Introduction
Osteosarcoma is a type of primary malignant tumor
that exhibits the highest morbidity of all neoplasms in the human
skeletal system, and often presents in the metaphysis of the long
tubal bones (1). Osteosarcoma often
affects young people between the age of 20 and 30 years old. The
mortality rate is high (2) and ~20%
of patients exhibit pulmonary metastasis prior to diagnosis.
Subsequent to diagnosis, the majority of patients succumb to the
disease within 2 years (2). At
present, there are no effective therapeutic treatments for early
osteosarcoma (3). Therefore, it is
important to investigate the causes of osteosarcoma occurrence,
development and invasion, the mechanisms of osteosarcoma
oncogenesis, and to identify effective diagnostic and therapeutic
techniques. The development of gene therapy has created novel
research targets in oncotherapy, including the identification of a
target gene (4).
Mitogen-activated protein kinase (MAPK) is the one
of the most important types of signal conduction pathway in humans,
and interacts with multiple signaling pathways (5). The tumor protein (p)38 MAPK pathway is
activated through phosphotyrosine and threonine and inflammatory
and growth factors, and activates downstream transcription factors
on target genes, increases the initiation of cancer cell
development, promotes protein synthesis, regulates cell surface
receptors and regulates the invasion and transfer of tumor cells
(6).
Reactive oxygen species (ROS) are secondary products
in the process of aerobic metabolism and include oxygen ions,
peroxide and oxygen radical molecules (7). The increase in the level of
intracellular ROS may promote cellular proliferation and
differentiation to some extent. However, excessive levels of ROS
results in damage to lipids, proteins and DNA, destroying numerous
normal cell signaling pathways, inducing apoptosis and autophagic
death, and ultimately results in cell death (8). Notably, previous research demonstrates
that the base value of ROS in lung cancer cells is higher than in
normal cells, and may be associated with oncogene activation, high
metabolic status and disordered mitochondrial functions (9). ROS in splenoma cells are easily induced
by external factors, such as inflammation, or bacterial infection,
resulting in cellular damage (9). In
addition, ROS affects multiple other cell signaling pathways, such
as MAPK (10). p38MAPK is one of the
important members of the MAPK family, and participates in numerous
important cell events subsequent to activation by extracellular
signals, such as cell proliferation, differentiation and transfer,
including apoptosis and autophagy (11). Therefore, the p38MAPK pathway may be a
worthwhile target in tumor cells.
Water-soluble components in Danshen serve a role in
cardiovascular disease and exhibit antineoplastic effects, as they
reduce blood pressure, provide anti-thrombotic activity, expand the
blood vessels, increase capillary permeability (12). Clinically, injections of Danshen may
expand blood vessels, increase coronary artery blood flow and
promote the recovery of ischemic or damaged myocardium, and may
treat multiple types of cardiovascular disease (13). In terms of antineoplastic research,
the water-soluble components of Danshen may inhibit the growth of
multiple types of tumor, promote tumor cell apoptosis and inhibit
tumor angiogenesis (14). Salvianolic
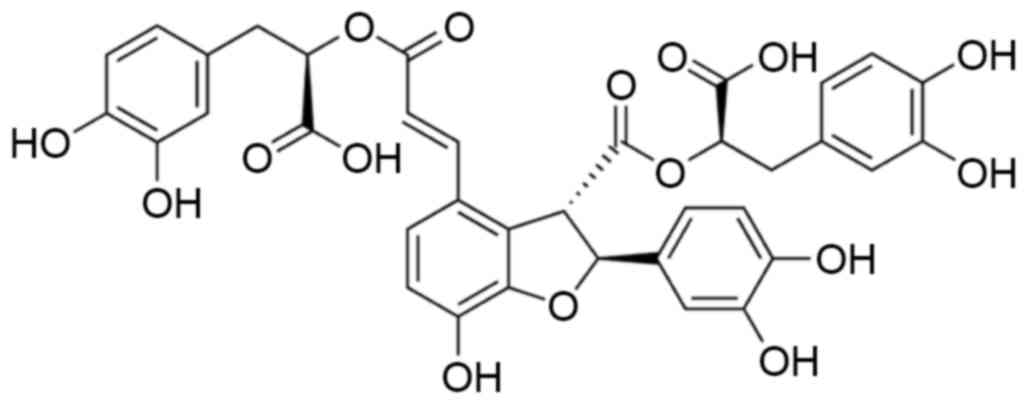
acid B, as illustrated in Fig. 1, is
a low-polymer compound with a relative molecular weight of 718.59
and comprises 1 molecule of caffeic acid and 3 molecules of
danshensu, with a molecular formula of
C36H36016 (15). Salvianolic acid B possess
anti-oxidative and anti-inflammation effects (16). At present, salvianolic acid B has been
demonstrated to exhibit the strongest pharmacological activity in
Danshen water-soluble substances (14). Recently, salvianolic acid B was the
focus of a study investigating myocardial regeneration,
angiogenesis, reversion ventricular remodeling and ischemic
peripheral vascular disease (17).
However, the molecular mechanism underlying the salvianolic acid
B-induced anticancer effect on osteosarcoma is not fully
understood, and the effect of salvianolic acid B on osteosarcoma
cells has not previously been determined. Based on these data, the
present study examined whether salvianolic acid B suppresses cell
proliferation and induces apoptosis of osteosarcoma through
p38-mediated ROS generation.
Materials and methods
Cell culture
The osteosarcoma MG63 cell lines were purchased from
Gansu University of Chinese Medicine (Lanzhou, China) and cultured
in RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA), supplemented with 10% fetal bovine serum (FBS; Hyclone;
GE Healthcare Life Sciences), 100 mg/ml streptomycin (Hyclone; GE
Healthcare Life Sciences) and 100 IU/ml penicillin (Hyclone; GE
Healthcare Life Sciences) in 5% CO2 at 37°C.
Small interfering (si)RNA and
transfection
Si-p38 was as follows: Forward,
5′-AUGAAUGAUGGACUGAAAUGGUCUG-3′, reverse,
5′-CAGACCAUUUCAGUCCAUCAUUCAU-3′ (Sangon Biotech Co., Ltd, Shanghai,
China). The cells were transfected with Si-p38 siRNA (100 nM) or
control (100 nM) together with Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 24 h.
Cell viability assay
The cells were plated in 96-well microplates and
cultured with 1, 10, 50 and 100 µM salvianolic acid B for 24 h or
with 50 µM salvianolic acid B for 0, 12, 24 and 48 h. The cellular
viability was assessed by MTT assay. A total of 20 µl of 5 mg/ml
MTT was added to each culture well, and the cells were incubated
for 4 h at 37°C. DMSO was then added into each well for 20 min at
37°C and the cell viability was quantified at 490 nm using an ELISA
reader (Infinite® 200 PRO; Tecan Schweiz, Männedorf,
Switzerland). The control group was treated with DMSO alone.
Flow cytometry
The cells were plated in 6-well microplates and
cultured with 50 µM salvianolic acid B or Si-p38 siRNA for 24 h.
The cells were resuspended in binding buffer and stained with 10 µl
Annexin V-fluorescein isothiocyanate (Beyotime Institute of
Biotechnology, Haimen, China) for 30 min in the dark. The cells
were then stained with 10 µl propidium iodide (Beyotime Institute
of Biotechnology) for 5 min in the dark. Cell apoptosis was
immediately detected using an EPICS® ALTRA™ flow
cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Western blot analysis
The cells were plated in 6-well microplates and
cultured with 50 µM salvianolic acid B or Si-p38 siRNA for 24 h at
37°C. The cells or tissues were harvested and lysed using ice-cold
radioimmunoprecipitation analysis buffer (Beyotime Institute of
Biotechnology). The proteins in the supernatants were collected
subsequent to centrifugation at 20,000 × g for 10 min at 4°C and
quantified with a bicinchoninic acid assay protein assay kit
(Beyotime Institute of Biotechnology). A total of 50 µg protein was
separated using 10% SDS-PAGE and transferred to a nitrocellulose
membrane (GE Healthcare Life Sciences, Chalfont, UK). The membranes
were blocked with 5% nonfat milk for 1 h at 37°C and incubated with
anti-cleaved caspase-3 (Asp175; cat. no., 9579; dilution, 1:3,000;
Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-phosphorylated-p38 mitogen-activated protein kinase (p-p38
MAPK; cat. no., 9211; dilution, 1:4,000; Cell Signaling Technology,
Inc.) anti-phosphorylated-p53 (cat. no., 2527,; dilution, 1:2,000,
Cell Signaling Technology, Inc.) and anti-GAPDH (cat. no., 5014;
dilution, 1:2,000, Cell Signaling Technology, Inc.) at 4°C
overnight. The membranes were incubated with horseradish
peroxidase-linked goat anti-rabbit IgG secondary antibodies (cat.
no., ab6721; dilution, 1:10,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). The bands were visualized using the enhanced
chemiluminescence kit (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and quantified using the Filmentwickler CP1000 Processor
(AGFA, Mortsel, Belgium).
Measurement of ROS production
The cells were plated into 6-well microplates and
cultured with 50 µM salvianolic Acid B or Si-p38 siRNA for 24 h.
The cells incubated with dichlorofluorescein diacetate for 1 h at
37°C in the dark and washed with PBS. ROS production was detected
using the fluorescence intensity of 2,7-dichlorodihydrofluorescein
diacetate (H2DCFDA) using an EPICS® ALTRA™ flow
cytometer (Beckman Coulter, Inc.).
Statistical analysis
SPSS 19.0 (IBM SPSS, Inc., Chicago, IL, USA) was
used for the statistical analysis. Data are presented as the mean ±
standard deviation (n=3). Statistical evaluation of the data was
performed by one-way analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
Salvianolic acid B suppresses cell
proliferation in the osteosarcoma MG63 cell line
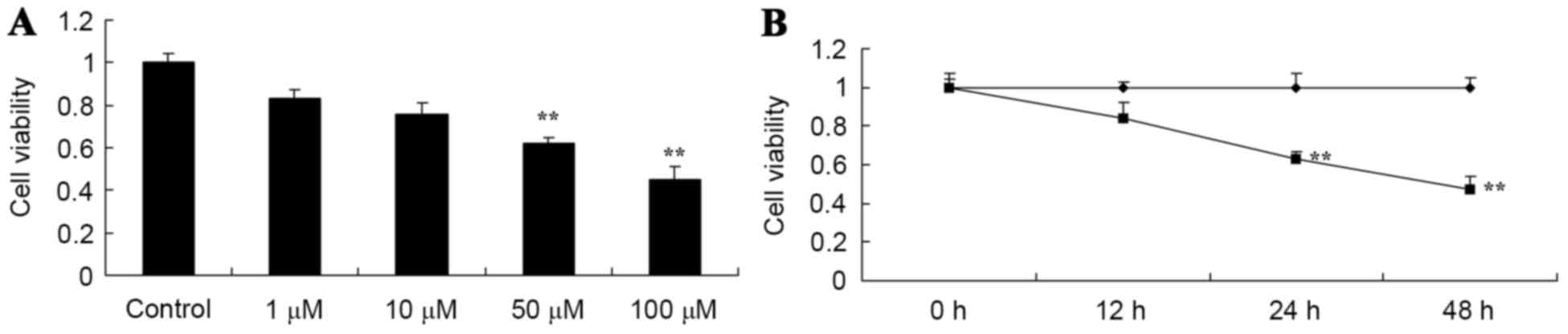
To investigate the anticancer effect of salvianolic
acid B on osteosarcoma MG63 cells, the cells were treated with 1,
10, 50 and 100 µM salvianolic acid B for 24 h or with 50 µM
salvianolic acid B for 0, 12, 24 and 48 h and cell viability was
measured by MTT assay. The cell proliferation of osteosarcoma MG63
cell was suppressed by treatment with salvianolic acid B in
dose-dependent manner compared with the control cells, as
illustrated in Fig. 2A. Subsequent to
treatment with 50 µM salvianolic acid B for 12, 24 and 48 h, the
cell proliferation of the osteosarcoma MG63 cells was suppressed in
a time-dependent manner, as demonstrated in Fig. 2B.
Salvianolic acid B induces the
apoptosis of osteosarcoma MG63 cells
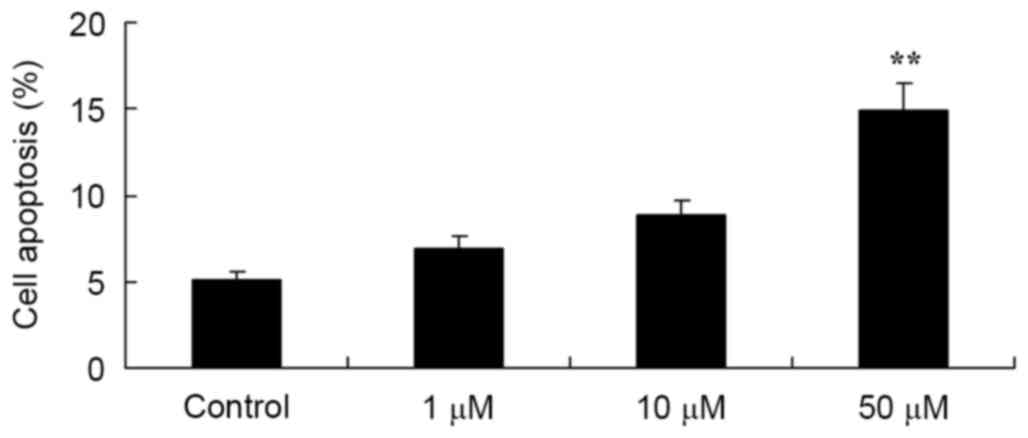
To determine whether the growth inhibition effect of
salvianolic acid B on the osteosarcoma MG63 cells was mediated
through the induction of apoptosis, flow cytometry was used to
examine levels of apoptotic cell death in the MG63 cells. As
demonstrated in Fig. 3, the rate of
apoptotic cells significantly increased with the treatment of 50 µM
salvianolic acid B compared with the control cells.
Salvianolic acid B activates cleaved
caspase 3 protein expression in the osteosarcoma MG63 cells
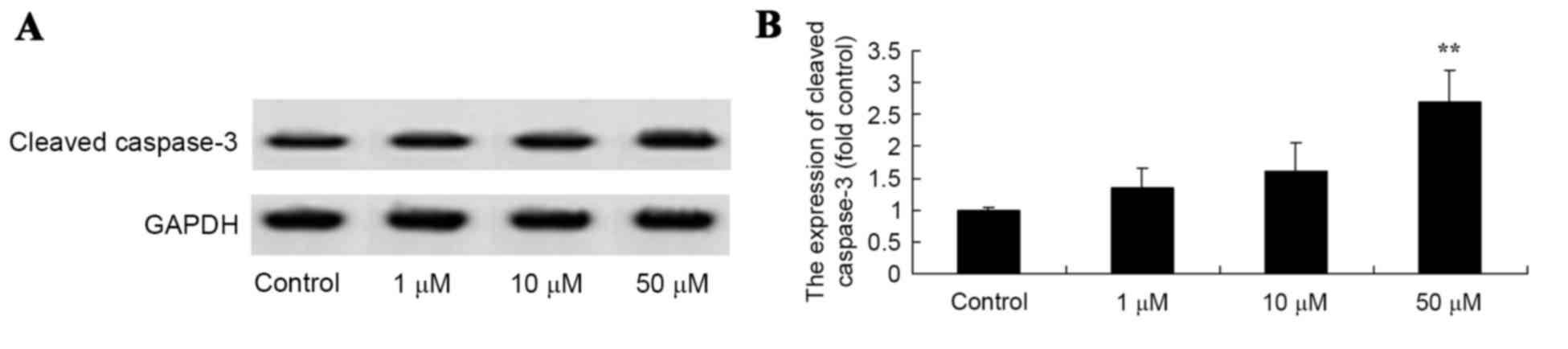
To determine the activation of apoptosis of
salvianolic acid B, a western blot analysis was used to examine
Asp175 expression levels in the MG63 cells. Treatment with 50 µM
salvianolic acid B significantly enhanced the levels of Asp175
protein expression in the MG63 cells compared with the control
cells, as illustrated in Fig. 4.
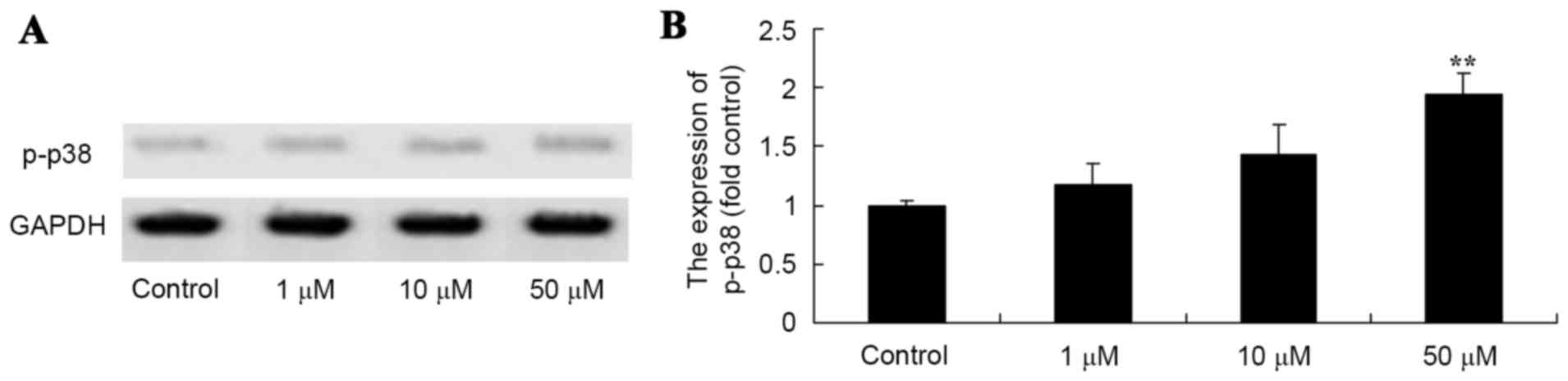
Salvianolic acid B activates p-p38
protein expression in the osteosarcoma MG63 cells
To determine the role of p-p38 in salvianolic acid
B-induced apoptosis, the level of p-p38 protein expression was
measured using a western blot analysis. The results from the
western blot analysis revealed that treatment with 50 µM
salvianolic acid B significantly increased the level of p-p38
protein in MG63 cells compared with the control, as demonstrated in
Fig. 5.
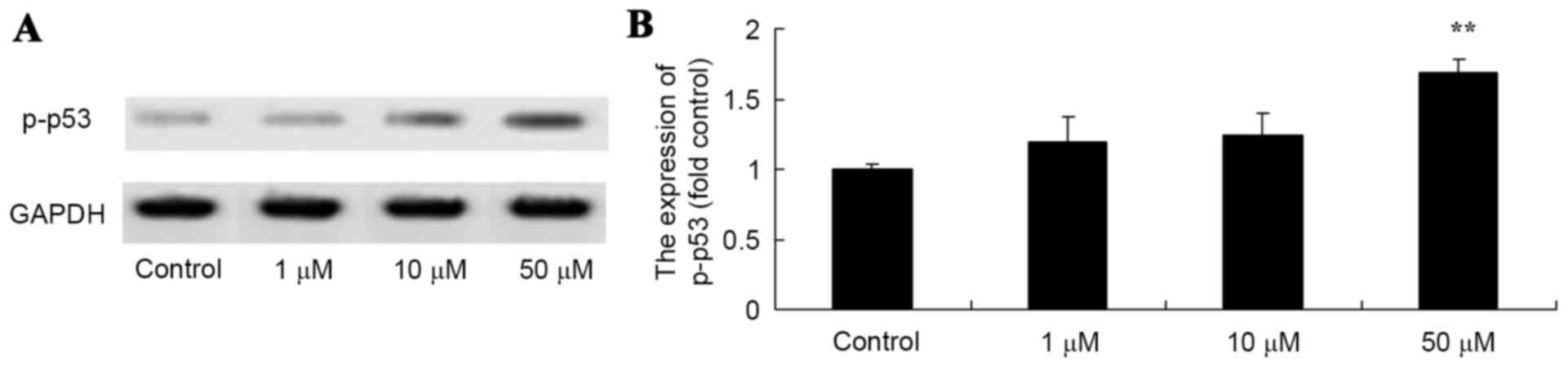
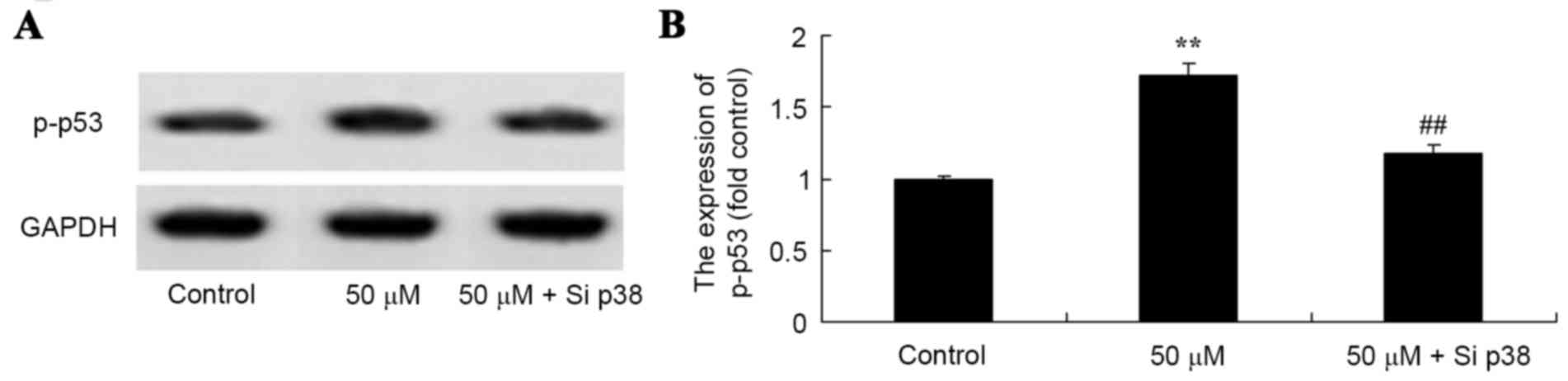
Salvianolic acid B activated p-p53
protein expression in the osteosarcoma MG63 cells
To quantify the activation of p-p53 protein
expression in the MG63 cells exposed to salvianolic acid B, western
blotting was used to detect the expression of p-p53 protein in the
MG63 cells. The results demonstrated that salvianolic acid B
increased the expression of p-p53 in a dose-dependent manner, as
illustrated in Fig. 6. in particular,
50 µM salvianolic acid B significantly increased the levels of
p-p53 protein in the MG63 cells compared with the control cells, as
demonstrated in Fig. 6.
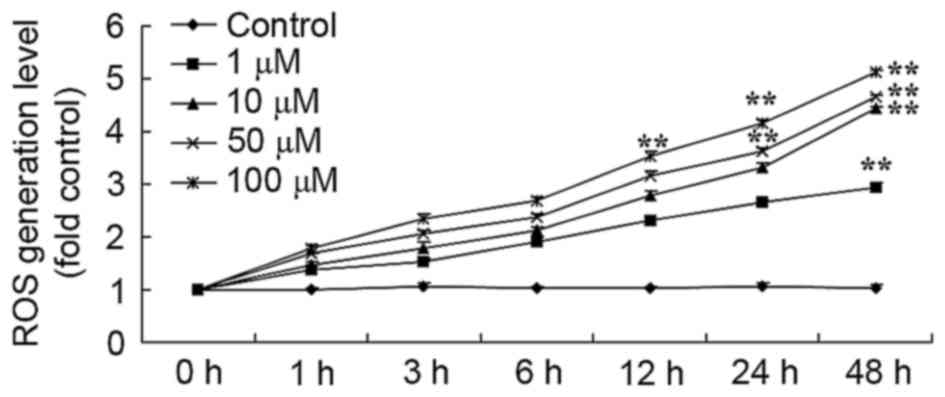
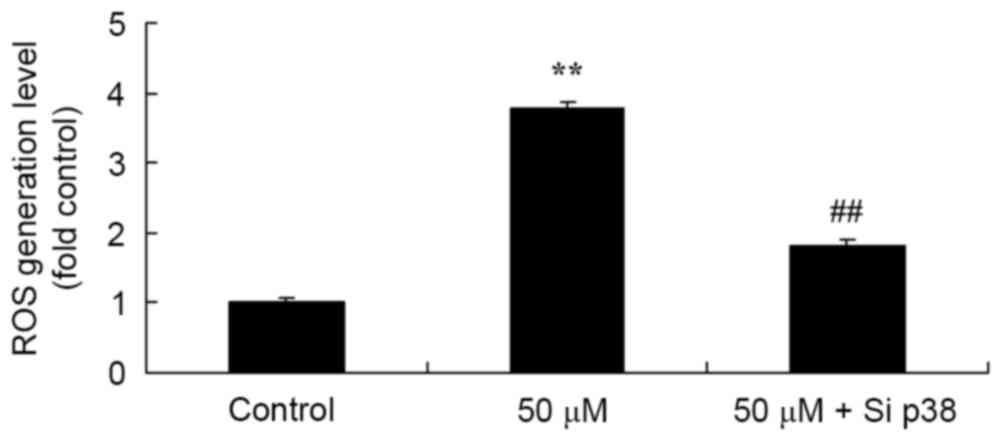
Salvianolic acid B-activated ROS
generation in the osteosarcoma MG63 cells
To confirm the antitumor effect of salvianolic acid
B on ROS generation in the osteosarcoma MG63 cell line, ROS
generation was measured using H2DCFDA. As demonstrated in Fig. 7, there was a significant increase in
ROS generation levels in the 50 µM salvianolic acid B treated group
compared with the control group.
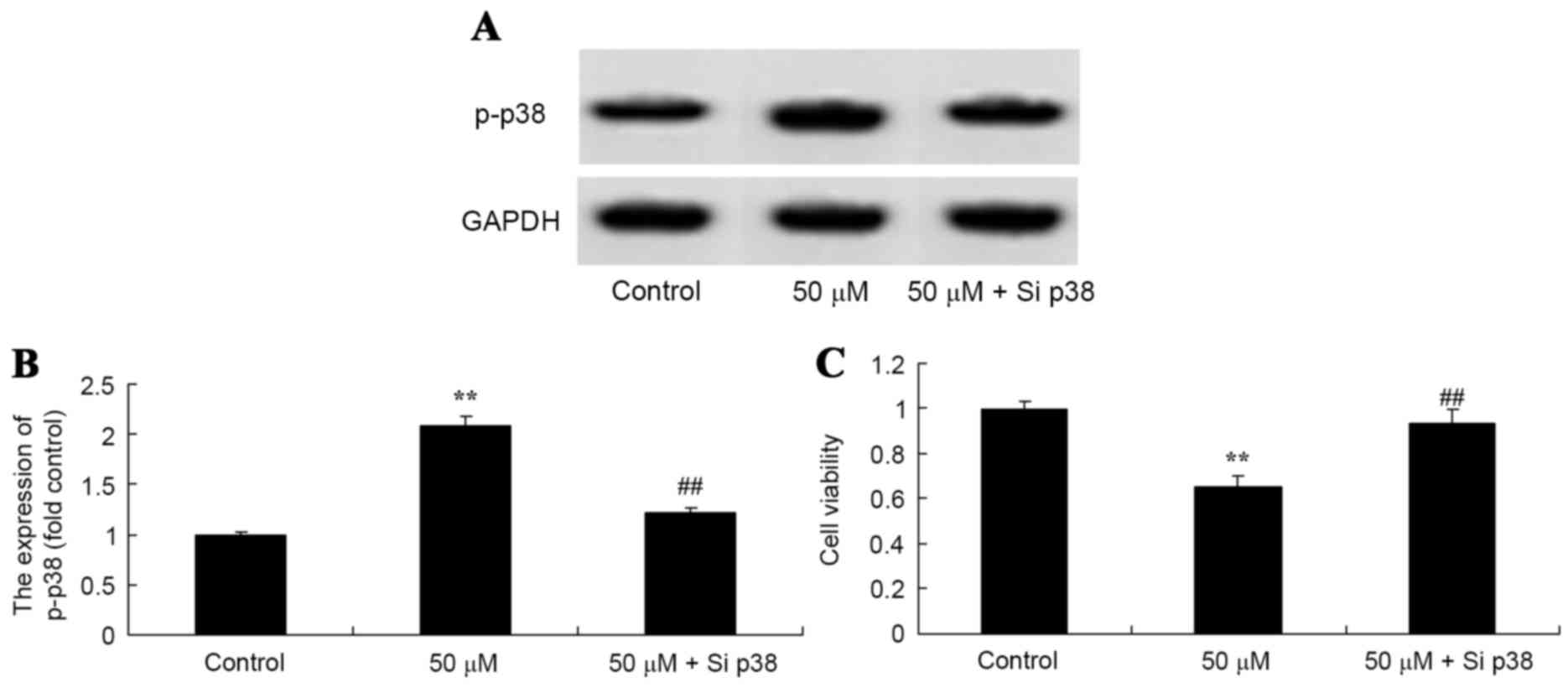
Salvianolic acid B suppresses cell
proliferation in osteosarcoma MG63 cells subsequent to the
knockdown of p38
To confirm that salvianolic acid B acts via p38 to
mediate its effects on osteosarcoma in vitro, si-p38RNAs
were transfected into MG63 cells to suppress the protein expression
of p38 and influence the anticancer effect of salvianolic acid B on
osteosarcoma. As illustrated in Fig.
8A, si-p38 significantly suppressed the p38 protein expression
in the MG63 cells treated with 50 µM salvianolic acid B compared
with the control cells. Additionally, si-p38 significantly
inhibited the anticancer effects of salvianolic acid B on the cell
proliferation of osteosarcoma MG63 cell compared with the 50 µM
salvianolic acid B treated group, as illustrated in Fig. 8B.
Salvianolic acid B activated p53
protein expression in osteosarcoma MG63 cells subsequent to
knockdown of p38
Salvianolic acid B acted via a P-38 mediated
mechanism to alter levels of p-p53 protein in osteosarcoma MG63
cells. Knockdown of p38 expression significantly suppressed the
levels of p-p53 protein phosphorylation in MG63 cells treated with
50 µM salvianolic acid B compared with 50 µM salvianolic acid B
treated group that was not knocked down (Fig. 9).
Salvianolic acid B activated ROS
generation in osteosarcoma MG63 cells subsequent to knockdown of
p38
To examine the p38-mediated mechanism of salvianolic
acid B on the ROS generation in osteosarcoma MG63 cells, ROS
generation was detected using H2DCFDA subsequent to knockdown of
p38. The knockdown of p38 expression significantly reduced ROS
generation in osteosarcoma MG63 cells treated with 50 µM
salvianolic acid B, as compared with the 50 µM salvianolic acid B
treated group that was not knocked down, as illustrated in Fig. 10.
Discussion
Osteosarcoma is the most common type of primary
malignant bone tumor in adolescents (18). According to statistical data from
China, the morbidity of osteosarcoma is the highest of all primary
malignant bone tumors (5). Levels of
malignancy in osteosarcoma are high, patient prognosis is poor, and
patients may exhibit lung metastases within several months.
Subsequent to amputation, the 3–5-year survival rates are only 60%
(19). The present study observed
that salvianolic acid B suppressed cell proliferation, induced
apoptosis and activated cleaved caspase 3 of the osteosarcoma MG63
cell. Wang et al (12)
suggested that salvianolic acid B induced apoptosis through
p38-mediated ROS generation in human glioma U87 cells and
suppressed the growth and angiogenic potential of oral squamous
carcinoma cells (20) and
neuroblastoma SH-SY5Y cell lines (16).
MAPK is a type of serine/threonine protein kinase,
is one of the most important types of signal transduction molecules
in humans, is involved in cell signal regulation through acting on
target genes by activating multiple nuclear transcription factors
and is equipped with multiple regulatory functions for
participating in cell proliferation, differentiation and
intercellular functional synchronization (21).
Gene sequencing analysis demonstrated that p38MAPK
is a tyrosine phosphorylation protein kinase comprising 360 amino
acids (22). Previous studies
revealed that the p38 MAPK pathway may be activated through the
double phosphorylation of tyrosine and threonine, act on multiple
downstream factors and possess an important role in regulating
tumor cell invasion (22,23). Through the p38MAPK signal transduction
pathway, vascular endothelial growth factor induces metastasis of
tumor cells. p38 proteins are present in invasive colorectal
cancers (24). The p38MAPK pathway
induces expression of MMPs promotes the invasion of osteosarcoma
cells. However, data indicate that p38 serves a protective role in
apoptosis caused by ultraviolet light, restrains p38MAPK and
promotes and induces keratinocyte apoptosis in healthy individuals
(24). Activated p38MAPK improves the
stability and phosphorylation of p53 and promotes the concentration
of p53 in the cytoplasm (25). In the
present study, it was revealed that salvianolic acid B activated
p-p38 and p-p53 protein expression in the MG63 cell line.
Additionally, the silencing of p38 expression
inhibited the anticancer effects of salvianolic acid B on cell
proliferation of MG63 cells. p38 was also indicated to be a target
of salvianolic acid B on the MG63 cell line through the regulation
of p53 expression in osteosarcoma cells. Hung et al
(26) suggested that salvianolic acid
B induces neointimal cell apoptosis through p53 expression in a
rabbit angioplasty model. Ma et al (17) reported that salvianolic acid B
inhibits tumor necrosis factor-α-induced human coronary artery
endothelial cells through matrix metalloproteinase-9 and p38
activity.
The generation of ROS may trigger multiple
biological process, including apoptosis and programmed cell death
(27). In a previous study on
apoptosis caused by ultraviolet A (UVA) light, it was demonstrated
that UVA triggers ROS-mediated apoptosis (27). In addition, the authors hypothesized
that singlet oxygen is the dominant ROS. A previous study also
revealed that antioxidants may effectively restrain ROS generation
and apoptosis caused by UVA (28).
The generated ROS activates the upstream activating agent and
apoptosis signal conditioning kinase 1 (AsKI) of p38 and c-Jun
N-terminal kinase, so as to activate p38, indicating that
ROS-ASKI-p38MAPK pathway is an important signal transduction
pathway in the carcinogenic effects of ultraviolet light (29). In the present study it was revealed
that salvianolic acid B increased ROS generation levels and the
silencing of p38 expression inhibited the anticancer effect of
salvianolic acid B on the level of ROS generation in the MG63 cell
line.
In conclusion, the present study demonstrated the
salvianolic acid B inhibits cell proliferation and tumor growth via
inducing apoptotic cell death in the osteosarcoma MG63 cell line.
Additionally, these results suggest that salvianolic acid B may act
as a novel type of chemotherapy in osteosarcoma therapy via the
p38-mediated generation of ROS.
References
|
1
|
Nataraj V, Batra A, Rastogi S, Khan SA,
Sharma MC, Vishnubhatla S and Bakhshi S: Developing a prognostic
model for patients with localized osteosarcoma treated with uniform
chemotherapy protocol without high dose methotrexate: A
single-center experience of 237 patients. J Surg Oncol.
112:662–668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ambroszkiewicz J, Gajewska J, Klepacka T,
Chelchowska M, Laskowska-Klita T and Woźniak W: Clinical utility of
biochemical bone turnover markers in children and adolescents with
osteosarcoma. Adv Med Sci. 55:266–272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luisi FA, Petrilli AS, Tanaka C and Caran
EM: Contribution to the treatment of nausea and emesis induced by
chemotherapy in children and adolescents with osteosarcoma. Sao
Paulo Med J. 124:61–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferguson WS, Harris MB, Goorin AM,
Gebhardt MC, Link MP, Shochat SJ, Siegal GP, Devidas M and Grier
HE: Presurgical window of carboplatin and surgery and multidrug
chemotherapy for the treatment of newly diagnosed metastatic or
unresectable osteosarcoma: Pediatric oncology group trial. J
Pediatr Hematol Oncol. 23:340–348. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu XF, Yang WL, Wan ZH, Li J and Bi ZG:
Glutathione S-transferase polymorphisms and bone tumor risk in
China. Asian Pac J Cancer Prev. 12:3357–3360. 2011.PubMed/NCBI
|
|
6
|
Basu M, Mukhopadhyay S, Chatterjee U and
Roy SS: FGF16 promotes invasive behavior of SKOV-3 ovarian cancer
cells through activation of mitogen-activated protein kinase (MAPK)
signaling pathway. J Biol Chem. 289:1415–1428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang K, Fu XT, Li Y, Hou YJ, Yang MF, Sun
JY, Yi SY, Fan CD, Fu XY, Zhai J and Sun BL: Induction of S-phase
arrest in human glioma cells by selenocysteine, a natural
selenium-containing agent via triggering reactive oxygen
species-mediated DNA damage and modulating MAPKs and Akt pathways.
Neurochem Res. 41:1439–1447. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu KH, Li WX, Sun MY, Zhang SB, Fan CX, Wu
Q, Zhu W and Xu X: Cadmium induced apoptosis in MG63 cells by
increasing ROS, activation of p38 MAPK and inhibition of ERK 1/2
pathways. Cell Physiol Biochem. 36:642–654. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen CY, Yi L, Jin X, Zhang T, Fu YJ, Zhu
JD, Mi MT, Zhang QY, Ling WH and Yu B: Inhibitory effect of
delphinidin on monocyte-endothelial cell adhesion induced by
oxidized low-density lipoprotein via ROS/p38MAPK/NF-κB pathway.
Cell Biochem Biophys. 61:337–348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu M, Zheng Y, Sun HX and Yu DJ:
Inhibitory effects of enalaprilat on rat cardiac fibroblast
proliferation via ROS/P38MAPK/TGF-beta1 signaling pathway.
Molecules. 17:2738–2751. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao D, Nong S, Huang X, Lu Y, Zhao H, Lin
Y, Man Y, Wang S, Yang J and Li J: The effects of palmitate on
hepatic insulin resistance are mediated by NADPH Oxidase 3-derived
reactive oxygen species through JNK and p38MAPK pathways. J Biol
Chem. 285:29965–29973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZS, Luo P, Dai SH, Liu ZB, Zheng XR
and Chen T: Salvianolic acid B induces apoptosis in human glioma
U87 cells through p38-mediated ROS generation. Cell Mol Neurobiol.
33:921–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo HD, Cui GH, Tian JX, Lu PP, Zhu QC, Lv
R and Shao SJ: Transplantation of salvianolic acid B pretreated
mesenchymal stem cells improves cardiac function in rats with
myocardial infarction through angiogenesis and paracrine
mechanisms. Int J Cardiol. 177:538–542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei J, Xie G, Ge S, Qiu Y, Liu W, Lu A,
Chen T, Li H, Zhou Z and Jia W: Metabolic transformation of
DMBA-induced carcinogenesis and inhibitory effect of salvianolic
acid b and breviscapine treatment. J Proteome Res. 11:1302–1316.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao W, Guo XW, Zheng HZ, Li DP, Jia GB and
Wang J: Current progress of research on pharmacologic actions of
salvianolic acid B. Chin J Integr Med. 18:316–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng G, Tang T, Wu HJ, You WH, Luo JK, Lin
Y, Liang QH, Li XQ, Huang X and Yang QD: Salvianolic acid B
protects SH-SY5Y neuroblastoma cells from
1-methyl-4-phenylpyridinium-induced apoptosis. Biol Pharm Bull.
33:1337–1342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma L, Guan YQ and Du ZD: Salvianolic acid
B down-regulates matrix metalloproteinase-9 activity and expression
in tumor necrosis factor-α-induced human coronary artery
endothelial cells. Chin Med J (Engl). 128:2658–2663. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Loeb DM, Hobbs RF, Okoli A, Chen AR, Cho
S, Srinivasan S, Sgouros G, Shokek O, Wharam MD Jr, Scott T and
Schwartz CL: Tandem dosing of samarium-153 ethylenediamine
tetramethylene phosphoric acid with stem cell support for patients
with high-risk osteosarcoma. Cancer. 116:5470–5478. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mir O, Ropert S, Babinet A, Alexandre J,
Larousserie F, Durand JP, Enkaoua E, Anract P and Goldwasser F:
Hyper-alkalinization without hyper-hydration for the prevention of
high-dose methotrexate acute nephrotoxicity in patients with
osteosarcoma. Cancer Chemother Pharmacol. 66:1059–1063. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Ge PJ, Jiang L, Li FL and Zhu QY:
Modulation of growth and angiogenic potential of oral squamous
carcinoma cells in vitro using salvianolic acid B. BMC Complement
Altern Med. 11:542011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mason WP, Belanger K, Nicholas G,
Vallières I, Mathieu D, Kavan P, Desjardins A, Omuro A and Reymond
D: A phase II study of the Ras-MAPK signaling pathway inhibitor
TLN-4601 in patients with glioblastoma at first progression. J
Neurooncol. 107:343–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang L, Shu T, Liang Y, Gu W, Wang C, Song
X, Fan C and Wang W: GDC-0152 attenuates the malignant progression
of osteosarcoma promoted by ANGPTL2 via PI3K/AKT but not p38MAPK
signaling pathway. Int J Oncol. 46:1651–1658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Williamson AJ, Dibling BC, Boyne JR, Selby
P and Burchill SA: Basic fibroblast growth factor-induced cell
death is effected through sustained activation of p38MAPK and
up-regulation of the death receptor p75NTR. J Biol Chem.
279:47912–47928. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berger S, Dyugovskaya L, Polyakov A and
Lavie L: Short-term fibronectin treatment induces endothelial-like
and angiogenic properties in monocyte-derived immature dendritic
cells: Involvement of intracellular VEGF and MAPK regulation. Eur J
Cell Biol. 91:640–653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen YY, Hsieh CY, Jayakumar T, Lin KH,
Chou DS, Lu WJ, Hsu MJ and Sheu JR: Andrographolide induces
vascular smooth muscle cell apoptosis through a
SHP-1-PP2A-p38MAPK-p53 cascade. Sci Rep. 4:56512014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hung HH, Chen YL, Lin SJ, Yang SP, Shih
CC, Shiao MS and Chang CH: A salvianolic acid B-rich fraction of
Salvia miltiorrhiza induces neointimal cell apoptosis in rabbit
angioplasty model. Histol Histopathol. 16:175–183. 2001.PubMed/NCBI
|
|
27
|
Lan A, Xu W, Zhang H, Hua X, Zheng D, Guo
R, Shen N, Hu F, Feng J and Liu D: Inhibition of ROS-activated
p38MAPK pathway is involved in the protective effect of H2S against
chemical hypoxia-induced inflammation in PC12 cells. Neurochem Res.
38:1454–1466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi DC, Lee JY, Lim EJ, Baik HH, Oh TH
and Yune TY: Inhibition of ROS-induced p38MAPK and ERK activation
in microglia by acupuncture relieves neuropathic pain after spinal
cord injury in rats. Exp Neurol. 236:268–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie D, Wu X, Lan L, Shangguan F, Lin X,
Chen F, Xu S, Zhang Y, Chen Z, Huang K, et al: Downregulation of
TFAM inhibits the tumorigenesis of non-small cell lung cancer by
activating ROS-mediated JNK/p38MAPK signaling and reducing cellular
bioenergetics. Oncotarget. 7:11609–11624. 2016. View Article : Google Scholar : PubMed/NCBI
|