Introduction
With improvements in socioeconomic conditions,
individuals have developed an increased concern for their personal
health. As a result, routine medical check-ups and use of highly
available imaging procedures have led to the increased diagnosis of
asymptomatic pancreatic cystic lesions worldwide (1). A cystic tumor of the pancreas is
pathologically heterogeneous and accounts for between 10 and 15% of
cystic lesions of the pancreas (2).
Pancreatic cystic tumors are biologically diverse and typically
categorized as a serous cystic neoplasm (SCN), a mucinous cystic
neoplasm (MCN), an intraductal papillary mucinous neoplasm (IPMN)
or a solid pseudopapillary tumor of the pancreas (SPT). MCN of the
pancreas is a notable pathological entity of the pancreas,
histologically composed of inner epithelial mucin-secreting cells
and a surrounding dense ovarian-type stroma. However, there has
been confusion between MCN and IPMN, as ovarian-type stroma was not
previously regarded as a diagnostic criterion for MCN (3). However, the World Health Organization
classification in 2000 emphasized the significance of ovarian
stroma for the diagnosis of MCNs (4,5) and the
consensus meeting of the International Association of
Pancreatology, held in Sendai Japan, restricted the term MCN to a
neoplasm with the presence of ovarian stroma (6).
According to a Korean multi-institutional survey
(7), MCN was identified to be the
second most common type of neoplastic cyst of the pancreas in
Korea. The majority of MCNs are identified in middle-aged females,
and are typically located in the body and tail of the pancreas
(8–10). MCNs have been demonstrated to exhibit
malignant potential, but a good prognosis. However, the diagnosis
of pancreatic MCN remains rare, comprising between 1 and 2% all
pancreatic tumors (11). Surgical
experience of 163 resected MCNs was previously analyzed by one
study (12), however, only a limited
number of other studies have described surgical experiences with
MCNs.
In the present study, surgical experience with MCN
of the pancreas was reviewed, on the basis of Korean (Yonsei
University College of Medicine; YUCM) and Japanese (Nippon Medical
School; NMS) bi-institutional collaboration. In addition, the
present study aimed to identify the clinicopathological
characteristics of pancreatic MCNs detected between January 1990
and December 2012. Furthermore, the pre-surgical clinical
parameters that are predictive of malignant transformation were
analyzed to identify effective surgical management of MCN of the
pancreas.
Materials and methods
Patients
Between January 1990 and December 2012, 68 patients
(YUCM, 45 patients; NMS, 23 patients) underwent pancreatectomy for
non-invasive MCN of the pancreas. Among patients who underwent
surgical resection of known MCN, cases that exhibited MCNs were
reviewed. A total of 55 out of 68 cases, those that exhibited
mucin-secreting epithelial cells and a dense ovarian stroma, were
selected for the present study. All patients included in the
present study were female, with a mean age of 47.9±13.3 years
(range, 24–75 years). All patients were diagnosed and classified
using the International Agency for Research on Cancer/World Health
Organization 2010 classification (13).
Medical records review
The available medical records were reviewed to
identify the general characteristics, clinical presentations,
surgical outcomes and pathological results of the patients.
Chronological alterations in MCNs of the pancreas were analyzed and
grouped as follows: Period 1, between January 1990 and December
1999; period 2, between January 2000 and December 2006; and period
3, between January 2007 and December 2012. In addition, patients
were divided into the following two groups: The minimally invasive
approach group (MIS), for those who underwent laparoscopic or
robot-assisted surgery; and the open surgery group (conventional).
Subsequently, clinicopathological characteristics of MCNs and
perioperative outcomes were reviewed. A postoperative pancreatic
fistula (POPF) was defined according to the guidelines of the
International Study Group on Pancreatic Fistulas (ISGPF) (14). Furthermore, postoperative bleeding was
defined by the criteria proposed by the ISGPF (15). Mortality was defined as mortality
within 30 days of surgery whether in or outside of the hospital.
Follow-up data was reviewed for recurrence or tumor-specific
mortality.
Statistical analysis
Categorical variables were presented as frequency
and percentage, and continuous variables as the mean ± standard
deviation. A receiver operating characteristic curve was used to
estimate the optimal tumor size and predict the malignant
transformation of MCN of the pancreas. The χ2 test
(Fisher's exact test), Mann-Whitney test and linear regression
analysis were used for statistical assessment of associations.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using IBM SPSS
v.22.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
For the 68 patients, 13 pathological specimens
(13/68, 19.1%) were revealed not to be MCNs (10/13, IPMNs; 3/13,
pancreatic intraepithelial neoplasia cases). Excluding the 13
patients, a total of 55 patients with non-invasive MCN of the
pancreas were analyzed. All patients were female, with a mean age
of 47.9±13.3 years. A total of 19 patients (19/55, 34.5%) were
identified to exhibit a pancreatic cystic tumor via routine medical
check-up. Abdominal discomfort (15/55, 27.3%) was the most common
clinical symptom. General weakness and weight loss was demonstrated
in 9 patients (16.4%) and an abdominal mass was identified in 3
patients (5.5%). Diabetes mellitus was demonstrated in 4 patients
(7.3%) and 6 patients (10.9%) exhibited an extra-pancreatic
malignant disease, including breast cancer in 2 patients, cervical
cancer in 1 patient, thyroid cancer in 1 patient, hepatocellular
carcinoma in 1 patient and early gastric cancer in 1 patient.
Preoperative imaging study
All patients received abdominal computed tomography,
whereas abdominal endoscopic ultrasound scans, magnetic resonance
imaging and endoscopic retrograde cholangiopancreatography were
selectively performed for preoperative evaluation to characterize
cystic tumors if mural nodules appeared in a preoperative computed
tomography scan. There was no definitive radiological evidence of
local invasiveness in all patients, but 8 patients (8/55, 14.5%)
exhibited an intracystic solid component (mural nodule). The mean
size of the surgical specimen was 6.1±4.2 cm and the majority of
cystic tumors (52/55, 94.5%) were located in the distal pancreas
(body and tail of the pancreas), with only 3 (3/55, 5.5%) in the
pancreatic head.
Selection of surgical procedures
It was demonstrated that distal pancreatectomy with
or without splenectomy was the most common type of surgical
procedure for treating MCN of the pancreas (46/55, 83.6%). Cyst
enucleation was performed in 5 patients (5/55, 9.1%), and
pancreatoduodenectomy was performed in 3 patients (3/55, 5.5%). A
laparoscopic or robot-assisted minimally invasive approach was
applied in 31 patients (31/55, 56.4%). When considering cases of
distal pancreatectomy, there were significant different clinical
characteristics between the two institutions (Table I). In NMS, the minimally invasive
surgical approach was performed more frequently compared with the
conventional surgical approach (P=0.005). However, the
spleen-preserving procedure was more frequent in YUCM (P=0.069).
Comparing the data from the two institutions, tumor size was
demonstrated to be similar; however, tumor location and associated
symptoms were identified to be significantly different (P<0.05;
Table I).
 | Table I.Clinical characteristics of distal
pancreatectomy in two institutions. |
Table I.
Clinical characteristics of distal
pancreatectomy in two institutions.
| Characteristic | YUCM (n=28) | NMS (n=18) | P-value |
|---|
| Age,
yearsa | 46.3±12.3 | 51.1±14.1 | 0.839 |
| Sex |
|
|
|
|
Female | 28 | 18 |
|
|
Male | 0 | 0 |
|
| Symptoms |
|
| 0.003 |
| No | 14 | 15 |
|
|
Yes | 14 | 3 |
|
| R-Tumor
sizea | 6.5±4.2 | 5.7±3.4 | 0.068 |
|
Spleen-preserving |
|
| 0.069 |
| No | 20 | 17 |
|
|
Yes | 8 | 1 |
|
| Tumor location |
|
| <0.001 |
|
Body | 13 | 6 |
|
|
Tail | 15 | 4 |
|
|
Body+tail | – | 8 |
|
| Surgery |
|
| 0.005 |
|
Conventional | 15 | 2 |
|
|
Laparoscopic | 13 | 16 |
|
| LOH,
daysa,b | 8.1±4.2 | 19.2±10.2 | <0.001 |
Postoperative outcomes
There was no postoperative mortality. A POPF was
developed in 10 patients (10/55, 18.2%; grade B, n=9; and grade C,
n=1) and postoperative hemorrhage was identified in 1 patient
(1/55, 1.8%). MCN-adenoma was identified in 15 patients (27.3%) and
MCN-borderline in 20 patients (36.4%). Focal non-invasive carcinoma
was identified in 9 patients (9/55, 16.4%).
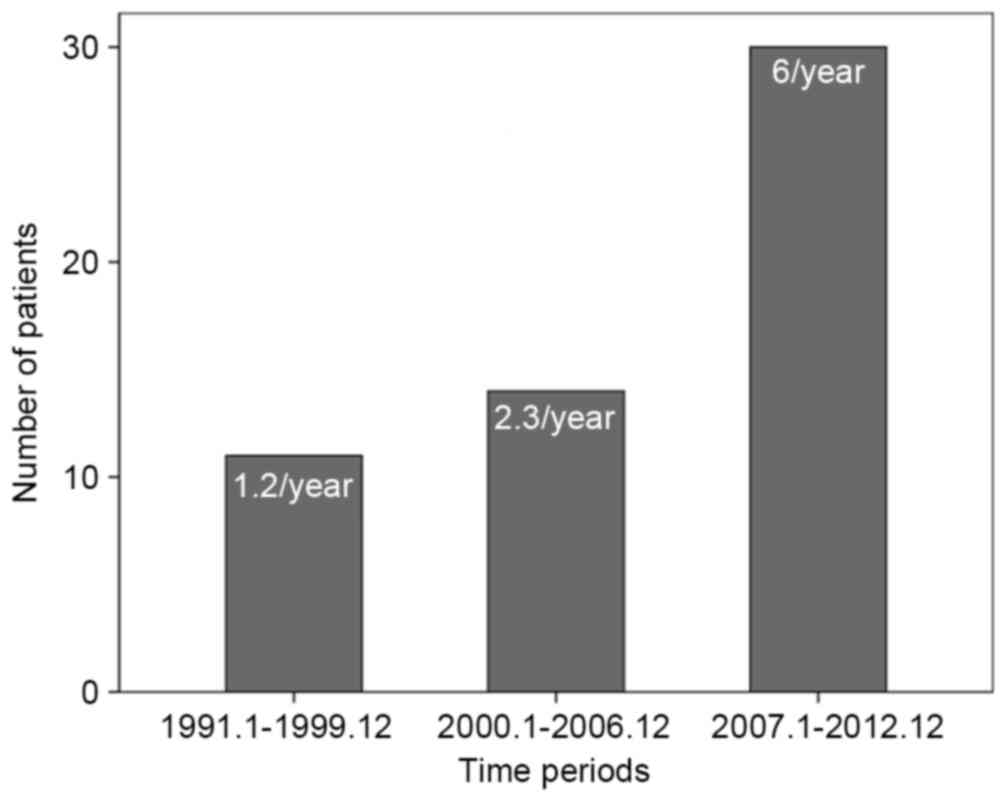
Chronological alterations in
surgically resected MCNs of the pancreas
A total of 11 patients (20%) were surgically treated
for MCN of the pancreas in period 1, 16 patients (29.1%) in period
2 and 28 patients (50.9%) in period 3. The number of treated
patients per year in period 3 increased by 5-fold compared with
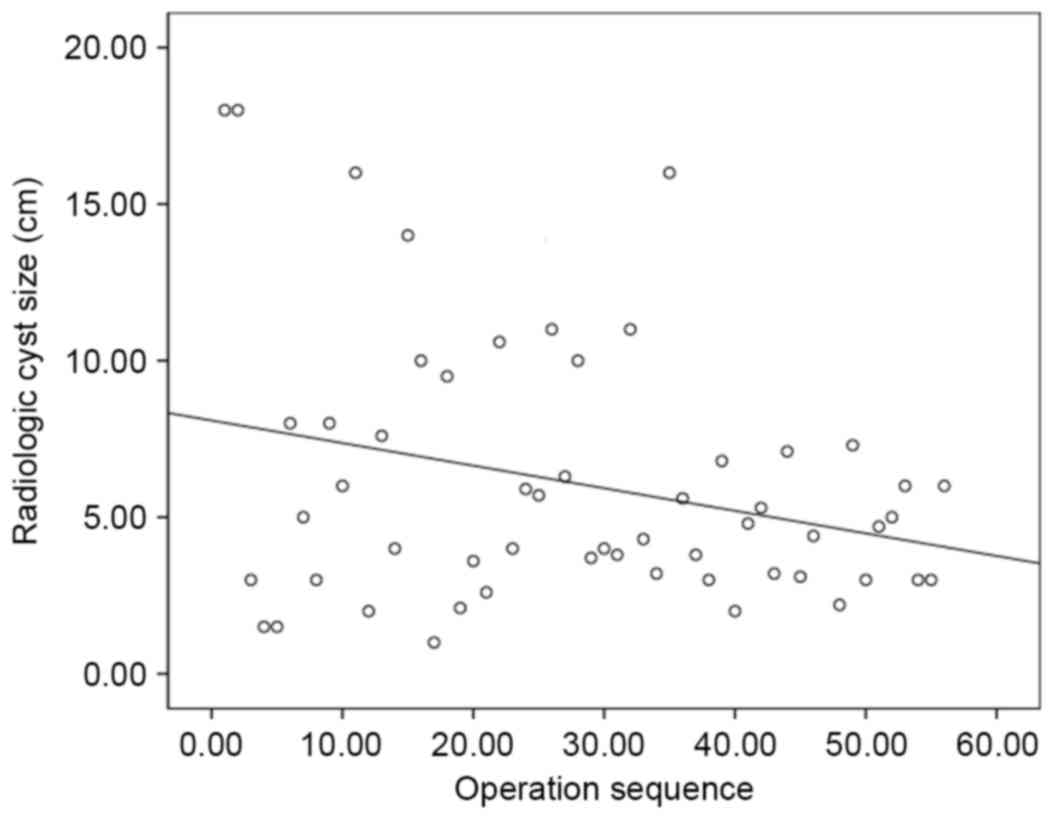
those in period 1 (Fig. 1). Tumor
size generally decreased over all periods. (Fig. 2) [tumor size=(−0.072) × case sequence
(order of operation) + 8.088; P=0.038]. Therefore, asymptomatic
patients were more frequently found through routine medical
check-up (P=0.022), and minimally invasive surgery, such as the
laparoscopic and robot-assisted approach, was frequently applied in
the recent surgical period (P<0.001). Furthermore, a significant
increase was demonstrated in spleen-preserving surgery, when
performing distal pancreatectomy, between periods (P=0.026;
Table II).
 | Table II.Alterations in the clinical
characteristics of pancreatic mucinous cystic neoplasms, according
to the time of surgery. |
Table II.
Alterations in the clinical
characteristics of pancreatic mucinous cystic neoplasms, according
to the time of surgery.
|
| Period |
|
|---|
|
|
|
|
|---|
| Characteristic | 1 | 2 | 3 | P-value |
|---|
| Age,
yearsa | 47.3±14.3 | 51.1±10.9 | 46.5±14.4 | 0.545 |
| Sex |
|
|
| 0.545 |
|
Male | 0 | 0 | 0 |
|
|
Female | 11 | 16 | 28 |
|
| Symptoms |
|
|
| 0.022 |
| No | 2 | 12 | 19 |
|
|
Yes | 9 | 4 | 9 |
|
| Tumor size,
cma | 8.0±6.4 | 6.2±3.8 | 5.2±3.0 | 0.250 |
| Malignant
transformation |
|
|
| 0.113 |
| No | 9 | 11 | 26 |
|
|
Yes | 2 | 5 | 2 |
|
| Surgery |
|
|
| <0.001 |
|
Conventional | 11 | 10 | 3 |
|
|
Minimally invasive | – | 6 | 25 |
|
|
Spleen-preservinga,b |
|
|
| 0.026 |
| No | 5 | 13 | 19 |
|
|
Yes | 0 | 0 | 9 |
|
Comparative analysis between
conventional and minimally invasive surgical approaches
A total of 31 patients (56.4%) underwent minimally
invasive pancreatectomy for MCN of the pancreas. Conventional
laparoscopic approach was performed in 26 patients (26/55, 47.3%)
and robot-assisted distal pancreatectomy was conducted in 5
patients (5/55, 9.1%). Compared with the conventional group, the
MIS group demonstrated an increased number of asymptomatic patients
(P=0.059), a smaller tumor size (radiological size, 7.4±5.32 vs.
4.9±2.8 cm, P=0.045; pathological size, 7.3±5.9 vs. 4.6±2.1 cm,
P=0.044) and an increased surgical duration (208.3±95.4 vs.
316.3±152.1 min, P=0.004). Notably, hospital stay was similar
between the two groups (12.7±6.7 vs. 13.8±9.6 days, P=0.412).
However, it was demonstrated that the post-surgical hospital stay
was distinct between the two groups when considering individual
institutional analysis (YUCM, 12.4±6.9 vs. 8.1±4.2 days, P=0.045;
NMS, 16.5±2.1 vs. 19.2±10.2 days, P=0.394) (Table III).
 | Table III.Comparative analysis between
conventional open and minimally invasive surgical approaches. |
Table III.
Comparative analysis between
conventional open and minimally invasive surgical approaches.
| Feature | Conventional | Minimally
invasive | P-value |
|---|
| Age,
yearsa | 48.1±12.9 | 48.1±14.4 | 0.773 |
| Sex |
|
|
|
|
Male | 0 | 0 |
|
|
Female | 24 | 31 |
|
| Symptom |
|
| 0.059 |
| No | 11 | 22 |
|
|
Yes | 13 | 9 |
|
| R tumor size,
cma | 7.4±5.2 | 4.9±2.8 | 0.045 |
| P tumor size,
cma | 7.3±5.9 | 4.6±2.1 | 0.044 |
| Location |
|
| 0.077 |
|
Proximal | 3 | – |
|
|
Distal | 21 | 31 |
|
| Type of
resection |
|
|
|
| EN | 3 | 2 |
|
|
SpDP | 1 | 8 |
|
|
DPS | 16 | 21 |
|
| PD | 3 | – |
|
| CP | 1 | – |
|
| Surgical duration,
mina | 208.3±95.4 | 316.3±152.1 | 0.004 |
| Bleeding amount,
mla | 726.8±211 | 331.6±426.3 | 0.059 |
| Transfusion |
|
| 0.863 |
| No | 22 | 28 |
|
|
Yes | 2 | 3 |
|
| POPF Grade B |
|
| 0.336 |
| No | 21 | 24 |
|
| Yes | 3 | 7 |
|
| LOH, days | 12.7±6.7 | 13.8±9.6 | 0.079 |
| YUCM | 12.4±6.9 | 8.1±4.2 | 0.45 |
| NMS | 16.5±2.1 | 19.2±10.2 | 0.941 |
Preoperative prediction of malignant
transformation
Malignant transformation (focal non-invasive
mucinous cystadenocarcinoma) was observed in 9 patients (9/55,
16.4%). Follow-up data was available for 51 patients (51/55, 92.7%)
and 4 patients were lost to follow-up. The mean follow-up duration
was 51.6 months (range, 1.1–242.8 months). Neither recurrence nor
tumor-associated mortality was observed in benign, borderline MCNs
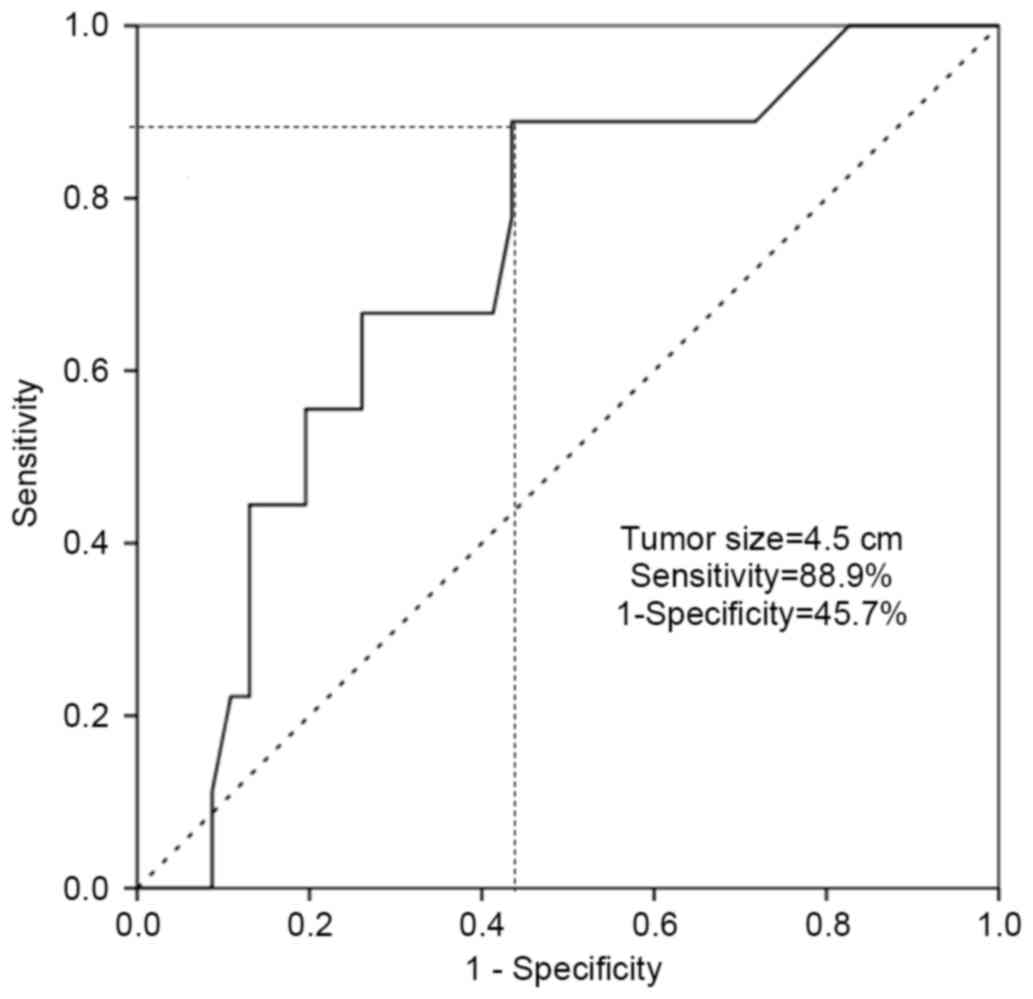
or MCNs with malignant transformation. A tumor size of >4.5 cm
was identified to predict malignant transformation of MCN with an
88.9% sensitivity and a 45.7% false-positive rate (1-specificity),
and this threshold value was statistically significant in the
preoperative prediction of malignant transformation [area under the
curve (AUC), 0.719; P=0.039; Fig. 3].
Radiological tumor size (≥4.5 cm; P=0.027) and intra-cystic solid
portions (mural nodule; P=0.002) were identified as important
preoperative clinical parameters to predict malignant
transformation (Table IV). In
particular, the significance of mural nodules in predicting
malignant transformation was significant in large MCNs of the
pancreas (≥4.5 cm; P=0.002; Table
V).
 | Table IV.Prediction of malignant
transformation of non-invasive mucinous cystic neoplasms of the
pancreas. |
Table IV.
Prediction of malignant
transformation of non-invasive mucinous cystic neoplasms of the
pancreas.
|
| Malignant
transformation |
|
|---|
|
|
|
|
|---|
| Characteristic | No | Yes | P-value |
|---|
| Tumor size <4.5
cm | 25 | 1 | 0.027 |
| Tumor size ≥4.5
cm | 21 | 8 |
|
| Mural nodule
(−) | 43 | 4 | 0.002 |
| Mural nodule
(+) | 3 | 5 |
|
| Total | 46 | 9 |
|
 | Table V.Significance of mural nodules in
predicting malignant transformation according to tumor size. |
Table V.
Significance of mural nodules in
predicting malignant transformation according to tumor size.
|
| Malignant
transformation |
|
|---|
|
|
|
|
|---|
| Tumor size, cm | No | Yes | P-value |
|---|
| <4.5 |
|
| 1.000 |
| Mural
nodule (−) | 22 | 1 |
|
| Mural
nodule (+) | 3 | 0 |
|
| ≥4.5 |
|
| 0.002 |
| Mural
nodule (−) | 21 | 3 |
|
| Mural
nodule (+) | 0 | 5 |
|
| Total | 46 | 9 |
|
Discussion
Pancreatic cancer is known to be one of the most
lethal gastrointestinal malignant diseases. Overall survival is
<5% and complete surgical resection is the most effective
treatment approach (16). However,
between 10 and 15% of patients with pancreatic cancer may be
surgical candidates and the long-term survival rate is between 10
and 20% with surgical resection (17–19).
Therefore, early detection and prevention are critical to improve
the outcome of pancreatic cancer. MCN of the pancreas is
potentially malignant and previous studies have suggested the
presence of adenoma-carcinoma sequences (9,20,21). Although the outcome of mucinous
cystadenocarcinoma is known to be superior to that of ductal
adenocarcinoma of the pancreas (22,23), the
5-year disease-specific survival rate for patients with invasive
mucinous cystadenocarcinoma is ~30% (10). Therefore, complete resection of the
tumor prior to the development of invasive cancer is considered an
important surgical goal in treating MCNs of the pancreas.
Pancreatic MCN is not an aggressive cancer (12,24). As
described in the present study, in the follow-up of patients
available (mean, 51.6 months (range, 1.1–242.8 months), neither
recurrence nor tumor-specific mortality was observed in all MCNs
with malignant transformation. Therefore, pancreatic surgeons
should consider a patient's quality of life following
pancreatectomy, as long-term survival is expected only if surgery
can be performed prior to the MCN developing invasive malignant
characteristics. In addition, function-preserving minimally
invasive surgical approaches would be ideal for a select group of
patients. Routine medical check-ups and the wider availability of
axial imaging procedures have contributed to the increasing numbers
of asymptomatic and smaller MCNs of the pancreas being found
(1), which is consistent with the
results in the present study. As the majority of MCNs of the
pancreas are located in the pancreatic body and tail, minimally
invasive pancreatectomy is an appropriate surgical approach; this
is since laparoscopic distal pancreatectomy, with or without
splenectomy, is regarded as a safe and effective treatment option
for benign and borderline malignant tumors of the pancreas
(25–28).
The perioperative outcomes of a minimally invasive
approach to MCNs of the pancreas are comparable to those of
conventional open surgery (Table
III). In the present study, MIS exhibited a significantly
increased duration of surgery (316.3±152.1) compared with that of
conventional surgery (208.3±95.4 min) (P<0.05). However, a
difference of 100 min may not be clinically significant,
considering the fact that attempts to achieve spleen-preserving are
increasing. When considering distal pancreatectomy, which was the
most frequent surgical procedure performed, it is particularly
noteworthy that there are several different clinical
characteristics between two institutions from different countries.
These observations may be due to different clinical situations,
such as the individual medical insurance systems of the two
institutions; however, despite the differences, the two
institutions dictate the use of a minimally invasive approach.
A previous study of 179 MCN cases revealed that a
large tumor size was associated with an increased risk for
malignancy (29). As demonstrated in
patients with intraductal papillary mucin-producing tumors of the
pancreas, the cyst size of the tumor was associated with malignant
transformation (6,30–32).
Typically, a size of 3–4 cm is suggested to be a cut-off value for
determining the malignant potential of MCN of the pancreas
(6,12,33).
Although there was a limited number of cases, the results of the
present study demonstrated that a tumor size of >4.5 cm was a
preoperative parameter that may be used to predict the malignant
transformation of MCN of the pancreas, with 88.9% sensitivity and a
45.7% false-positive rate (AUC, 0.719; P=0.039; Fig. 3). In addition, it was suggested that
preoperative detectable mural nodules were a reliable preoperative
parameter for the prediction malignant transformation in MCN of the
pancreas (P=0.002). A tumor >4.5 cm combined with an intracystic
solid portion (mural nodule) were associated with malignant
transformation (P=0.002; Table V).
Yamao et al (24) demonstrated
that the presence of a large cystic tumor (60,1±38.0 vs. 90.0±45.5
mm, P<0.001) and the presence of a nodule (28/129 vs. 14/27,
P=0.003) were observed at a higher frequency in mucinous
cystadenocarcinoma compared with that in mucinous cyst adenoma.
Therefore, it may be appropriate to consider the tumor size and the
presence of mural nodules together in predicting the malignant
potential in MCNs of the pancreas.
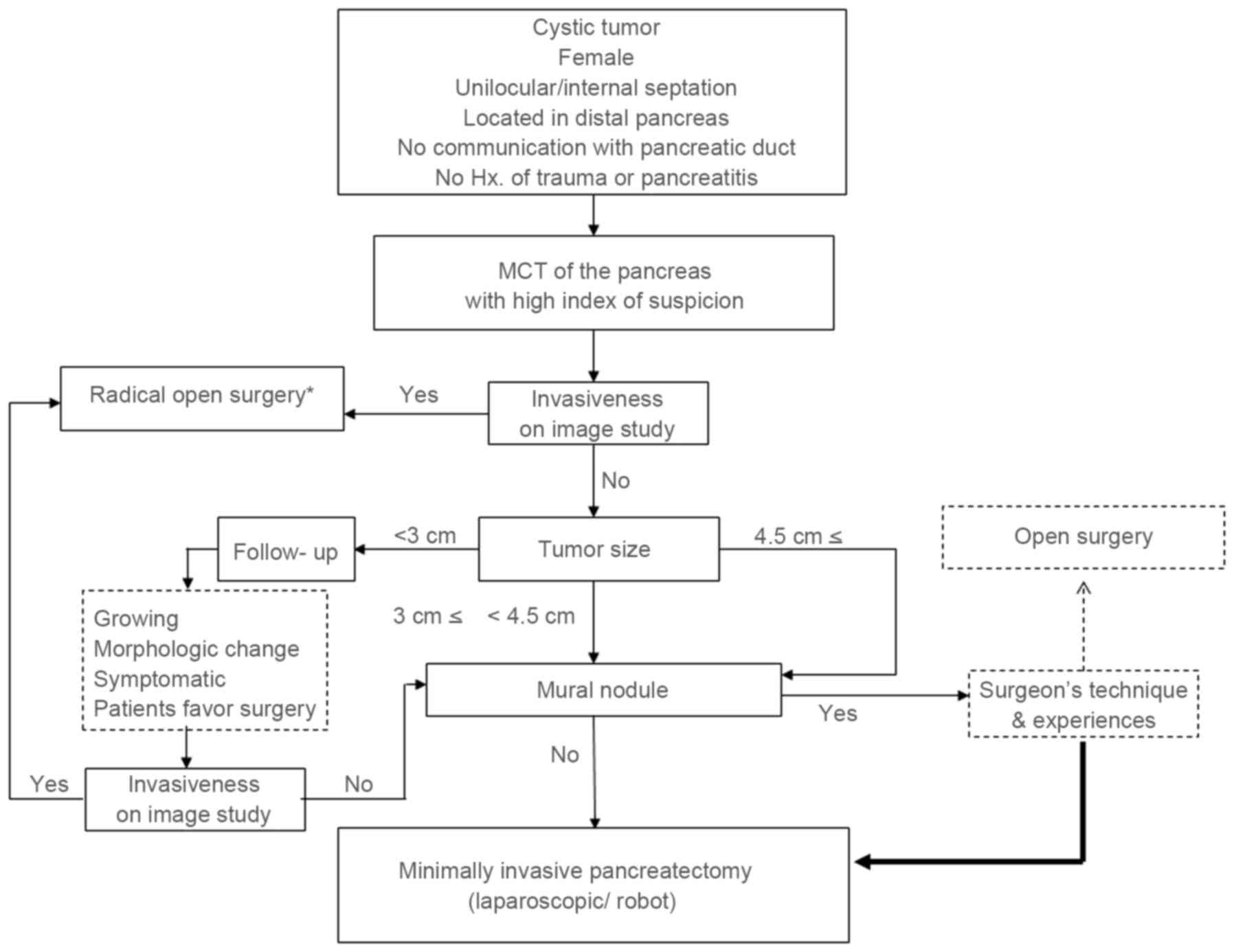
On the basis of the present study, minimally
invasive pancreatectomy may be selected for small MCNs (<4.5 cm)
without an invasive component in preoperative imaging studies. The
minimally invasive approach may additionally be considered for
radical pancreatectomy in selected cases (34–37).
According to the technique and experience of the surgeon involved,
active minimally invasive pancreatectomy can be applied in
non-invasive MCN of the pancreas regardless of tumor size. If there
are no definitive invasive characteristics identified in
preoperative images, even a large tumor (≥4.5 cm) with a mural
nodule may be a candidate for the minimally invasive approach,
according to results of the present study (Figs. 4 and 5).
However, the risk of a rupture of the cyst during the laparoscopic
approach may increase in cases of large MCNs and may lead to tumor
metastasis. Nakamura et al (38) demonstrated a safe surgical technique
for laparoscopic distal pancreatectomy involving a large cystic
tumor based on securing sufficient working space and minimizing
intraperitoneal cystic fluid spillage. Previous studies have
suggested a surgical technique for spleen-preserving laparoscopic
or robotic subtotal pancreatectomy with segmental resection of
whole splenic vessels to avoid cystic rupture during dissection
(39,40). Therefore, patient selection and the
surgical approach are important.
In summary, pancreatic cystic tumors are a
heterogeneous disease. However, unique clinical presentations and
advanced radiological experience can reveal an appropriate
preoperative diagnosis of MCNs of the pancreas. A tumor size of
>4.5 cm with the presence of a mural nodule may predict the
malignant change of pancreatic MCNs. However, the majority of
cystic tumors of the pancreas with well-defined boundaries of
cystic walls without local invasion in preoperative imaging are
considered to be benign or borderline MCNs with or without focal
non-invasive carcinomatous transformation. Therefore, minimally
invasive and function-preserving pancreatectomy may be initially
recommended.
Acknowledgements
This abstract was presented at the 26th World
Congress of the International Association of Surgeons,
Gastroenterologists and Oncologists, September 8–10, 2016 in Seoul,
Republic of Korea, and was published as Abstract no. PP2-012 in Dig
Surg 33 (Suppl 1): 1–232, 2016.
References
|
1
|
Edirimanne S and Connor SJ: Incidental
pancreatic cystic lesions. World J Surg. 32:2028–2037. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fernández-del Castillo C and Warshaw AL:
Cystic tumors of the pancreas. Surg Clin North Am. 75:1001–1016.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lester T, Robert B, Ronald P, et al:
Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade
malignant potential) of the pancreas: A clinicopathologic study of
130 cases. Am J Surg Pathol. 23:1–16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kloppel G, Solcia E, Longnecker DS,
Capella L and Sobin LH: Histological typing of tumor of the
exocrine pancreas. In: World Health OrganizationInternational
Histological Classification of Tumors. Springer; Berlin,
Heidelberg, New York, NY: 1996
|
|
5
|
Zamboi G KG, Hruban RH, Longnecker DA and
Adler G: Mucinous cystic neoplasms of the pancreas. In: Pathology
and Genetics of Tumors of the Digestie SystemWorld Health
Organization Classification of Tumors. Hamilton SR and Aaltonen LA:
IARC press; Lyon: pp. 234–236. 2000
|
|
6
|
Tanaka M, Chari S, Adsay V, Fernandez-del
Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K and Matsuno
S: International Association of Pancreatology: International
consensus guidelines for management of intraductal papillary
mucinous neoplasms and mucinous cystic neoplasms of the pancreas.
Pancreatology. 6:17–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoon WJ, Lee JK, Lee KH, Ryu JK, Kim YT
and Yoon YB: Cystic neoplasms of the exocrine pancreas: An update
of a nationwide survey in Korea. Pancreas. 37:254–258. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zamboni G, Scarpa A, Bogina G, Iacono C,
Bassi C, Talamini G, Sessa F, Capella C, Solcia E, Rickaert F, et
al: Mucinous cystic tumors of the pancreas: Clinicopathological
features, prognosis, and relationship to other mucinous cystic
tumors. Am J Surg Pathol. 23:410–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarr MG, Carpenter HA, Prabhakar LP,
Orchard TF, Hughes S, van Heerden JA and DiMagno EP: Clinical and
pathologic correlation of 84 mucinous cystic neoplasms of the
pancreas: Can one reliably differentiate benign from malignant (or
premalignant) neoplasms? Ann Surg. 231:205–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilentz RE, Albores-Saavedra J, Zahurak M,
Talamini MA, Yeo CJ, Cameron JL and Hruban RH: Pathologic
examination accurately predicts prognosis in mucinous cystic
neoplasms of the pancreas. Am J Surg Pathol. 23:1320–1327. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilentz RE, Albores-Saavedra J and Hruban
RH: Mucinous cystic neoplasms of the pancreas. Semin Diagn Pathol.
17:31–42. 2000.PubMed/NCBI
|
|
12
|
Crippa S, Salvia R, Warshaw AL, Domínguez
I, Bassi C, Falconi M, Thayer SP, Zamboni G, Lauwers GY,
Mino-Kenudson M, et al: Mucinous cystic neoplasm of the pancreas is
not an aggressive entity: Lessons from 163 resected patients. Ann
Surg. 247:571–579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hruban R, Kloppel G, Boffetta P, et al:
Tumours of the pancreasWHO Classification of Tumours of the
Digestive System. Bosman FT, Carneiro F, Hruban RH and Theise ND:
4th edition. IARC press; Lyon: pp. 280–330. 2010
|
|
14
|
Bassi C, Dervenis C, Butturini G,
Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W
and Buchler M: International Study Group on Pancreatic Fistula
Definition: Postoperative pancreatic fistula: An international
study group (ISGPF) definition. Surgery. 138:8–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wente MN, Veit JA, Bassi C, Dervenis C,
Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr
MG, et al: Postpancreatectomy hemorrhage (PPH): An International
Study Group of Pancreatic Surgery (ISGPS) definition. Surgery.
142:20–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simoes PK, Olson SH, Saldia A and Kurtz
RC: Epidemiology of pancreatic adenocarcinoma. Chin Clin Oncol.
6:242017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsumoto Y, Yamada E, Kamei S, Iwahara M
and Ueoka R: High affinity of hybrid liposomes for normal human
epidermal keratinocytes in vitro. Biol Pharm Bull. 17:1299–1300.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pingpank JF, Hoffman JP, Ross EA, Cooper
HS, Meropol NJ, Freedman G, Pinover WH, LeVoyer TE, Sasson AR and
Eisenberg BL: Effect of preoperative chemoradiotherapy on surgical
margin status of resected adenocarcinoma of the head of the
pancreas. J Gastrointest Surg. 5:121–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Le Borgne J, de Calan L and Partensky C:
Cystadenomas and cystadenocarcinomas of the pancreas: A
multiinstitutional retrospective study of 398 cases. French
surgical association. Ann Surg. 230:152–161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jimenez RE, Warshaw AL, Z'graggen K,
Hartwig W, Taylor DZ, Compton CC and Fernández-del Castillo C:
Sequential accumulation of K-ras mutations and p53 overexpression
in the progression of pancreatic mucinous cystic neoplasms to
malignancy. Ann Surg. 230:501–511. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD,
Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, et
al: Six hundred fifty consecutive pancreaticoduodenectomies in the
1990s: Pathology, complications, and outcomes. Ann Surg.
226:248–260. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ridder GJ, Maschek H and Klempnauer J:
Favourable prognosis of cystadeno-over adenocarcinoma of the
pancreas after curative resection. Eur J Surg Oncol. 22:232–236.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamao K, Yanagisawa A, Takahashi K, Kimura
W, Doi R, Fukushima N, Ohike N, Shimizu M, Hatori T, Nobukawa B, et
al: Clinicopathological features and prognosis of mucinous cystic
neoplasm with ovarian-type stroma: A multi-institutional study of
the Japan pancreas society. Pancreas. 40:67–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vijan SS, Ahmed KA, Harmsen WS, Que FG,
Reid-Lombardo KM, Nagorney DM, Donohue JH, Farnell MB and Kendrick
ML: Laparoscopic vs open distal pancreatectomy: A
single-institution comparative study. Arch Surg. 145:616–621. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baker MS, Bentrem DJ, Ujiki MB, Stocker S
and Talamonti MS: A prospective single institution comparison of
peri-operative outcomes for laparoscopic and open distal
pancreatectomy. Surgery. 146:635–645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kooby DA, Gillespie T, Bentrem D, Nakeeb
A, Schmidt MC, Merchant NB, Parikh AA, Martin RC II, Scoggins CR,
Ahmad S, et al: Left-sided pancreatectomy: A multicenter comparison
of laparoscopic and open approaches. Ann Surg. 248:438–446.
2008.PubMed/NCBI
|
|
28
|
Palanivelu C, Shetty R, Jani K,
Sendhilkumar K, Rajan PS and Maheshkumar GS: Laparoscopic distal
pancreatectomy: Results of a prospective non-randomized study from
a tertiary center. Surg Endosc. 21:373–377. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suzuki Y, Atomi Y, Sugiyama M, Isaji S,
Inui K, Kimura W, Sunamura M, Furukawa T, Yanagisawa A, Ariyama J,
et al: Cystic neoplasm of the pancreas: A Japanese
multiinstitutional study of intraductal papillary mucinous tumor
and mucinous cystic tumor. Pancreas. 28:241–246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Farnell MB: Surgical management of
intraductal papillary mucinous neoplasm (IPMN) of the pancreas. J
Gastrointest Surg. 12:414–416. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jang JY, Kim SW, Lee SE, Yang SH, Lee KU,
Lee YJ, Kim SC, Han DJ, Choi DW, Choi SH, et al: Treatment
guidelines for branch duct type intraductal papillary mucinous
neoplasms of the pancreas: When can we operate or observe? Ann Surg
Oncol. 15:199–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okabayashi T, Kobayashi M, Nishimori I,
Sugimoto T, Namikawa T, Okamoto K, Okamoto N, Kosaki T, Onishi S
and Araki K: Clinicopathological features and medical management of
intraductal papillary mucinous neoplasms. J Gastroenterol Hepatol.
21:462–467. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reddy RP, Smyrk TC, Zapiach M, Levy MJ,
Pearson RK, Clain JE, Farnell MB, Sarr MG and Chari ST: Pancreatic
mucinous cystic neoplasm defined by ovarian stroma: Demographics,
clinical features, and prevalence of cancer. Clin Gastroenterol
Hepatol. 2:1026–1031. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi SH, Kang CM, Hwang HK, Lee WJ and Chi
HS: Robotic anterior RAMPS in well-selected left-sided pancreatic
cancer. J Gastrointest Surg. 16:868–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi SH, Kang CM, Lee WJ and Chi HS:
Multimedia article. Laparoscopic modified anterior RAMPS in
well-selected left-sided pancreatic cancer: Technical feasibility
and interim results. Surg Endosc. 25:2360–2361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fernández-Cruz L, Cosa R, Blanco L, Levi
S, López-Boado MA and Navarro S: Curative laparoscopic resection
for pancreatic neoplasms: A critical analysis from a single
institution. J Gastrointest Surg. 11:1607–1622. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song KB, Kim SC, Park JB, Kim YH, Jung YS,
Kim MH, Lee SK, Seo DW, Lee SS, Park DH and Han DJ: Single-center
experience of laparoscopic left pancreatic resection in 359
consecutive patients: Changing the surgical paradigm of left
pancreatic resection. Surg Endosc. 25:3364–3372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakamura Y, Matsumoto S, Tajiri T and
Uchida E: Safe technique for laparoscopic distal pancreatectomy
involving a large cystic tumor. J Nippon Med Sch. 78:374–378. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi SH, Kang CM, Kim JY, Hwang HK and Lee
WJ: Laparoscopic extended (subtotal) distal pancreatectomy with
resection of both splenic artery and vein. Surg Endosc.
27:1412–1413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim DH KC, Hwang HK, Lee WJ and Chi HS:
‘Extended’ distal pancreatectomy with segmental resection of both
splenic vessels: Extended Warshaw's procedure. Korean J
Hepatobiliary Pancreat Surg. 14:52010.
|