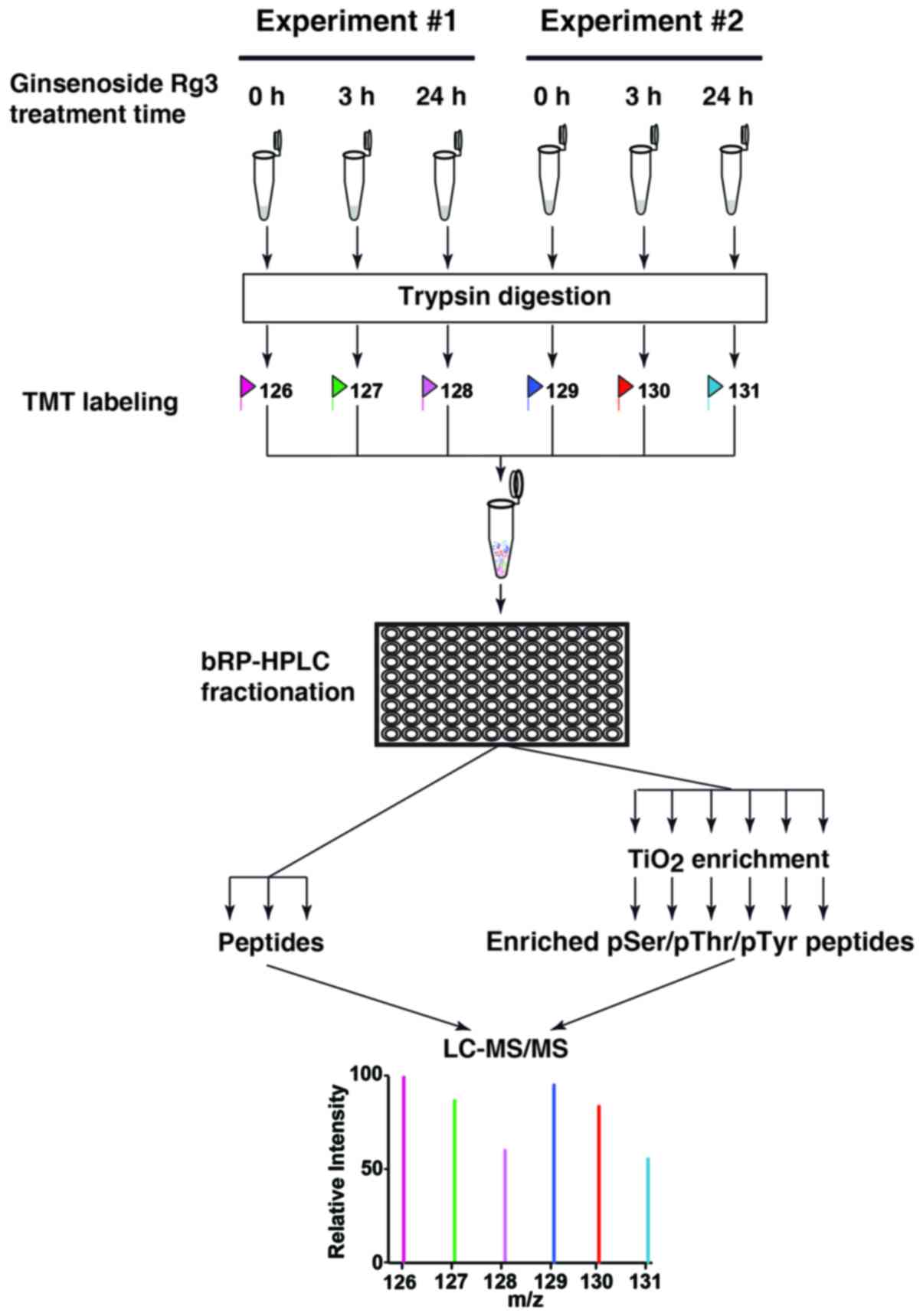
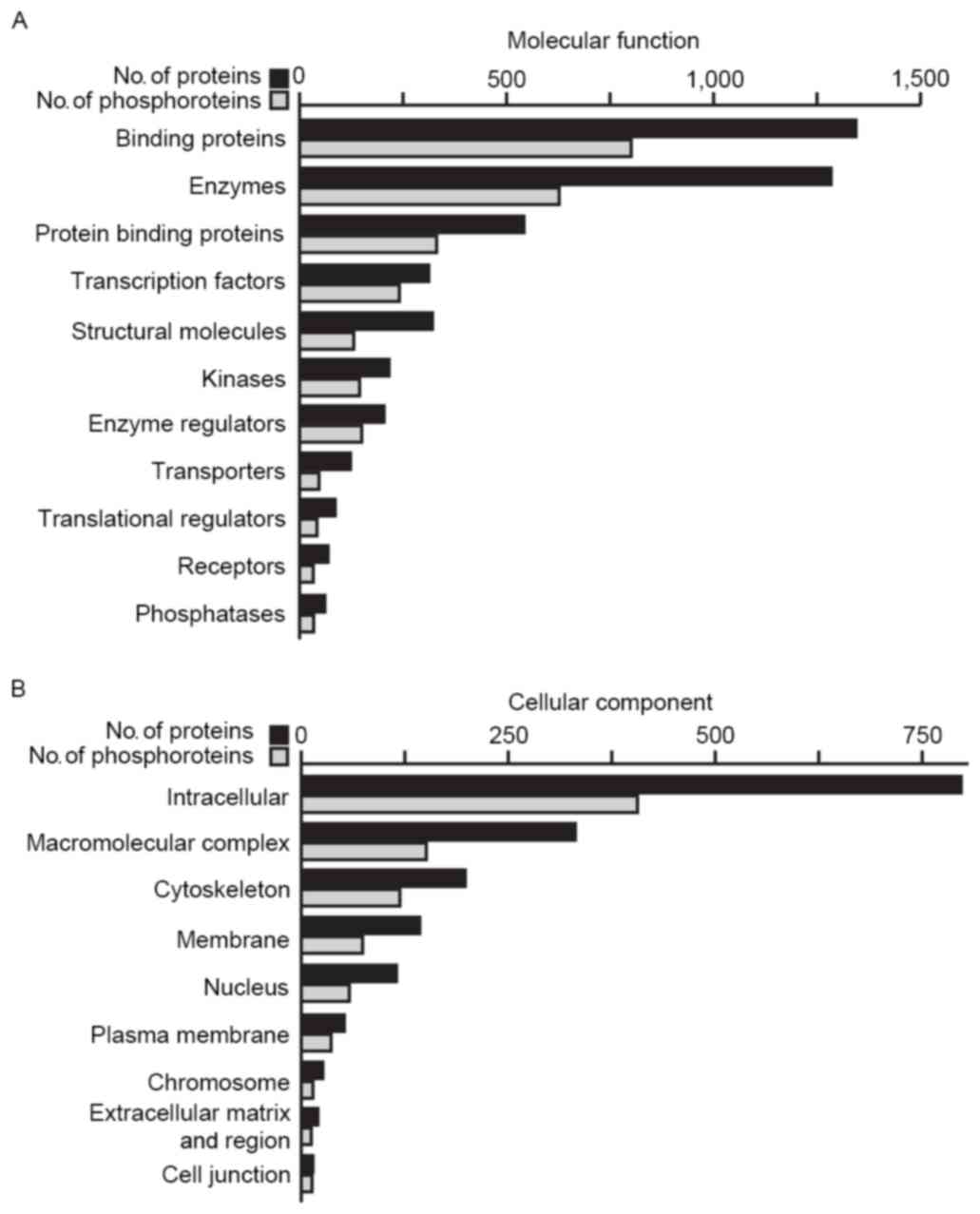
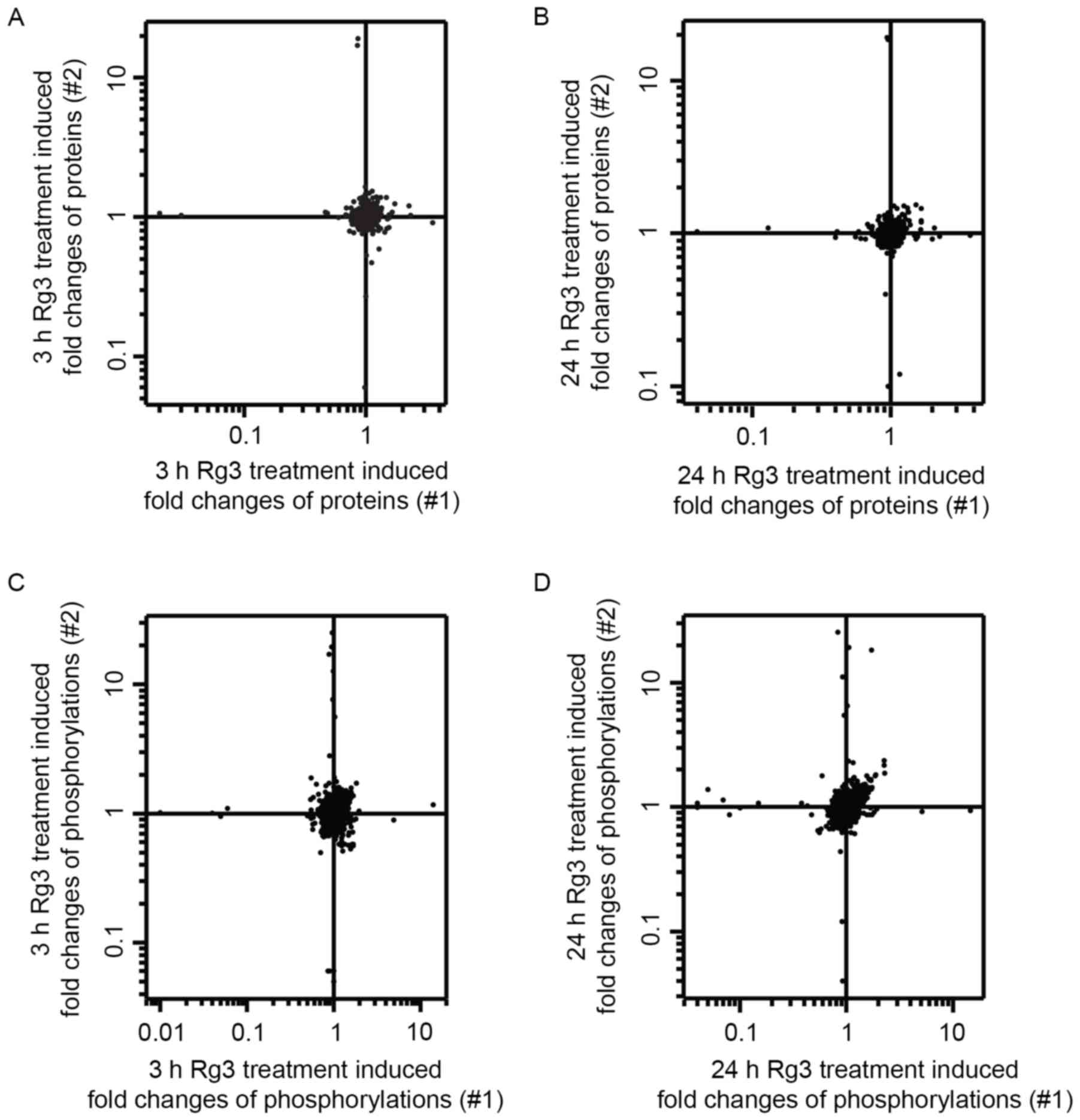
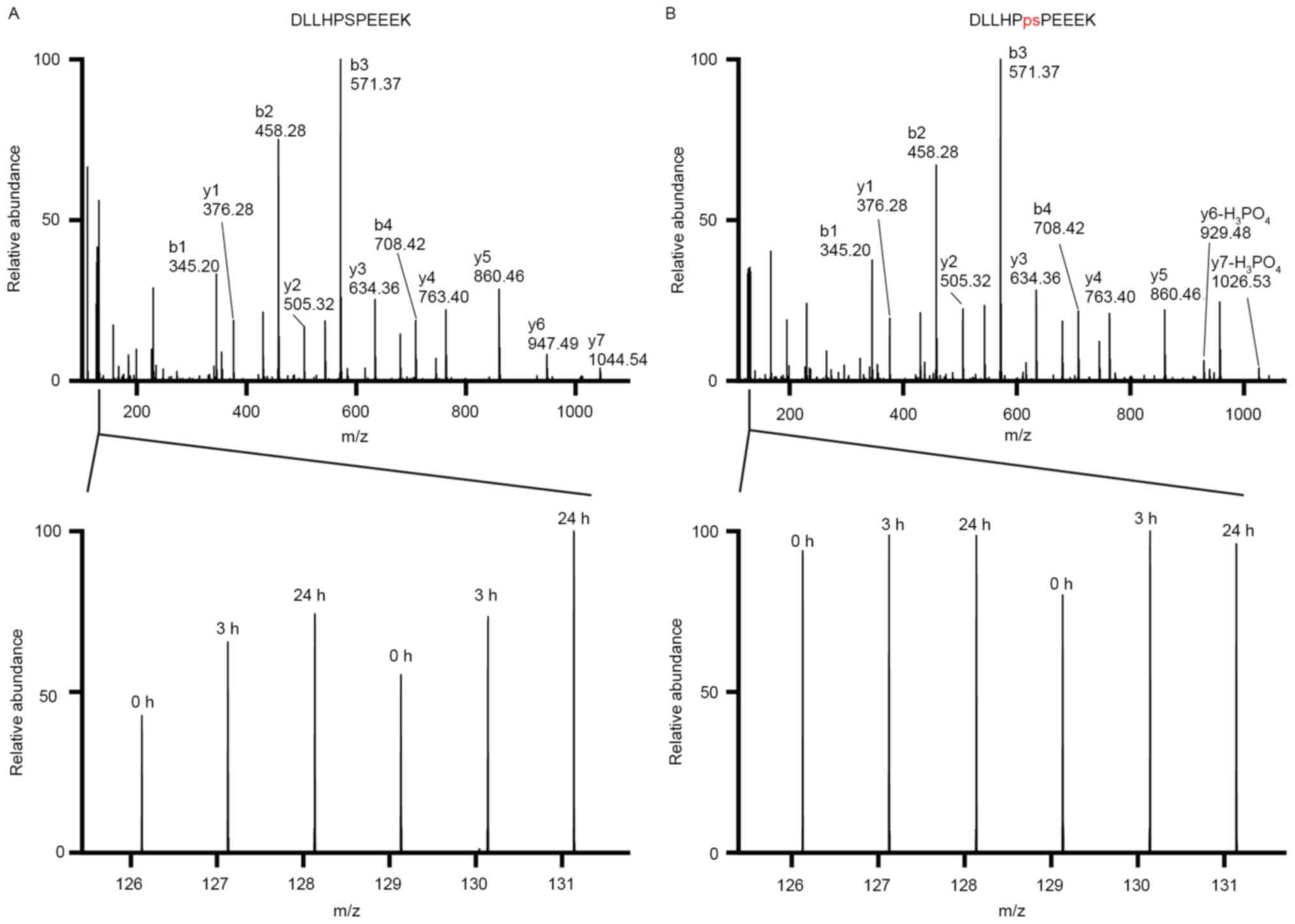
|
1
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song QK, Wang XL, Zhou XN, Yang HB, Li YC,
Wu JP, Ren J and Lyerly HK: Breast cancer challenges and screening
in China: Lessons from current registry data and population
screening studies. Oncologist. 20:773–779. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma R, Sun L, Chen X, Mei B, Chang G, Wang
M and Zhao D: Proteomic analyses provide novel insights into plant
growth and ginsenoside biosynthesis in forest cultivated panax
ginseng (F. Ginseng). Front Plant Sci. 7:12016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colzani M, Altomare A, Caliendo M, Aldini
G, Righetti PG and Fasoli E: The secrets of Oriental panacea: Panax
ginseng. J Proteomics. 130:150–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie G, Wang CZ, Yu C, Qiu Y, Wen XD, Zhang
CF, Yuan CS and Jia W: Metabonomic profiling reveals cancer
chemopreventive effects of american ginseng on colon carcinogenesis
in Apc (Min/+) mice. J Proteome Res. 14:3336–3347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gillis CN: Panax ginseng pharmacology: A
nitric oxide link? Biochem Pharmacol. 54:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen XP, Qian LL, Jiang H and Chen JH:
Ginsenoside Rg3 inhibits CXCR4 expression and related migrations in
a breast cancer cell line. Int J Clin Oncol. 16:519–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim BM, Kim DH, Park JH, Na HK and Surh
YJ: Ginsenoside Rg3 induces apoptosis of human breast cancer
(MDA-MB-231) cells. J Cancer Prev. 18:177–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim BM, Kim DH, Park JH, Surh YJ and Na
HK: Ginsenoside Rg3 inhibits constitutive activation of NF-κB
signaling in human breast cancer (MDA-MB-231) cells: ERK and Akt as
potential upstream targe. J Cancer Prev. 19:23–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thompson A, Schäfer J, Kuhn K, Kienle S,
Schwarz J, Schmidt G, Neumann T, Johnstone R, Mohammed AK and Hamon
C: Tandem mass tags: A novel quantification strategy for
comparative analysis of complex protein mixtures by MS/MS. Anal
Chem. 75:1895–1904. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ross PL, Huang YN, Marchese JN, Williamson
B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, et
al: Multiplexed protein quantitation in Saccharomyces cerevisiae
using amine-reactive isobaric tagging reagents. Mol Cell
Proteomics. 3:1154–1169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nirujogi RS, Wright JD Jr, Manda SS, Zhong
J, Na CH, Meyerhoff J, Benton B, Jabbour R, Willis K, Kim MS, et
al: Phosphoproteomic analysis reveals compensatory effects in the
piriform cortex of VX nerve agent exposed rats. Proteomics.
15:487–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roitinger E, Hofer M, Kocher T, Pichler P,
Novatchkova M, Yang J, Schlögelhofer P and Mechtler K: Quantitative
phosphoproteomics of the ataxia telangiectasia-mutated (ATM) and
ataxia telangiectasia-mutated and rad3-related (ATR) dependent DNA
damage response in Arabidopsis thaliana. Mol Cell Proteomics.
14:556–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang Y, Zhang Q, Wang X, Yang X, Wang X,
Huang Z, Jiao Y and Wang J: Quantitative phosphoproteomics reveals
genistein as a modulator of cell cycle and DNA damage response
pathways in triple-negative breast cancer cells. Int J Oncol.
48:1016–1028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wisniewski JR, Zougman A, Nagaraj N and
Mann M: Universal sample preparation method for proteome analysis.
Nat Methods. 6:359–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Larsen MR, Thingholm TE, Jensen ON,
Roepstorff P and Jørgensen TJ: Highly selective enrichment of
phosphorylated peptides from peptide mixtures using titanium
dioxide microcolumns. Mol Cell Proteomics. 4:873–886. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rappsilber J, Ishihama Y and Mann M: Stop
and go extraction tips for matrix-assisted laser
desorption/ionization, nanoelectrospray, and LC/MS sample
pretreatment in proteomics. Anal Chem. 75:663–670. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cox J and Mann M: MaxQuant enables high
peptide identification rates, individualized p.p.b.-range mass
accuracies and proteome-wide protein quantification. Nat
Biotechnol. 26:1367–1372. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vizcaino JA, Deutsch EW, Wang R, Csordas
A, Reisinger F, Rios D, Dianes JA, Sun Z, Farrah T, Bandeira N, et
al: ProteomeXchange provides globally coordinated proteomics data
submission and dissemination. Nat Biotechnol. 32:223–226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mi H, Muruganujan A and Thomas PD: PANTHER
in 2013: modeling the evolution of gene function, and other gene
attributes, in the context of phylogenetic trees. Nucleic Acids
Res. 41(Database issue): D377–D386. 2013.PubMed/NCBI
|
|
21
|
Mi H, Muruganujan A, Casagrande JT and
Thomas PD: Large-scale gene function analysis with the PANTHER
classification system. Nat Protoc. 8:1551–1566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shan X, Aziz F, Tian LL, Wang XQ, Yan Q
and Liu JW: Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation
inhibits melanoma cell proliferation by decreasing FUT4/LeY
expression. Int J Oncol. 46:1667–1676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang JH, Nao JF, Zhang M and He P:
20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer
HO-8910 cells through PI3K/Akt and XIAP pathways. Tumour Biol.
35:11985–11994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shan X, Fu YS, Aziz F, Wang XQ, Yan Q and
Liu JW: Ginsenoside Rg3 inhibits melanoma cell proliferation
through down-regulation of histone deacetylase 3 (HDAC3) and
increase of p53 acetylation. PLoS One. 9:e1154012014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu T, Zhao L, Zhang Y, Chen W, Liu D, Hou
H, Ding L and Li X: Ginsenoside 20(S)-Rg3 targets HIF-1α to block
hypoxia-induced epithelial-mesenchymal transition in ovarian cancer
cells. PLoS One. 9:e1038872014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim BJ, Nah SY, Jeon JH, So I and Kim SJ:
Transient receptor potential melastatin 7 channels are involved in
ginsenoside Rg3-induced apoptosis in gastric cancer cells. Basic
Clin Pharmacol Toxicol. 109:233–239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SY, Kim GT, Roh SH, Song JS, Kim HJ,
Hong SS, Kwon SW and Park JH: Proteomic analysis of the anti-cancer
effect of 20S-ginsenoside Rg3 in human colon cancer cell lines.
Biosci Biotechnol Biochem. 73:811–816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo Y, Zhang P, Zeng HQ, Lou SF and Wang
DX: Ginsenoside Rg3 induces apoptosis in human multiple myeloma
cells via the activation of Bcl-2-associated X protein. Mol Med
Rep. 12:3557–3562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cox J, Matic I, Hilger M, Nagaraj N,
Selbach M, Olsen JV and Mann M: A practical guide to the MaxQuant
computational platform for SILAC-based quantitative proteomics. Nat
Protoc. 4:698–705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Liang S, Lian X, Liu L, Zhao S,
Xuan Q, Guo L, Liu H, Yang Y, Dong T, et al: Identification of
proteins responsible for adriamycin resistance in breast cancer
cells using proteomics analysis. Sci Rep. 5:93012015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fernandez-Pol JA: Increased serum level of
RPMPS-1/S27 protein in patients with various types of cancer is
useful for the early detection, prevention and therapy. Cancer
Genomics Proteomics. 9:203–256. 2012.PubMed/NCBI
|
|
32
|
Stack BC Jr, Dalsaso TA, Lee C Jr, Lowe
VJ, Hamilton PD, Fletcher JW and Fernandez-Pol JA: Overexpression
of MPS antigens by squamous cell carcinomas of the head and neck:
Immunohistochemical and serological correlation with FDG positron
emission tomography. Anticancer Res. 19:5503–5510. 1999.PubMed/NCBI
|
|
33
|
Ganger DR, Hamilton PD, Klos DJ, Jakate S,
McChesney L and Fernandez-Pol JA: Differential expression of
metallopanstimulin/S27 ribosomal protein in hepatic regeneration
and neoplasia. Cancer Detect Prev. 25:231–236. 2001.PubMed/NCBI
|
|
34
|
Santa Cruz DJ, Hamilton PD, Klos DJ and
Fernandez-Pol JA: Differential expression of metallopanstimulin/S27
ribosomal protein in melanocytic lesions of the skin. J Cutan
Pathol. 24:533–542. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ganger DR, Hamilton PD, Fletcher JW and
Fernandez-Pol JA: Metallopanstimulin is overexpressed in a patient
with colonic carcinoma. Anticancer Res. 17:1993–1999.
1997.PubMed/NCBI
|
|
36
|
Fernandez-Pol JA, Fletcher JW, Hamilton PD
and Klos DJ: Expression of metallopanstimulin and oncogenesis in
human prostatic carcinoma. Anticancer Res. 17:1519–1530.
1997.PubMed/NCBI
|
|
37
|
Yang ZY, Qu Y, Zhang Q, Wei M, Liu CX,
Chen XH, Yan M, Zhu ZG, Liu BY, Chen GQ, et al: Knockdown of
metallopanstimulin-1 inhibits NF-κB signaling at different levels:
The role of apoptosis induction of gastric cancer cells. Int J
Cancer. 130:2761–2770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang YW, Qu Y, Li JF, Chen XH, Liu BY, Gu
QL and Zhu ZG: In vitro and in vivo evidence of
metallopanstimulin-1 in gastric cancer progression and
tumorigenicity. Clin Cancer Res. 12:4965–4973. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dai Y, Pierson SE, Dudney WC and Stack BC
Jr: Extraribosomal function of metallopanstimulin-1: Reducing
paxillin in head and neck squamous cell carcinoma and inhibiting
tumor growth. Int J Cancer. 126:611–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim S, Yamamoto J, Chen Y, Aida M, Wada T,
Handa H and Yamaguchi Y: Evidence that cleavage factor Im is a
heterotetrameric protein complex controlling alternative
polyadenylation. Genes Cells. 15:1003–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Millevoi S, Loulergue C, Dettwiler S,
Karaa SZ, Keller W, Antoniou M and Vagner S: An interaction between
U2AF 65 and CF I(m) links the splicing and 3′ end processing
machineries. EMBO J. 25:4854–4864. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Verghese ET, Drury R, Green CA, Holliday
DL, Lu X, Nash C, Speirs V, Thorne JL, Thygesen HH, Zougman A, et
al: MiR-26b is down-regulated in carcinoma-associated fibroblasts
from ER-positive breast cancers leading to enhanced cell migration
and invasion. J Pathol. 231:388–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Trinkle-Mulcahy L and Lamond AI: Mitotic
phosphatases: No longer silent partners. Curr Opin Cell Biol.
18:623–631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ito M, Nakano T, Erdodi F and Hartshorne
DJ: Myosin phosphatase: Structure, regulation and function. Mol
Cell Biochem. 259:197–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Matsumura F and Hartshorne DJ: Myosin
phosphatase target subunit: Many roles in cell function. Biochem
Biophys Res Commun. 369:149–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vallenius T, Vaahtomeri K, Kovac B,
Osiceanu AM, Viljanen M and Mäkelä TP: An association between NUAK2
and MRIP reveals a novel mechanism for regulation of actin stress
fibers. J Cell Sci. 124:384–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Surks HK, Riddick N and Ohtani K: M-RIP
targets myosin phosphatase to stress fibers to regulate myosin
light chain phosphorylation in vascular smooth muscle cells. J Biol
Chem. 280:42543–42551. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Matsumura F, Yamakita Y and Yamashiro S:
Myosin phosphatase-targeting subunit 1 controls chromatid
segregation. J Biol Chem. 286:10825–10833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xia D, Stull JT and Kamm KE: Myosin
phosphatase targeting subunit 1 affects cell migration by
regulating myosin phosphorylation and actin assembly. Exp Cell Res.
304:506–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Estey MP, Di Ciano-Oliveira C, Froese CD,
Bejide MT and Trimble WS: Distinct roles of septins in cytokinesis:
SEPT9 mediates midbody abscission. J Cell Biol. 191:741–749. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
van Alfen N, Hannibal MC, Chance PF and
van Engelen BGM: Hereditary neuralgic amyotrophyPagon RA, Adam MP,
Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT,
Smith RJH and Stephens K: GeneReviews (R). Seattle (WA): University
of Washington; 1993
|
|
52
|
Pierre SC, Häusler J, Birod K, Geisslinger
G and Scholich K: PAM mediates sustained inhibition of cAMP
signaling by sphingosine-1-phosphate. EMBO J. 23:3031–3040. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hebbar N, Shrestha-Bhattarai T and
Rangnekar VM: Cancer-selective apoptosis by tumor suppressor par-4.
Adv Exp Med Biol. 818:155–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hutten S, Walde S, Spillner C, Hauber J
and Kehlenbach RH: The nuclear pore component Nup358 promotes
transportin-dependent nuclear import. J Cell Sci. 122:1100–1110.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wälde S, Thakar K, Hutten S, Spillner C,
Nath A, Rothbauer U, Wiemann S and Kehlenbach RH: The nucleoporin
Nup358/RanBP2 promotes nuclear import in a cargo- and transport
receptor-specific manner. Traffic. 13:218–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tatsumi K, Sou YS, Tada N, Nakamura E,
Iemura S, Natsume T, Kang SH, Chung CH, Kasahara M, Kominami E, et
al: A novel type of E3 ligase for the Ufm1 conjugation system. J
Biol Chem. 285:5417–5427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu J, Lei G, Mei M, Tang Y and Li H: A
novel C53/LZAP-interacting protein regulates stability of C53/LZAP
and DDRGK domain-containing Protein 1 (DDRGK1) and modulates
NF-kappaB signaling. J Biol Chem. 285:15126–15136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim CH, Nam HS, Lee EH, Han SH, Cho HJ,
Chung HJ, Lee NS, Choi SJ, Kim H, Ryu JS, et al: Overexpression of
a novel regulator of p120 catenin, NLBP, promotes lung
adenocarcinoma proliferation. Cell Cycle. 12:2443–2453. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang L, Li X, Song YM, Wang B, Zhang FR,
Yang R, Wang HQ and Zhang GJ: Ginsenoside Rg3 sensitizes human
non-small cell lung cancer cells to γ-radiation by targeting the
nuclear factor-κB pathway. Mol Med Rep. 12:609–614. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kim SM, Lee SY, Cho JS, Son SM, Choi SS,
Yun YP, Yoo HS, Yoon DY, Oh KW, Han SB and Hong JT: Combination of
ginsenoside Rg3 with docetaxel enhances the susceptibility of
prostate cancer cells via inhibition of NF-kappaB. Eur J Pharmacol.
631:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lorain S, Quivy JP, Monier-Gavelle F,
Scamps C, Lécluse Y, Almouzni G and Lipinski M: Core histones and
HIRIP3, a novel histone-binding protein, directly interact with WD
repeat protein HIRA. Mol Cell Biol. 18:5546–5556. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Papageorgio C, Brachmann R, Zeng J,
Culverhouse R, Zhang W and McLeod H: MAGED2: A novel
p53-dissociator. Int J Oncol. 31:1205–1211. 2007.PubMed/NCBI
|
|
63
|
Tseng HY, Chen LH, Ye Y, Tay KH, Jiang CC,
Guo ST, Jin L, Hersey P and Zhang XD: The melanoma-associated
antigen MAGE-D2 suppresses TRAIL receptor 2 and protects against
TRAIL-induced apoptosis in human melanoma cells. Carcinogenesis.
33:1871–1881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Redpath NT, Price NT, Severinov KV and
Proud CG: Regulation of elongation factor-2 by multisite
phosphorylation. Eur J Biochem. 213:689–699. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Horman S, Browne G, Krause U, Patel J,
Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C and Rider M:
Activation of AMP-activated protein kinase leads to the
phosphorylation of elongation factor 2 and an inhibition of protein
synthesis. Curr Biol. 12:1419–1423. 2002. View Article : Google Scholar : PubMed/NCBI
|