacqua G:
Vascular endothelial growth factor is associated with
neovascularization and influences progression of non-small cell
lung carcinoma. Clin Cancer Res. 3:861–865. 1997.PubMed/NCBI
|
|
17
|
Han H, Silverman JF, Santucci TS, Macherey
RS, d'Amato TA, Tung MY, Weyant RJ and Landreneau RJ: Vascular
endothelial growth factor expression in stage I non-small cell lung
cancer correlates with neoangiogenesis and a poor prognosis. Ann
Surg Oncol. 8:72–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
International Association for the Study of
Lung Cancer and Peter GoldstrawIASLC Staging Manual in Thoracic
Oncology. Orange Park: Editorial Rx Press; 2009
|
|
19
|
Janković M, Samarzija M, Jakopović M,
Kulis T and Znaor A: Trends in lung cancer incidence and mortality
in Croatia, 1988–2008. Croat Med J. 53:93–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuyama K, Chiba Y, Sasaki M, Tanaka H,
Muraoka R and Tanigawa N: Tumor angiogenesis as a prognostic marker
in operable non-small cell lung cancer. Ann Thorac Surg.
65:1405–1409. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Macchiarini P, Fontanini G, Hardin MJ,
Squartini F and Angeletti CA: Relation of neovascularization to
metastasis of non-small cell lung cancer. Lancet. 340:145–146.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giatromanolaki A, Koukourakis MI,
Theodossiou D, Barbatis K, O'Byrne K, Harris AL and Gatter KC:
Comparative evaluation of angiogenesis assessment with
anti-factor-VIII and anti-CD31 immunostaining in non-small cell
lung cancer. Clin Cancer Res. 3:2485–9224. 1997.PubMed/NCBI
|
|
23
|
Angeletti CA, Lucchi M, Fontanini G, Mussi
A, Chella A, Ribechini A, Vignati S and Bevilacqua G: Prognostic
significance of tumoral angiogenesis in completely resected late
stage lung carcinoma (stage IIIA-N2). Impact of adjuvant therapies
in a subset of patients at high risk of recurrence. Cancer.
78:409–415. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chandrachud LM, Pendleton N, Chisholm DM,
Horan MA and Schor AM: Relationship between vascularity, age and
survival in non-small-cell lung cancer. Br J Cancer. 76:1367–1375.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pastorino U, Andreola S, Tagliabue E,
Pezzella F, Incarbone M, Sozzi G, Buyse M, Menard S, Pierotti M and
Rilke F: Immunocytochemical markers in stage I lung cancer:
Relevance to prognosis. J Clin Oncol. 15:2858–2865. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Imoto H, Osaki T, Taga S, Ohgami A,
Ichiyoshi Y and Yasumoto K: Vascular endothelial growth factor
expression in non-small-cell lung cancer: Prognostic significance
in squamous cell carcinoma. J Thorac Cardiovasc Surg.
115:1007–1014. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwasaki A, Kuwahara M, Yoshinaga Y and
Shirakusa T: Basic fibroblast growth factor (bFGF) and vascular
endothelial growth factor (VEGF) levels, as prognostic indicators
in NSCLC. Eu J Cardio-Thoracic Surg. 25:443–448. 2004. View Article : Google Scholar
|
|
28
|
Bonnesen B, Pappot H, Holmstav J and Skov
BG: Vascular endothelial growth factor A and vascular endothelial
growth factor receptor 2 expression in non-small cell lung cancer
patients: Relation to prognosis. Lung Cancer. 66:314–318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yano T, Tanikawa S, Fujie T, Masutani M
and Horie T: Vascular endothelial growth factor expression and
neovascularisation in non-small cell lung cancer. Eur J Cancer.
36:601–609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Decaussin M, Sartelet H, Robert C, Moro D,
Claraz C, Brambilla C and Brambilla E: Expression of vascular
endothelial growth factor (VEGF) and its two receptors
(VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung
carcinomas (NSCLCs): Correlation with angiogenesis and survival. J
Pathol. 188:369–377. 1999. View Article : Google Scholar : PubMed/NCBI
|