Introduction
Actin is one of the most abundant proteins
identified in almost all eukaryotic cells, and it is a highly
conserved protein during evolution (1). Actin-binding proteins (ABPs) refer to
proteins that contain actin-binding domains that interact with
actin. They can bind to actin monomers, actin polymers or both
(2).
Early studies have focused on the biological
features and physiological mechanisms of cytoplasmic actin.
Therefore, ABPs were considered to be distributed only in the
cytoplasm and associated with the organization of actin
cytoskeleton (3). In the cytoplasm,
actin is associated with numerous cellular activities, including
sustaining cellular morphology, determining cellular organelle
distribution, mediating intracellular transfer, endocytosis and
exocytosis, cell division, cell migration and adhesion (4–8).
Meanwhile, ABPs regulate actin cytoskeletal structure by modulating
actin filament cross-linking into networks or depolymerizing into
monomers, allowing actin to switch between the polymeric (F-actin
form, filamentous actin) and monomeric state (G-actin form,
globular actin) (2,9).
However, recent studies (10,11)
indicate that a great number of actin and ABPs exist in the
nucleus. Nuclear actin and nuclear ABPs exhibit nuclear-specific
functions that are different from those in the cytoplasm. Although
the precise biological mechanisms remain elusive, we are fortunate
to uncover several observations (12). This review aims to present up-to-date
discoveries of nuclear actin and nuclear ABPs in the field of
cancer research.
Actin and actin-binding proteins (ABPs) in
the nucleus
Studies in the recent decades provide a plethora of
evidence that has broadened our horizon on the functions of nuclear
actin and nuclear ABPs in the eukaryotic cell life (12–14). Since
the existence of nuclear actin was confirmed, subsequent studies
also established the presence of ABPs in the nucleus (12,14,15). The
very first nuclear ABP was reported as early as 1987, henceforth,
the rest of the ABP family in the nucleus has come to light
comprising of proflilin, anillin, flightless I (Fli I), filamin α
(FLNα), α-actinins, myosins, gelsolin and ezrin-radixin-moesin
proteins (12,13,16,17).
Although these ABPs are primarily in the cytoplasm, they can
translocate into the nucleus under certain circumstances, for
example, extracellular stimuli (stress), hormone stimulation and
intracellular signaling (16).
In the nucleus, actin is associated with chromatin
remodeling, DNA replication, DNA repair, gene transcriptional
regulation, RNA processing, nuclear protein transportation and
maintenance of nuclear structure, for instance, the nuclear
envelope assembly (12,18–20).
Nuclear ABPs are closely associated to nuclear actin and implicated
in various nuclear activities. ABPs promote actin filament
nucleation or sequestering, manipulate nuclear actin dynamics and
determine the ratio of nuclear to cytoplasmic actin (13). Therefore, ABPs directly or indirectly
associate with chromatin remodeling, DNA replication,
transcription, DNA repair, nucleocytoplasmic transport and
maintenance of nuclear structure integrity (12,13,21).
Furthermore, nuclear actin is required by all three RNA polymerases
in transcriptional activation (22–24). The
study by Miyamoto and Gurdon (25)
mentions the major function of nuclear actin and ABPs in
transcriptional regulation and nuclear reprogramming.
In eukaryotic cells, the cytoplasm and nucleus are
separated by the nuclear envelope, which is a double membrane
barrier. Trafficking of proteins and other molecules between these
two compartments occurs by passing through the nuclear pore
complex. Additionally, cytoplasmic ABPs can interact with the
nuclear receptor in the cytoplasm, form complexes and facilitate
nuclear translocation. Therefore, ABPs mediate transcriptional
activation of nuclear receptors. These nuclear receptors include
the glucocorticoid and estrogen receptor, androgen receptor (AR),
thyroid receptor and peroxisome proliferator-activated receptor-c
(26–28). Furthermore, they are associated with
transcriptional activation of multiple genes and are involved in a
spectrum of functions, including cell proliferation,
differentiation and apoptosis (21,29,30).
Nuclear ABPs in cancer cells
Numerous ABPs demonstrate the ability to shuttle
between the cytoplasm and nucleus via different mechanisms. The
question remains whether it is the shuttling of ABPs between
different cellular compartments that is linked to pathological
behaviors, including abnormal cell development and differentiation,
carcinogenesis and metastasis. However, this needs to be clarified.
The theory that ABPs in the nucleus affect the aforementioned
pathological processes is intriguing. In the sections, the nuclear
ABPs that are associated with chromatin remodeling, transcriptional
regulation, DNA damage repair, protein nucleocytoplasmic shuttling
and nuclear structure maintenance in human cancer cells are
summarized in Table I and Fig. 1.
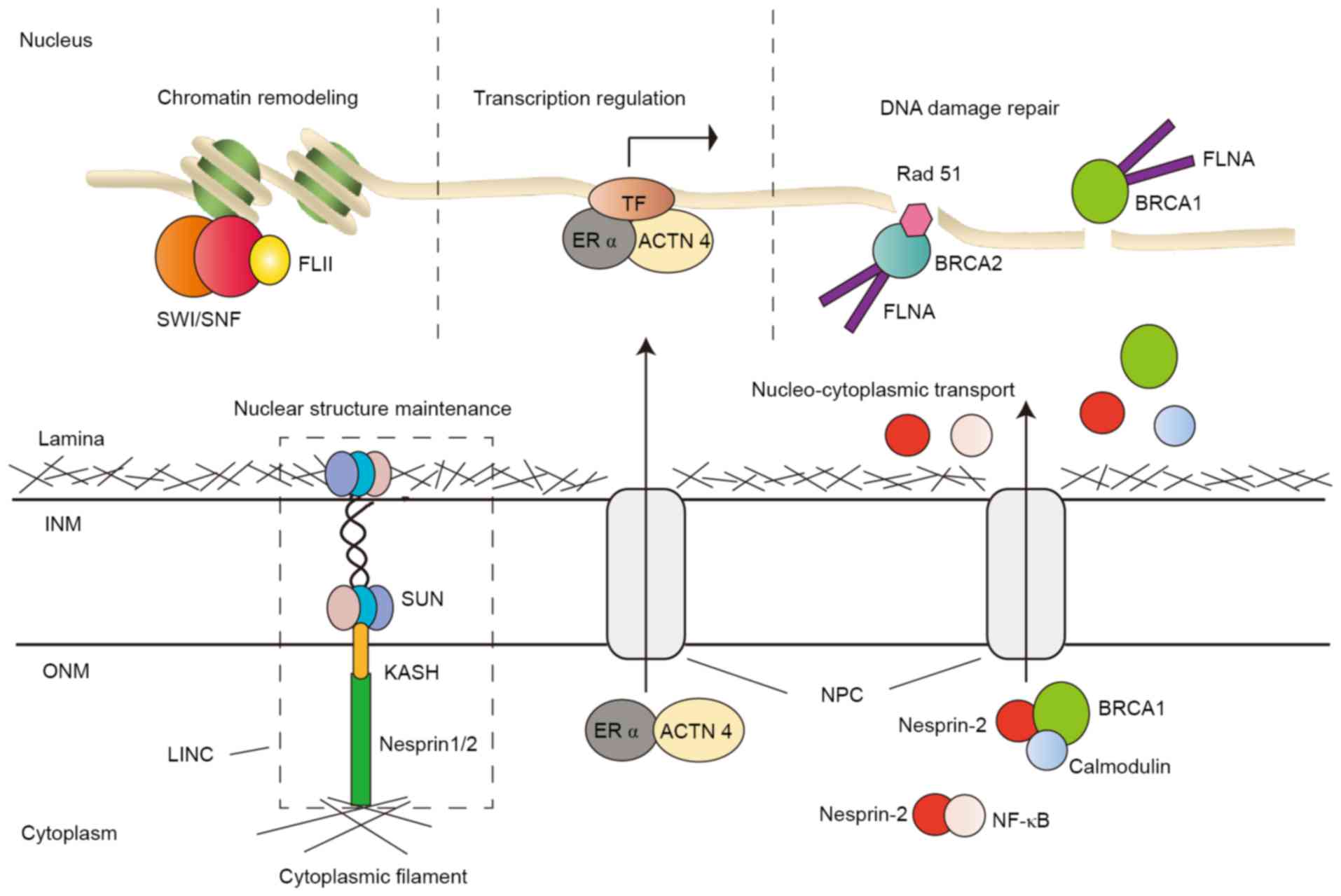 | Figure 1.Illustrations of ABPs in the nucleus.
ABPs are involved in chromatin remodeling, gene transcriptional
regulation, DNA damage repair, nucleocytoplasmic transport and
nuclear structure maintenance. ABP, actin-binding protein; INM,
inner nuclear membrane; ONM, outer nuclear membrane; NPC, nuclear
pore complex; LINC, linker of nucleoskeleton and cytoskeleton
complex; SUN, SUN domain proteins; KASH, KASH domain; FLII,
flightless I homolog; SWI/SNF, SWItch/Sucrose Non-Fermentable
chromatin remodeling complex; ACTN 4, α-actinin 4; ERα, estrogen
receptor α; TF, transcription factor; FLNα, filamin α; NF-κB,
nuclear factor κ-light-chain enhancer of activated B-cells; BRCA2,
breast cancer type 2; BRCA1, breast cancer type 1. |
 | Table I.Summary of nuclear functions of
actin-binding proteins in human cancer. |
Table I.
Summary of nuclear functions of
actin-binding proteins in human cancer.
| Nuclear
function | ABP | Mechanism | Tumor | (Refs.) |
|---|
| Chromatin
remodeling | Flightless I
homolog | Recruits chromatin
remodeling complex | Breast cancer | (31,32) |
| Transcriptional
regulation | Villin | Interacts with
transcriptional corepressor | Lung, gastric,
colorectal, pancreatic, biliary, liver, renal, cervical and
endometrioid cancer | (33–36) |
|
| α-actinin 4 | Interacts with
nuclear receptor (act as a coactivator); interacts with
transcriptional corepressor | Breast, prostate,
ovarian, colorectal, pancreatic and esophageal cancer, and salivary
gland carcinoma | (37–39,41–46) |
|
| Filamin α | Interact with
transcription factors | Colon, breast and
prostate cancer | (47–49) |
|
| Transgelin | Speculated to act
as a transcriptional regulator | Colorectal
cancer | (57,58) |
| DNA damage
response | Filamin α | Participates in
double strand break repair (DSBR) | Breast cancer,
certain melanomas | (61,63) |
|
| Nesprin-1 | Participates in the
DNA damage response (DDR), DNA mismatch repair (MMR) | Liver cancer | (64) |
| Protein nuclear
translocation | Nesprin-2 | Participates in
protein nuclear localization | Breast cancer | (65–67) |
| Nuclear structure
maintenance | Nesprin-1 | Constitutes the
linker of nucleoskeleton and cytoskeleton (LINC) complex | Lung and pancreatic
cancer | (69) |
|
| Nesprin-2 | Constitutes LINC
complex | Breast and
colorectal cancer, head and neck squamous cell carcinoma | (70–72) |
Fli I homolog (FLII), with a C-terminal
gelsolin-like actin-binding domain, is a member of the gelsolin
protein superfamily. FLII interacts with BAF53, a subunit of the
SWItch/Sucrose Non-Fermentable chromatin-remodeling complex, and
recruits the latter to the promoter and enhancer regions of the
trefoil factor 1 gene, an estrogen receptor (ERα) target gene
(31) (Fig.
1). FLII also regulates chromatin accessibility for the binding
of RNA polymerase II and other transcriptional coactivators to the
promoter and enhancer of other ERα target genes, including growth
regulation by estrogen in breast cancer 1, continuous traumatic
stress disorder and MYC in MCF-7 breast cancer cells. In addition,
FLII promotes the hormone-dependent growth of breast cancer cells
(32).
Villin is a tissue-specific ABP predominantly
expressed in the epithelium, including the gastrointestinal tract
and digestive organs. Overexpression of villin is reported in
tissues of Barrett's metaplasia, gastric and colorectal
adenocarcinoma (33,34). Furthermore, villin is distributed in
the cytoplasm and nucleus and cytoplamic-nuclear transport of
villin keeps the system dynamically stable. It migrates to the
nucleus upon stimulation, including hypoxia and injury. Another
postulation is that tyrosine phosphorylation of villin may prompt
its gathering in the nucleus. A study reveals that villin in the
nucleus can interact with ZBRK1 [also called zinc finger and breast
cancer type 1 (BRCA1)-interaction protein], a transcriptional
corepressor and also a ligand of the human Slug promoter, thus
regulating the activity of Slug and gene expression (34).
The villin-ZBRK1 complex eliminates the corepressor
effect of ZBRK1 and upregulates Slug expression. Slug is a crucial
transcriptional regulator of epithelial-mesenchymal transition
(EMT). Therefore, nuclear villin performs an important role in
inducing EMT, which is considered essential in tumorigenesis
(34), invasiveness and metastasis. A
previous study demonstrates that severe combined immunodeficiency
mice injected with xenografts derived from five colon cancer cell
lines developed tumors in 21 days at a 100% frequency, and by
staining with villin antibodies, the xenografts reveal strong
nuclear accumulation of villin (34).
Furthermore, villin expression is observed in gastric, colorectal,
pancreatic, biliary, liver, renal, cervical, endometrioid, lung and
other types of cancer (34–36), particularly with propensity for
metastasis and poor prognosis.
α-actinin 4 (ACTN4), a member of the ABP family, is
a regulator of gene transcription that is presumably mediated by
nuclear hormone receptors. Although the majority of ACTN4 is
located in the cytoplasm, the proportion of ACTN4 in the nucleus is
unneglectable. ACTN4 binds to the nuclear receptors in a
hormone-dependent manner. Furthermore, ACTN4 is a coactivator of
the estrogen receptor α (ER-α) that regulates target gene
transcription in MCF-7 breast cancer cells, subsequently promoting
tumor cell proliferation (37).
Overexpression of ACTN4 in the nucleus increases the expression of
progesterone receptors and antigen related to ER (pS2), and target
genes of ER-α (Fig. 1). Furthermore,
it can interact with the pS2 promoter and potentiate estradiol
(E2)-induced transcription. Additionally, it interacts with the AR
and functions as a co-regulator of AR-mediated transcription
(38).
Nuclear ACTN4 serves as a transcriptional
coactivator for nuclear factor κ-light-chain enhancer of activated
B-cell (NF-κB) (39). NF-κB
activation in ERα-negative breast cancer promotes cancer cell
proliferation (40). Furthermore,
ACTN4 also interacts with histone deacetylase 7 (HDAC7), a
transcriptional corepressor of myocyte enhancer factor 2 (MEF2),
competitively inhibiting the repressing effect of HDAC7 and
potentiating MEF2 transcription activity (41). ACTN4 interacts with HDAC7 by its
C-terminal calmodulin (CaM) -like domain, and activates ERα
transcription, which contributes to tumorigenesis in breast cancer
(42). In addition, elevated levels
of ACTN4 are widely identified in other malignancies, including
colorectal, pancreatic, prostate and ovarian cancer, salivary gland
carcinoma and esophageal cancer (38,42–46).
FLNα, also called ABP-280, is a scaffold protein.
Filamin deficit is prevalent among carcinomas, including colon,
prostate and breast cancer. It is verified that FLNα acts as a
promoter in cancer metastasis and invasion in the cytoplasm, while
it functions as a tumor suppressor in the nucleus (47). Furthermore, cytoplasmic FLNα interacts
with a number of proteins, for example, β1-integrin,
phosphatidylinositols and small GTPases, which facilitate cell
adhesion and migration. Furthermore, nuclear FLNα interacts with
transcription factors and the associate transcription machinery
subsequently restrains cell migration and represses cell growth. A
previous study demonstrates that nuclear FLNα prohibits ribosome
RNA transcription by interacting with RNA polymerase I (48). In addition, nuclear FLNα binds to the
AR and modulates the nuclear translocation of the latter, thus
regulates AR-induced gene expression. AR is a steroid nuclear
receptor and closely associated with prostate cancer. Nuclear FLNα
inhibits AR target gene transcription, thus negatively regulating
cancer development (49).
Transgelin is an ABP mostly distributed in the
cytoplasm of fibroblasts and several epithelial cells (50). Its expression is often altered in
human types of cancer (51–55). Previous research by this group
(56) revealed that activated AKT and
c-jun NH2-terminal kinases promote the expression of transgelin in
the cytoplasm and contribute to colorectal cancer progression.
Additionally, it is revealed that transgelin was located in the
cytoplasm and nucleus of colorectal cancer cells (57). Overexpression of transgelin in human
colon cancer cells affects the expression of ~250 other transcripts
and enhances the metastatic behavior (58). Thus, it is speculated that transgelin
is another transcriptional regulator within the ABP family.
The recovery of DNA damage is essential in the
maintenance of genome integrity. The nuclear FLNα is associated
with DNA damage repair (59). By
contacting BRCA1 and breast cancer type 2 (BRCA2), nuclear FLNα is
involved in the process of homologous recombinational and
non-homologous DNA repair (60).
Furthermore, FLNα interacts with BRCA1 with its extreme C-terminus
and mediates BRCA1 and Rad51 foci formation following DNA damage
(Fig. 1). In addition, the lack of
FLNα may contribute to predisposition to breast cancer (61). In double strand break repair (DSBR),
FLNα interacts with BRCA2 and subsequently forms the repair complex
(Fig. 1). The deficiency in FLNα
makes the cells susceptible to ionizing radiation and delays the
recovery from G2/M arrest, which may trigger the incidence of
cancer (60–63). Measurement of FLNα expression reveals
that FLNα is negative in several melanomas (63). In the absence of FLNα, cells impair to
recover from DNA damage and incline to accumulate genetic mutations
and initiate tumorigenesis.
Nesprin-1, also known as Enaptin, is a nuclear
envelope protein, consisting of a C-terminal KASH domain, a long
spectrin repeat region and an N-terminal F-actin binding domain.
Novel observations indicate that Nesprin-1 is involved in the DNA
damage response and DNA mismatch repair (MMR), thus maintaining
genetic stability (64). The study
indicates that Nesprin-1 interacts with the DNA MMR proteins, MSH2
and MSH6, and regulates the expression level and function of these
proteins, which are associated to DSBR. Reduction of Nesprin-1 may
lead to deficiency in correcting DNA damage, therefore triggering
tumorigenesis and accelerating tumor progression. Consistent with
this, Nesprin-1 expression significantly decreases in liver cancer
and numerous other types of human cancer (64). Nesprin-2 is a nuclear membrane protein
of the nuclear envelope spectrin-repeat (nesprin) family, which
contains an actin-binding domain. Loss of Nesprin-2 is associated
with less nuclear accumulation of c-Fos, mothers against
decapentaplegic homolog (SMAD) 2, 3 and 4, holding back the course
of nuclear translocation (65,66).
Furthermore, the latest studies imply that Nesprin-2 is a
prerequisite for the nuclear transport of certain proteins, for
example, BRCA1 and NF-κB (Fig. 1).
The nuclear localization of BRCA1 is regulated by a RAN-independent
Ca2+/CaM mediated machinery in which Nesprin-2 is
necessary (66). Additionally,
abnormality in Nesprin-2 nuclear trafficking results in impaired
nuclear translocation and mislocalization of BRCA1. Downregulation
of Nesprin-2 is revealed in breast cancer tissue (67). Therefore, disturbance of nuclear
translocation of certain proteins by the ABPs may be associated to
a number of diseases, including cancer (66).
Nesprins, a family of nuclear envelope proteins,
together with SUN domain proteins, form the core of the linker of
nucleoskeleton and cytoskeleton (LINC) complex. As a key component
of the LINC complex, nesprins tether nuclei to the cytoskeleton and
are essential in the maintenance of the nuclear architecture
(Fig. 1). Loss of nesprins leads to
risk of nuclear structural instability, including nuclear shape,
size and chromatin organization, which is implicated in
tumorigenesis (68). Furthermore,
nesprin-1 downregulation is reported in lung cancer, and synaptic
nuclear envelope protein 1 gene mutation is observed in pancreatic
cancer with metastasis (69).
Spectrin repeat containing, nuclear envelope protein
2 abnormality is revealed in breast and colorectal cancer, and head
and neck squamous cell carcinoma (70–72). One
possible mechanism is that nesprin downregulation modulates nuclear
stiffness via the LINC complex, and increases nuclear malleability
for cells to migrate through restricted tissue spaces (69).
Conclusion and perspectives
ABPs have been revealed in both the cytoplasm and
nucleus of eukaryotic cells. These proteins share an actin-binding
calponin homology domain, exhibit different functions and are
involved in diverse activities in the two cellular compartments.
Numerous studies observe a significant proportion of ABP shuttle
between the cytoplasm and the nucleus. Cytoplasmic ABPs may
translocate into the nucleus in response to the alternation of the
extracellular microenvironment, including hypoxia, inflammation and
injury. The subcellular localization of ABPs is associated with the
onset and development of various pathogenesis and carcinogenesis
processes. Recent observations (73–75)
demonstrate that nuclear ABPs may be involved in chromatin
remodeling, function as transcriptional regulators, are involved in
the DNA damage response and DNA mismatch repair, mediate protein
nuclear translocation and maintain nuclear structural stability.
Altogether, the nuclear ABPs maintain the genomic integrity and
reduce cellular oncogenic potential; whereas mutations and
deficiencies of nuclear ABPs contribute to tumorigenesis and
metastasis.
Although the comprehensive molecular mechanism of
specific nuclear ABPs remains elusive, the understanding of the
association between nuclear ABPs and relevant diseases is extended.
The investigation of ABPs' nuclear function and their effects on
cancer remains underway and lots of questions remain to be
answered. These studies will help to identify novel therapeutic
targets in fighting against cancer in the near future.
Acknowledgements
The National Natural Science Foundation of China
(grant no. 81641179, YL) and the Natural Science Foundation of
Guangdong Province (grant no. 2017A030313603, YL) supported the
present study. Grant (2013) 163 from the Key Laboratory of
Malignant Tumor Molecular Mechanism and Translational Medicine of
Guangzhou Bureau of Science and Information Technology, and grant
no. KLB09001 from the Key Laboratory of Malignant Tumor Gene
Regulation and Target Therapy of Guangdong Higher Education
Institutes also supported the present study.
References
|
1
|
Pollard TD and Cooper JA: Actin and
actin-binding proteins. A critical evaluation of mechanisms and
functions. Annu Rev Biochem. 55:987–1035. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
dos Remedios CG, Chhabra D, Kekic M,
Dedova IV, Tsubakihara M, Berry DA and Nosworthy NJ: Actin binding
proteins: Regulation of cytoskeletal microfilaments. Physiol Rev.
83:433–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartwig JH, Tyler J and Stossel TP:
Actin-binding protein promotes the bipolar and perpendicular
branching of actin filaments. J Cell Biol. 87:841–848. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amann KJ and Pollard TD: Cellular
regulation of actin network assembly. Curr Biol. 10:R728–R730.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vasioukhin V, Bauer C, Yin M and Fuchs E:
Directed actin polymerization is the driving force for epithelial
cell-cell adhesion. Cell. 100:209–219. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winder SJ and Ayscough KR: Actin-binding
proteins. J Cell Sci. 118:651–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pollard TD and Cooper JA: Actin, a central
player in cell shape and movement. Science. 326:1208–1212.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weston L, Coutts AS and La Thangue NB:
Actin nucleators in the nucleus: An emerging theme. J Cell Sci.
125:3519–3527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fairley EA, Kendrick-Jones J and Ellis JA:
The Emery-Dreifuss muscular dystrophy phenotype arises from
aberrant targeting and binding of emerin at the inner nuclear
membrane. J Cell Sci. 112:2571–2582. 1999.PubMed/NCBI
|
|
11
|
Tse WT, Tang J, Jin O, Korsgren C, John
KM, Kung AL, Gwynn B, Peters LL and Lux SE: A new spectrin, beta
IV, has a major truncated isoform that associates with
promyelocytic leukemia protein nuclear bodies and the nuclear
matrix. J Biol Chem. 276:23974–23985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castano E, Philimonenko VV, Kahle M,
Fukalová J, Kalendová A, Yildirim S, Dzijak R, Dingová-Krásna H and
Hozák P: Actin complexes in the cell nucleus: New stones in an old
field. Histochem Cell Biol. 133:607–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kristo I, Bajusz I, Bajusz C, Borkuti P
and Vilmos P: Actin, actin-binding proteins and actin-related
proteins in the nucleus. Histochem Cell Biol. 145:373–388. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hofmann WA: Cell and molecular biology of
nuclear actin. Int Rev Cell Mol Biol. 273:219–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Lanerolle P and Serebryannyy L: Nuclear
actin and myosins: Life without filaments. Nat Cell Biol.
13:1282–1288. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gettemans J, Van Impe K, Delanote V,
Hubert T, Vandekerckhove J and De Corte V: Nuclear actin-binding
proteins as modulators of gene transcription. Traffic. 6:847–857.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rando OJ, Zhao K and Crabtree GR:
Searching for a function for nuclear actin. Trends Cell Biol.
10:92–97. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bettinger BT, Gilbert DM and Amberg DC:
Actin up in the nucleus. Nat Rev Mol Cell Biol. 5:410–415. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blessing CA, Ugrinova GT and Goodson HV:
Actin and ARPs: Action in the nucleus. Trends Cell Biol.
14:435–442. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miralles F and Visa N: Actin in
transcription and transcription regulation. Curr Opin Cell Biol.
18:261–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng B, Han M, Bernier M and Wen JK:
Nuclear actin and actin-binding proteins in the regulation of
transcription and gene expression. FEBS J. 276:2669–2685. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Philimonenko VV, Zhao J, Iben S, Dingová
H, Kyselá K, Kahle M, Zentgraf H, Hofmann WA, de Lanerolle P, Hozák
P and Grummt I: Nuclear actin and myosin I are required for RNA
polymerase I transcription. Nat Cell Biol. 6:1165–1172. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hofmann WA, Stojiljkovic L, Fuchsova B,
Vargas GM, Mavrommatis E, Philimonenko V, Kysela K, Goodrich JA,
Lessard JL, Hope TJ, et al: Actin is part of pre-initiation
complexes and is necessary for transcription by RNA polymerase II.
Nat Cell Biol. 6:1094–1101. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu P, Wu S and Hernandez N: A role for
beta-actin in RNA polymerase III transcription. Genes Dev.
18:3010–3015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyamoto K and Gurdon JB: Transcriptional
regulation and nuclear reprogramming: Roles of nuclear actin and
actin-binding proteins. Cell Mol Life Sci. 70:3289–3302. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ting HJ, Yeh S, Nishimura K and Chang C:
Supervillin associates with androgen receptor and modulates its
transcriptional activity. Proc Natl Acad Sci USA. 99:pp. 661–666.
2002; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishimura K, Ting HJ, Harada Y, Tokizane
T, Nonomura N, Kang HY, Chang HC, Yeh S, Miyamoto H, Shin M, et al:
Modulation of androgen receptor transactivation by gelsolin: A
newly identified androgen receptor coregulator. Cancer Res.
63:4888–4894. 2003.PubMed/NCBI
|
|
28
|
Yang Z, Chang YJ, Miyamoto H, Ni J, Niu Y,
Chen Z, Chen YL, Yao JL, di Sant'Agnese PA and Chang C: Transgelin
functions as a suppressor via inhibition of ARA54-enhanced androgen
receptor transactivation and prostate cancer cell growth. Mol
Endocrinol. 21:343–358. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baek SH, Ohgi KA, Nelson CA, Welsbie D,
Chen C, Sawyers CL, Rose DW and Rosenfeld MG: Ligand-specific
allosteric regulation of coactivator functions of androgen receptor
in prostate cancer cells. Proc Natl Acad Sci USA. 103:pp.
3100–3105. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang F, Liu XQ, Li H, Liang KN, Miner JN,
Hong M, Kallel EA, van Oeveren A, Zhi L and Jiang T: Structure of
the ligand-binding domain (LBD) of human androgen receptor in
complex with a selective modulator LGD2226. Acta Crystallogr Sect F
Struct Biol Cryst Commun. 62:1067–1071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Won Jeong K, Chodankar R, Purcell DJ,
Bittencourt D and Stallcup MR: Gene-specific patterns of
coregulator requirements by estrogen receptor-alpha in breast
cancer cells. Mol Endocrinol. 26:955–966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jeong KW: Flightless I (Drosophila)
homolog facilitates chromatin accessibility of the estrogen
receptor α target genes in MCF-7 breast cancer cells. Biochem
Biophys Res Commun. 446:608–613. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khurana S: Structure and function of
villinAspects of the Cytoskeleton. Khurana S: Elsevier; New York:
pp. 89–1159. 2006, View Article : Google Scholar
|
|
34
|
Patnaik S, George SP, Pham E, Roy S, Singh
K, Mariadason JM and Khurana S: By moonlighting in the nucleus,
villin regulates epithelial plasticity. Mol Biol Cell. 27:535–548.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shillingford NM, Calicchio ML, Teot LA,
Boyd T, Kurek KC, Goldsmith JD, Bousvaros A, Perez-Atayde AR and
Kozakewich HP: Villin immunohistochemistry is a reliable method for
diagnosing microvillus inclusion disease. Am J Surg Pathol.
39:245–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang Z: The utility of villin and
mammaglobin in the differential diagnosis between intrahepatic
cholangiocarcinoma and breast cancer. Appl Immunohistochem Mol
Morphol. 23:19–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khurana S, Chakraborty S, Cheng X, Su YT
and Kao HY: The actin-binding protein, actinin alpha 4 (ACTN4), is
a nuclear receptor coactivator that promotes proliferation of MCF-7
breast cancer cells. J Biol Chem. 286:1850–1859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jasavala R, Martinez H, Thumar J, Andaya
A, Gingras AC, Eng JK, Aebersold R, Han DK and Wright ME:
Identification of putative androgen receptor interaction protein
modules: Cytoskeleton and endosomes modulate androgen receptor
signaling in prostate cancer cells. Mol Cell Proteomics. 6:252–271.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao X, Hsu KS, Lim JH, Bruggeman LA and
Kao HY: α-Actinin 4 potentiates nuclear factor
κ-light-chain-enhancer of activated B-cell (NF-κB) activity in
podocytes independent of its cytoplasmic actin binding function. J
Biol Chem. 290:338–349. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Biswas DK, Shi Q, Baily S, Strickland I,
Ghosh S, Pardee AB and Iglehart JD: NF-kappa B activation in human
breast cancer specimens and its role in cell proliferation and
apoptosis. Proc Natl Acad Sci USA. 101:pp. 10137–10142. 2004;
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chakraborty S, Reineke EL, Lam M, Li X,
Liu Y, Gao C, Khurana S and Kao HY: Alpha-actinin 4 potentiates
myocyte enhancer factor-2 transcription activity by antagonizing
histone deacetylase 7. J Biol Chem. 281:35070–35080. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsu KS and Kao HY: Alpha-actinin 4 and
tumorigenesis of breast cancer. Vitam Horm. 93:323–351. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hara T, Honda K, Shitashige M, Ono M,
Matsuyama H, Naito K, Hirohashi S and Yamada T: Mass spectrometry
analysis of the native protein complex containing actinin-4 in
prostate cancer cells. Mol Cell Proteomics. 6:479–491. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamamoto S, Tsuda H, Honda K, Kita T,
Takano M, Tamai S, Inazawa J, Yamada T and Matsubara O: Actinin-4
expression in ovarian cancer: A novel prognostic indicator
independent of clinical stage and histological type. Mod Pathol.
20:1278–1285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kikuchi S, Honda K, Tsuda H, Hiraoka N,
Imoto I, Kosuge T, Umaki T, Onozato K, Shitashige M, Yamaguchi U,
et al: Expression and gene amplification of actinin-4 in invasive
ductal carcinoma of the pancreas. Clin Cancer Res. 14:5348–5356.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Honda K: The biological role of actinin-4
(ACTN4) in malignant phenotypes of cancer. Cell Biosci. 5:412015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Savoy RM and Ghosh PM: The dual role of
filamin A in cancer: Can't live with (too much of) it, can't live
without it. Endocr Relat Cancer. 20:R341–R356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Deng W, Lopez-Camacho C, Tang JY,
Mendoza-Villanueva D, Maya-Mendoza A, Jackson DA and Shore P:
Cytoskeletal protein filamin A is a nucleolar protein that
suppresses ribosomal RNA gene transcription. Proc Natl Acad Sci
USA. 109:pp. 1524–1529. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Loy CJ, Sim KS and Yong EL: Filamin-A
fragment localizes to the nucleus to regulate androgen receptor and
coactivator functions. Proc Natl Acad Sci USA. 100:pp. 4562–4567.
2003; View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lawson D, Harrison M and Shapland C:
Fibroblast transgelin and smooth muscle SM22alpha are the same
protein, the expression of which is down-regulated in many cell
lines. Cell Motil Cytoskeleton. 38:250–257. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shields JM, Rogers-Graham K and Der CJ:
Loss of transgelin in breast and colon tumors and in RIE-1 cells by
Ras deregulation of gene expression through Raf-independent
pathways. J Biol Chem. 277:9790–9799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sitek B, Lüttges J, Marcus K, Klöppel G,
Schmiegel W, Meyer HE, Hahn SA and Stühler K: Application of
fluorescence difference gel electrophoresis saturation labelling
for the analysis of microdissected precursor lesions of pancreatic
ductal adenocarcinoma. Proteomics. 5:2665–2679. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mikuriya K, Kuramitsu Y, Ryozawa S,
Fujimoto M, Mori S, Oka M, Hamano K, Okita K, Sakaida I and
Nakamura K: Expression of glycolytic enzymes is increased in
pancreatic cancerous tissues as evidenced by proteomic profiling by
two-dimensional electrophoresis and liquid chromatography-mass
spectrometry/mass spectrometry. Int J Oncol. 30:849–855.
2007.PubMed/NCBI
|
|
54
|
Huang Q, Huang Q, Chen W, Wang L, Lin W,
Lin J and Lin X: Identification of transgelin as a potential novel
biomarker for gastric adenocarcinoma based on proteomics
technology. J Cancer Res Clin Oncol. 134:1219–1227. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sun X, Zhang H, Luo L, Zhong K, Ma Y, Fan
L, Fu D and Wan L: Comparative proteomic profiling identifies
potential prognostic factors for human clear cell renal cell
carcinoma. Oncol Rep. 36:3131–3138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou H, Zhang Y, Chen Q and Lin Y: AKT and
JNK signaling pathways increase the metastatic potential of
colorectal cancer cells by altering transgelin expression. Dig Dis
Sci. 61:1091–1097. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lin Y, Buckhaults PJ, Lee JR, Xiong H,
Farrell C, Podolsky RH, Schade RR and Dynan WS: Association of the
actin-binding protein transgelin with lymph node metastasis in
human colorectal cancer. Neoplasia. 11:864–873. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhou HM, Fang YY, Weinberger PM, Ding LL,
Cowell JK, Hudson FZ, Ren M, Lee JR, Chen QK, Su H, et al:
Transgelin increases metastatic potential of colorectal cancer
cells in vivo and alters expression of genes involved in cell
motility. BMC Cancer. 16:552016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yuan Y and Shen Z: Interaction with BRCA2
suggests a role for filamin-1 (hsFLNa) in DNA damage response. J
Biol Chem. 276:48318–48324. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yue J, Huhn S and Shen Z: Complex roles of
filamin-A mediated cytoskeleton network in cancer progression. Cell
Biosci. 3:72013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Velkova A, Carvalho MA, Johnson JO,
Tavtigian SV and Monteiro AN: Identification of Filamin A as a
BRCA1-interacting protein required for efficient DNA repair. Cell
cycle. 9:1421–1433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Meng X, Yuan Y, Maestas A and Shen Z:
Recovery from DNA damage-induced G2 arrest requires actin-binding
protein filamin-A/actin-binding protein 280. J Biol Chem.
279:6098–6105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yue J, Wang Q, Lu H, Brenneman M, Fan F
and Shen Z: The cytoskeleton protein filamin-A is required for an
efficient recombinational DNA double strand break repair. Cancer
Res. 69:7978–7985. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sur I, Neumann S and Noegel AA: Nesprin-1
role in DNA damage response. Nucleus. 5:173–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rashmi RN, Eckes B, Glöckner G, Groth M,
Neumann S, Gloy J, Sellin L, Walz G, Schneider M, Karakesisoglou I,
et al: The nuclear envelope protein Nesprin-2 has roles in cell
proliferation and differentiation during wound healing. Nucleus.
3:172–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kelkar P, Walter A, Papadopoulos S, Mroß
C, Munck M, Peche VS and Noegel AA: Nesprin-2 mediated nuclear
trafficking and its clinical implications. Nucleus. 6:479–489.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Matsumoto A, Hieda M, Yokoyama Y, Nishioka
Y, Yoshidome K, Tsujimoto M and Matsuura N: Global loss of a
nuclear lamina component, lamin A/C, and LINC complex components
SUN1, SUN2 and nesprin-2 in breast cancer. Cancer Med. 4:1547–1557.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Neumann S and Noegel AA: Nesprins in cell
stability and migration. Adv Exp Med Biol. 773:491–504. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cartwright S and Karakesisoglou I:
Nesprins in health and disease. Semin Cell Dev Biol. 29:169–179.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sjöblom T, Jones S, Wood LD, Parsons DW,
Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, et al:
The consensus coding sequences of human breast and colorectal
cancers. Science. 314:268–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chittenden TW, Howe EA, Culhane AC,
Sultana R, Taylor JM, Holmes C and Quackenbush J: Functional
classification analysis of somatically mutated genes in human
breast and colorectal cancers. Genomics. 91:508–511. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Stransky N, Egloff AM, Tward AD, Kostic
AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C,
McKenna A, et al: The mutational landscape of head and neck
squamous cell carcinoma. Science. 333:1157–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang P, Sridharan D and Lambert MW:
Nuclear α spectrin differentially affects monoubiquitinated versus
non-ubiquitinated FANCD2 function after DNA interstrand cross-link
damage. J Cell Biochem. 117:671–683. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Almuzzaini B, Sarshad AA, Farrants AK and
Percipalle P: Nuclear myosin 1 contributes to a chromatin landscape
compatible with RNA polymerase II transcription activation. Bmc
Biol. 13:352015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Savoy RM, Chen L, Siddiqui S, Melgoza FU,
Durbin-Johnson B, Drake C, Jathal MK, Bose S, Steele TM, Mooso BA,
et al: Transcription of Nrdp1 by the androgen receptor is regulated
by nuclear filamin A in prostate cancer. Endocr Relat Cancer.
22:369–386. 2015. View Article : Google Scholar : PubMed/NCBI
|















