Introduction
Papillary thyroid carcinoma (PTC) is the most common
type of endocrine malignancy (1). PTC
accounts for ~80% of thyroid carcinomas in adults and 90% in
children; patients are usually 20–50 years old, but individuals of
any age may be affected (1). PTC is
more common in females, with a prevalence 2.9-times higher in women
than in men (2). The incidence of
this human tumor type has been grown rapidly over the past three
decades with a 2.3-fold increase in the total number of patients, a
trend that has been observed in numerous countries across Europe,
Asia, Oceania, North and South America (3). Well differentiated papillary thyroid
carcinomas (WDPTC) are associated with superior survival statistics
when treated appropriately with surgery, radioiodine ablation and
thyroid suppression therapy; however, the occurrence of aggressive
WDPTC is not a rare event (4) and
there is an increasing requirement for more accurate prognostic
tools to predict the possible disease outcome. Risk stratification
is important for identifying patients at a higher risk of
recurrence or distant metastasis so more aggressive therapy and
monitoring can be implemented.
In addition to the known clinical and
histopathological risk factors, recent advances in the molecular
genetics of thyroid cancer may be applied to identify certain novel
biomarkers useful for understanding the tumor behavior. B-Raf
proto-oncogene, serine/threonine kinase (BRAF) mutations are known
to have an important role in PTC tumorigenesis and appear to be
correlated with the outcome of the disease (5–7). BRAF is a
serine/threonine protein kinase, encoded on chromosome 7q34, which
activates the mitogen activated protein kinase (MAPK)/extracellular
signal-related kinase-signaling pathway, which affects cell
division, proliferation and differentiation. The BRAF mutation
exists in PTCs and PTC-derived anaplastic thyroid cancer (ATCs),
but it has not been described in follicular (FTC) or medullary
(MTC) thyroid carcinoma, or in normal thyroid tissue (6). Occasionally, a few uncommon BRAF
mutations have been reported in benign thyroid neoplasia (8,9). The BRAF
mutation has been identified in ~29–69% of PTCs and in >80% of
PTCs of the tall cell variant, and the correlation between the BRAF
mutation and the poorer clinicopathological characteristics of PTC
has been demonstrated (5,7). Approximately 90% of BRAF somatic
mutations consist of a T to A substitution at codon 600 (p.V600E)
(10). Rat sarcoma (RAS) mutations
have also been studied; RAS mutations are identified with variable
frequency in all types of thyroid follicular cell-derived tumors
(11). They occur in 10–20% of PTCs,
especially in the follicular variant, in 40–50% of
conventional-type follicular carcinomas and 20–40% of
conventional-type follicular adenomas (6). In general, previous studies have
demonstrated that the presence of RAS mutations in a thyroid nodule
provides evidence for neoplasia; however, it does not establish a
diagnosis of malignancy (6,11).
Beyond BRAF and RAS, additional studies regarding
non-coding mutations in cancer have underlined the importance of
the human telomerase reverse transcriptase (hTERT) promoter
mutation (12,13). Telomerase is a ribonucleoprotein
polymerase that maintains the telomere repeat TTAGGG at the ends of
chromosomes, and consists of a protein with reverse transcriptase
activity, TERT, and an RNA component that serves as a template
(13). Initially identified in
melanoma, TERT promoter mutations appear to have a vital role in
the pathogenesis of other types of neoplasm, including bladder
cancer, hepatocellular carcinoma, liposarcoma, central nervous
system tumors and thyroid cancer [WDPTC or poorly differentiated
(PDTC) and ATC] (14). Recent studies
have revealed an association between the TERT mutation and certain
clinical features of patients, including an older age at diagnosis,
male sex and tumor size, particularly in WDPTCs (13). In addition, the coexistence of TERT
and BRAF/RAS mutations has been taken into consideration and
certain authors emphasized how TERT mutations appear to be more
frequent in BRAF-mutated WDPTC, in particular in those with a V600E
mutation, as compared with in BRAF-wild type (WT) carcinoma
(13). It is not currently clear
whether this association has a clinical impact: Liu et al
(15) revealed that TERT mutations in
thyroid cancer are particularly prevalent in BRAF-V600E mutated
PTC, having a role in the de-differentiation, progression and
aggressiveness of the tumor (14).
Conversely, additional studies identified no significant
differences in the outcome among cancer cases with TERT/BRAF
mutations, and those cases with only TERT mutations (13).
The present study focused on the expression of TERT
in a large series of thyroid cancer cases, investigating a possible
correlation between its expression and certain features of the
tumor. In addition, the expression of TERT according to BRAF and
RAS mutations was evaluated.
Materials and methods
Patients and tumor specimens
A large series of consecutive primary papillary
thyroid tumors were obtained from 145 patients (106 females and 39
males) who underwent surgical resection at the University Hospital
of Pisa (Pisa, Italy) between January and December 2010. Formalin
fixed and paraffin-embedded thyroid specimens from all the 145
patients were sliced with a microtome, and routine hematoxylin and
eosin staining was performed. They were stored in archives and were
retrospectively reviewed. All 145 cases were WDPTCs; in particular,
79 were follicular variants (FVPTC), 47 were classic variants
(CVPTC), 15 were tall cell variants (TCPTC), two were
solid/trabecular variants, one was a macrofollicular variant and
one was a Hurthle cell variant. The age of the patients ranged
between 10 and 78, with a mean age of 46. All the specimens were
reviewed by two pathologists (A.C.I. and A.P.) to confirm the
diagnosis. This study was approved by the institutional review
board, and informed consent was obtained from all patients.
DNA purification
Serial 10 µm-thick tissue sections were obtained
from paraffin blocks for DNA extraction from the primary tumor
tissue. Hematoxylin and eosin reference slides were marked to
identify the area of interest with neoplastic cells; afterwards
neoplastic areas were macro-dissected. For the extraction of the
DNA, embedded sections were deparaffinized in xylene, dehydrated
through a graded series of alcohols, and processed using a
diaminobenzidine detection system (Ventana Medical Systems, Inc.,
Tucson, AZ, USA), following the manufacturer's protocol. DNA was
purified using the Qiagen RNeasy FFPE kit (Qiagen GmbH, Hilden,
Germany) as described by the manufacturer. DNA concentration and
quality were assessed by a spectrophotometer (NanoDrop ND-1000,
NanoDrop Technologies; Thermo Fisher Scientific, Inc., Waltham, MA,
USA); the instrument measures absorbance at 260 nm (A260) to
quantify DNA in samples, at 280 nm (A280) to verify protein
contamination and at 230 nm (A230) for determining contamination by
phenol. Ratio between A260/A280 and A260/A230 are parameters to
evaluate DNA purity (A260/A280 should be >1.7; A260/A280 should
be >1.8).
TERT, BRAF and RAS gene family
mutational analysis
The DNA was evaluated for the TERT, BRAF and RAS
gene family. The two more frequently identified variations of the
promoter of the TERT gene (located on chromosome 5) at positions
1295228 and 1295250 are known as C228T and C250T, respectively.
These mutations are located at −124 and −146-bp upstream of the ATG
start codon (13,16). Additionally, two low-frequency
CC>TT tandem variations have been described at positions
1295228/1295229 (−124/-125 from the ATG site) and 1295242/1295243
(−138/-139 from the ATG site), which were named C228T/C229T and
C242T/C243T, respectively (13,16).
Consequently, the TERT promoter target region was amplified via PCR
using the following primer pair: TERT promoter forward
5′-CAGCGCTGCCTGAAACTC−3′; reverse 5′-GTCCTGCCCCTTCACCTT−3′
(1,2).
Consequently, the TERT promoter target region was amplified via PCR
using the following primer pair: TERT promoter forward
5′CAGCGCTGCCTGAAACTC3′; reverse 5′GTCCTGCCCCTTCACCTT3′ and the
following reagents mixture: 10 mM Tris-HCl, 50 mM KCl, 1.5 mM
MgCl2 (pH 8.3), 0.2 mM dNTPs, 8 pmol primers and 1.25 U
AmpliTaq Gold DNA Polymerase (Thermo Fisher Scientific, Inc.) in a
final volume of 20 µl. A total of 5 µl DNA were added, and reaction
was performed on a thermal cycler (SensoQuest, Gottingen, Germany)
with the following protocol: Hold at 95°C for 5 min 50 cycles 95°C
for 30 sec, 55°C for 30 sec and 72°C for 45 sec. A total of 2 µl
PCR products were used as template for reaction sequencing
containing labeled nucleotide terminators 1 µl, sequencing buffer 2
µl, 1 µM primer 3 µl and water in a final volume of 20 µl (BigDye
Terminator Cycle Sequencing kit; Applied Biosystem, Foster City,
CA, USA). The reaction conditions were the following: Hold at 96°C
for 10 min, 24 cycles at 96°C for 10 sec, 50°C for 5 sec, and 60°C
for 4 min. Then, the reaction was run for direct sequencing on a
AbiPrism 3130 Genetic Analyzer (Applied Biosystem, Foster City, CA,
USA). BRAF and RAS genes (NRAS, HRAS, KRAS) were analyzed by
high-resolution melt analysis (HRMA) followed by direct sequencing.
Approximately 80 ng DNA was amplified in a final volume of 25 µl
containing 12.5 µl Master Mix (Qiagen GmbH, Hilden, Germany) 0.8
µmol/l of each primer and 1 µl EvaGreen 20X. PCR and HRMA were
performed on a Rotorgene 6000™ Real Time Analyzer (Qiagen GmbH).
Post-amplification fluorescence melting curve analysis was
performed by gradual heating of samples at a rate of 1°C/sec from
45–95°C. A HRMA was immediately performed from 75–85°C rising at
0.1°C/sec. The resulting data were analyzed using Rotor-Gene Series
software version 1.7 (Qiagen GmbH). PCR products of all samples
with altered melting curves, together with a number of non-altered
ones, were sequenced as described for TERT promoter analysis.
Immunohistochemical studies
Samples with the best tumor/normal tissue ratio
and/or samples without factors that may invalidate
immunohistochemistry (necrosis, calcifications, fibrosis) from the
paraffin block as the best representation of the tumors was
selected for analysis for each case. The immunohistochemical
analyses were performed automatically using the Ventana Bench-mark
immunostaining system (Ventana Medical Systems, Inc.).
Paraffin-embedded tissue sections (3–5 µm) were deparaffinized in
xylene, rehydrated through a graded series of ethanol and processed
using a diaminobenzidine detection system (Ventana Medical Systems,
Inc.), following the manufacturer's instructions. TERT
immunostaining was performed using a rabbit polyclonal antibody
produced by repeated immunizations with a synthetic peptide
corresponding to a region near the carboxy-terminal end of hTERT
(no. AF018167; Rockland Immunochemicals, Inc., Limerick, PA, USA)
at a dilution of 1:300. In order to test the specificity and
sensitivity of the antibody, control neoplastic tissues
(astrocytoma) from the archives were also stained. The
immunostaining of all the samples was evaluated independently by
two surgical pathologists; each pathologist assessed the intensity
and extent of immunoreactivity for each case. Disparate scores
between the two investigators were observed in <10% of the
samples and a consensus was achieved in all cases following
discussion. In case of disagreement the slides were evaluated again
with a multi-ocular microscope. All the samples were scored based
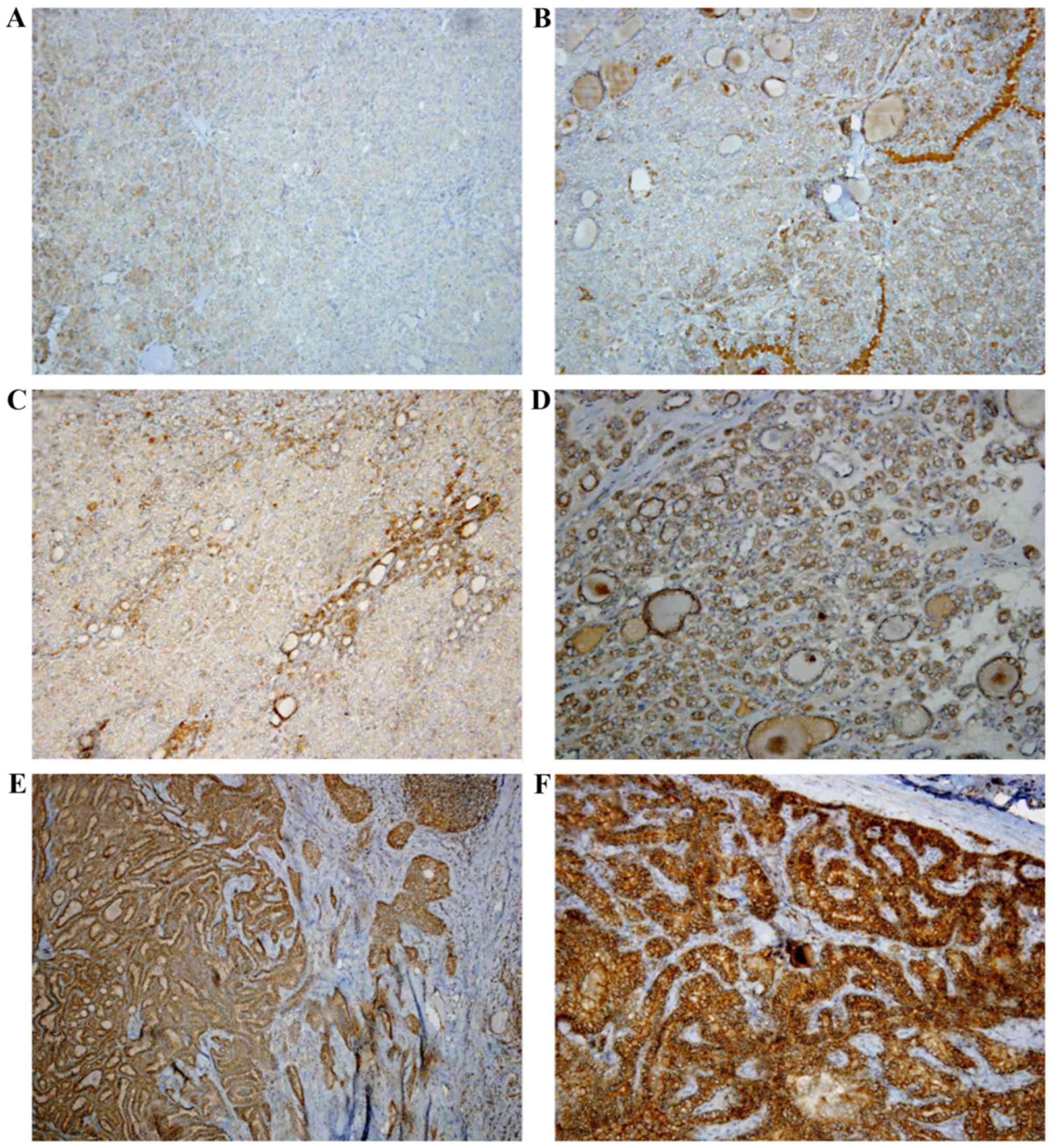
on the percentage and intensity of the positive neoplastic cells.
The extension of the positive reaction was classified into three
grades (1, 2 or 3) as follows: Grade 1, a positive reaction was
detected in <20% of the neoplasm; grade 2, a positive reaction
between 21–50%; grade 3, a positive reaction was detected in
>50% of the neoplasm. The staining intensity was also classified
in three grades, as follows: Grade 1, weak intensity; grade 2,
moderate intensity; grade 3, strong intensity (Table I). Subsequently, a total score ranging
from 2–6 was obtained by adding the scores of the two categories
analyzed (Fig. 1).
 | Table I.Classification of IHC grade and stain
intensity. |
Table I.
Classification of IHC grade and stain
intensity.
| Grade | Positive area
extension, % | Intensity |
|---|
| 1 | <20 | Weak |
| 2 | 21–50 | Moderate |
| 3 | >50 | Strong |
A z-score was also calculated, taking into account
the intensity of staining and the percentage of immunoreactive
cells. All the cases were divided into two groups, defined as
positive or negative, depending on the z-score
obtained: All the cases with a z-score >0 were defined as
positive; all the cases with a z-score <0 were defined as
negative.
Statistical analysis
Statistical analysis was performed using IBM SPSS
Statistical software (version 17.0.1; IBM SPSS, Armonk, NY, USA). A
Shapiro-Wilk test was performed to verify the normality of
distributions. Correlations between clinical features were
evaluated using Fisher's exact test, χ2 test or a
two-tailed t-test, as appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical-pathological features of 145
cases of PTC
Several clinical features were analyzed for all
tumors. A total of 76 cases were capsulated tumors and 64 out of
these 76 capsulated tumors resulted FVPTC. Among the other 69
non-capsulated cases, 36 exhibited infiltration of the
perithyroidal soft tissues; in particular, 21 were CVPTCs, 11 were
TCPTCs and 4 were FVPTCs. A total of 16/145 cases had lymph node
metastasis at the time of diagnosis (12 CVPTCs, 3 TCPTCs and 1
FVPTC). Finally, 7/145 cases had embolization at the diagnosis, of
which 6 were CVPTCs and 1 was TCPTC.
BRAF and RAS mutation
Among all 145 tumors analyzed, a BRAF mutation was
detected in 53 cases, corresponding to 36.6% of the entire cohort;
in particular, 31 BRAF mutated tumors were CVPTCs, 12 were TCPTCs
and 10 were FVPTCs (Table II). All
the CVPTCs, as well as all the TCPTCs, carried a V600E mutation;
instead, among the 10 BRAF-mutated FVPTCs, 6 had a V600E mutation,
2 a K601E mutation, 1 presented with a deletion (V600_K601delinsE)
and 1 with a T599I mutation. Comparing the presence of BRAF
mutation with clinical-pathological features we observed 27 cases
with involvement of the perithyroidal tissues, 6 cases with
embolization and 11 with lymph nodes metastasis. RAS mutation was
detected in 23 cases, corresponding to 15.9% of the entire cohort
and all were FVPTCs. In particular, an NRAS mutation occurred in
18/23 cases, while the remaining 5 cases were HRAS mutated
(Table II). All 23 RAS mutated
tumors were limited to the parenchyma without involving the
periglandular soft tissues and, in particular, 22/23 presented as
capsulated nodules. None of the tumors with embolization or lymph
node metastasis were revealed to be RAS mutated.
 | Table II.Clinicopathological features of 145
cases of PTC. |
Table II.
Clinicopathological features of 145
cases of PTC.
| Clinicopathological
characteristic | CVPTC (47/145-32.4%)
(%) | FVPTC (79/145-54.5%)
(%) | TCPTC (15/145-10.3%)
(%) | OTHERSa (4/145-2.8%) (%) |
|---|
| Sex |
|
|
|
|
| Female
(106/145-73.1%) | 29/47 (61.7) | 61/79 (77.2) | 13/15 (86.7) | 3/4 (75) |
| Male
(39/145-26.9%) | 18/47 (38.3) | 18/79 (22.8) | 2/15 (13.3) | 1/4 (25) |
| Size |
|
|
|
|
| ≤1 cm
(44/145-30.3%) | 17/47 (36.2) | 24/79 (30.4) | 3/15 (20) | – |
| >1 cm
(101/145-69.7%) | 30/47 (63.8) | 55/79 (69.6) | 12/15 (80) | 4/4 (100) |
| Capsule |
|
|
|
|
|
Capsulated (76/145-52.4%) | 8/47 (17.1) | 64/79 (81) | – | 4/4 (100) |
| Not
Capsulated (69/145-47.6%) | 39/47 (82.9) | 15/79 (19) | 15/15(100) | – |
| Tumor capsule
invasion |
|
|
|
|
| Yes
(8/76-10.5%) | 1/47 (2.1) | 7/79 (8.9) | – | – |
| No
(68/76-89.5%) | 46/47 (97.9) | 72/79 (91.1) | 15/15 (100) | 4/4 (100) |
| Thyroid capsule
invasion |
|
|
|
|
| Yes
(17/69-24.6%) | 9/47 (19.2) | 6/79 (7.6) | 2/15 (13.4) | – |
| No
(52/69-75.4%) | 38/47 (80.8) | 73/79 (92.4) | 13/15 (86.6) | 4/4 (100) |
| Perithyroid soft
tissue invasion |
|
|
|
|
| Yes
(36/69-52.2%) | 21/47 (44.7) | 4/79 (5.1) | 11/15 (73.4) | – |
| No
(33/69-47.8%) | 26/47 (55.3) | 75/79 (94.9) | 4/15 (26.6) | 4/4 (100) |
| Embolization |
|
|
|
|
| Yes
(7/145-4.8%) | 6/47 (12.8) | – | 1/15 (6.7) | – |
| No
(138/145-95.2%) | 41/47 (87.2) | 79/79 (100) | 14/15 (93.3) | 4/4 (100) |
| Lymph node
metastasis |
|
|
|
|
| Yes
(16/145-11%) | 12/47 (25.5) | 1/79 (1.3) | 3/15 (20) | – |
| No
(129/145-89%) | 35/47 (74.5) | 78/79 (98.7) | 12/15 (80) | 4/4 (100) |
| BRAF |
|
|
|
|
| Mutated
(53/145-36.6%) | 31/47 (66) | 10/79 (12.7) | 12/15 (80) | – |
| WT
(92/145-63.4%) | 16/47 (34) | 69/79 (87.3) | 3/15 (20) | 4/4 (100) |
| RAS |
|
|
|
|
| Mutated
(23/145-15.9%) | – | 23/79 (29.1) | – | – |
| WT
(122/145-84.1%) | 47/47 (100) | 56/79 (70.9%) | 15/15 (100) | 4/4 (100) |
TERT promoter mutation
In our study, TERT mutations were identified in
9/145 (5 CVPTCs, 2 FVPTCs and 1 TCPTC) corresponding to the 6.2% of
the overall cohort. ‘C228T’ mutation was the most frequent
alteration observed, present in 8/9 cases; the remaining one
exhibited a ‘C250T’ mutation. All the 145 patients were divided
according to the TERT mutation in ‘mutated’/‘non-mutated’ and
several clinical features were studied, the most important reported
in Table III. We identified a
significant association with sex (66.7% males and 33.3% females).
All the other parameters analyzed did not exhibit any association
with TERT mutation.
 | Table III.Clinicopathological features of the
145 TERT mutated and WT PTC. |
Table III.
Clinicopathological features of the
145 TERT mutated and WT PTC.
| Clinicopathological
characteristic |
TERT(MUT)/TERT(WT) | P-value |
|---|
| Sex |
| <0,05 |
|
Male | 6/33 |
|
|
Female | 3/103 |
|
| Cap | 3/73 | NS |
| TCI | 1/16 | NS |
| PSTI | 3/33 | NS |
| Lymph | 2/14 | NS |
Association of TERT promoter mutation
with BRAF and RAS mutation
We analyzed the possible correlation of TERT
promoter mutation with BRAF and RAS mutation. TERT mutations were
identified in 5/53 BRAF mutated PTCs (9.4%, 4 CVPTCs and 1 TCPTC):
In these co-mutated cases, all the 5 BRAF mutations were V600E,
while regarding TERT mutations, 4 were C228T and 1 was C250T. The
other 4 TERT mutated cases were identified among the remaining 92
BRAF-WT tumors (4.4%) and all of them exhibited a C228T mutation
(Table IV). All five PTCs with BRAF
and TERT mutation revealed features of a more aggressive behavior:
None of them were capsulated, 4/5 were infiltrating the thyroidal
capsule and 3/5 the extra-thyroidal soft tissues, while 2/5 had
lymph node metastasis (Table V). Only
one case presented with RAS and TERT mutations; it was an
encapsulated, well-differentiated FVPTC with no lymph node
metastasis or vascular invasion (Table
VI).
 | Table IV.Correlation between TERT and BRAF
mutations. |
Table IV.
Correlation between TERT and BRAF
mutations.
|
| TERT Mutation |
|
|---|
|
|
|
|
|---|
| BRAF status | Present (9)
(%) | Absent (136)
(%) | P-value |
|---|
| BRAF |
|
| NS |
| Mutated
(53) | 5 (55.6) | 48 (35.3) |
|
| WT
(92) | 4 (44.4) | 88 (64.7) |
|
 | Table V.Clinicopathological features of the
five BRAF and TERT mutated PTCs. |
Table V.
Clinicopathological features of the
five BRAF and TERT mutated PTCs.
| BRAF/TERT
ratio | Cap | TCI | PSTI | Lymph |
|---|
|
BRAF(MUT)/TERT(MUT) | 0/5 | 4/5 | 3/5 | 2/5 |
 | Table VI.Correlation between TERT and RAS
mutations. |
Table VI.
Correlation between TERT and RAS
mutations.
|
| TERT Mutation |
|
|---|
|
|
|
|
|---|
| Phenotype | Present (9)
(%) | Absent (136)
(%) | P-value |
|---|
| RAS |
|
| NS |
| Mutated
(23) | 1 (11.2) | 19
(14) |
|
| WT
(122) | 8 (88.8) | 117 (86) |
|
TERT Immunohistochemical
expression
The expression of TERT protein was evaluated in
neoplastic and in normal peritumoral tissues. All the neoplastic
tissue samples analyzed exhibited a cytoplasmic pattern of
immunoreactivity; normal tissues had the same cytoplasmic
positivity, but 2 cases also had nuclear positivity. In tumors, the
mean cytoplasmic intensity of staining was 1.7; the mean percentage
of immunoreactive cells was 1.7 as well, in accordance to similar
data reported in literature (13). No
significant differences in TERT protein expression were observed
between mutated and WT tumors (Table
VII). According to the Z-score calculated, 69 cases out of 145
were considered positive; 36 cases out of 69 had a total
score (intensity plus extension) of 5 or 6: 19 were FVPTCs, 11
CVPTCs, 5 TCPTCs and 1 was a macrofollicular carcinoma. The TERT
protein expression evaluated in these cases did not appear to
correlate significantly with any clinical pathological aspect.
Among these 36 positive cases, 16 were BRAF mutated (9 CVPTCs, 4
TCPTCs and 3 FVPTCs) while 4 were RAS mutated. Only one case
resulted to be TERT mutated; this case, which presented with BRAF
and TERT mutation, was a CVPTC, limited to the thyroid and with
neither the presence of embolization nor lymph node metastasis.
 | Table VII.Correlation between TERT IHC
expression, BRAF and RAS mutations. |
Table VII.
Correlation between TERT IHC
expression, BRAF and RAS mutations.
|
| TERT IHC
Expression |
|
|---|
|
|
|
|
|---|
| Phenotype | Positive (>0;
69) | Negative (<0;
76) | P-value |
|---|
| BRAF |
|
| NS |
| Mutated
(53) | 28 (40.6) | 25 (32.9) |
|
| WT
(92) | 41 (59.4) | 51 (67.1) |
|
| RAS |
|
| NS |
| Mutated
(20) | 11 (15.9) | 9 (11.8) |
|
| WT
(125) | 58 (84.1) | 67 (88.2) |
|
Discussion
Thyroid tumor is the most common endocrine malignant
cancer, with an increasing incidence all around the world. WDPTCs
are usually indolent lesions with low metastatic potential and a
5-year survival rate of approximately 98% (17), but a small significant percentage
behave aggressively, developing metastases and possibly leading the
patient to death (18). Numerous
studies have been carried on to identify prognostic markers able to
discriminate aggressive PTCs from those with a more indolent
outcome, to identify patients at higher risk and to better define
the therapeutic approaches, avoiding overtreatments (5,19). BRAF
mutation is already known to have an important role in PTC
tumorigenesis and prognosis, in particular with his variant V600E
that occurs in almost the 45% of patients. The presence of this
latter mutation appears to correlate with a higher risk of
recurrence and probably with a more aggressive behavior of the
tumor, while a association between BRAF mutation status and
PTC-related mortality is still under debate (5,17).
According to the literature, in our study we observed the presence
of a BRAF mutation in the 36.6% of cases and, among all the
BRAF-mutated tumors, the 94.3% had a V600E mutation. At the
histological examination, the majority of these V600E-mutated cases
(83%) were CVPTCs or TCPTCs and all these tumors exhibited a
tendency to behave in a more aggressive way. In particular, several
tumors with evidence of extrathyroidal extension (27/36, 75%) were
V600E mutated as well as numerous cases with lymph nodes metastasis
(11/15, 73.4%) or embolization (6 in 7, 85.7%). Among all the BRAF
mutated cases, four presented with a rare BRAF mutation: 2 cases
with a K601E mutation, 1 with the ‘V600_K601delinsE’ deletion and 1
with a T599I mutation. As reported in a recent study, rare BRAF
mutations appear to be more frequent in thyroid tumors with a more
indolent behavior, such as FVPTCs and the prevalence of aggressive
clinicopathological features is lower in these tumors than in V600E
mutated ones. According to these data, all our PTCs with a rare
BRAF mutation were encapsulated FVPTC and none of them exhibited
signs of clinical aggressiveness like extrathyroidal extension,
vascular invasion or lymph node metastasis (20). The role of RAS oncogene in thyroid
tumorigenesis has been established, as well: RAS mutations occur up
to 45% of follicular thyroid cancer cases as well as in 30–40% of
follicular variant papillary thyroid carcinoma; in addition, they
have also been described in anaplastic thyroid cancer cases and,
occasionally, in benign adenomas (6,11). Several
studies have demonstrated that RAS mutated WDPTCs, without other
coexisting genetic alterations, generally lack aggressive behavior
(6,11). In our study, all the 23 RAS-mutated
neoplasms were a follicular variant of papillary thyroid carcinoma:
22 out of 23 were encapsulated forms of cancer and only two cases
exhibited a clear invasion of the neoplastic capsule; embolization
or lymph node metastasis were absent in all the 23 cases analyzed.
These data appear to confirm that RAS mutated cancer tends to
behave in a less aggressive manner. One of the 23 RAS mutated cases
also presented with a TERT (C228T) mutation but, differently from
what observed in BRAF-TERT co-mutated cases, the RAS-TERT
co-mutated one did not exhibit any sign of aggressiveness. Beyond
BRAF and RAS, numerous researchers have started to focus on certain
new genes mutations, such as the TERT promoter one. This mutation
has been described in numerous different kinds of tumors, such as
melanoma, bladder cancer, hepatocellular carcinoma, squamous cell
carcinoma, liposarcoma, a subset of central nervous tumors and,
recently, thyroid tumors (10,14). The
TERT promoter mutations have been reported in PTCs and also in
FTCs, while they do not appear to have a role in the tumorigenesis
of MTCs (13,15). In particular, the average TERT
mutation percentage for WDPTCs reported in the literature amount to
13,7%, ranging from 8–25%; the average percentage for PDTCs and
ATCs are respectively of 34,8% and 38,4% (13). The C228T mutation is the most common
one, compared with the C250T (12,13,15). In
our study, we analyzed a large series of 145 consecutive PTCs
considering the BRAF, RAS and TERT mutation and evaluating a
possible correlation of these alterations with certain clinical
features. All the BRAF mutations detected were V600E, except for
four FVPTCs (2 K601E, 1 deletion and 1 T599I). In addition, 23
cases out of 145 were RAS mutated, 18 N-RAS and 5 H-RAS and all of
them were FVPTCs. According to the results obtained, our cohort
appeared to be representative of the general population, with data
in line with the literature. Nonetheless, we observed only 9 cases
out of 145 with TERT mutation, corresponding to the 6.2%. In these
last few years, certain authors have pointed out an association
between the TERT mutation and clinical aspects such as the older
age at diagnosis, male sex and tumor size, especially in WDPTC. In
this cohort of PTCs, TERT promoter mutations were not identified to
correlate significantly with any clinical feature, except for the
sex, confirming a prevalence in males. certain further studies
underlined how TERT mutations appear to be more frequent in
BRAF-mutated (particularly V600E) than in BRAF-WT WDPTC (13). However, it is not clear whether this
association has a clinical impact: Liu et al (15) for instance, demonstrated that TERT
mutations in thyroid cancer are particularly prevalent in
BRAF-V600E mutated PTCs, having a role in the de-differentiation,
progression and aggressiveness of the tumor (15). Instead, additional studies revealed no
differences in the outcome among cancer cases with mutations
(TERT/BRAF) and those with only TERT mutation (13). According to these studies, in our
group the coexistence of BRAF and TERT mutations was not
significantly associated with any clinical parameter, probably due
to the small number of cases analyzed. However, we have noticed how
all the 5 PTCs with BRAF and TERT mutations had a tendency to
behave in a more aggressive way. Considering our results and all
the data reported in literature, it is reasonable to think that
TERT mutations are likely to have a role in de-differentiation of
WDPTCs but their contribution in the initial tumorigenesis could be
uncertain. Furthermore, as suggested by certain authors, the
possible implication of TERT mutation in the aggressiveness of
WDPTCs could concern only certain particular histotypes (15). The hypothesis that TERT mutation could
correlate more with the aggressiveness than the tumorigenesis of
the thyroid tumor appears also to be supported by the preferential
occurrence reported of TERT promoter mutations in BRAF mutated PTCs
and, over all, in PDTCs and ATCs (15,18,21,22);
in particular, it has been supposed how TERT promoter mutation may
join the mechanism involving the MAPK signaling, supporting the
action of the BRAF mutation (11). In
our study, among the few TERT mutated cases reported, the 55.6%
were associated with a BRAF mutation and, even if the co-mutated
PTCs had different clinical features, all of them appeared to have
a tendency to behave in a more aggressive way presenting the
infiltration of the extra-thyroidal soft tissues or lymph nodes
metastasis. However, immunohistochemical results concerning the
TERT protein expression did not appear to correlate significantly
neither with BRAF nor RAS mutation.
The purpose of this study was to establish a
possible clinical impact of the TERT promoter mutation in thyroid
cancer cases. Although a cohort of cases representative of the
general population, as demonstrated by the data regarding BRAF and
RAS mutation, we identified a rate of TERT mutation far less than
the average reported in literature. In addition, TERT mutations
lonely and the coexistence of BRAF or RAS and TERT mutations were
not significantly associated with any clinical parameter, probably
due to the small number of mutated cases observed. The evaluation
of the TERT protein expression did not reveal any particular
correlation with clinical pathological aspects, as well. However,
we observed how cancer cases with TERT and BRAF mutation appeared
to behave in a more aggressive way, suggesting a possible role of
TERT mutation in the aggressiveness of the neoplasia. On the
contrary, in our opinion, its role in the initial tumorigenesis
should be more investigated.
References
|
1
|
Erickson Lori A: Papillary thyroid
carcinoma. Atlas of Endocrine Pathology. 1–50. 2014. View Article : Google Scholar
|
|
2
|
Rahbari R, Zhang L and Kebebew E: Thyroid
cancer gender disparity. Future Oncol. 6:1771–1779. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sipos JA and Mazzaferri EL: Thyroid cancer
epidemiology and prognostic variables. Clin Oncol (R Coll Radiol).
22:395–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caron NR and Clark OH: Papillary thyroid
cancer. Curr Treat Options Oncol. 7:309–319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xing M, Westra WH, Tufano RP, Cohen Y,
Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, et
al: BRAF mutation predicts a poorer clinical prognosis for
papillary thyroid cancer. J Clin Endocrinol Metab. 90:6373–6379.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nikiforov YE: Molecular diagnostics of
thyroid tumors. Arch Pathol Lab Med. 135:569–577. 2011.PubMed/NCBI
|
|
7
|
Pelizzo MR, Dobrinja C, Casal Ide E, Zane
M, Lora O, Toniato A, Mian C, Barollo S, Izuzquiza M, Guerrini J,
et al: The role of BRAF(V600E) mutation as poor prognostic factor
for the outcome of patients with intrathyroid papillary thyroid
carcinoma. Biomed Pharmacother. 68:413–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Afkhami M, Karunamurthy A, Chiosea S,
Nikiforova MN, Seethala R, Nikiforov YE and Coyne C:
Histopathologic and clinical characterization of thyroid tumors
carrying the BRAF K601E mutation. Thyroid. 26:242–247. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jara SM, Bhatnagar R, Guan H, Gocke CD,
Ali SZ and Tufano RP: Utility of BRAF mutation detection in
fine-needle aspiration biopsy samples read as ‘suspicious for
papillary thyroid carcinoma’. Head Neck. 37:1788–1793. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Macerola E, Loggini B, Giannini R,
Garavello G, Giordano M, Proietti A, Niccoli C, Basolo F and
Fontanini G: Coexistence of TERT promoter and BRAF mutations in
cutaneous melanoma is associated with more clinicopathological
features of aggressiveness. Virchows Arch. 467:177–184. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xing M: Clinical utility of RAS mutations
in thyroid cancer: A blurred picture now emerging clearer. BMC Med.
14:122016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vinagre J, Almeida A, Pópulo H, Batista R,
Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, et al:
Frequency of TERT promoter mutations in human cancers. Nat Commun.
4:21852013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muzza M, Colombo C, Rossi S, Tosi D,
Cirello V, Perrino M, De Leo S, Magnani E, Pignatti E, Vigo B, et
al: Telomerase in differentiated thyroid cancer: Promoter
mutations, expression and localization. Mol Cell Endocrinol.
399:288–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park CK, Lee SH, Kim JY, Kim JE, Kim TM,
Lee ST, Choi SH, Park SH and Kim IH: Expression level of hTERT is
regulated by somatic mutation and common single nucleotide
polymorphism at promoter region in glioblastoma. Oncotarget.
5:3399–3407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Bishop J, Shan Y, Pai S, Liu D,
Murugan AK, Sun H, El-Naggar AK and Xing M: Highly prevalent TERT
promoter mutations in aggressive thyroid cancers. Endocr Relat
Cancer. 20:603–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horn S, Figl A, Rachakonda PS, Fischer C,
Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al:
TERT promoter mutations in familial and sporadic melanoma. Science.
339:959–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yarchoan M, LiVolsi VA and Brose MS: BRAF
mutation and thyroid cancer recurrence. J Clin Oncol. 33:7–8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gandolfi G, Ragazzi M, Frasoldati A, Piana
S, Ciarocchi A and Sancisi V: TERT promoter mutations are
associated with distant metastases in papillary thyroid carcinoma.
Eur J Endocrinol. 172:403–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Esserman LJ, Thompson IM Jr and Reid B:
Overdiagnosis and overtreatment in cancer: An opportunity for
improvement. JAMA. 310:797–798. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Torregrossa L, Viola D, Sensi E, Giordano
M, Piaggi P, Romei C, Materazzi G, Miccoli P, Elisei R and Basolo
F: Papillary thyroid carcinoma with rare exon 15 BRAF mutation has
indolent behavior: A single-institution experience. J Clin
Endocrinol Metab. 101:4413–4420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi X, Liu R, Qu S, Zhu G, Bishop J, Liu
X, Sun H, Shan Z, Wang E, Luo Y, et al: Association of TERT
promoter mutation 1,295,228 C>T with BRAF V600E mutation, older
patient age and distant metastasis in anaplastic thyroid cancer. J
Clin Endocrinol Metab. 100:E632–E637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xing M, Liu R, Liu X, Murugan AK, Zhu G,
Zeiger MA, Pai S and Bishop J: BRAF V600E and TERT promoter
mutations cooperatively identify the most aggressive papillary
thyroid cancer with highest recurrence. J Clin Oncol. 32:2718–2726.
2014. View Article : Google Scholar : PubMed/NCBI
|