Introduction
Costimulatory signal is necessary to stimulate the
response of effective cellular immune (1–3). The B7
family and other costimulatory molecules play an important role in
the process of costimulatory signal transmission (4,5). The
binding of CD28 and B7 molecules on the surface of T cells seems to
provide the primary costimulatory signal for T cell activation
(6–8).
Various membrane surface molecules are located onto T cell surface.
These molecules guarantee the antigen recognition of T cell,
interactions with other immune cells and receiving signal
stimulation (8–12). Also, they provide an important
fundamental for the identification and isolation of T cells and T
cell subsets (7,11,13). Due
to the generation and transmission of costimulatory signals, the
apoptosis of target cells can regulate the immune response
artificially (14–16). Tim-3 might act as a ligand for reverse
transmission of signals to affect tumor immunity correspondingly
(17,18). In order to investigate this new
mechanism of Tim-3 molecules, hepatoma Hepa1-6 cell strain of ICR
mouse was used to build a solid tumor model in thigh muscle to
study the inhibitory effect of Tim-3 molecule on Hepa1-6 solid
tumor and the influence on immune system of tumor bearing ICR
mice.
Materials and methods
Materials
The cell strain of Hepa1-6 hepatocarcinoma was
purchased from BeNa Culture Collection (Guangzhou, China). Male ICR
mice (age: 4–6 weeks, weight: 18–22 g) were purchased from Animal
Center of Jilin Medical University. The study was approved by the
Academic Committee on the Ethics of Animal Experiments of Jilin
Medical University [Jilin, China; permit no. SCXK (Jilin)
2007–0003]. All animals were treated in accordance with the
Guidelines and Regulations for the Use and Care of Laboratory
Animals of Jilin Laboratory Animal Monitoring Institute under the
National Laboratory Animal Monitoring Institute of China. All
animals were at the Animal Center of Jilin Medical University
(Jilin, China) and acclimatized for two weeks at 24–28°C and 50–60%
humidity. Trizol was purchased from Thermo Fisher Scientific. The
reverse transcriptase and RNasin were purchased from Tiangen
Biochemical Tech Co Ltd (Beijing). Taq DNA polymerase was purchased
from Shanghai Haoran Company. 5-Carboxylfluorescein diacetate
succinimidyl ester (CFSE) and propidium bromide were purchased from
Sigma-Aldrich (China). When the tumor was measurable and the
treatment was begun.
Tim-3 expression and detection
Twelve ICR mice were taken to conduct the
measurements. The plasmid Tim-3 was dissolved in saline solution
and injected into the muscle of left thigh of the mice using in
situ injection method (0.1 mg per ICR mouse). After injection
of Tim-3 solution, each three mice were killed after 12, 24, 36,
and 48 h, respectively. RT-PCR method was applied to detect the
expression of Tim-3 in the muscle tissue of ICR mice.
Tumor cell inoculation
Hepa1-6 hepatoma cells (1×106/ml) were inoculated
into the muscle of left thigh of mice (0.1 ml per mouse). The mice
were randomly divided into normal saline group (the blank control
group), pcDNA group (the plasmid control group) and Tim-3 group
(the treatment group) with 20 mice in each group. Plasmid was
injected on alternate day after the second day of inoculation with
each injection of 0.1 ml (1 mg/ml), the injection site was the
position of inoculated tumor cells. All mice inoculated with cells
developed the tumor, when the mice were sacrificed.
Based on above experimental groups, TAP1 group (the
treatment group) and Tim-3/TAP1 group (the combined treatment
group, injection was half for each plasmid) were added with 20 mice
in each group, Hepa1-6 hepatoma cells were inoculated with the same
method and the same treatment was conduct for examination of
synergistic effect of Tim-3 and TAP1.
Gene expression in tumor
micro-environment
After 3rd and 6th day of inoculation, five mice that
were randomly selected from normal saline group, pcDNA group and
Tim-3 group, respectively were killed for tumor tissue sampling.
The total RNA was extracted with Trizol reagent for reverse
transcriptase reaction. Then 5 µl samples were taken as template
from 60 µl reverse transcriptase product to amplify IL-13, fup-1
and TAP1 by PCR, respectively. There were 40 cycles at the
conditions of 85°C for 40 sec, 60°C for 60 sec, 40°C for 90 sec,
and 25°C for 120 sec. Gel imaging analysis system was used to
analyze the expression level of mRNA and relative expression value
was calculated by the following equation: relative expression value
(%) = (gray value of amplified bands of the gene to be test / gray
value of β-actin gene amplification band) × 100%.
In vitro proliferation activity of
spleen cells
After 4th day of tumor cells inoculation, six mice
from normal saline group, pcDNA group and Tim-3 group, respectively
were randomly selected for spleen sampling. Single spleen cell was
prepared under sterile conditions for the following two
experiments: The first experiment is proliferation of spleen cells
in vitro. The concentration of mice spleen cell was adjusted
to 1×106/ml, suspended in culture medium containing 15%
fetal calf serum followed by stained with CFSE. The solution was
divided into two samples, one added Hepa1-6 antigen peptide and
TAP1 protein complex (the final concentration was 0.58 µg/ml). Both
samples were transferred to a hole plate (100 µl per hole) at 37°C
in an incubator for 7 days. Flow cytometry was used to detect
spleen cell proliferation index. The second experiment is spleen
cell killing in vitro. Three samples of prepared single
spleen cell suspension were taken (without CFSE staining), along
with the spleen cells taken from a normal mouse which was used to
determine the non-specific killing rate. All samples were cultured
in culture medium containing 15% fetal calf serum. Hepa1-6 antigen
peptide and TAP1 protein complex (the final concentration was 0.58
µg/ml) was added and cultured in vitro for 7 days as
effector cells. Hepa1-6 cells were taken from mouse ascites and
cultured in the same culture medium for overnight followed by
stained with CFSE as target cells. The effector cells were mixed
with the target cells according to a ratio of 30:1 at 37°C, the
mixture was cultured in an incubator for 6 h, followed by propidium
bromide second staining. Flow cytometry was used to detect the
death rate of target cells (Hepa1-6 cells).
Statistical method
The in vitro experiments were repeated for 3
times. Single-factor ANOVA was used to compare the difference
between the control group and the experimental group. P<0.05
indicates statistical significance. The experimental data were
analyzed by statistical software package SPSS13.
Results
Inhibitory effect of Tim-3 expression
on tumor growth in vivo
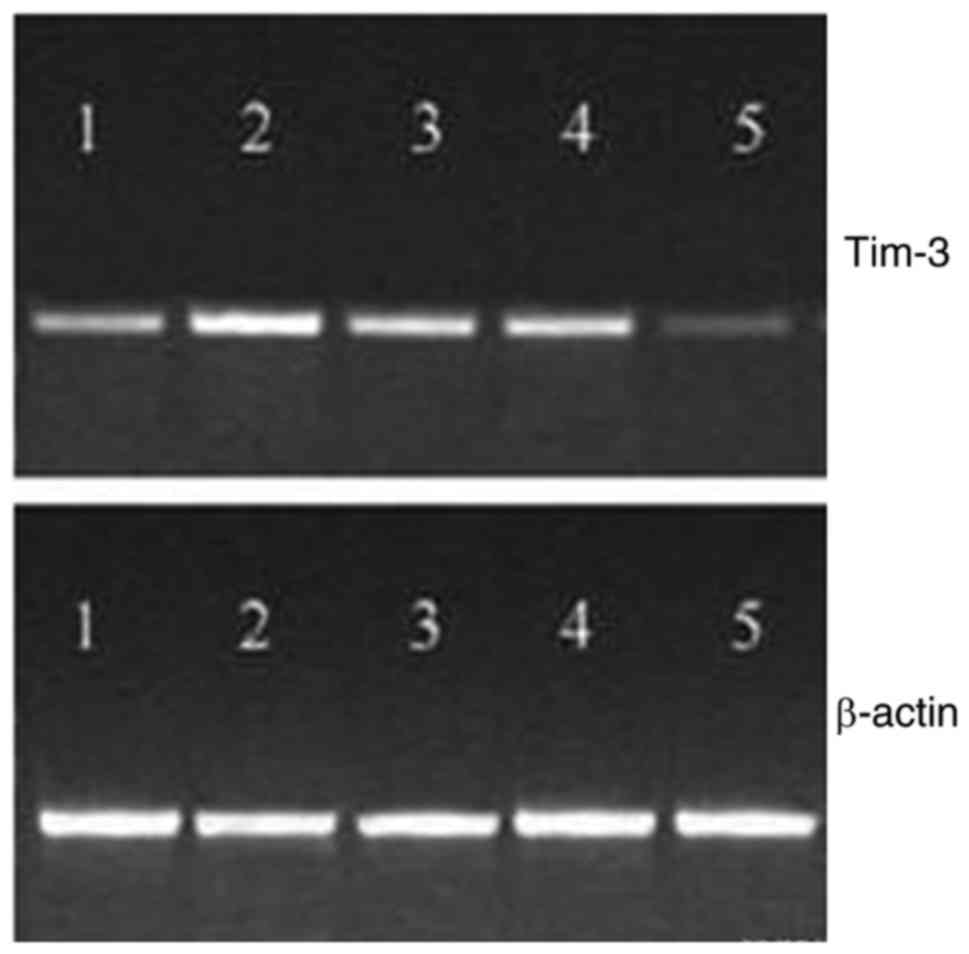
Tim-3 plasmid was injected into the muscle of mice,
and the expression of Tim-3 mRNA was detected in muscle tissue
after 12 h, and the peak value reached to peak at 24–48 h, which
could not be detected after 60 h (Fig.
1). After inoculation for 12 days, the average tumor weight
(0.84 g) of Tim-3 group was less than that of pcDNA group (1.57 g)
and normal saline group (1.61 g), which has significant difference
(P<0.05). However, there was no significant difference between
pcDNA group and normal saline group, suggesting that the expression
of Tim-3 could be inhibited by gene transfection in T cells, which
could probably inhibit the growth of tumor cells.
Expression of immune related
genes
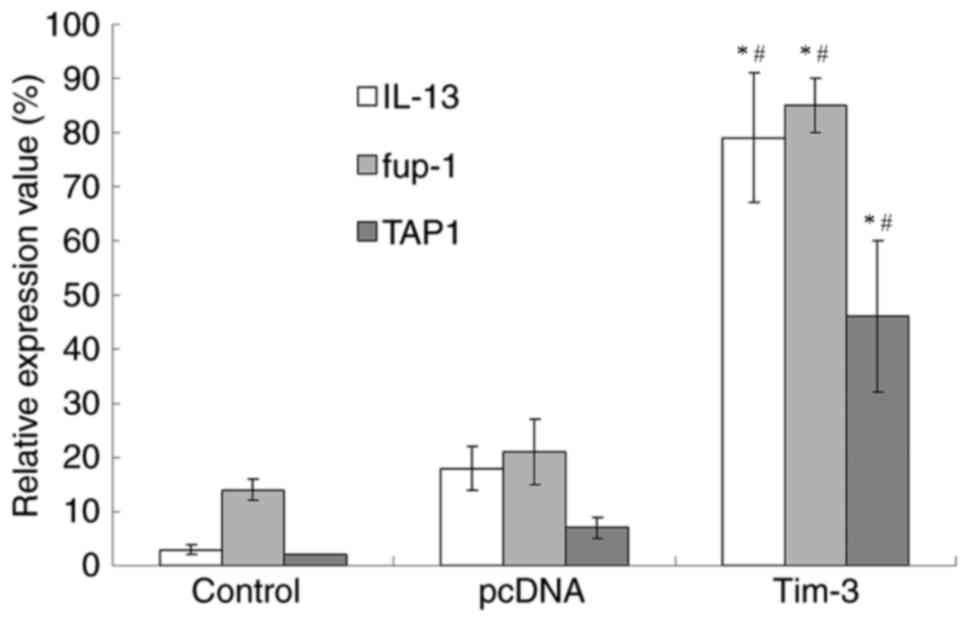
After the 5th day of inoculation, IL-13, fup-1 and
TAP1 of saline group and pcDNA group only exhibited trace
expression, while the Tim-3 group displayed the expression of all
three genes (Fig. 2). The expression
of fup-1 was higher than IL-13, while the expression of TAP1 was
markedly lower than the other two genes.
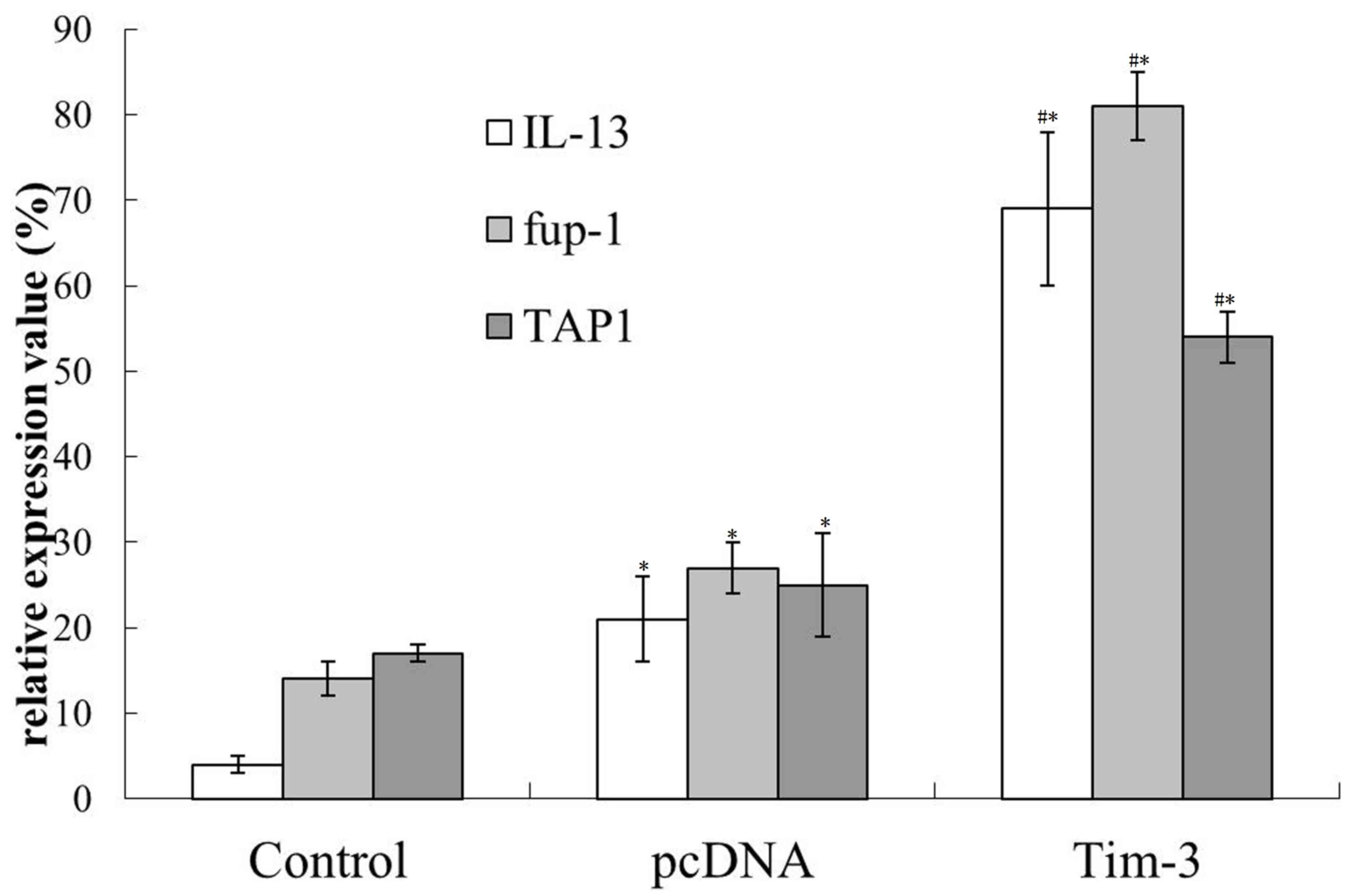
After the 10th day of inoculation, the expression of
IL-13 and fup-1 in Tim-3 group did not show further increase
compared with the results obtained above. However, the TAP1
expression in normal saline group, pcDNA group and Tim-3 group was
all enhanced (Fig. 3). Therefore, it
can be concluded that Tim-3 can promote the expression of positive
immune related genes in the early stage of tumor growth.
Effects of Tim-3 on enhancement of
splenocytes proliferation and cytotoxicity
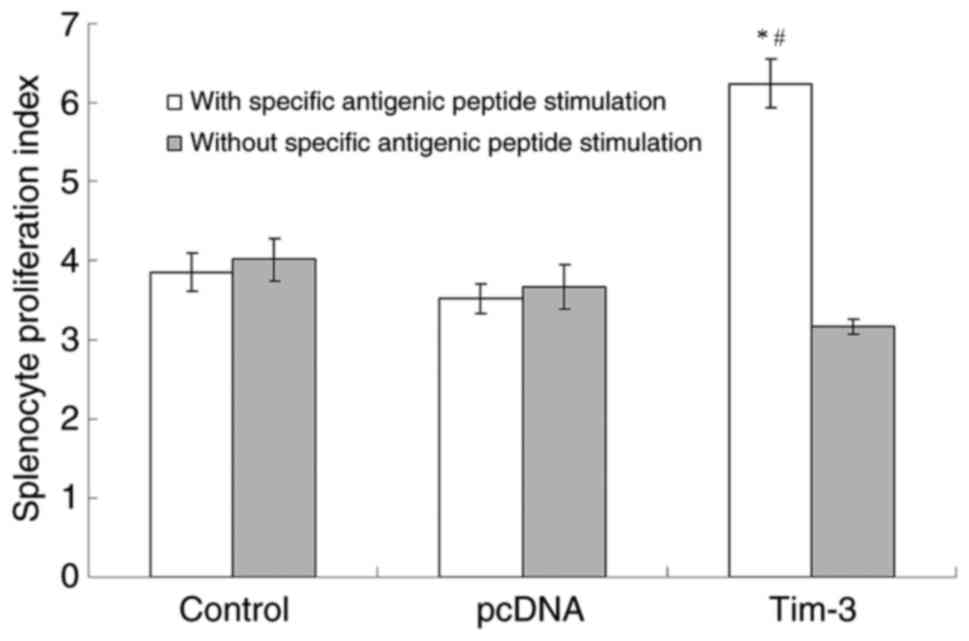
After inoculation for one week, the spleen cells
were taken for proliferation experiment. Under the conditions of
adding specific antigenic peptide stimulation and without specific
antigenic peptide stimulation, flow cytometry was used to detect
the mice splenocyte proliferation index of each experimental group.
The results (Fig. 4) showed that the
Tim-3 group had a significant difference (P<0.05) compared with
the saline control group and the pcDNA group with specific
antigenic peptide stimulation. Also, the mice splenocyte
proliferation index of the Tim-3 group without specific antigenic
peptide stimulation did not have significant difference comparing
the other two groups. The ratios of proliferation index obtained by
adding specific antigenic peptide stimulation and without specific
antigenic peptide stimulation for the three groups were 0.97, 0.99,
and 1.62, respectively. This result indicates that the larger the
ratio is, the higher the activity of spleen cell proliferation is
under antigen stimulation.
The killing activity experiment of spleen cells
further confirmed the specific killing activity of T cells. The
specific killing function of Tim-3 group was stronger than pcDNA
group (P<0.05) and saline control group (P<0.01). The killing
rate was 32, 23, and 14%, respectively.
Synergistic effect of Tim-3 with
TAP1
So far, we have demonstrated that transfection of
Tim-3 into tumor-bearing mice significantly inhibited the growth of
tumor. Furthermore, the growth was further inhibited when Tim-3 and
TAP1 were simultaneously transfected. After inoculation for two
weeks, of the average weight of tumor was only 0.42 g,
significantly lower than that of Tim-3 transfected mice (0.84 g) or
TAP1 transfected mice (1.57 g) and normal saline group (1.61 g).
The experimental group treated with combined Tim-3 and TAP1 has
significant difference (P<0.05), suggesting that Tim-3 and TAP1
have stronger synergistic antitumor effect.
Discussion
So far, Tim-3 has been used as the receptor molecule
on the surface of T3 cells in the studies of membrane-type Tim-3
(5–7).
Generally, TAP1 is considered as the ligand of Tim-3 (8,9). The
signal produced by synergistic effect of TAP1 and membrane-type
Tim-3 can prevent the activation of the cell and negatively
regulate the cell immunity. However, if membrane-type Tim-3
inhibits receptor only, soluble Tim-3 should enhance the immune
response by blocking effect. Previous studies have found that
soluble Tim-3 does not produce an immune enhancing effect, but
generates an immunosuppressive effect (10–12).
In this study, it was found that T cells can enhance
the immune function by transfecting the recombinant membrane-type
Tim-3 eukaryotic expression plasmid into Hepa1-6 hepatoma cells of
vaccinated mice, which had significant antitumor effect. This
result is not consistent with a previous publication which reported
that membrane-type Tim-3 can induce T cell immune tolerance. The
membrane-type Tim-3 is a receptor of the T cell surface, after
binding with ligand for signal transduction. Tim-3 produced a
series of changes within the cell. In current work, intramuscular
injection of recombinant carrier of membrane-type Tim-3 was
directly conducted. Which carrier effectively expressed in muscle
cells but was difficult to transfect T cells. The possibility that
membrane-type Tim-3 as cell surface receptor expression in mature
muscle cell surface to induce the produce of positive immune effect
by muscle cells is very small. Another possibility is that the
membrane-type Tim-3 has ligand properties, which produced positive
immune regulatory effect through the interaction with receptor of
immune cells surface. Because only TAP1 molecules are known to bind
with membrane-type Tim-3, the existence of other receptor molecules
remains unclear. In this research, the method of local transfection
was taken to allow the expression of membrane-type Tim-3 only in
specific position - the thigh muscle cells of ICR mice. We
speculate that in this case the membrane-type Tim-3 may not have a
direct effect on T cells, but indirectly activate T cells via
activation of macrophages or antigen cells. Because TAP1 that
combined with Tim-3 can express in a variety of tissue cells
including macrophages, the Tim-3 molecule will react with these
cells. The RT-PCR results obtained with pure Hepa1-6 cell samples
showed very weak expression of TAP1 only, the possiblity that Tim-3
molecules reacted with the Hepa1-6 cells locally inoculated can be
excluded.
In this study, we have found that the membrane-type
Tim-3 and TAP1 could have synergistic effect. TAP1 as a molecule
having clear physiological functions can interact with its receptor
(expressed on the surface of activated T cells) to maintain the
activity of T cells or further enhance the activity and turn it
into memory T cells. The transformation is independent and not
restricted by other molecules, suggesting that the mechanisms of
membrane-type Tim-3 signal is activation of T cells eventually, and
produce synergistic effect with TAP1 based on the activation. After
2 weeks of tumor cells inoculation, TAP1 molecule started to
function. Although T cells were activated at this moment, the
effect of Tim-3 molecules is not further strengthened, suggesting
that Tim-3 molecules do not influence the functions of T cells
after activation.
References
|
1
|
Monney L, Sabatos CA, Gaglia JL, Ryu A,
Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel
RA, et al: Th1-specific cell surface protein tim-3 regulates
macrophage activation and severity of an autoimmune disease.
Nature. 415:536–541. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sabatos CA, Chakravarti S, Cha E, Schubart
A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ and
Kuchroo VK: Interaction of Tim-3 and Tim-3 ligand regulates T
helper type 1 responses and induction of peripheral tolerance. Nat
Immunol. 4:1102–1110. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakuishi K, Apetoh L, Sullivan JM, Blazar
BR, Kuchroo VK and Anderson AC: Targeting Tim-3 and PD-1 pathways
to reverse T cell exhaustion and restore anti-tumor immunity. J Exp
Med. 207:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones RB, Ndhlovu LC, Barbour JD, Sheth
PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman
JM, et al: Tim-3 expression defines a novel population of
dysfunctional T cells with highly elevated frequencies in
progressive HIV-1 infection. J Exp Med. 205:2763–2779. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin HT, Anderson AC, Tan WG, West EE, Ha
SJ, Araki K, Freeman GJ, Kuchroo VK and Ahmed R: Cooperation of
Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral
infection. Proc Natl Acad Sci USA. 107:14733–14738. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fourcade J, Sun Z, Benallaoua M, Guillaume
P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V and Zarour HM:
Upregulation of Tim-3 and PD-1 expression is associated with tumor
antigen-specific CD8+ T cell dysfunction in melanoma
patients. J Exp Med. 207:2175–2786. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Golden-Mason L, Palmer BE, Kassam N,
Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N,
Kuchroo V, Gretch DR and Rosen HR: Negative immune regulator Tim-3
is overexpressed on T cells in hepatitis C virus infection and its
blockade rescues dysfunctional CD4+ and CD8+
T cells. J Virol. 83:9122–9130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sehrawat S, Reddy PB, Rajasagi N,
Suryawanshi A, Hirashima M and Rouse BT: Galectin-9/TIM-3
interaction regulates virus-specific primary and memory CD8 T cell
response. PLoS Pathog. 6:e10008822010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khademi M, Illés Z, Gielen AW, Marta M,
Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris
RA, et al: T Cell Ig- and mucin-domain-containing molecule-3
(TIM-3) and TIM-1 molecules are differentially expressed on human
Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear
cells in multiple sclerosis. J Immunol. 172:7169–7176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McMahan RH, Golden-Mason L, Nishimura MI,
McMahon BJ, Kemper M, Allen TM, Gretch DR and Rosen HR: Tim-3
expression on PD-1+ HCV-specific human CTLs is
associated with viral persistence, and its blockade restores
hepatocyte-directed in vitro cytotoxicity. J Clin Invest.
120:4546–4557. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiba S, Baghdadi M, Akiba H, Yoshiyama H,
Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan
JD, et al: Tumor-infiltrating DCs suppress nucleic acid-mediated
innate immune responses through interactions between the receptor
TIM-3 and the alarmin HMGB1. Nat Immunol. 13:832–842. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagahara K, Arikawa T, Oomizu S, Kontani
K, Nobumoto A, Tateno H, Watanabe K, Niki T, Katoh S, Miyake M, et
al: Galectin-9 increases Tim-3+ dendritic cells and
CD8+ T cells and enhances antitumor immunity via
galectin-9-Tim-3 interactions. J Immunol. 181:7660–7669. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sehrawat S, Suryawanshi A, Hirashima M and
Rouse BT: Role of Tim-3/galectin-9 inhibitory interaction in
viral-induced immunopathology: shifting the balance toward
regulators. J Immunol. 182:3191–3201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ju Y, Hou N, Meng J, Wang X, Zhang X, Zhao
D, Liu Y, Zhu F, Zhang L, Sun W, et al: T cell immunoglobulin- and
mucin-domain-containing molecule-3 (Tim-3) mediates natural killer
cell suppression in chronic hepatitis B. J Hepatol. 52:322–329.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oikawa T, Kamimura Y, Akiba H, Yagita H,
Okumura K, Takahashi H, Zeniya M, Tajiri H and Azuma M:
Preferential involvement of Tim-3 in the regulation of hepatic
CD8+ T cells in murine acute graft-versus-host disease.
J Immunol. 177:4281–4287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kozdağ G, Ertaş G, Kiliç T, Acar E, Ağir
A, Sahin T, Cetin M, Bildirici U and Ural D: Elevated level of
high-sensitivity C-reactive protein is important in determining
prognosis in chronic heart failure. Med Sci Monit. 16:CR156–CR161.
2010.PubMed/NCBI
|
|
18
|
Sakhdari A, Mujib S, Vali B, Yue FY,
MacParland S, Clayton K, Jones RB, Liu J, Lee EY, Benko E, et al:
Tim-3 negatively regulates cytotoxicity in exhausted
CD8+ T cells in HIV infection. PLoS One. 7:e401462012.
View Article : Google Scholar : PubMed/NCBI
|