Introduction
Low tissue specificity and efficiency of exogenous
gene expression are two major obstacles to tumor-targeted gene
therapy. Previous studies have suggested that the regulation of a
tumor-specific promoter (TSP) and two-step transcriptional
amplification system (TSTA) is able to markedly improve the
specificity and efficiency of expression of a target gene in tumor
cells (1,2). Although numerous promoters have been
used in targeted gene therapy for ovarian cancer, including
secretory leukocyte protease inhibitor, ovarian-specific promoter
and human epithelial tissue-specific transcription factor promoter,
these promoters are neither ovarian cancer-specific nor
epithelium-specific, and may even be active in normal cells
(3). By contrast, the human
telomerase reverse transcriptase (hTERT) promoter is only activated
in ovarian cancer cells with high telomerase activity, and
therefore is highly suitable for the gene therapy of ovarian cancer
(4,5).
However, the activity of tumor-specific promoters is often too weak
to mediate the desired gene therapy (6). Recent studies have shown that the
recombinant TSTA containing a transcriptional activator (RTA) may
effectively enhance the activity of tumor-specific promoters
(7,8).
RTA is an important transcription factor that controls the switch
from the latent to the lytic cycle and regulates immediate-early
gene expression (9). The TSTA system,
composed of a transcriptional activator GAL4-VP16 (Activator) and
an end-biotinylated G5E4T regulatory element (a small promoter that
is responsive to GAL4), has markedly enhanced the activities of the
corresponding TSP (8,10).
The selection of an appropriate target gene is
crucial for efficient gene therapy. The Fas cell-surface death
receptor gene (Fas) regulates cell apoptosis primarily through the
Fas/Fas ligand (FasL) signaling pathway, and thus is associated
with the occurrence and development of ovarian cancer (11), and the chemosensitivity of cancer
cells to certain chemotherapy reagents, including cisplatin,
epirubicin and paclitaxel (12,13).
Therefore, increasing the expression of Fas may directly activate
tumor cell apoptosis. It is known that the immune effector γδ T
cells, with abundant surface FasL, are able to specifically target
and kill Fas-expressing cells by activating the Fas/FasL apoptotic
pathway (7). However, Fas is
expressed at markedly low levels, or not at all, in certain ovarian
cancer cells, leading to decreased Fas-mediated cell apoptosis and
drug resistance in these cells. Enhancing intracellular Fas levels
may be an efficient approach for ovarian cancer gene therapy.
In a preliminary study, the recombinant adenoviral
vectors Ad5-hTERT-Fas and Ad5-hTERT-TSTA-Fas that markedly express
Fas under the regulation of hTERT promoter and TSTA system,
respectively, were successfully constructed in SKOV3 cells
transfected with these adenoviruses (7). The marked killing effect of γδ T cells
on SKOV3 cells with high Fas expression was also confirmed
(7). The present study further
measured the therapeutic effect of Fas-expressing adenoviruses
combined with γδ T cell-mediated killing in a mouse xenograft model
of human ovarian cancer. In recent years, with increasingly more
clinical studies on tumor gene therapy, the safety of gene therapy
has received considerable attention (14–16). In
the present study, the safety of the combined therapy of adenoviral
Fas expression and γδ T cells was therefore evaluated in mice with
human ovarian cancer xenografts.
Materials and methods
Materials and animals
The plasmid pBCVP2G5-luc-NSN carrying GAL4VP2 and
G5E4TATA elements and pBTdel279 carrying the hTERT core promoter
were provided by Dr Yue Song, ShengJing Hospital of China Medical
University (Shenyang, China). EcoRV restriction enzyme (cat.
no. R0195L), EcoRI restriction enzyme (cat. no. R0101S),
BglII restriction enzyme (cat. no. R0144S), SalI restriction
enzyme (cat. no. R3138S), SacI restriction enzyme (cat. no.
R3156S), NotI restriction enzyme (cat. no. R3189S) and
SpeI restriction enzyme (cat. no. SpeI; all from New
England Biolabs, Beverly, MA, USA). The plasmid vector pMD18-T was
purchased from Takara Biotechnology Co., Ltd. (Dalian, China). The
shuttle plasmid pDC316 and recombinant adenovirus backbone plasmid
pBHGloxdelE13cre were purchased from AGTC, Gene Technology Company,
Ltd. (Beijing, China). The AdEasy adenoviral vector systems were
purchased from Applied Biological Materials, Inc. (Richmond, BC,
Canada). For co-transfection, 293 cells were purchased from AGTC
Gene Biotech. The human ovarian carcinoma SKOV3 cell line and γδ T
cells were provided by the Gynecological Oncology Laboratory in the
Beijing Union Medical College Hospital (Beijing, China). Female
BALB/c nude mice were provided by the Experimental Animal Center in
the Beijing Union Medical College Hospital.
Cell culture
SKOV3 cells were cultured in RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 2 mM
L-glutamine. A total of 293 cells were cultured in Dulbecco's
Modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) with 10% FBS. All cells were incubated in an atmosphere of 5%
CO2 at 37°C.
Construction of recombinant plasmid
vectors carrying hTERT promoter and/or TSTA regulatory element and
Fas gene
Plasmid pBCVP2G5-luc-NSN, pBTdel279, pCDNA3-Fas
carrying the Fas gene and shuttle plasmid pDC316 were transfected
into Escherichia coli JM109 competent cells (Promega
Corporation, Madison, WI, USA) using Lipofectamine 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h, according to
the manufacturer's protocol. Following amplification, bacterial
plasmids were extracted and purified using the Wizard Plus SV
Minipreps DNA Purification system (Promega Corporation). To verify
transfection of pBTdel279, using plasmid DNA as the template, the
target fragment was amplified using Qiagen Multiplex PCR kit
(Qiagen China Co., Ltd., Shanghai, China) with the following primer
sequences: 5′-TTGATATCGACCCCCGGGTCCGCCCGGAGCA-3′; and
5′-CTGAATTCGCTGCCTGAAACTCGCGCCGCGAG-3′, containing an
EcoRV/EcoRI restriction enzyme sites. Verification of
pCDNA3-Fas was performed using plasmid DNA as template and the T7
promoter sequence 5′-TAATACGACCTACTATAGGG-3′ as a primer, starting
prior to the insertion site of the Fas gene, the target fragment
was sequenced using the terminal ending method (17). At the same time, given that the
inserted Fas gene has XbaI and KpnI enzyme sites, it
was possible to obtain a fragment of ~1,000 bp upon enzyme
digestion.
From the analysis of the plasmid profile, it was
known that it is possible to obtain two fragments (2,284 and 6,487
bp) following enzyme digestion using the enzymes NheI and
Bsu36I. PDC316-hTERT and pDC316-G5E4T were constructed using
a series of enzyme digestion and ligation reactions. Double digest
samples were set up with 6 µl DNA, 1 µl 10× Buffer, 0.5 µl
restriction endonuclease, and distilled water to 10 µl reaction
volumes. Following the double digest, the samples were incubated at
37°C for 3 h. Digested products were ligated with T4 DNA ligase
(New England BioLabs, Inc., Ipswich, MA, USA) using a 5:1 insert
DNA to vector DNA ratio. A total of 10 µl reaction volumes were set
up, including 1 µl T4 DNA ligase, 1 µl 10× Ligase Buffer (New
England Biolabs) and 20 ng plasmid DNA in distilled water. Tubes
were incubated overnight at 4°C, and products were visualized by
gel electrophoresis and stored at −20°C. The shuttle plasmid
pDC316-hTERT-GAL4VP2 and pDC316-G5E4T-Fas were constructed as
previously described (7).
Construction of shuttle plasmids
As presented in Table
I, plasmid DNA with target sequences were used as a template
and primer pairs containing corresponding restriction enzyme sites
to amplify target sequences using the polymerase chain reaction
(PCR). The thermocycling conditions were: 94°C for 5 min followed
by 35 cycles of 94°C for 45 sec, 64°C for 45 sec and 72°C for 60
sec, and finally 72°C for 7 min. The PCR product of the target
sequence fragment was electrophoresed and collected using an
Agarose Gel DNA Purification kit (Takara Biotechnology Co., Ltd.),
and then ligated into a connection vector pMD18-T using Solution I
of a DNA Ligation kit (Takara Biotechnology Co., Ltd.), according
to the manufacturer's protocol. The connection vector was used to
transfect competent JM109 cells and select bacterial colony for
clone culture. For verification of the target sequence in the
pMD18-T vector, the RV-M sequence (5′-GAGCGGATAACAATTTCACACAGG-3′)
and M13-47 sequence (5′-CGCCAGGGTTTTCCCAGTCACGAC-3′) were used as
primers to amplify positive clones using 2X Taq PCR Master
Mix (Qiagen) and the fragment was sequenced. The PCR reaction
protocol was programmed as: Initial pre-denaturation step at 94°C
for 5 min, followed by 34 cycles of denaturation at 94°C for 30
sec, an annealing step at 59°C for 40 sec, an extension at 72°C for
45 sec and a final extension at 72°C for 10 min. Next, the obtained
connection vector plasmid containing the target sequence was
constructed using a series of enzyme digestion and ligation
reactions as described above. The target sequence was
electrophoresed and collected. At the same time, the vector plasmid
was digested using the corresponding restriction enzyme, and then
electrophoresed and collected. The target sequence was ligated into
the vector plasmid and the constructed plasmid was transfected into
competent JM109 cells using Lipofectamine 3000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) for 48 h, according to the
manufacturer's protocol. The plasmid-positive clone culture was
collected and verified by fragment PCR sequencing or enzyme
digestion.
 | Table I.Construction of shuttle plasmids. |
Table I.
Construction of shuttle plasmids.
| Plasmid | Target sequence | Template plasmid | PCR primers for
target sequence, forward and reverse | Restriction enzymes
used | Verification
method | Vector plasmid | Restriction enzyme
processing of vector plasmid | Verification of
constructed plasmid |
|---|
| pDC316-hTERT | hTERT promoter | pBTdel279 |
5′-TTGATATCGACCCCCGGGTCCGCCCGGAGCA-3′ and
5′-CTGAATTCGCTGCCTGAAACTCGCGCCGCGAG-3′ | EcoRV and
EcoRI | Sequencing using
RV-M/M13-47 primer pair | pDC316 | Complement terminals
following XbaI digestion and cut MCMV promoter using
EcoRI following ethanol precipitation | Sequencing using PCR
primers and enzyme cutting using EcoRV/EcoRI |
|
pDC316-hTERT-GAL4VP2 | GAL4VP2+PA gene | pBCVP2G5-luc-NSN |
5′-GTAGATCTGAAGCTAGCCTCCTGAAAGATG-3′ and
5′-TAGTCGACTAGTGCGGCCGCGATCCAGACAT-3′ | BglII and SalI
(NotI-SpeI) | Sequencing using P1
primer 5′-AAGTGCGACATCATCATC-3′ | pDC316-hTERT | BglII and
SacI | Sequencing using P2
primer 5′-TTCTAGCCTTGATTCCAC-3′ and enzyme cutting using
XbaI/EcoRI |
| pDC316-G5E4T | G5E4T fragment | pBCVP2G5-luc-NSN |
5′-TAGATATCAGGTGACACTATAGAATACAAG-3′ and
5′-GTGAATTCAACAGTACCGGAATGC-3′ | EcoRV and
EcoRI | Sequencing using
M13-47 primer | pDC316 | Complement terminals
following XbaI digestion and cut MCMV promoter using
EcoRI | Sequencing using
PCR primers |
|
pDC316-G5E4T-Fas | Fas gene | pCDNA3-Fas |
5′-TATGAATTCGCCGCCACCATGCTGGGCATCTGGAC-3′
and 5′-GCTGAGCTCTAGACCAAGCTTTGGATTTC-3′ | EcoRI and
SacI | Sequencing using
RV-M/M13-47 primer pair | pDC316-G5E4T | EcoRI and
SacI | Sequencing using P3
primer |
Adenoviral packaging and
purification
As described previously (7), the packaging and purification of
adenovirus vectors Ad5-hTERT-GAL4VP2 and Ad5-G5E4T-Fas (Benyuan
Zhengyang Gene Technology Co., Ltd., Beijing, China) was performed
using the AdEasy adenoviral vector systems (cat. no. 240009;
Agilent Technologies, Inc., Santa Clara, CA, USA) according to the
manufacturer's protocol. Briefly, 293 cells which obtained 60–70%
confluency in fresh DMEM containing 10% fetal bovine serum (FBS;
Gibco) were co-transfected with recombinant shuttle plasmid
(pDC316-hTERT-Fas, pDC316-hTERT-GAL4VP2 or pDC316-G5E4T-Fas) and
adenoviral backbone plasmid pBHGloxdelE13cre, using Lipofectamine
3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
plaques were scraped off at 7–10 days following transfection,
collected by centrifugation (30,000 × g at 4°C for 16 h) and
resuspended in PBS. The suspension was frozen in liquid nitrogen
and thawed at 37°C three times to release the virus. The
supernatant containing recombinant adenoviruses was collected by
centrifugation (12,000 × g at 4°C for 15 min), purified by
chromatography, and stored at −20°C in PBS (cat. no. P5368-10PAK;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The target gene in
the recombinant adenoviruses was confirmed using 2X Taq PCR
Master Mix (Qiagen) (Table II). The
PCR reaction protocol was programmed as described above. The viral
titer and half-maximal tissue culture infective dose of each viral
stock was measured (Table II).
 | Table II.Summary of target gene
identification, viral titer and TCID50 of the
recombinant adenoviruses. |
Table II.
Summary of target gene
identification, viral titer and TCID50 of the
recombinant adenoviruses.
| Adenovirus | Viral titer,
VP/ml | Target gene
identification by polymerase chain reaction | TCID50,
IU/ml |
|---|
|
Ad5-htert-GAL4VP2 |
1.4×1011 | Correct |
5.0×109 |
| Ad5-G5E4T-Fas |
2.7×1011 | Correct |
5.6×109 |
Construction of mouse xenograft model
of human ovarian cancer
SKOV3 cells in the exponential growth phase were
collected and diluted into a 2.5×107/ml cell suspension.
A total of 38, 4-week-old female BALB/c mice weighing 14–16 g were
purchased from the Laboratory Animal Centre of Guangxi Medical
University. All mice were housed in a specific pathogen-free clean
room in a temperature-controlled (24–26°C) room at 60±5% humidity
under a 12-h light/dark cycle. Mice were provided with distilled
water ad libitum and fed with OVA-free food. Mice were given
a subcutaneous injection of 0.2 ml SKOV3 cell suspension at the
back of the neck. The tumor xenografts were measured every 4 days
until their diameters reached 5–6 mm. The Institutional Review
Board of Liuzhou Maternal and Child Health Hospital approved the
present study.
Treatment
A total of 35 mice were randomly selected from the
38 mice with xenograft tumors, and randomly divided into five
groups (n=7), which were injected with PBS, γδ T cells,
Fas-expressing adenoviruses (TSTA group), taxol, and Fas-expressing
adenovirus with γδ T cells (TSTA+γδ T group) (Table III). The total weight and tumor size
of each mouse were measured, and tumor volume was calculated prior
to treatment.
 | Table III.Summary of treatment groups of mice
with ovarian xenograft tumors. |
Table III.
Summary of treatment groups of mice
with ovarian xenograft tumors.
| Group | Treatment |
|---|
| PBS | Multi-point
intratumoral injection of PBS twice a week, 200 µl/injection four
times (days 1, 4, 7 and 11) |
| Taxol | Multi-point
intratumoral injection of taxol twice a week, 200 mg/kg/injection
four times (days 1, 4, 7 and 11) |
| TSTA | Multi-point
intratumoral injection of Ad5-hTERT-GAL4VP2 and Ad5-G5E4T-Fas once
a week, TCID50 1×109 IU/injection twice (days
1 and 7) |
| γδ T | Multi-point
intratumoral injection of γδ T cells once a week, 2×107
cells/injection twice (days 1 and 7) |
| TSTA+γδ T | Multi-point
intratumoral injection of Ad5-hTERT-GAL4VP2 and Ad5-G5E4T-Fas once
a week, TCID50 1×109 IU/injection twice (days
1 and 7) and multi-point intratumoral injection of γδ T cells once
a week on the following day of adenoviral injection,
2×107 cells/injection twice (days 2 and 8) |
Comparison of therapeutic effects
The therapeutic effects of different treatments were
compared according to the following. i) Morphological observation
of the surface morphology and texture of the xenograft, and
activity and other conditions of mice. ii) Tumor growth curve: the
major axis (a) and minor axis (b) of the xenograft tumors were
measured with a Vernier caliper every 4 days. The mean tumor volume
in each group was calculated as V = a × b2/2, and the
tumor growth curve was created. iii) Volume and weight inhibitory
rates: All mice were sacrificed at 24 days after the start of
treatment. The size and weight of the xenograft tumors were
measured, and volume and weight inhibitory rates were calculated
using the following formulae: Weight inhibition rate = (mean tumor
weight in PBS group - mean tumor weight in the treatment
group)/mean tumor weight in PBS group ×100, and volume inhibition
rate = (mean tumor volume in PBS group - mean tumor volume in the
treatment group)/mean tumor volume in the PBS group ×100.
Safety evaluation of TSTA+γδ T
cells
The safety of the combined treatment of
Fas-expressing adenovirus+γδ T cells was evaluated by pathological
observation. Briefly, tissue samples of xenograft, liver, kidney,
spleen, intestines, heart and ovary of mice in the TSTA+γδ T cell
group were collected, fixed with 10% neutral buffered formalin
overnight at 4°C, embedded in paraffin and cut into 4 mm sections.
The sections in the TSTA+γδ T cell group were compared with those
in the PBS control group to evaluate the safety of using
Fas-expressing adenovirus with γδ T cells in the treatment of human
ovarian cancer xenograft.
Statistical analysis
All statistical analyses were performed using SPSS
(version 13.0; SPSS, Inc., Chicago, IL, USA). The difference in
volume and weight inhibitory rates among the treatment groups was
compared by univariate analysis of variance. The difference between
a treatment group and the PBS group was further compared using the
Fisher's least significant difference test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Construction of mouse xenograft model
of human ovarian cancer
At 2 weeks after the injection of SKOV3 cells,
subcutaneous xenograft tumors with a diameter of 4–6 mm developed.
The oval hard xenograft tumors had a uniform nodule size and smooth
surface and exhibited slow expansive growth.
General conditions of mice
During the course of treatment, there was no
significant difference in the general conditions of mice, including
eating and mental status. No significant loss of appetite or weight
was observed in any mouse. No mice succumbed to disease throughout
the 24-day observation period. At 24 days, mice were sacrificed and
autopsy analysis identified no ascites or pleural/abdominal
metastasis of the tumor. A large necrotic area was detected in the
center of the xenograft tumors in the TSTA+γδ T group, but no
morphological changes in the tumor were observed in any other
group.
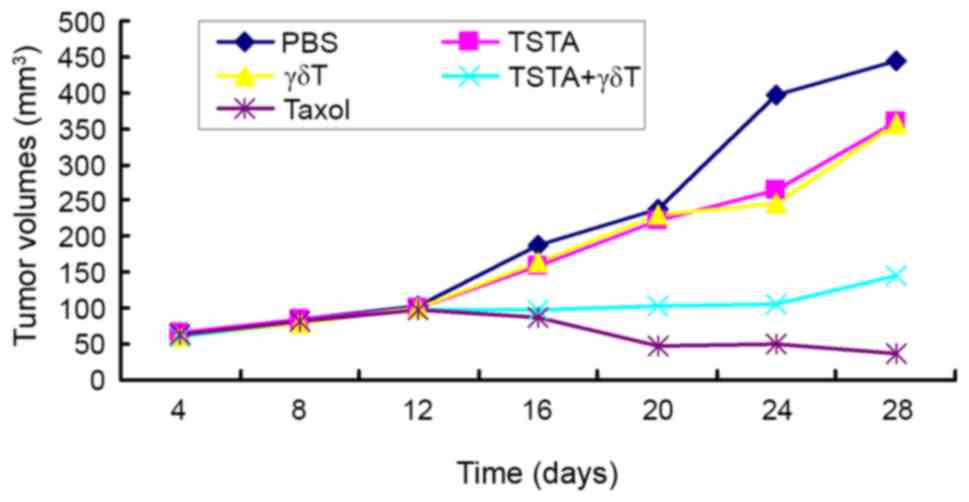
Tumor growth curve
The tumor growth curves for all treatment groups are
presented in Fig. 1. The tumors grew
slowly in the first 12 days in all groups. The tumor in the PBS,
TSTA and γδ T groups began to grow rapidly after 12 days, whereas
the growth of tumors in the TSTA+γδ T group remained slow and the
tumor volume in the taxol group was decreased.
Combination of TSTA and γδ T inhibits
tumor weight
To investigate the effects of TSTA and γδ T on the
tumor growth, the tumor weight was observed and the
weight-inhibiting rate was analyzed. The results revealed that the
tumor weight in the TSTA+γδ T and taxol groups were significantly
increased compared with the TSTA or γδ T single-treatment groups
(P<0.05; Table IV). Furthermore,
the tumor weight inhibitory rates of TSTA+γδ T (50.9%) and taxol
group (79.0%) were obviously increased compared with those of the
single-treatment groups (Table
IV).
 | Table IV.Comparison of weight inhibitory rates
in different treatment groups. |
Table IV.
Comparison of weight inhibitory rates
in different treatment groups.
| Group | Mean tumor weight,
g | Weight inhibitory
rate, % |
|---|
| PBS | 0.573±0.015 | – |
| TSTA | 0.507±0.012 | 10.5 |
| γδ T | 0.497±0.013 | 14.0 |
| TSTA+γδ T |
0.276±0.016a,b | 50.9 |
| Taxol |
0.120±0.011a,b | 79.0 |
Combination of TSTA and γδ T inhibits
tumor volume
The effects of TSTA and γδ T on the tumor volume
were also observed in the present study. The results indicated that
the tumor volume in the TSTA+γδ T and taxol groups were
significantly increased compared with the TSTA or γδ T
single-treatment groups (P<0.05; Table
V). Furthermore, the tumor volume inhibitory rates of TSTA+γδ T
(60.3%) and taxol (88.6%) groups were markedly increased compared
with those of the single-treatment groups (Table V).
 | Table V.Comparison of volume inhibitory rates
in different treatment groups. |
Table V.
Comparison of volume inhibitory rates
in different treatment groups.
| Group | Mean tumor volume,
mm3 | Volume inhibitory
rate, % |
|---|
| PBS | 402.29±9.83 | – |
| TSTA | 361.43±6.87 | 10.2 |
| γδ T | 360.00±8.32 | 10.5 |
| TSTA+γδ T |
159.71±11.93a,b | 60.3 |
| Taxol |
45.86±4.87a,b | 88.6 |
Safety evaluation of Fas-expressing
adenovirus and γδ T cells
Tissue sections of the xenograft, liver, kidney,
spleen, intestines, heart and ovary of mice in the TSTA+γδ T group
were compared with those in the PBS group to evaluate the safety of
the combined treatment for human ovarian cancer xenograft. None of
the vital organs in TSTA+γδ T group developed any evident
morphological changes during the treatment when compared with the
PBS controls. A large necrotic area was detected in the center of
the xenograft tumors in the TSTA+γδ T group, but was not observed
in the PBS group.
Discussion
The Fas gene is one of the most important regulatory
genes for apoptosis, and the abnormalities in the Fas/FasL
signaling pathway is associated with the occurrence and development
of tumor and the sensitivity of tumor cells to certain
chemotherapeutic reagents (18–20).
However, the level of Fas gene expression is low in or even absent
from certain ovarian cancer cells, which decreases the Fas-mediated
apoptosis and chemosensitivity of these cells (1,12).
Introducing exogenous Fas gene expression into tumor cells has been
suggested as an effective method to improve the targeted gene
therapy of ovarian cancer (7).
However, the low expression of the exogenous Fas gene in tumor
cells has been a major obstacle in tumor gene therapy. The
tumor-specific hTERT promoter has been successfully used in the
gene therapy of ovarian cancer (21,22).
Nevertheless, the activity of the hTERT promoter in ovarian tumor
cells is often too low for effective targeted gene therapy
(5). The TSTA system, including the
transcriptional activator GAL4-VP16 and G5E4T regulatory element (a
small promoter that is responsive to GAL4), has markedly enhanced
the activity of the corresponding TSP (23,24).
Consistent with a previous study, preliminary results demonstrated
that the Fas gene was markedly expressed in SKOV3 cells following
co-transfection with Ad5-hTERT-GAL4VP2 and Ad5-G5E4T-Fas, whereas
Fas expression in the control lung fibroblast cell line HELF was
not altered following transfection, indicating the efficient
targeted expression of Fas in human ovarian cancer cells using the
TSTA system (7).
As a subgroup of T cells, γδ T cells are able to
directly bind to antigens, including polypeptides and lipids, owing
to the rich surface expression of FasL, and thus can effectively
target Fas-expressing tumor cells, initiating the Fas/FasL
apoptotic pathway (25–29). Preliminary results confirmed the
strong killing activity of γδ T cells against adenovirus-mediated
Fas-expressing SKOV3 cells (7). The
killing activity of γδ T cells blocked with anti-human FasL-IgG1
monoclonal antibody against SKOV3 cells was markedly decreased. In
the present study, the therapeutic effect of targeted
Fas-expressing adenoviruses combined with γδ T cells was evaluated
in a mouse xenograft model of human ovarian cancer. Since nude mice
are T cell-immunodeficient animals and have no immune effector
cells against tumor cells overexpressing Fas, no inhibitory effect
was observed in the PBS group. By contrast, the weight and volume
inhibition rates (50.9 and 60.3%, respectively) were significantly
increased compared with in the PBS group. Furthermore, a large
necrotic area was detected in the center of the xenograft tumors in
TSTA+γδ T group, but this was not observed in the PBS group,
indicating that the treatment activated the Fas/FasL apoptotic
pathway and directly induced the necrotic lysis of tumor cells.
In recent years, the safety of tumor gene therapy
has become a major focus of research (30). There are three main issues concerning
the clinical application of replication-defective adenoviral
vectors in tumor gene therapy: The construction of recombinant
adenovirus with replication ability, the activation of the immune
response against adenovirus, and the cytotoxic effect of the
adenovirus (31). In the present
study, the reporter gene of the injected adenovirus was identified
in other regions of the body, despite the local injection of the
virus, which might be due to the non-targeted infection of host
cells by adenovirus. The safety of the combined treatment of
Fas-expressing adenovirus with γδ T cells was further assessed by
the pathological examination of the xenograft, liver, kidney,
spleen, intestine, heart and ovary tissue of mice in the TSTA+γδ T
and PBS groups. None of the vital organs in the TSTA+γδ T group
developed any evident morphological changes during the treatment
compared with the PBS controls, suggesting that treatment with
Fas-expressing adenovirus and γδ T cells was safe in mice. However,
further studies are required to validate the results of the present
study, which include the measurement of serum biochemical
indicators in treated mice.
To conclude, the combination of Fas-expressing
adenoviruses and γδ T cell therapy is efficient and safe for the
treatment of mouse human ovarian carcinoma xenografts, which may
provide a novel strategy for tumor gene therapy. The therapeutic
effect may be improved further when target genes that are more
specific for ovarian cancer are identified.
References
|
1
|
Chen W, Delongchamps NB, Mao K, Beuvon F,
Peyromaure M, Liu Z and Dinh-Xuan AT: High RhoA expression at the
tumor front in clinically localized prostate cancer and association
with poor tumor differentiation. Oncol Lett. 11:1375–1381. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryu CJ, Whitehurst CE and Chen J:
Expression of Gal4-VP16 and Gal4-DNA binding domain under the
control of the T lymphocyte-specific lck proximal promoter in
transgenic mice. BMB Rep. 41:575–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamashita Y: Ovarian cancer: New
developments in clear cell carcinoma and hopes for targeted
therapy. Jpn J Clin Oncol. 45:405–407. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song Y, Xin X, Zhai X, Xia Z and Shen K:
Sequential combination therapy with flavopiridol and autocatalytic
caspase-3 driven by amplified hTERT promoter synergistically
suppresses human ovarian carcinoma growth in vitro and in mice. J
Ovarian Res. 7:1212014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie X, Hsu JL, Choi MG, Xia W, Yamaguchi
H, Chen CT, Trinh BQ, Lu Z, Ueno NT, Wolf JK, et al: A novel hTERT
promoter-driven E1A therapeutic for ovarian cancer. Mol Cancer
Ther. 8:2375–2382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu L, Johnson M and Sato M:
Transcriptionally targeted gene therapy to detect and treat cancer.
Trends Mol Med. 9:421–429. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin J, Zeng D, He H, Tan G, Lan Y, Jiang F
and Sheng S: Gene therapy for human ovarian cancer cells using
efficient expression of Fas gene combined with γδ T cells. Mol Med
Rep. 16:3791–3798. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song Y, Xin X, Xia Z, Zhai X and Shen K:
Selective suppression of autocatalytic caspase-3 driven by two-step
transcriptional amplified human telomerase reverse transcriptase
promoter on ovarian carcinoma growth in vitro and in mice. Oncol
Rep. 32:225–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun R, Lin SF, Gradoville L, Yuan Y, Zhu F
and Miller G: A viral gene that activates lytic cycle expression of
Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA.
95:10866–10871. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iyer M, Salazar FB, Lewis X, Zhang L, Wu
L, Carey M and Gambhir SS: Non-invasive imaging of a transgenic
mouse model using a prostate-specific two-step transcriptional
amplification strategy. Transgenic Res. 14:47–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura M, Nagano H, Sakon M, Yamamoto T,
Ota H, Wada H, Damdinsuren B, Noda T, Marubashi S, Miyamoto A, et
al: Role of the Fas/FasL pathway in combination therapy with
interferon-alpha and fluorouracil against hepatocellular carcinoma
in vitro. J Hepatol. 46:77–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guillermo LV, Silva EM, Ribeiro-Gomes FL,
De Meis J, Pereira WF, Yagita H, DosReis GA and Lopes MF: The Fas
death pathway controls coordinated expansions of type 1 CD8 and
type 2 CD4 T cells in Trypanosoma cruzi infection. J Leukoc Biol.
81:942–951. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brenes O, Arce F, Gätjens-Boniche O and
Díaz C: Characterization of cell death events induced by
anti-neoplastic drugs cisplatin, paclitaxel and 5-fluorouracil on
human hepatoma cell lines: Possible mechanisms of cell resistance.
Biomed Pharmacoth. 61:347–355. 2007. View Article : Google Scholar
|
|
14
|
Collins M and Thrasher A: Gene therapy:
Progress and predictions. Proc Biol Sci. 282:201430032015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naldini L: Gene therapy returns to centre
stage. Nature. 526:351–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang D and Gao G: State-of-the-art human
gene therapy: Part II. Gene therapy strategies and clinical
applications. Discov Med. 18:151–161. 2014.PubMed/NCBI
|
|
17
|
Hoshida H, Murakami N, Suzuki A, Tamura R,
Asakawa J, Abdel-Banat BM, Nonklang S, Nakamura M and Akada R:
Non-homologous end joining-mediated functional marker selection for
DNA cloning in the yeast Kluyveromyces marxianus. Yeast. 31:29–46.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mitsiades N, Yu WH, Poulaki V, Tsokos M
and Stamenkovic I: Matrix metalloproteinase-7-mediated cleavage of
Fas ligand protects tumor cells from chemotherapeutic drug
cytotoxicity. Cancer Res. 61:577–581. 2001.PubMed/NCBI
|
|
19
|
Villa-Morales M and Fernández-Piqueras J:
Targeting the Fas/FasL signaling pathway in cancer therapy. Expert
Opin Ther Targets. 16:85–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu HL, Wang YZ, Yu L, Li B, Yao SQ and
Lou FD: The expression of Fas, FasL and Bcl-2 on RMA cells during
the process of apoptosis induced by chemotherapeutic drugs.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 10:35–39. 2002.(In Chinese).
PubMed/NCBI
|
|
21
|
Lin T, Gu J, Zhang L, Huang X, Stephens
LC, Curley SA and Fang B: Targeted expression of green fluorescent
protein/tumor necrosis factor-related apoptosis-inducing ligand
fusion protein from human telomerase reverse transcriptase promoter
elicits antitumor activity without toxic effects on primary human
hepatocytes. Cancer Res. 62:3620–3625. 2002.PubMed/NCBI
|
|
22
|
Zhang Y, Ma H, Zhang J, Liu S, Liu Y and
Zheng D: AAV-mediated TRAIL gene expression driven by hTERT
promoter suppressed human hepatocellular carcinoma growth in mice.
Life Sci. 82:1154–1161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iyer M, Wu L, Carey M, Wang Y, Smallwood A
and Gambhir SS: Two-step transcriptional amplification as a method
for imaging reporter gene expression using weak promoters. Proc
Natl Acad Sci USA. 98:14595–14600. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe M, Ueki H, Ochiai K, Huang P,
Kobayashi Y, Nasu Y, Sasaki K, Kaku H, Kashiwakura Y and Kumon H:
Advanced two-step transcriptional amplification as a novel method
for cancer-specific gene expression and imaging. Oncol Rep.
26:769–775. 2011.PubMed/NCBI
|
|
25
|
Merritt RE, Mahtabifard A, Yamada RE,
Crystal RG and Korst RJ: Cisplatin augments cytotoxic
T-lymphocyte-mediated antitumor immunity in poorly immunogenic
murine lung cancer. J Thorac Cardiovasc Surg. 126:1609–1617. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park IJ, Kim MJ, Park OJ, Park MG, Choe W,
Kang I, Kim SS and Ha J: Cryptotanshinone sensitizes DU145 prostate
cancer cells to Fas(APO1/CD95)-mediated apoptosis through Bcl-2 and
MAPK regulation. Cancer Lett. 298:88–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanagawa N, Yanagawa T, Nakagawa T, Okada
N and Nakagawa S: Tumor vessel-injuring ability improves antitumor
effect of cytotoxic T lymphocytes in adoptive immunotherapy. Cancer
Gene Ther. 20:57–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Habib HM, Taher TE, Isenberg DA and Mageed
RA: Enhanced propensity of T lymphocytes in patients with systemic
lupus erythematosus to apoptosis in the presence of tumour necrosis
factor alpha. Scand J Rheumatol. 38:112–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie J, Xiong L, Tao X, Li X, Su Y, Hou X
and Shi H: Antitumor effects of murine bone marrow-derived
dendritic cells infected with xenogeneic livin alpha recombinant
adenoviral vectors against Lewis lung carcinoma. Lung cancer.
68:338–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matthews KS, Alvarez RD and Curiel DT:
Advancements in adenoviral based virotherapy for ovarian cancer.
Adv Drug Deliv Rev. 61:836–841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mahasreshti PJ, Kataram M, Wu H,
Yalavarthy LP, Carey D, Fisher PB, Chada S, Alvarez RD, Haisma HJ,
Dent P and Curiel DT: Ovarian cancer targeted adenoviral-mediated
mda-7/IL-24 gene therapy. Gynecol Oncol. 100:521–532. 2006.
View Article : Google Scholar : PubMed/NCBI
|