Introduction
Gliomas represent the most common type of primary
tumors of the central nervous system (1). The highly invasive feature of malignant
gliomas renders them refractory to surgery, radiation and
chemotherapy (2). Despite improvement
in treatment modalities, the median survival time for malignant
gliomas is only between 1 and 2 years (3,4).
Therefore, it is of importance to develop novel effective therapies
against invasive gliomas.
Rho GTPases are a family of small signaling
G-proteins that perform critical roles in cancer cell migration,
invasion and metastasis (5). They act
as molecular switches through cycling between an inactive GDP-bound
and active GTP-bound form. Rac1 is one of the most studied Rho
GTPase family members (5). Several
lines of evidence indicate that Rac1 activation contributes to
glioma cell invasion (6–8). Nakada et al (6) reported that the ephrin-B3 ligand
facilitates the invasion of glioma cells through activation of
Rac1. Tumor necrosis factor receptor superfamily member 19
(TNFRSF19) overexpression has been found to activate Rac1 signaling
to drive glioma cell invasion and migration, and inactivation of
Rac1 may abrogate TNFRSF19-induced glioma cell invasion (8). ZINC69391, a specific Rac1 inhibitor, has
been reported to exhibit anti-invasive activity on glioma cells
(9). These studies indicate a pivotal
role for Rac1 signaling in glioma invasion. Matrix
metalloproteinases (MMPs), which are important regulators of
extracellular matrix degradation, have been documented to be
implicated in Rac1-induced cell invasion (10).
Bergamottin, a natural furanocoumarin abundantly
present in grapefruit juice, has been demonstrated to exhibit
anticancer effects in a variety of human cancers, including chronic
myelogenous leukemia (11), multiple
myeloma (12), skin cancer (13) and breast cancer (14). It has been reported that bergamottin
enhances tumor necrosis factor-induced apoptosis in human chronic
myelogenous leukemia via inactivation of nuclear factor-κB
signaling (11). Kim et al
(12) reported that bergamottin
exhibits chemosensitizing activity in multiple myeloma cells via
inhibition of the signal transducer and activator of transcription
3 signaling pathway. Hwang et al (15) demonstrated that bergamottin may
prevent phorbol 12-myristate 13-acetate-induced tumor cell invasion
via downregulation of MMP-9 expression. Despite these studies,
relatively little is known about the effect of bergamottin in human
glioma.
Therefore, in the present study, the potential
anticancer effect of bergamottin on human glioma cells was
explored. Considering the importance of Rac1 in glioma invasion,
the involvement of Rac1 in the action of bergamottin was also
investigated.
Materials and methods
Cell culture and treatment
Human U87 and U251 glioma cells were purchased from
the American Type Culture Collection (Manassas, VA, USA). Cells
were maintained in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS) and
1% penicillin (100 IU/ml)/streptomycin (100 µg/ml; all from
Invitrogen; Thermo Fisher Scientific, Inc.), at 37°C in a
humidified atmosphere containing 5% CO2.
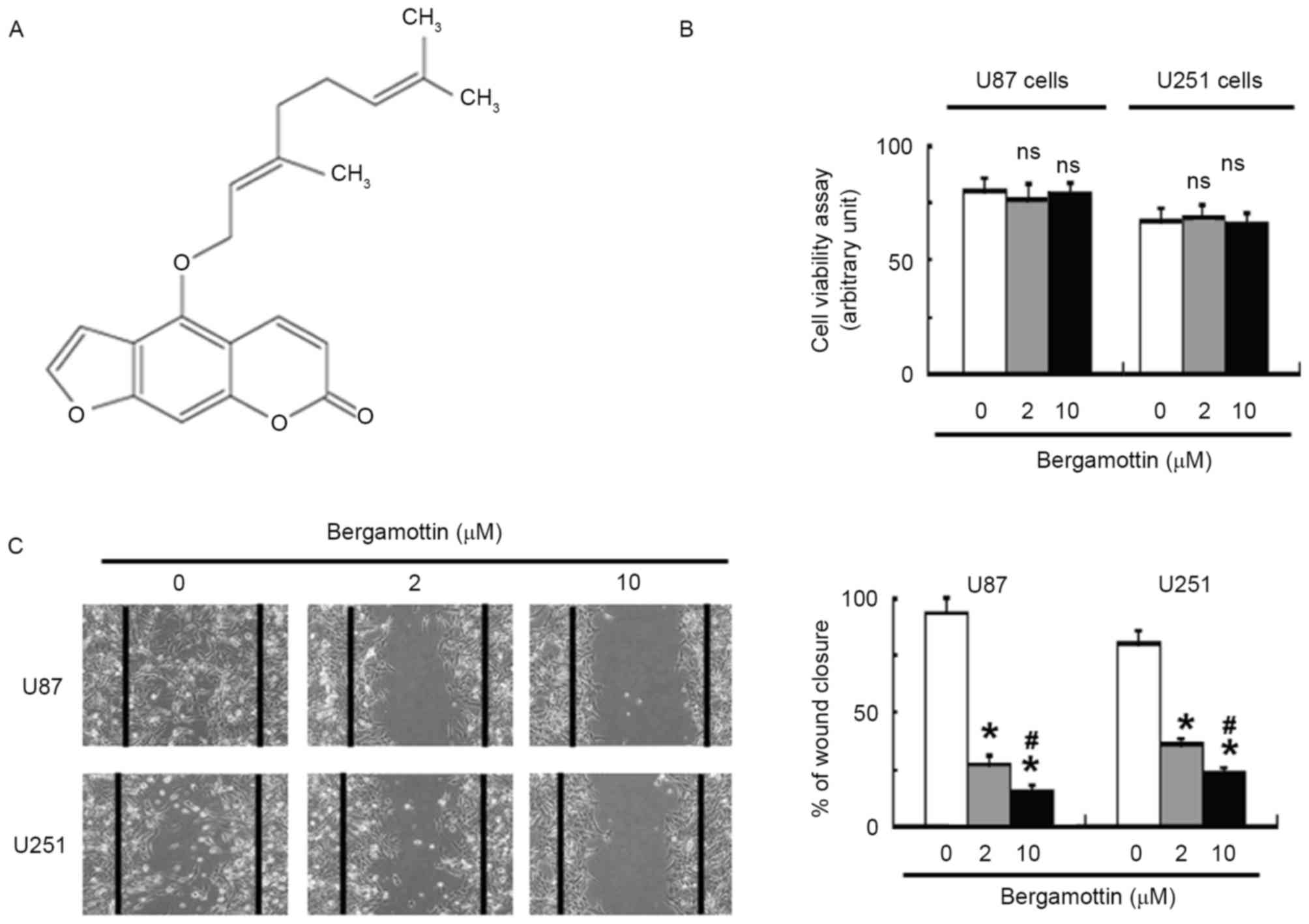
Bergamottin (≥96.9% in purity; Fig. 1A) was purchased from ChromaDex
(Irvine, CA, USA). The cells were incubated with 2 and 10 µM
bergamottin for 48 h at 37°C and collected for subsequent
analyses.
Plasmid transfection
A constitutively active Rac1 construct
(pcDNA3-EGFP-Rac1-Q61L) was purchased from Addgene, Inc.
(Cambridge, MA, USA). Transient transfection of
pcDNA3-EGFP-Rac1-Q61L or empty vector was performed using Fugene
reagent (Roche Diagnostics, Basel, Switzerland), according to the
manufacturer's protocol. At 24 h post-transfection, cells were
exposed to bergamottin for an additional 48 h and examined for cell
migration, invasion and MMP-9 expression. To estimate transfection
efficiency, parallel cell cultures were transfected with a green
fluorescent protein (GFP)-encoding plasmid (pEGFP-C1; Clontech
Laboratories, Inc., Mountain View, CA, USA). In the present study,
transfection efficiency was >85%.
Cell viability assay
The cells in serum-free medium were seeded at
5×103 cells/well in 96-well plates and treated with
bergamottin for 48 h. Cell proliferation was assessed using the
Cell Titer 96 aqueous non-radioactive cell proliferation assay kit
(Promega Corporation, WI, USA), according to the manufacturer's
protocol. Absorbance was read at 490 nm using a microplate
reader.
Wound-healing assay
Cells were seeded in 6-well plates and grown to 100%
confluence. A scratch wound was made in the cell monolayer using a
10 µl pipette tip. Detached and damaged cells were carefully washed
with PBS. The adherent cells were then cultured in DMEM containing
1% FBS with or without bergamottin. Following incubation for 48 h
at 37°C, images of cells were captured using a light microscope
(DM6000 B; Leica Microsystems GmbH, Wetzlar, Germany) in 5 random
fields (magnification, ×40). The percentage of wound closure was
calculated as follows: [(Initial wound area-post-migration
area)/initial wound area] ×100.
Transwell invasion assay
Transwell chambers in 24-well plates were coated
with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) in DMEM and
incubated at 37°C for 45 min to allow the gel to solidify. The
lower chambers were filled with DMEM with 10% FBS as a
chemoattractant. The upper chambers were seeded with
2×104 cells/well in serum-free DMEM containing 2 or 10
µM bergamottin. Following incubation for 48 h at 37°C, non-invading
cells (upper chamber) were gently removed with a cotton swab.
Invading cells (lower chamber) were fixed with 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), stained with 0.2%
crystal violet (Sigma-Aldrich; Merck KGaA) and counted under a
light microscope (DM6000 B; Leica Microsystems GmbH). A total of 10
random microscopic fields (magnification, ×200) were examined for
each well.
Western blot analysis of MMP-9
protein
Following treatment, cells were collected and lysed
in ice-cold radioimmunoprecipitation assay buffer (Cell Signaling
Technology, Inc., Danvers, MA, USA) containing 50 mM sodium
fluoride, 1 mM phenylmethylsulfonyl fluoride, and 10 µg/ml
aprotinin and leupeptin (Sigma-Aldrich; Merck KGaA). The lysates
were clarified and quantified using the Bradford Protein assay
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's protocol. Equal amounts of total protein (40 µg per
lane) were resolved by 10% SDS-PAGE and transferred onto
nitrocellulose membranes. Following blocking with 10% fat-free
milk, the membrane was incubated with rabbit anti-MMP-9 antibody
(1:500; cat. no. sc-393859) or anti-β-actin antibody (1:1,000; cat.
no. sc-130301; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at
4°C overnight, followed by incubation at room temperature for 1 h
with horseradish peroxidase-conjugated secondary antibody (1:3,000;
cat. no. sc-2005; Santa Cruz Biotechnology, Inc.). Protein bands
were visualized using enhanced chemiluminescence (GE Healthcare
Life Sciences, Chalfont, UK) and quantified using Quantity One
software (version 4.6.2; Bio-Rad Laboratories Inc.).
Rho GTPase activity assays
U87 and U251 cells were treated for 48 h with or
without bergamottin, and the Rac1 Activation assay kit was
performed according to the manufacturer's protocol (Cell Biolabs,
Inc., San Diego, CA, USA). Following treatment, cells were lysed in
tissue culture plates and cell lysates were clarified by
centrifugation at 12,000 × g for 10 min at 4°C. The protein
concentration was quantified using the Bradford protein assay.
Lysates (50 µg/lane) were diluted and incubated for 1 h with
glutathione transferase-fusion proteins containing the p21-binding
domain of p21-activated protein kinase 1. Bound complexes were
washed and subjected to western blot analysis using mouse
monoclonal antibody anti-Rac1 (1:300; cat. no., 610650; BD
Biosciences) or anti-cell division cycle (Cdc) 42 (1:300; cat. no.,
610929; BD Biosciences). The intensity of immunoreactive signals
was quantified using Quantity One software (version 4.6.2; Bio-Rad
Laboratories, Inc.).
ELISA
Cells were treated with bergamottin for 48 h and the
supernatants were collected and centrifuged at 12,000 × g for 10
min at 4°C. The protein concentrations of MMP-9 in the supernatants
were determined using the human MMP-9 Quantikine ELISA kit
according to the manufacturer's protocol (R&D Systems, Inc.,
Minneapolis, MN, USA).
Statistical analysis
All data are presented as the mean ± standard error.
The significance of differences among groups was determined using
one-way analysis of variance followed by Tukey's test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Bergamottin inhibits migration and
invasion of human glioma cells
Exposure to bergamottin <10 µM in serum-free
medium had no significant effect on the viability of U87 and U251
cells, compared with control cells (Fig.
1B). Wound-healing assays demonstrated that bergamottin
suppressed the migration of U87 and U251 cells into the wound in
cell monolayers (Fig. 1C).
Quantification analysis indicated that the percentage of wound
closure was significantly decreased in bergamottin-treated cells
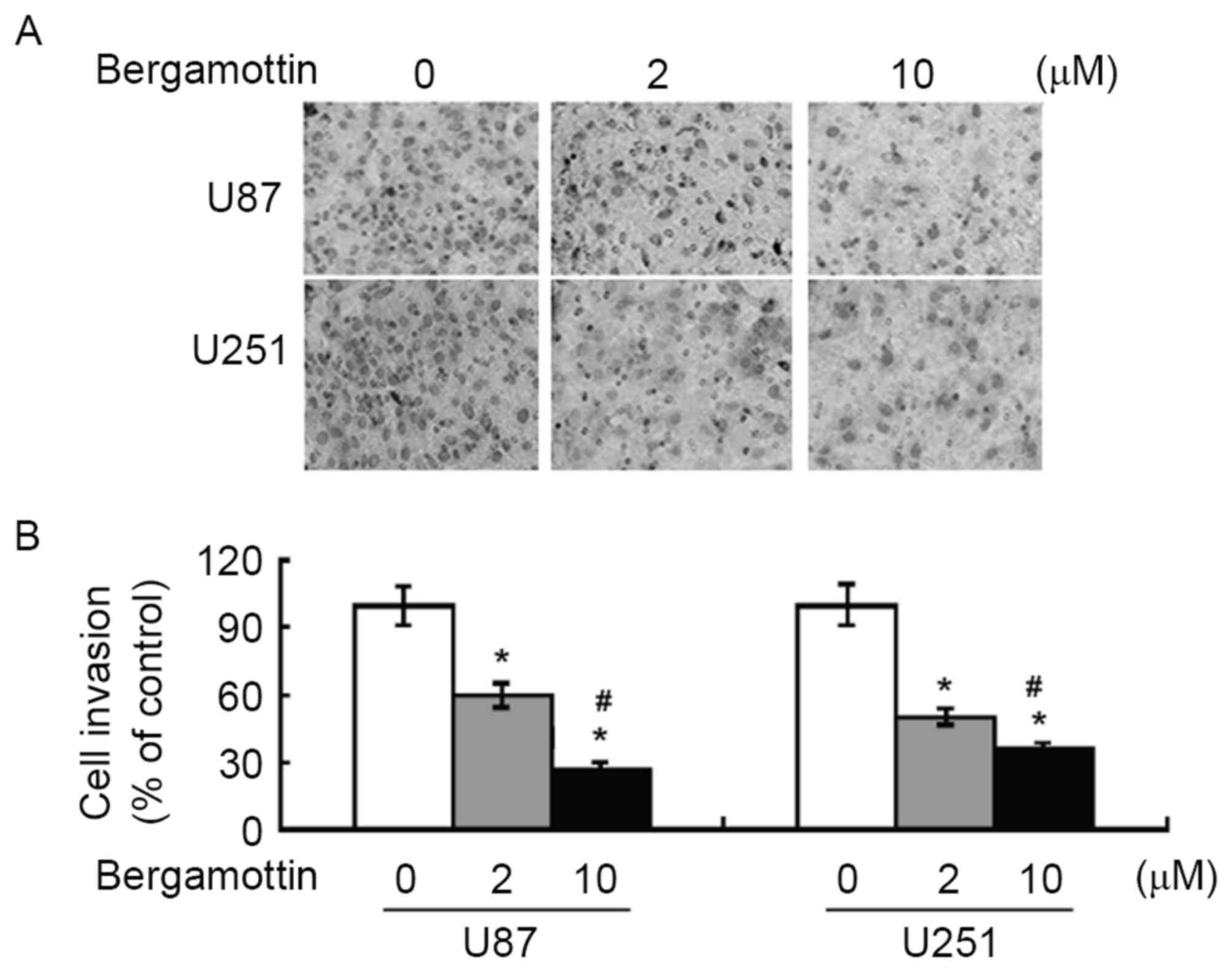
compared with untreated control cells (P<0.05). The invasive
ability of glioma cells was assessed using Matrigel-coated
Transwell assays. Following bergamottin treatment for 48 h, the
number of invading cells was decreased between 40 and 70% in
comparison with control cells (P<0.05; Fig. 2).
Bergamottin suppresses the expression
and secretion of MMP-9
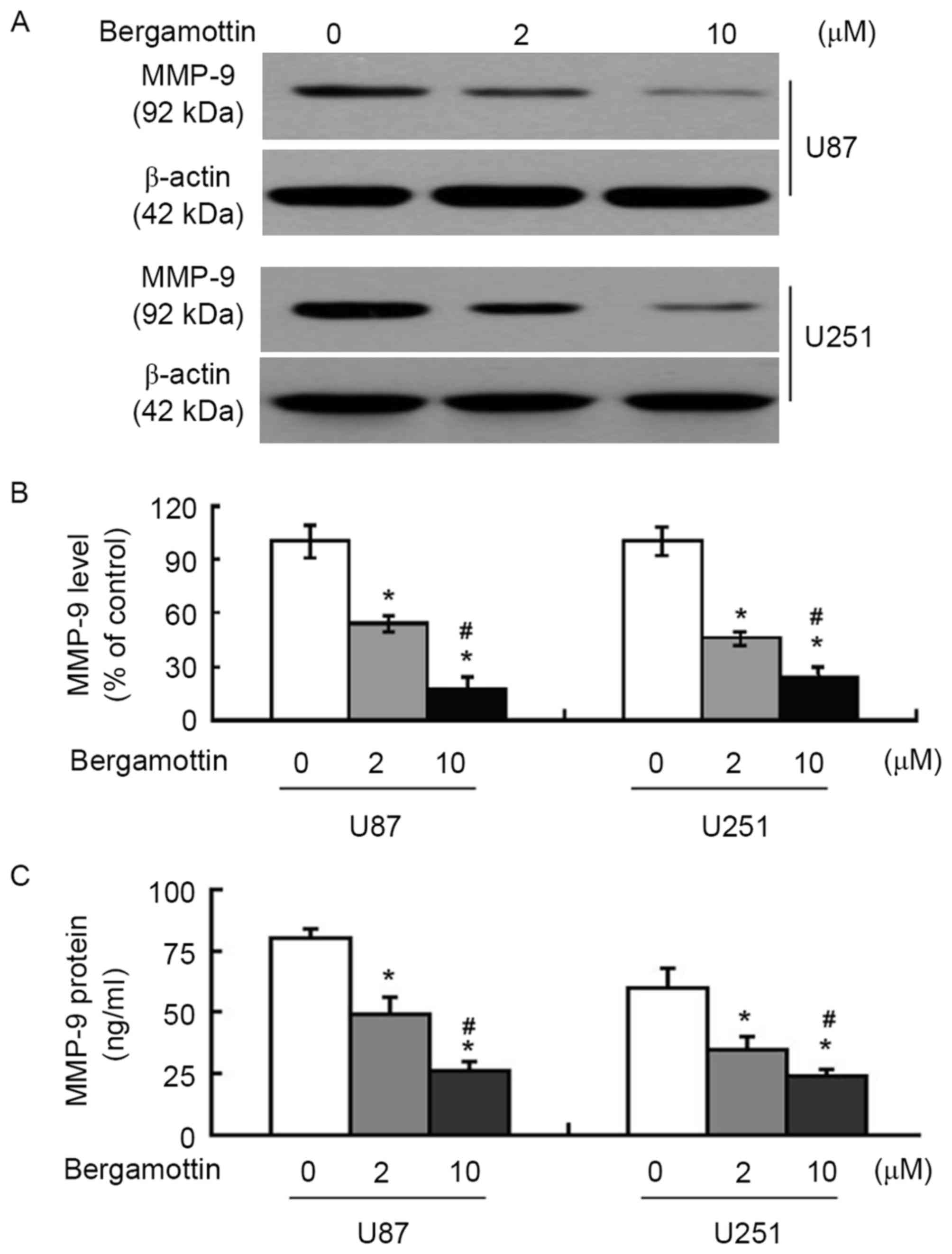
The effects of bergamottin on the expression and
secretion of MMP-9 were then assessed. Bergamottin at 2 and 10 µM
decreased MMP-9 protein expression in U87 and U251 cells (Fig. 3A), which was determined to be
significant (P<0.05; Fig. 3B).
ELISA confirmed that the supernatants from bergamottin-treated
cells expressed significantly decreased levels of MMP-9 compared
with those from control cells (P<0.05; Fig. 3C).
Bergamottin interferes with the
activation of Rac1
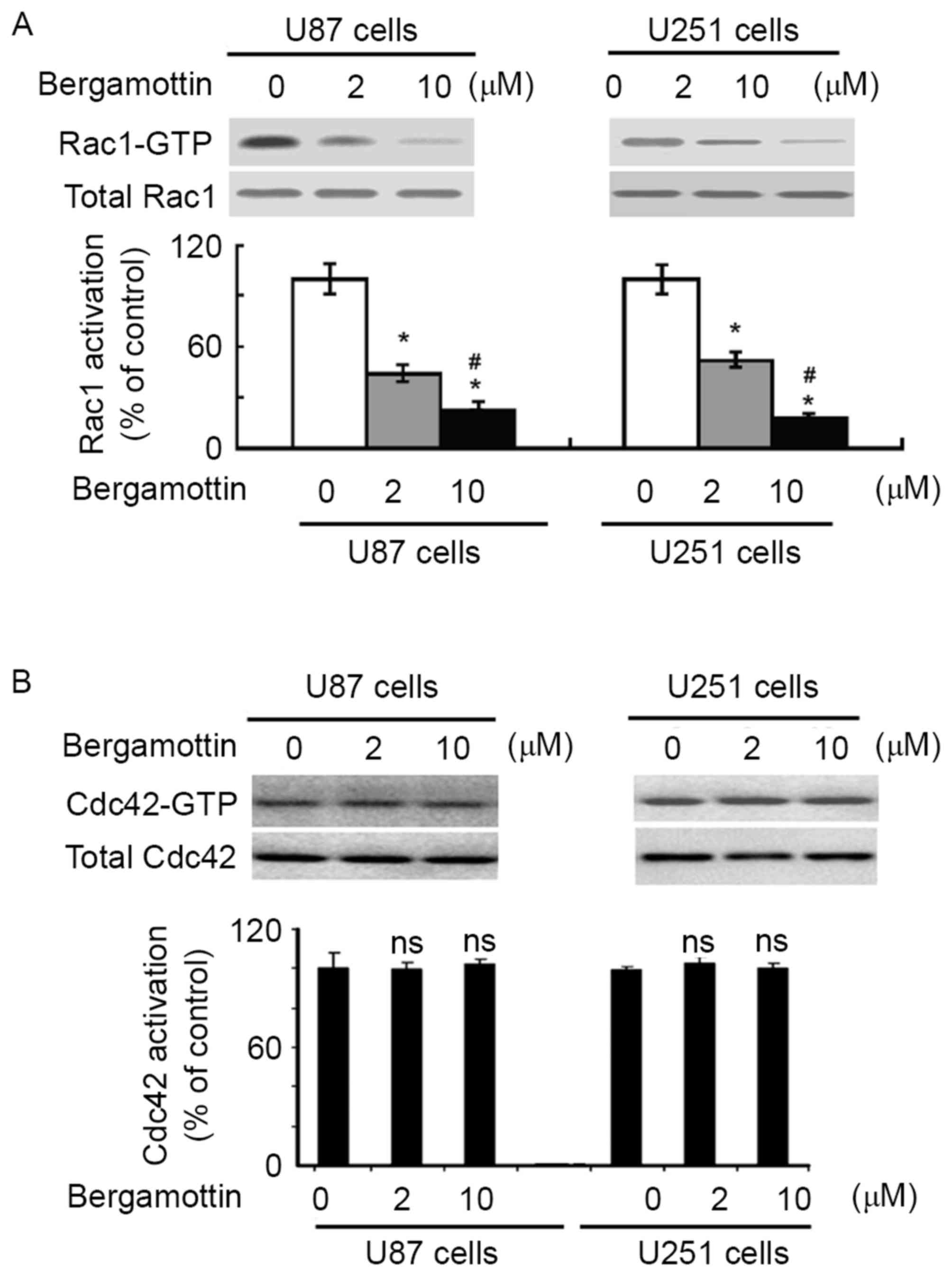
To examine the effect of bergamottin on Rac1
activation, Rac1-GTP pull-down assays were performed in U87 and
U251 cells treated with or without bergamottin. Bergamottin
treatment caused a significant decrease decline in the level of
active Rac1-GTP, compared with untreated cells (P<0.05; Fig. 4A). However, no change in Cdc42
activity was detected (Fig. 4B).
These results indicated that bergamottin is specific for inhibition
of Rac1.
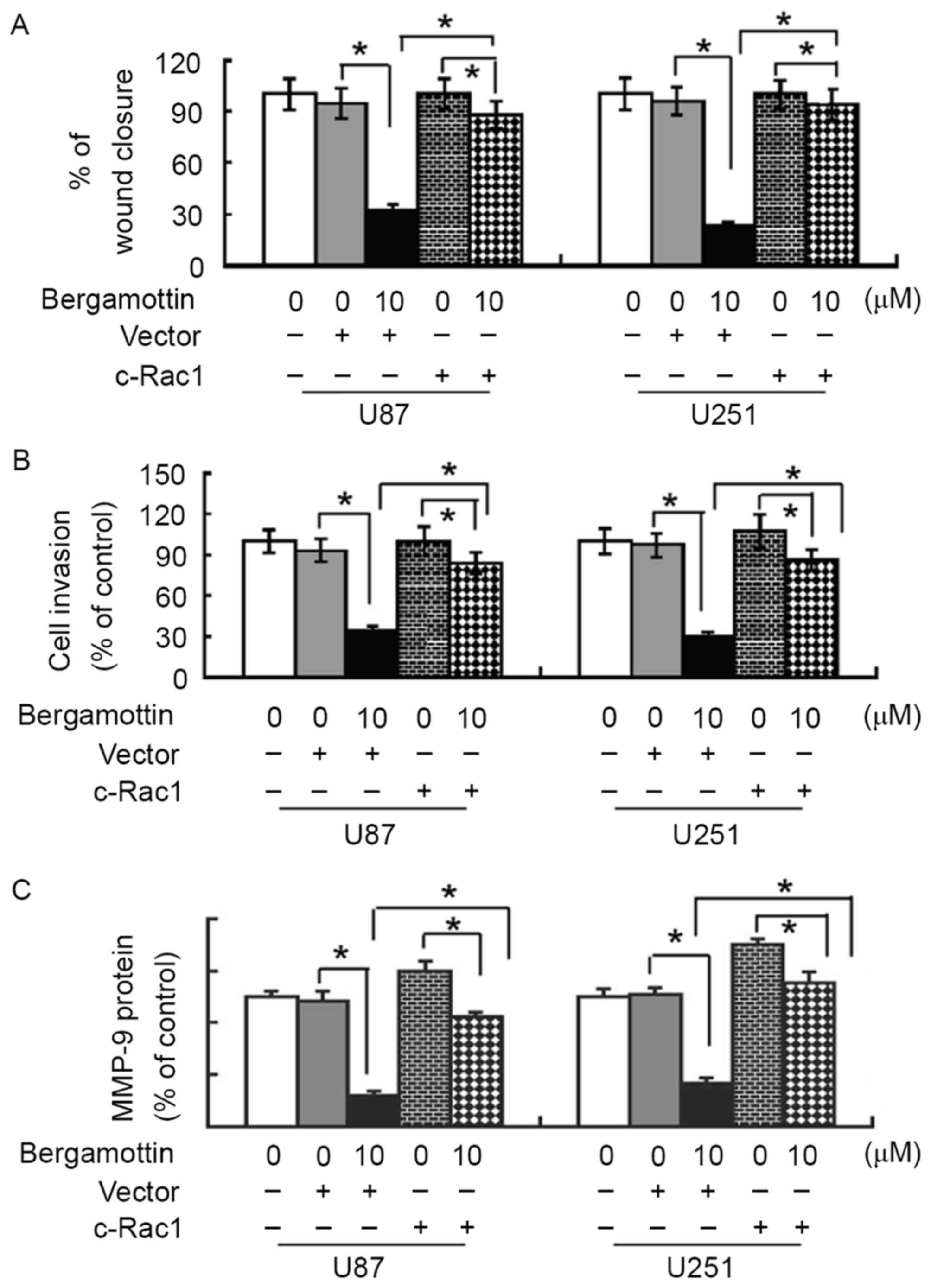
Rac1 inactivation is involved in the
anti-invasive activity of bergamottin
To validate whether the anti-invasive effect of
bergamottin is mediated through Rac1 inactivation, glioma cells
were pre-transfected with constitutively activated Rac1 or empty
vector prior to exposure to 10 µM bergamottin, and cell migration
and invasion were assessed. Notably, the presence of constitutively
activated Rac1 almost completely restored the migration and
invasion of bergamottin-treated U87 and U251 cells (Fig. 5A and B). In addition,
bergamottin-induced suppression of MMP-9 protein expression was
significantly prevented by exogenous activated Rac1 (P<0.05;
Fig. 5C).
Discussion
There is growing interest in the application of
plant phytochemicals as anticancer agents (16–18).
Racoma et al (17) reported
that thymoquinone, a bioactive compound of the Nigella
sativa seed oil, exerts inhibitory effects on the clonogenicity
of glioblastoma cells, but not of normal human astrocytes.
Quercetin (a dietary flavonoid) has been demonstrated to promote
apoptotic death in human glioma cells (18). Bergamottin has been reported to induce
an apoptotic response in tumor cells (11,12). To
the best of our knowledge, the present study provides the first
evidence for the anticancer potential of bergamottin in human
glioma. The results of the present study demonstrated that
bergamottin treatment causes a significant suppression of the
migration and invasion of human glioma cells. The anti-invasive
activity of bergamottin has also been described previously
(15). These results indicated that
bergamottin affects multiple aspects of cancer development and
progression.
Mechanistically, it was revealed that bergamottin
treatment significantly impaired the activation of Rac1 and
downregulated the expression of MMP-9. Rac1 has been identified as
a key regulator of cancer cell invasion (6–8). C6 glioma
cells with high Rac1 activity possess increased invasiveness
compared with those with low Rac1 activity (19), indicating a favorable role for Rac1 in
glioma invasion. Targeting Rac1 abrogates cancer progression and
metastasis (20,21). Rac1 small-molecule inhibitors
(ZINC69391 and 1A-116) have been documented to decrease cell
proliferation, trigger apoptotic death and inhibit cell migration
and invasion in malignant glioma cells (9). These studies indicated that Rac1 is a
potential target for controlling cancer invasion and metastasis. To
confirm the role of Rac1 in bergamottin-mediated anti-invasive
effects, active Rac1 was constitutively expressed in glioma cells
and changes in the invasiveness of glioma cells were evaluated. The
results of the present study demonstrated that U87 and U251 cells
with constitutively activated Rac1 maintained their invasiveness in
the presence of bergamottin, suggesting that the anti-invasive
activity of bergamottin in glioma cells is largely mediated through
inactivation of Rac1.
The pro-invasive activity of Rac1 is associated with
enhanced expression and secretion of MMPs (22–24). Zhang
et al (23) reported that
extracellular ATP facilitates the invasion of prostate cancer cells
through the activation of Rac1 and Cdc42 and the upregulation of
MMP-3 and MMP-13. The results of the present study demonstrated
that enforced expression of activated Rac1 prevented the
suppression of MMP-9 expression in glioma cells by bergamottin.
MMP-9 performs a critical role in glioma cell invasion.
Downregulation of MMP-3 and MMP-9 has been demonstrated to
contribute to inhibition of glioma cell invasion by glycitein, a
bacterial metabolite of the isoflavone glycitin (25). Targeting MMP-9 via small interfering
RNA leads to decreased invasiveness of glioma cells (26). Taken together, bergamottin-mediated
suppression of glioma cell invasion is associated with inactivation
of Rac1 and a subsequent decrease in MMP-9 expression. However, the
signaling pathways involved in bergamottin-induced inactivation of
Rac1 remain unclear.
To the best of the knowledge of the authors, the
present study provided the first evidence that bergamottin has
anti-invasive activity in human glioma cells, which is primarily
associated with inhibition of Rac1 activation and downregulation of
MMP-9. These results indicated that bergamottin has therapeutic
potential for the treatment of metastatic glioma.
References
|
1
|
Altieri R, Agnoletti A, Quattrucci F,
Garbossa D, Calamo Specchia FM, Bozzaro M, Fornaro R, Mencarani C,
Lanotte M, Spaziante R and Ducati A: Molecular biology of gliomas:
Present and future challenges. Transl Med UniSa. 10:29–37.
2014.PubMed/NCBI
|
|
2
|
Siebzehnrubl FA, Silver DJ, Tugertimur B,
Deleyrolle LP, Siebzehnrubl D, Sarkisian MR, Devers KG, Yachnis AT,
Kupper MD, Neal D, et al: The ZEB1 pathway links glioblastoma
initiation, invasion and chemoresistance. EMBO Mol Med.
5:1196–1212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanase CP, Enciu AM, Mihai S, Neagu AI,
Calenic B and Cruceru ML: Anti-cancer therapies in high grade
gliomas. Curr Proteomics. 10:246–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fortin Ensign SP, Mathews IT, Symons MH,
Berens ME and Tran NL: Implications of Rho GTPase signaling in
glioma cell invasion and tumor progression. Front Oncol. 3:2412013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakada M, Drake KL, Nakada S, Niska JA and
Berens ME: Ephrin-B3 ligand promotes glioma invasion through
activation of Rac1. Cancer Res. 66:8492–8500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu B, Shi B, Jarzynka MJ, Yiin JJ,
D'Souza-Schorey C and Cheng SY: ADP-ribosylation factor 6 regulates
glioma cell invasion through the IQ-domain GTPase-activating
protein 1-Rac1-mediated pathway. Cancer Res. 69:794–801. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paulino VM, Yang Z, Kloss J, Ennis MJ,
Armstrong BA, Loftus JC and Tran NL: TROY (TNFRSF19) is
overexpressed in advanced glial tumors and promotes glioblastoma
cell invasion via Pyk2-Rac1 signaling. Mol Cancer Res. 8:1558–1567.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cardama GA, Gonzalez N, Ciarlantini M,
Gandolfi Donadío L, Comin MJ, Alonso DF, Menna PL and Gomez DE:
Proapoptotic and antiinvasive activity of Rac1 small molecule
inhibitors on malignant glioma cells. Onco Targets Ther.
7:2021–2033. 2014.PubMed/NCBI
|
|
10
|
Shin I, Kim S, Song H, Kim HR and Moon A:
H-Ras-specific activation of Rac-MKK3/6-p38 pathway: Its critical
role in invasion and migration of breast epithelial cells. J Biol
Chem. 80:14675–14683. 2005. View Article : Google Scholar
|
|
11
|
Kim SM, Lee EJ, Lee JH, Yang WM, Nam D,
Lee JH, Lee SG, Um JY, Shim BS and Ahn KS: Simvastatin in
combination with bergamottin potentiates TNF-induced apoptosis
through modulation of NF-κB signalling pathway in human chronic
myelogenous leukaemia. Pharm Biol. 54:2050–2060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SM, Lee JH, Sethi G, Kim C, Baek SH,
Nam D, Chung WS, Kim SH, Shim BS and Ahn KS: Bergamottin, a natural
furanocoumarin obtained from grapefruit juice induces
chemosensitization and apoptosis through the inhibition of STAT3
signaling pathway in tumor cells. Cancer Lett. 354:153–163. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kleiner HE, Vulimiri SV, Reed MJ,
Uberecken A and DiGiovanni J: Role of cytochrome P450 1a1 and 1b1
in the metabolic activation of 7,12-dimethylbenz[a]anthracene and
the effects of naturally occurring furanocoumarins on skin tumor
initiation. Chem Res Toxicol. 15:226–235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kleiner HE, Reed MJ and DiGiovanni J:
Naturally occurring coumarins inhibit human cytochromes P450 and
block benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene DNA adduct
formation in MCF-7 cells. Chem Res Toxicol. 16:415–422. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang YP, Yun HJ, Choi JH, Kang KW and
Jeong HG: Suppression of phorbol-12-myristate-13-acetate-induced
tumor cell invasion by bergamottin via the inhibition of protein
kinase Cdelta/p38 mitogen-activated protein kinase and JNK/nuclear
factor-kappaB-dependent matrix metalloproteinase-9 expression. Mol
Nutr Food Res. 54:977–990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Øverby A, Zhao CM and Chen D: Plant
phytochemicals: Potential anticancer agents against gastric cancer.
Curr Opin Pharmacol. 19:6–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Racoma IO, Meisen WH, Wang QE, Kaur B and
Wani AA: Thymoquinone inhibits autophagy and induces
cathepsin-mediated, caspase-independent cell death in glioblastoma
cells. PLoS One. 8:e728822013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan HC, Jiang Q, Yu Y, Mei JP, Cui YK and
Zhao WJ: Quercetin promotes cell apoptosis and inhibits the
expression of MMP-9 and fibronectin via the AKT and ERK signalling
pathways in human glioma cells. Neurochem Int. 80:60–71. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yukinaga H, Shionyu C, Hirata E, Ui-Tei K,
Nagashima T, Kondo S, Okada-Hatakeyama M, Naoki H and Matsuda M:
Fluctuation of Rac1 activity is associated with the phenotypic and
transcriptional heterogeneity of glioma cells. J Cell Sci.
127:1805–1815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao X, Zhang L, Shi Y, Sun Y, Dai S, Guo
C, Zhu F, Wang Q, Wang J, Wang X, et al: Human tumor necrosis
factor (TNF)-alpha-induced protein 8-like 2 suppresses
hepatocellular carcinoma metastasis through inhibiting Rac1. Mol
Cancer. 12:1492013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao H, Dong T, Zhou H, Wang L, Huang A,
Feng B, Quan Y, Jin R, Zhang W, Sun J, et al: miR-320a suppresses
colorectal cancer progression by targeting Rac1. Carcinogenesis.
35:886–895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhuge Y and Xu J: Rac1 mediates type I
collagen-dependent MMP-2 activation. Role in cell invasion across
collagen barrier. J Biol Chem. 276:16248–16256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Gong LH, Zhang HQ, Du Q, You JF,
Tian XX and Fang WG: Extracellular ATP enhances in vitro invasion
of prostate cancer cells by activating Rho GTPase and upregulating
MMPs expression. Cancer Lett. 293:189–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen QY, Zheng Y, Jiao DM, Chen FY, Hu HZ,
Wu YQ, Song J, Yan J, Wu LJ and Lv GY: Curcumin inhibits lung
cancer cell migration and invasion through Rac1-dependent signaling
pathway. J Nutr Biochem. 25:177–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee EJ, Kim SY, Hyun JW, Min SW, Kim DH
and Kim HS: Glycitein inhibits glioma cell invasion through
down-regulation of MMP-3 and MMP-9 gene expression. Chem Biol
Interact. 185:18–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gondi CS, Lakka SS, Dinh DH, Olivero WC,
Gujrati M and Rao JS: Downregulation of uPA, uPAR and MMP-9 using
small, interfering, hairpin RNA (siRNA) inhibits glioma cell
invasion, angiogenesis and tumor growth. Neuron Glia Biol.
1:165–176. 2004. View Article : Google Scholar : PubMed/NCBI
|