Introduction
Thymic epithelial tumors (TETs), well known for
their variability in morphological appearance and for the
heterogeneity of their neoplastic epithelial cells, are epithelial
neoplasms originating from the thymus (1,2). TETs
account for 50% of the anterior mediastinal masses occurring in the
adult population and represent the most common tumors of the
anterior portion of the mediastinum (3). However, the overall incidence of TETs is
rare, at 3.2 cases/1,000,000 individuals per year (4). The majority of patients are between the
ages of 40 and 60 years at the time of diagnosis, with no
significant difference between males and females with respect to
the incidence of this disease (5).
Furthermore, previous studies have demonstrated that TETs are
associated with a variety of autoimmune disorders, including
myasthenia gravis, pure red cell aplasia and hypogammaglobulinemia
(3,5).
According to the widely-accepted World Health
Organization (WHO) classification system, which is based upon the
morphological features and atypia of epithelial cells as well as
the lymphocyte-to-epithelial cell ratio, TETs are subdivided into
thymomas (types A, AB, B1, B2 and B3) and thymic carcinomas (TCs)
(6). Thymomas are generally
considered to have an indolent growth pattern with malignant
transformation potential. Certain thymomas demonstrate local
invasion, pleural dissemination and systemic metastasis during
advanced stages, despite being indolent and non-invasive during
their early stages (1). Furthermore,
previous studies have documented the recurrence and metastasis of
thymomas following resection (7–9),
suggesting that even unaggressive, non-invasive thymomas possess
the fundamental features of a malignant tumor. Therefore, thymomas
require consideration as a potentially malignant disease requiring
prolonged follow-up, despite the fact that the majority are low
grade and indolent (10). TCs, as the
most aggressive subtype of TETs, usually exhibit a more invasive
phenotype with a worse survival rate, as well as a greater
potential for relapse and metastasis compared with the majority of
thymomas (11). Surgical resection
remains the optimal treatment for early-stage TETs, while
advanced-stage, unresectable or recurrent thymic malignancies are
usually treated with palliative chemotherapy (12).
However, due to the rarity and morphological
complexity of TETs, the biological mechanisms that facilitate TET
tumorigenesis and development remain unclear at present.
Additionally, biomarkers that are associated with clinical behavior
and prognosis are urgently required.
Tumor invasion and metastasis are complex processes
involving interactions between cancer cells and the extracellular
matrix (ECM), including alterations in cell adhesion and motility
that permit tumor cells to invade and migrate through the ECM
(13). A number of these alterations
develop at the contact points between cells and the ECM, which are
known as focal adhesions. Focal adhesion kinase (FAK), originally
identified in v-Src-transformed chicken embryo fibroblasts
(14), is a highly conserved 125-kDa
cytoplasmic, non-receptor protein tyrosine kinase that is activated
and localized at the sites of cellular focal adhesions (15). As a critical mediator of signaling
events between cells and the ECM, FAK serves a pivotal function in
growth factor receptor- and integrin-mediated signal transduction
pathways (16). The activation of FAK
by integrin clustering, cell adhesion or growth factor receptors
induces the rapid phosphorylation of FAK at Tyr-397, which has been
identified as the major autophosphorylation site. Once
phosphorylated, Tyr-397 generates a high-affinity binding site for
SH2-domain-containing proteins, including Src-family kinases,
phospholipase Cg and growth factor receptor-bound protein 7
(17). The subsequent binding of FAK
to Src likely contributes to Src kinase activation, which in turn
promotes the phosphorylation of FAK at additional tyrosine residues
to stimulate maximal FAK kinase activity (18).
FAK has been implicated in the regulation of a
diverse set of cellular functions, including survival, migration,
proliferation, angiogenesis and apoptosis, in a variety of cell
types (16), suggesting that FAK may
contribute to tumor formation and malignant progression (19). Previously, FAK has also been
demonstrated to serve an important role in the regulation of cancer
stem cells, the epithelial-to-mesenchymal transition and the tumor
microenvironment (16,20). In addition, accumulating evidence has
demonstrated that FAK is overexpressed in a wide range of human
tumors, including colon, breast, oral, liver, head and neck tumors,
gastric carcinomas and neuroblastomas (21–28).
Furthermore, the overexpression of FAK has been reported to serve
as an independent prognostic factor for various types of
malignancies, including ovarian, esophageal and hepatocellular
cancer, as well as acute myeloid leukemia (29–32).
However, despite the gradually-increasing research
into various malignancies, there is insufficient data available
regarding FAK expression in TETs. Therefore, the present study
aimed to examine FAK expression in TETs and to determine whether
FAK expression is associated with the clinical behavior and
prognosis of TETs.
Materials and methods
Patients and specimens
TET tissue specimens were obtained from 100 patients
who had undergone tumor resection without preoperative chemotherapy
or radiotherapy at the Department of Thoracic Surgery, Affiliated
Hospital of Qingdao University (Qingdao, China) between January
2002 and December 2006. Of the 100 patients who were included in
the study, 55 were male and 45 were female (male-to-female ratio of
1.22:1) and the mean age of the patients was 52.1±13.7 years
(range, 19–80 years). Additionally, 28 patients (28.0%) suffered
from myasthenia gravis. Tumor diameters ranged between 1.5 and 11.0
cm, with a mean diameter of 5.8±2.2 cm. TETs were classified into
84 thymomas (9 type A, 24 type AB, 15 type B1, 18 type B2 and 18
type B3) and 16 TCs, according to the 2015 WHO criteria (6). Furthermore, clinical stages were based
upon the newly revised Masaoka-Koga staging system (10), with 37 stage I, 27 stage II, 23 stage
III and 13 stage IV cases in the study cohort. In addition, 58
normal thymus tissues were obtained from different patients with
mediastinal cysts who had undergone mediastinal cystectomy at the
same institution between January 2002 and December 2006. The normal
thymus tissues were used as the control group. The healthy thymus
cohort consisted of 28 men and 30 women, aged 18–72 years (mean,
48.6±13.2 years). The medical records and histopathological
archives of all patients were complete. Diagnoses were confirmed
histologically in all specimens, based upon the examination of
sections stained with hematoxylin and eosin at the Department of
Pathology of the Affiliated Hospital of Qingdao University.
Following surgical resection, all specimens were fixed in 10%
buffered formalin overnight at room temperature prior to being
embedded in paraffin. Postoperative follow-up data were obtained
from all patients, with a mean follow-up period of 101.3±31.1
months (range, 3–120 months). One patient with type AB thymoma did
not complete the follow-up. The present study was performed in
accordance with the Declaration of Helsinki, and was approved by
the Medical Ethics Committees of the Affiliated Hospital of Qingdao
University. Written informed consent was obtained from all
participants included in the study.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections
were cut into 4-µm thick sections. Following routine
deparaffinization and rehydration in descending ethanol series
(100, 95, 85 and 70%) for 5 min, respectively, the sections were
autoclaved in citrate buffer (pH 6.0; 0.1 M; 1 l; Zhongshan
Goldenbridge Biotechnology Co., Ltd., Beijing, China) for 5 min at
120°C for antigen retrieval. Sections were then washed in PBS three
times. Endogenous peroxidase activity was blocked in methanol
containing 0.3% H2O2 for 30 min at room
temperature. The sections were subsequently washed in PBS (3 times,
for 3 min each time), prior to being incubated with a mouse
anti-FAK monoclonal primary antibody 4.47 (dilution 1:200; cat. no.
05–537; EMD Millipore, Billerica, MA, USA) overnight at 4°C.
Following 3 cycles of washing with PBS (for 3 min each time), the
sections were incubated with the Polink-1 horseradish peroxidase
mouse for DAB Bulk kit (cat. no. D12-110; Golden Bridge
International, Inc., Bothell, WA, USA), at room temperature for 15
min. All procedures were performed according to the manufacturer's
instructions. Sections were washed again with PBS (3 times for 3
min each time), prior to being treated at room temperature with a
diaminobenzidine working solution for 10 min and then
counterstained with hematoxylin at room temperature for 5 min. The
negative controls underwent the same procedures; however, the
primary antibody was omitted or replaced with normal serum.
Evaluation of
immunohistochemistry
The presence of staining was evaluated by two
board-certified pathologists who were blinded to the clinical data
of the patients. FAK expression was determined using a scoring
system that measured staining intensity (0, negative; 1, weak; 2,
moderate; and 3, intense) and the proportion of positively-stained
cells among the tumor epithelial cells (0, none; 1, 1–25%; 2,
26–50%; 3, 51–75%; and 4, 76–100%) in ≥5 areas under a light
microscope (Olympus BX41; Olympus Corporation, Tokyo, Japan) at
×400 magnification. The overall staining score was obtained by
multiplying the staining intensity score by the score representing
the proportion of positive-stained cells. Specimens were separated
into high expression (overall score ≥6) and low expression (overall
score <6) groups to more accurately determine FAK
expression.
Statistical analysis
The χ2 test and the Kruskal-Wallis test
were used to assess the association between FAK expression and
different clinicopathological parameters. Overall survival rates
were calculated using the Kaplan-Meier method, and the differences
between the survival curves were evaluated using the log-rank test.
A Cox proportional hazards multivariate regression model was
developed to identify independent significant prognostic factors.
P<0.05 was considered to indicate a statistically significant
difference. SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was
used to perform all analyses.
Results
FAK is expressed in TETs but not in
the normal thymus
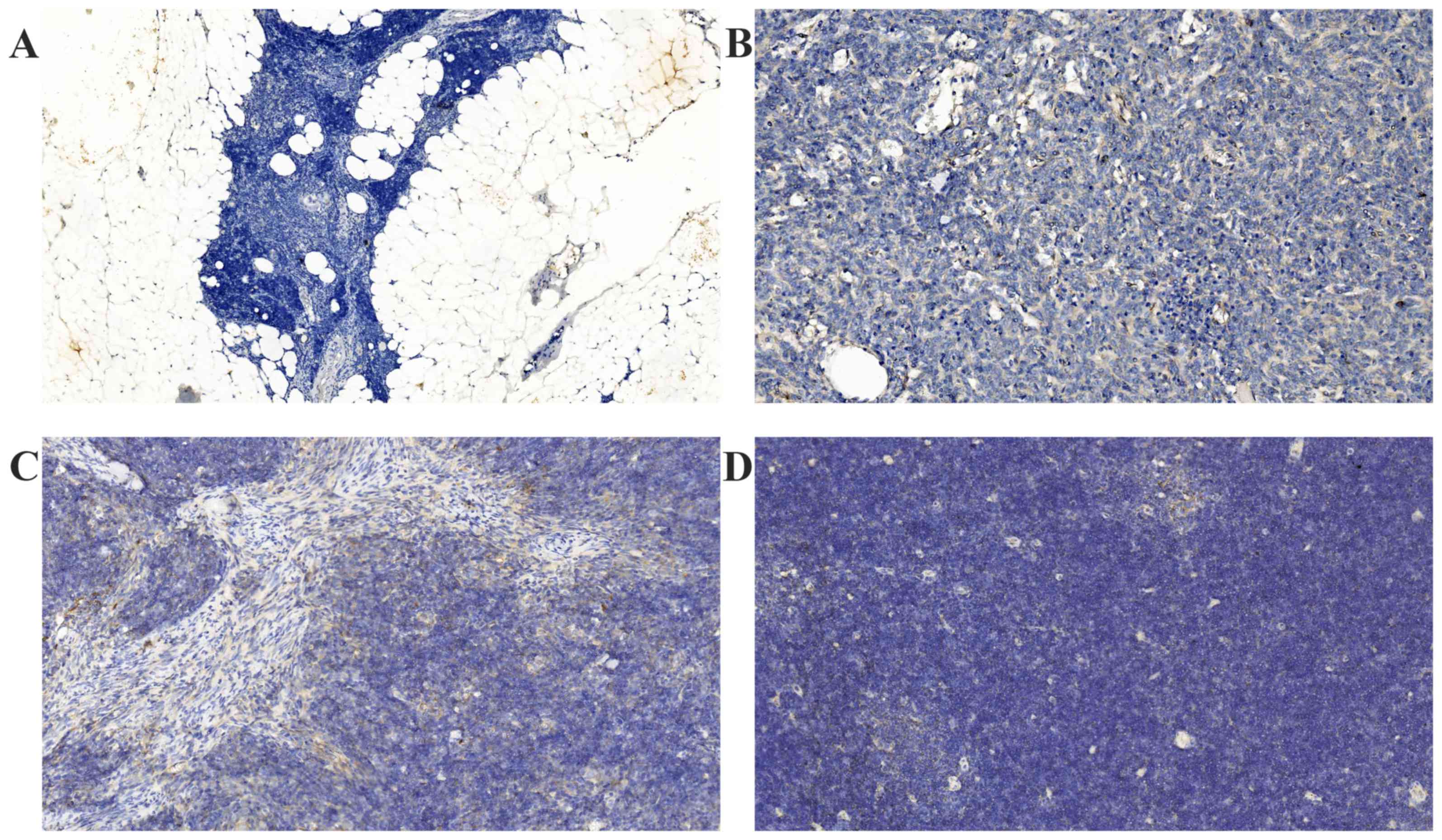
All normal thymus tissues were negative for FAK
expression (Fig. 1A). Positive
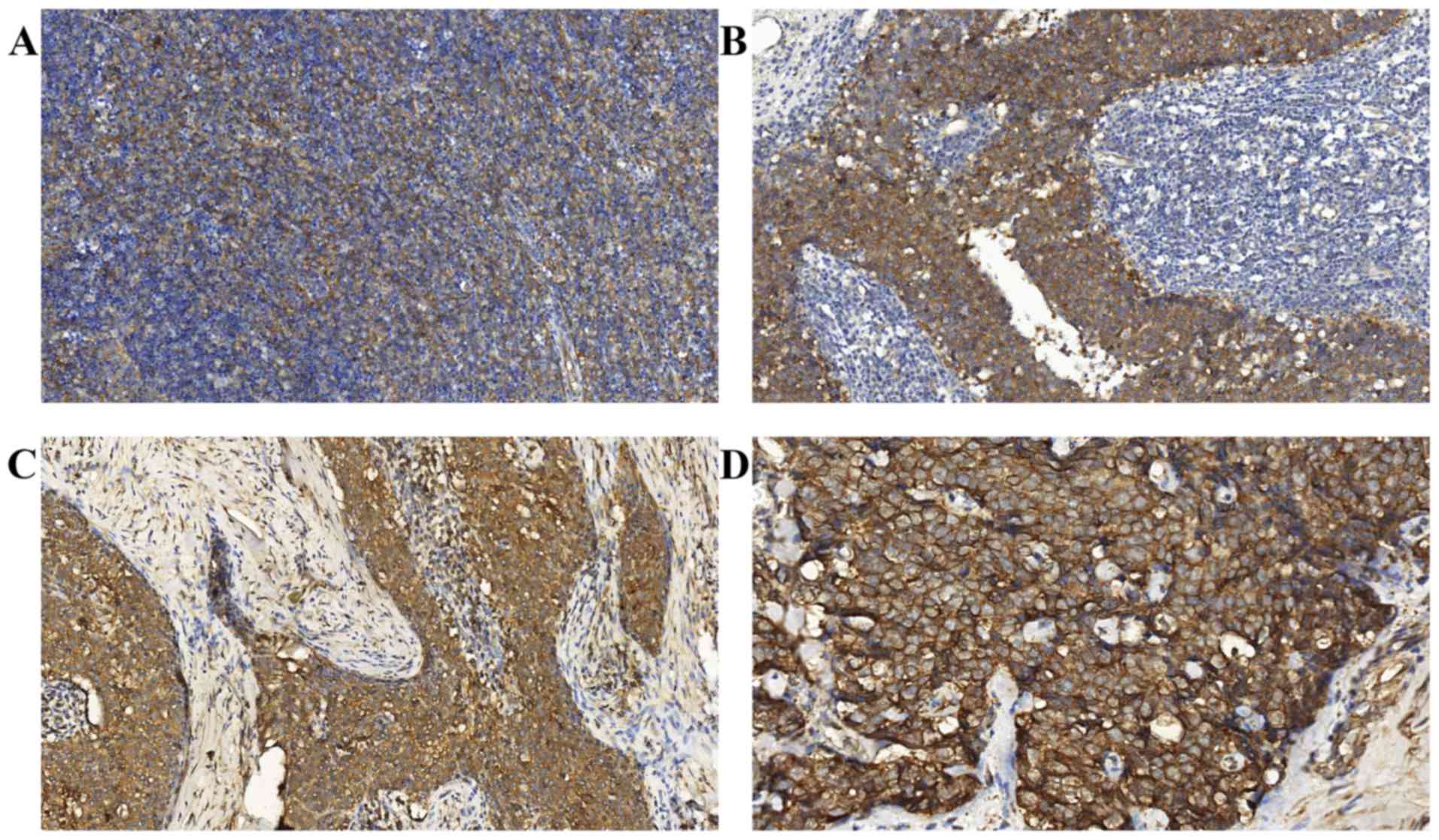
immunostaining of FAK was detected at varying levels in all the TET
specimens. FAK staining was localized to the cytoplasm and the
membrane of the epithelial cells of the thymic tumors (Figs. 1B-D and 2A-D). Notably, the tumor lymphocytes did not
express FAK. Significantly higher FAK expression was observed in
52.0% of TETs (52/100) compared with in the normal thymus, as
determined using the χ2 test (P<0.001). Furthermore,
FAK was weakly expressed in the majority of the less aggressive TET
subtypes (types A, AB and B1 thymomas), but was upregulated in the
majority of highly aggressive tumor subtypes (types B2 and B3
thymomas, and TCs).
Associations between FAK
overexpression and clinicopathological parameters in TETs
The associations between the clinicopathological
characteristics of patients with TETs and FAK overexpression are
summarized in Table I. A
statistically significant association was observed between FAK
overexpression and the Masaoka-Koga stage and WHO classification
(both P<0.001). Notably, 33/36 (91.7%) high-stage (stages III
and IV) tumors overexpressed FAK, compared with only 19/64 (29.7%)
low-stage (stages I and II) tumors (P<0.001). In the less
aggressive TET subtypes, the majority of tumors exhibited low FAK
expression, with only 10/48 tumors (20.8%) exhibiting high FAK
expression. By contrast, in the highly aggressive subtypes of TETs,
42/52 tumors (80.8%) exhibited high FAK expression. This difference
between the two groups was statistically significant (P<0.001).
However, FAK expression was not associated with age, sex, tumor
size or myasthenia gravis (Table
I).
 | Table I.Association between FAK expression and
clinicopathological parameters in 100 patients with thymic
epithelial tumors. |
Table I.
Association between FAK expression and
clinicopathological parameters in 100 patients with thymic
epithelial tumors.
|
|
| FAK expression |
|
|---|
|
|
|
|
|
|---|
| Parameter | Total, n | High (n=52) | Low (n=48) | P-value |
|---|
| Age, years |
|
|
| 0.986 |
| ≤60 | 73 | 38 | 35 |
|
|
>60 | 27 | 14 | 13 |
|
| Sex |
|
|
| 0.520 |
| Male | 55 | 27 | 28 |
|
|
Female | 45 | 25 | 20 |
|
| Tumor size |
|
|
| 0.097 |
| ≤6
cm | 56 | 25 | 31 |
|
| >6
cm | 44 | 27 | 17 |
|
| MG |
|
|
| 0.844 |
|
Present | 28 | 15 | 13 |
|
|
Absent | 72 | 37 | 35 |
|
| Classification |
|
|
| <0.001 |
| A, AB,
B1 | 48 | 10 | 38 |
|
| B2, B3,
TC | 52 | 42 | 10 |
|
| Stage |
|
|
|
<0.001a |
| I | 37 | 7 | 30 |
|
| II | 27 | 12 | 15 |
|
|
III | 23 | 20 | 3 |
|
| IV | 13 | 13 | 0 |
|
Univariate and multivariate analyses
of prognostic factors for patients with TETs
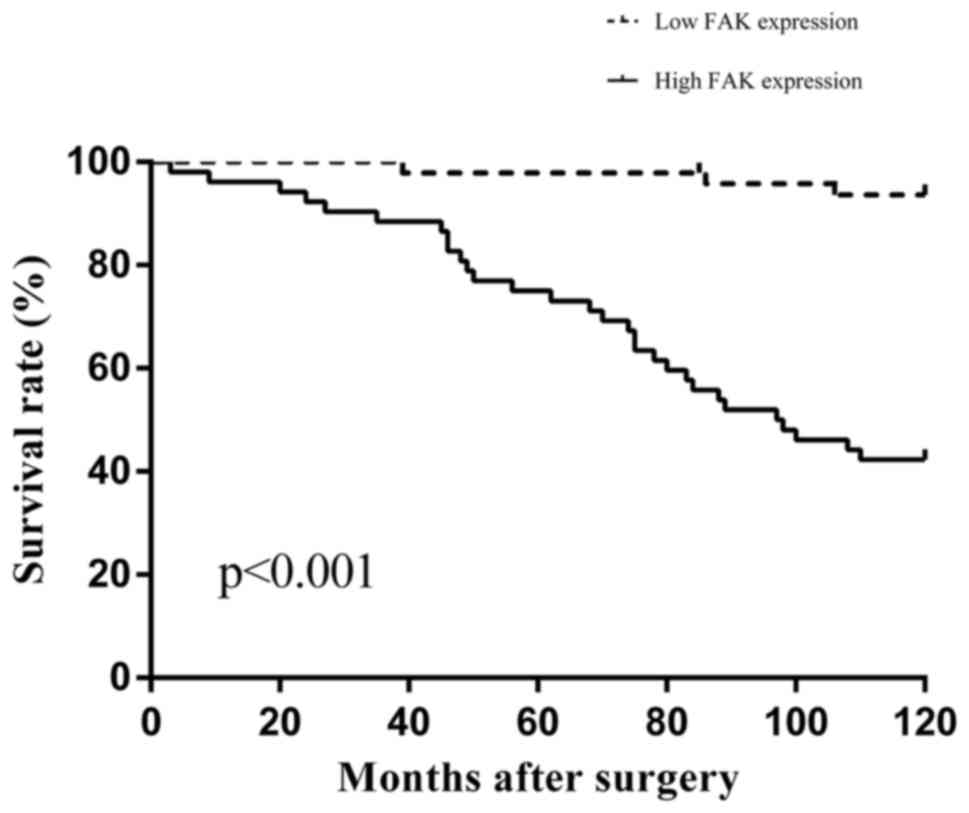
The 10-year overall survival rates of patients were
analyzed in order to assess the prognostic significance of FAK
expression, as well as that of other clinicopathological parameters
(Table II). The 10-year survival
rate of the high FAK expression group was significantly lower than
that for the low FAK expression group in patients with TETs, as
determined using the log rank test (42.3 vs. 93.7%; P<0.001;
Fig. 3). Multivariate analysis, using
the Cox proportional hazards model, of the WHO classification,
Masaoka-Koga stage, tumor size and FAK expression revealed that
only high FAK expression and the Masaoka-Koga stage were
significant independent prognostic factors of TETs (P=0.034 and
P=0.005, respectively; Table
III).
 | Table II.Univariate analyses of prognostic
factors in patients with thymic epithelial tumors. |
Table II.
Univariate analyses of prognostic
factors in patients with thymic epithelial tumors.
| Parameter | Total, n | 10-year OS, % | P-value |
|---|
| Age, years |
|
| 0.100 |
|
≤60 | 73 | 71.2 |
|
|
>60 | 27 | 54.9 |
|
| Sex |
|
| 0.645 |
|
Male | 55 | 65.5 |
|
|
Female | 45 | 68.6 |
|
| Tumor size |
|
| 0.023 |
| ≤6
cm | 56 | 76.7 |
|
| >6
cm | 44 | 54.5 |
|
| FAK expression |
|
| <0.001 |
|
Low | 48 | 93.7 |
|
|
High | 52 | 42.3 |
|
| MG |
|
| 0.278 |
|
Absent | 72 | 63.7 |
|
|
Present | 28 | 75.0 |
|
| Classification |
|
| <0.001 |
| A, AB,
B1 | 48 | 91.5 |
|
| B2, B3,
TC | 52 | 44.2 |
|
| Stage |
|
| <0.001 |
| I and
II | 64 | 89.0 |
|
| III and
IV | 36 | 27.8 |
|
 | Table III.Multivariate analyses of prognostic
factors in patients with thymic epithelial tumors. |
Table III.
Multivariate analyses of prognostic
factors in patients with thymic epithelial tumors.
| Parameter | P-value | HR | 95% CI |
|---|
| FAK expression
(low/high) | 0.034 | 4.080 | 1.110–15.005 |
| Stage
(I+II/III+IV) | 0.005 | 3.824 | 1.506–9.713 |
| Classification
(A+AB+B1/B2+B3+TC) | 0.110 | 2.548 | 0.808–8.032 |
| Tumor size
(≤6/>6 cm) | 0.161 | 1.653 | 0.818–3.338 |
Discussion
FAK, an important mediator between cells and the
extracellular matrix, is considered to serve an important function
in a number of biological processes, including cell adhesion,
spreading, motility, proliferation, survival, apoptosis and
migration (33). Given that the
dysregulation of these processes is often associated with the
development and progression of tumors, it is understandable that
FAK is highly involved in human malignancies. Increased expression
and elevated activity of FAK have been demonstrated by previous
studies to be associated with clinicopathological features in a
wide range of human cancer types (21–23,27,30,32,34),
thereby suggesting a role for FAK in carcinogenesis. However, to
the best of our knowledge, there has been no previous study on FAK
expression in TETs, which are rare and histologically complex
tumors. Therefore, the present study analyzed FAK expression and
assessed its clinical value as a prognostic marker in TETs.
The results of the present study provided definitive
evidence that FAK is not expressed in the normal thymus but is
significantly upregulated in TETs, as determined by
immunohistochemical analysis, indicating that FAK overexpression is
associated with the tumorigenesis of TETs. The present study also
evaluated the association between FAK expression and
clinicopathological parameters in TETs. Notably, the results of the
present study demonstrated that FAK overexpression was not only
strongly associated with advanced stages of TETs, but was also
associated with highly aggressive subtypes. Therefore, it was
hypothesized that FAK may serve important functions in the invasion
and metastasis of TETs. However, no statistical association was
observed between FAK expression and tumor size, sex, age or
myasthenia gravis in the present study.
The observation that elevated levels of FAK are
detected in TETs is consistent with reports from previous studies
of FAK overexpression in other types of solid tumor. For example,
Cance et al (21) analyzed the
expression of FAK in normal, pre-invasive and invasive human breast
and colon tissues from individual patients using
immunohistochemistry. In this study, FAK was weakly expressed in
the majority of benign breast and colon epithelia but was
overexpressed in the majority of invasive breast carcinomas and
colon cancer tissues, suggesting that FAK overexpression may occur
in the early stages of tumorigenesis (21). Additionally, Lark et al
(23) observed elevated levels of FAK
expression in primary colorectal carcinomas and liver metastases
compared with matched healthy colorectal tissues, using
quantitative polymerase chain reaction (qPCR) and
immunohistochemical analyses. The results of the aforementioned
study suggested that the increased expression of FAK may suppress
apoptosis and promote the survival and metastasis of cancer cells
(23). Furthermore, Itoh et al
(22) used western blotting and
immunohistochemical analyses to demonstrate that FAK expression was
upregulated in hepatocellular carcinoma (HCC), and that it was
significantly associated with portal venous invasion, indicating
that FAK overexpression serves an important function in HCC
progression and invasion (22).
Another study used immunohistochemistry and reverse transcription
qPCR to demonstrate that FAK was overexpressed in non-small cell
lung cancer (NSCLC) and that its overexpression was associated with
nodal metastasis and advanced stages of NSCLC, suggesting that FAK
serves an important role in lung cancer progression and metastasis
(35).
Notably, the present study also used univariate and
multivariate analyses to demonstrate that the overexpression of FAK
in patients with TETs is significantly associated with a worse
overall survival rate. Therefore, these results indicated that FAK
overexpression may be an independent prognostic biomarker for TETs.
The results of the present study are consistent with those of
previous studies that have demonstrated FAK protein overexpression
to be an independent unfavorable prognostic factor in various types
of tumor (22,29–32). By
contrast, it has also been reported that FAK expression was not
associated with prognosis in certain types of tumor (25,26,34). Taken
together, the results of these studies suggest that FAK may serve
distinct roles in different types of malignancies and during
different stages of tumor progression.
In the present study, the marked upregulation of FAK
in TETs, particularly in the advanced stages and highly aggressive
tumor subtypes, combined with the negative expression of FAK in
normal thymus tissues, suggests that FAK may be a potential
therapeutic target for TETs. Recently, a FAK-targeting therapeutic
approach has focused primarily on blocking its kinase enzymatic
activity and targeting its scaffolding function using
pharmacological agents; a number of FAK-directed small molecule
inhibitors, including PF-00562271, VS-4718, GSK2256098, BI853520
and PF-04554878, are undergoing clinical trials in patients with
cancer (16). Furthermore, a
combination therapy approach using FAK inhibitors against FAK
signaling pathways (e.g., the phosphatidylinositol-3-kinase,
protein kinase B, epidermal growth factor receptor, human epidermal
growth factor receptor-2, Src, mitogen-activated protein kinase and
cellular-mesenchymal-to-epithelial transition factor signaling
pathways) was employed to sensitize cancer cells to chemotherapy
and to increase the efficacy of drugs (16,33).
Considering this, the therapeutic utility of FAK inhibitors in the
advanced stages of TETs remains promising. However, future clinical
trials are required in order to evaluate the clinical efficacy of
these approaches in the treatment of TETs.
In the present study, immunohistochemistry was
performed using a monoclonal FAK-specific 4.47 antibody, which was
revealed to be highly specific to human FAK in formalin-fixed,
paraffin-embedded tissue sections (21). This antibody is able to recognize the
FAK-specific epitope at the amino terminus of a molecule in order
to avoid consensus sequences within the kinase domain and
cross-reactivity with the FAK-related non-kinase, an autonomously
expressed carboxyl-terminal fragment. Furthermore, patients who had
received adjuvant preoperative chemotherapy or radiotherapy were
excluded from the present study in order to eliminate the potential
effects of these treatments on the immunohistochemistry results.
However, the sample sizes were relatively small in the present
study due to the low incidence of TETs. Therefore, further studies
comprising larger cohorts in multiple treatment centers are
required in order to confirm these results.
In conclusion, to the best of our knowledge, the
present study is the first to provide definitive evidence that FAK
expression is upregulated in TETs. FAK overexpression may serve an
important role in the tumorigenesis and progression of TETs.
Furthermore, the results of the present study indicated that FAK
overexpression may be used as a prognostic biomarker for TET.
Additionally, FAK may be a promising therapeutic target for TETs,
but the precise role of FAK in TETs remains to be confirmed by
further in vivo and in vitro studies.
References
|
1
|
Masaoka A, Monden Y, Nakahara K and
Tanioka T: Follow-up study of thymomas with special reference to
their clinical stages. Cancer. 48:2485–2492. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen G, Marx A, Chen WH, Yong J, Puppe B,
Stroebel P and Mueller-Hermelink HK: New WHO histologic
classification predicts prognosis of thymic epithelial tumors: A
clinicopathologic study of 200 thymoma cases from China. Cancer.
95:420–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Venuta F, Anile M, Diso D, Vitolo D,
Rendina EA, De Giacomo T, Francioni F and Coloni GF: Thymoma and
thymic carcinoma. Eur J Cardiothoracic Surg. 37:13–25. 2010.
View Article : Google Scholar
|
|
4
|
de Jong WK, Blaauwgeers JL, Schaapveld M,
Timens W, Klinkenberg TJ and Groen HJ: Thymic epithelial tumours: A
population-based study of the incidence, diagnostic procedures and
therapy. Eur J Cancer. 44:123–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wright CD: Management of thymomas. Crit
Rev Oncol Hematol. 65:109–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marx A, Chan JK, Coindre JM, Detterbeck F,
Girard N, Harris NL, Jaffe ES, Kurrer MO, Marom EM, Moreira AL, et
al: The 2015 World Health Organization Classification of tumors of
the thymus: Continuity and changes. J Thorac Oncol. 10:1383–1395.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gamboa EO, Sawhney V, Lanoy RS, Haller NA,
Powell AT and Hazra SV: Widespread metastases after resection of
noninvasive thymoma. J Clin Oncol. 26:1752–1755. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jain RK, Mehta RJ, Henley JD, Kesler KA,
Loehrer PJ and Badve S: WHO types A and AB thymomas: Not always
benign. Mod Pathol. 23:1641–1649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okumura M, Shiono H, Inoue M, Tanaka H,
Yoon HE, Nakagawa K, Matsumura A, Ohta M, Iuchi K and Matsuda H:
Outcome of surgical treatment for recurrent thymic epithelial
tumors with reference to world health organization histologic
classification system. J Surg Oncol. 95:40–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Detterbeck FC, Nicholson AG, Kondo K, Van
Schil PV and Moran C: The Masaoka-Koga stage classification for
thymic malignancies: Clarification and definition of Terms. J
Thorac Oncol. 6(7 Suppl 3): S1710–S1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ogawa K, Toita T, Uno T, Fuwa N,
Kakinohana Y, Kamata M, Koja K, Kinjo T, Adachi G and Murayama S:
Treatment and prognosis of thymic carcinoma: A retrospective
analysis of 40 cases. Cancer. 94:3115–3119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas A, Rajan A, Berman A, Tomita Y,
Brzezniak C, Lee MJ, Lee S, Ling A, Spittler AJ, Carter CA, et al:
Sunitinib in patients with chemotherapy-refractory thymoma and
thymic carcinoma: An open-label phase 2 trial. Lancet Oncol.
16:177–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liotta LA and Stetler-Stevenson WG: Tumor
invasion and metastasis: An imbalance of positive and negative
regulation. Cancer Res. 51 Suppl 18:5054S–5059S. 1991.PubMed/NCBI
|
|
14
|
Schaller MD, Borgman CA, Cobb BS, Vines
RR, Reynolds AB and Parsons JT: pp125FAK a structurally distinctive
protein-tyrosine kinase associated with focal adhesions. Proc Natl
Acad Sci USA. 89:pp. 5192–5196. 1992; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanks SK, Calalb MB, Harper MC and Patel
SK: Focal adhesion protein-tyrosine kinase phosphorylated in
response to cell attachment to fibronectin. Proc Natl Acad Sci USA.
89:pp. 8487–8491. 1992; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee BY, Timpson P, Horvath LG and Daly RJ:
FAK signaling in human cancer as a target for therapeutics.
Pharmacol Ther. 146:132–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: In command and control of cell motility. Nat
Rev Mol Cell Biol. 6:56–68. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dwyer SF, Gao L and Gelman IH:
Identification of novel focal adhesion kinase substrates: Role for
FAK in NFκB signaling. Int J Biol Sci. 11:404–410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer - a new therapeutic opportunity. Nat Rev Cancer.
5:505–515. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sulzmaier FJ, Jean C and Schlaepfer DD:
FAK in cancer: Mechanistic findings and clinical applications. Nat
Rev Cancer. 14:598–610. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cance WG, Harris JE, Iacocca MV, Roche E,
Yang X, Chang J, Simkins S and Xu L: Immunohistochemical analyses
of focal adhesion kinase expression in benign and malignant human
breast and colon tissues: Correlation with preinvasive and invasive
phenotypes. Clin Cancer Res. 6:2417–2423. 2000.PubMed/NCBI
|
|
22
|
Itoh S, Maeda T, Shimada M, Aishima S,
Shirabe K, Tanaka S and Maehara Y: Role of expression of focal
adhesion kinase in progression of hepatocellular carcinoma. Clin
Cancer Res. 10:2812–2817. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lark AL, Livasy CA, Calvo B, Caskey L,
Moore DT, Yang X and Cance WG: Overexpression of focal adhesion
kinase in primary colorectal carcinomas and colorectal liver
metastases: Immunohistochemistry and real-time PCR analyses. Clin
Cancer Res. 9:215–222. 2003.PubMed/NCBI
|
|
24
|
Owens LV, Xu L, Craven RJ, Dent GA, Weiner
TM, Kornberg L, Liu ET and Cance WG: Overexpression of the focal
adhesion kinase (p125FAK) in invasive human tumors. Cancer Res.
55:2752–2755. 1995.PubMed/NCBI
|
|
25
|
Beierle EA, Massoll NA, Hartwich J,
Kurenova EV, Golubovskaya VM, Cance WG, McGrady P and London WB:
Focal adhesion kinase expression in human neuroblastoma:
Immunohistochemical and real-time PCR analyses. Clin Cancer Res.
14:3299–3305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Canel M, Secades P, Rodrigo JP, Cabanillas
R, Herrero A, Suarez C and Chiara MD: Overexpression of focal
adhesion kinase in head and neck squamous cell carcinoma is
independent of fak gene copy number. Clin Cancer Res. 12:3272–3279.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schneider GB, Kurago Z, Zaharias R, Gruman
LM, Schaller MD and Hendrix MJ: Elevated focal adhesion kinase
expression facilitates oral tumor cell invasion. Cancer.
95:2508–2515. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lai IR, Chu PY, Lin HS, Liou JY, Jan YJ,
Lee JC and Shen TL: Phosphorylation of focal adhesion kinase at
Tyr397 in gastric carcinomas and its clinical significance. Am J
Pathol. 177:1629–1637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Recher C, Ysebaert L, Beyne-Rauzy O,
Mansat-De Mas V, Ruidavets JB, Cariven P, Demur C, Payrastre B,
Laurent G and Racaud-Sultan C: Expression of focal adhesion kinase
in acute myeloid leukemia is associated with enhanced blast
migration, increased cellularity, and poor prognosis. Cancer Res.
64:3191–3197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sood AK, Coffin JE, Schneider GB, Fletcher
MS, DeYoung BR, Gruman LM, Gershenson DM, Schaller MD and Hendrix
MJ: Biological significance of focal adhesion kinase in ovarian
cancer: Role in migration and invasion. Am J Pathol. 165:1087–1095.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujii T, Koshikawa K, Nomoto S, Okochi O,
Kaneko T, Inoue S, Yatabe Y, Takeda S and Nakao A: Focal adhesion
kinase is overexpressed in hepatocellular carcinoma and can be
served as an independent prognostic factor. J Hepatol. 41:104–111.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyazaki T, Kato H, Nakajima M, Sohda M,
Fukai Y, Masuda N, Manda R, Fukuchi M, Tsukada K and Kuwano H: FAK
overexpression is correlated with tumour invasiveness and lymph
node metastasis in oesophageal squamous cell carcinoma. Br J
Cancer. 89:140–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Golubovskaya VM: Targeting FAK in human
cancer: From finding to first clinical trials. Front Biosci
(Landmark Ed). 19:687–706. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ocak S, Chen H, Callison C, Gonzalez AL
and Massion PP: Expression of focal adhesion kinase in small-cell
lung carcinoma. Cancer. 118:1293–1301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ji HF, Pang D, Fu SB, Jin Y, Yao L, Qi JP
and Bai J: Overexpression of focal adhesion kinase correlates with
increased lymph node metastasis and poor prognosis in
non-small-cell lung cancer. J Cancer Res Clin Oncol. 139:429–435.
2013. View Article : Google Scholar : PubMed/NCBI
|