Introduction
Many statistical reports indicated that malignant
tumors that are associated with lung cancer are the leading cause
of death globally (1,2). Based on its biological characteristics
and clinical manifestations, lung cancer can be classified into
small cell lung cancer (SCLC) and non-small cell lung cancer
(NSCLC) (3). Small-cell lung tumors
undergo metastasis more rapidly than NSCLC to other organs, such as
the brain, skeleton, and lymph tissues (4). NSCLC can be divided into adenocarcinoma,
squamous cell carcinoma, and large cell carcinoma. Large cell
carcinoma is the most difficult to treat owing to its potential
presence in any part of the lungs as well as its rapid growth and
migration (5).
Antidepressants are frequently dispensed to cancer
patients who are suffering from depressive disorders that develop
in later stages. A population-based cohort study found that
neuroleptic medications are associated with a reduced cancer risk
(6), including a reduced lung cancer
(7). An increasing number of
retrospective studies are finding that selective serotonin reuptake
inhibitors (SSRIs) have an anti-proliferative or cytotoxic effect
on various cancers (8). Fluoxetine, a
commonly used SSRI, has been demonstrated to slow the cell cycle
progression in A549 cells by reducing cyclin D1 and cyclin A
expressions and inducing P53 and P21 protein expressions (9). These findings reveal that
antidepressants such as SSRIs may have therapeutic potential
against NSCLC.
Escitalopram oxalate, also known as
Cipralex® (H. Lundbeck A/S, Copenhagen, Denmark) and
Lexapro® (Forest Laboratories, Inc., St. Louis, MO,
USA), is a SSRI that is used for the treatment of major depressive
disorder (MDD) and anxiety disorder by selectively binding to the
human serotonin transporter (10).
Based on a meta-analysis and a pooled analysis, escitalopram
oxalate is more effective than other antidepressants (11). The relevant results indicated that
escitalopram oxalate was superior to a placebo, and almost as
effective as, or superior to, other SSRIs, including citalopram,
paroxetine, fluoxetine and sertraline, and serotonin-noradrenaline
reuptake inhibitors, including duloxetine and sustained-release
venlafaxine. Escitalopram oxalate also exhibits favorable
tolerability and causes generally mild and temporary adverse events
(11). Since few studies of reports
on the effects of escitalopram oxalate in NSCLC cells have been
published, the present study attempts to examine the effects of
escitalopram oxalate on NSCLC and the underlying mechanism
involved.
Materials and methods
Cell line and escitalopram
oxalate
NSCLC cell lines, A549 and H460, purchased from
Taiwan Food Industry Development Research Institute (Hsinchu,
Taiwan) were cultured in RPMI-1640 medium containing 5% FBS (Gibco;
Life Technologies Co., Grand Island, NY, USA). Human bronchus
epithelial cell line BEAS-2B, kindly provided by Dr Gow-Tarng Sheu
(Graduate Institute of Medicine, Chung Shan Medical University,
Taichung, Taiwan) was cultured in RPMI-1640 medium containing 10%
FBS as control cell line. The SSRI, escitalopram oxalate (Lexapro;
Sigma, St. Louis, MO, USA), was provided by Chiayi Chang Gung
Memorial Hospital.
MTT assay
A total of 5×103 cells was seeded in each
well of a 96-well plate and cultured overnight for cell adhesion.
The culture medium was then removed and replaced with medium
containing different concentrations of escitalopram oxalate.
Triplicate treatments were conducted for each concentration. After
incubation with different concentrations of escitalopram oxalate
for 24 or 48 h, the culture medium was removed and 0.2 ml MTT
reagent (0.5 mg/ml) was added to each well for another 2 h. A 0.2
ml DMSO was then added to each well of the plate to dissolve the
crystal and absorbance was measured at 570 nm with an ELISA reader.
The relative cell survival rate was calculated based on the ratio
of the absorbance of the sample treatment relative to the
absorbance of the control treatment.
Cell-migration assay
To measure the effects of escitalopram oxalate, a
modified Boyden chamber assay using cell culture inserts with a
12-µm pore size polycarbonate filter in a 48-well format was used
to perform an in vitro migration assay. Cells were seeded on
the upper part of the chamber at a density of 2×104
cells/well in 50 µl of serum free medium. For the invasion assay,
10 µl Matrigel (BD Biosciences, Bedford, MA, USA) was applied to
12-µm-pore size polycarbonate membrane filters, with the bottom
chamber of the apparatus containing standard medium and then
incubated for 16 h at 37°C. The cells that had invaded to the lower
surface of the membrane were fixed with methanol, washed with
dd-H2O, and then stained with Giemsa. Ten random fields
were counted for each experiment under a light microscope at ×200
magnification per filter.
Flow cytometric analysis
A total number of 2×106 cells per 100
mm2 were seeded in culture plates for 24 h at 37°C in a
5% CO2 incubator. The cells were then incubated with
various concentrations of escitalopram oxalate for 24 h. After
incubation, the cells were harvested, washed with PBS, and fixed
with 70% alcohol for 16 h at 4°C. The cells were then washed using
PBS and transferred into 12×75 mm tubes. A total of 10 µl of
propidium iodide (PI) staining solution was added to each tube, and
the contents were gently mixed. The mixture was incubated in an ice
bath in the dark. Following filtration through a 40 µm nylon
screen, the stained cells were analyzed using a FACSCalibur
analyzer (Becton Dickinson, Bedford, MA, USA).
Caspase-3 activity assay
Analysis of caspase-3 activity was performed in
triplicate using the caspase-3, active form, ELISA pair kit (BD
Biosciences, San Diego, CA, USA) according to the manufacturer's
protocol.
Protein extraction and western
blotting
Cell lysates were obtained by homogenizing the cells
in lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM
Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4 and 1 µg/ml leupeptin). The
homogenates were then centrifuged at 12,000 × g for 40 min and the
supernatants were collected and stored at −80°C for further
experiments. Western blotting was performed as described elsewhere
(12). Protein samples were denatured
for 10 min in boiling water with sample buffer (0.0625 M Tris-HCl
buffer, pH 6.8, containing 2.3% SDS, 5% 2-mercaptoethanol, and 10%
glycerol). Samples were applied to a 12.5% Sodium dodecyl
sulfate-polyacrylamide gel (SDS-PAGE) and electrophoresis at
100–150 V for 1.5 h and then electrophoretically transferred to a
nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ,
USA). The membrane was then soaked in PBS with 5% nonfat dry milk
for 30 min at room temperature. Antibodies against Bax, tBid,
cytochrome c, Apaf-1, cleaved caspase-9, phosphorylated
IκB-α (p-IκB-α) and NF-κB (p-p65) and β-actin (Upstates,
Charlottesville, VA, USA; Santa Cruz Biotechnology, Santa Cruz, CA,
USA) were diluted in PBS with 2.5% BSA and incubated for 1.5 h with
gentle agitation at room temperature. The membranes were washed
twice with PBS-Tween for 1 h, and a secondary antibody conjugated
with horseradish peroxidase (HRP) was added. Pierce's SuperSignal
West Dura HRP Detection kit (Pierce Biotechnology Inc., Rockford,
IL, USA) was used to detect antigen-antibody complexes. The blots
were scanned and quantified by densitometry (Appraise,
Beckman-Coulter, Brea, CA, USA).
Statistical analysis
All statistical analyses were performed using SPSS
10.0 software (SPSS Inc., Chicago, IL, USA). Three independent
experiments were repeated. Statistical analyses were performed
using the analysis of variance plus posterior multiple comparison
test to determine the difference. P<0.05 was considered
statistically significant. The significant differences were
stressed with symbols as shown in figures.
Results
Escitalopram oxalate attenuates
proliferation and invasive ability in A549 and H460 cells
To evaluate the effects of escitalopram oxalate on
NSCLC, A549 and H460 cell lines were treated with various
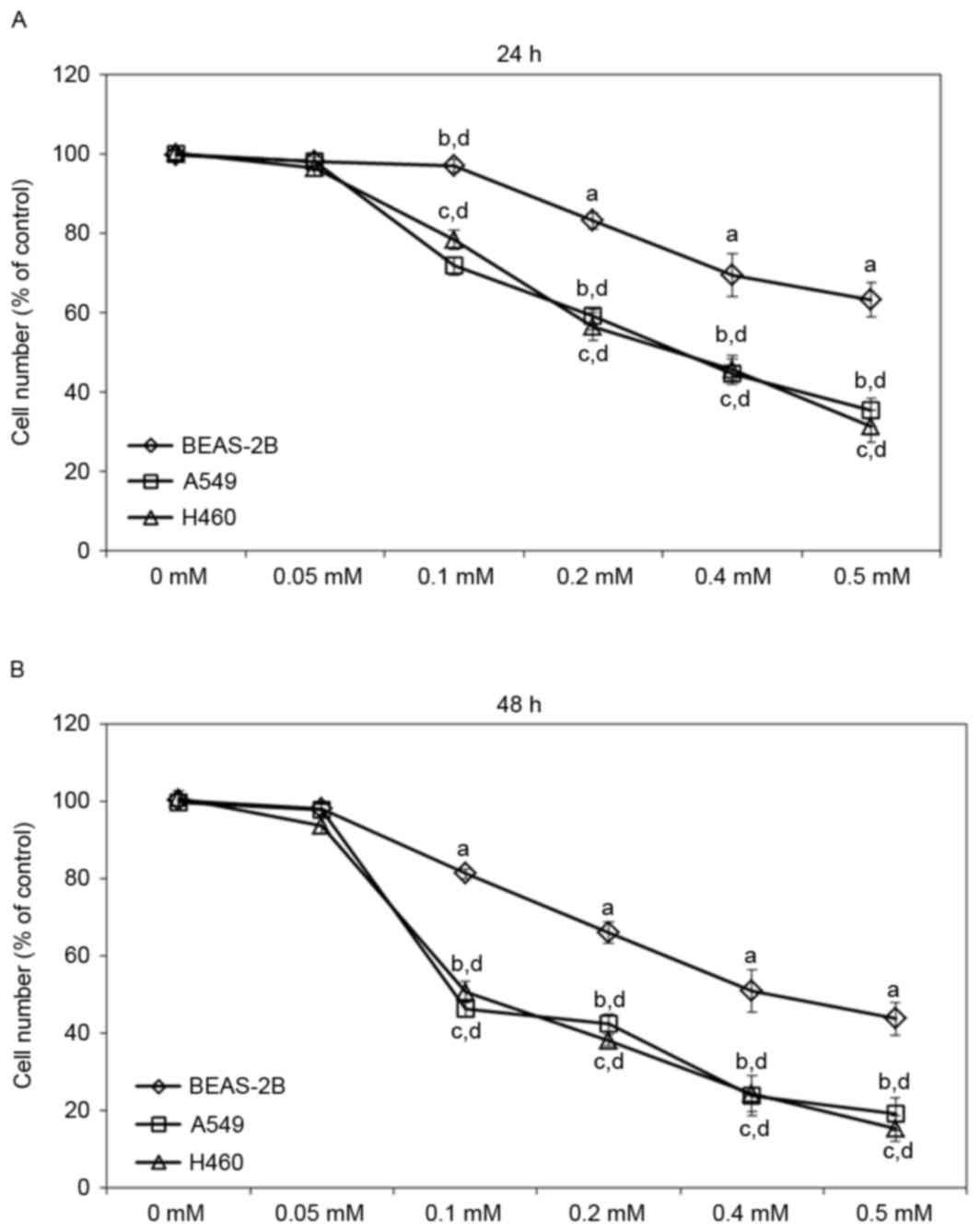
concentrations of escitalopram oxalate. Treatment with 0.1, 0.2,
0.4 or 0.5 mM escitalopram oxalate for 24 h significantly reduced
the viability of both A549 and H460 cells relative to those in the
control group and the control cell line, BEAS-2B, respectively
(Fig. 1A). Significantly reduced cell
viability was also detected in A549 and H460 cells that were
treated with 0.1, 0.2, 0.4 or 0.5 mM escitalopram oxalate for 48 h
relative to those in the control group and the control cell line,
BEAS-2B, respectively (Fig. 1B).
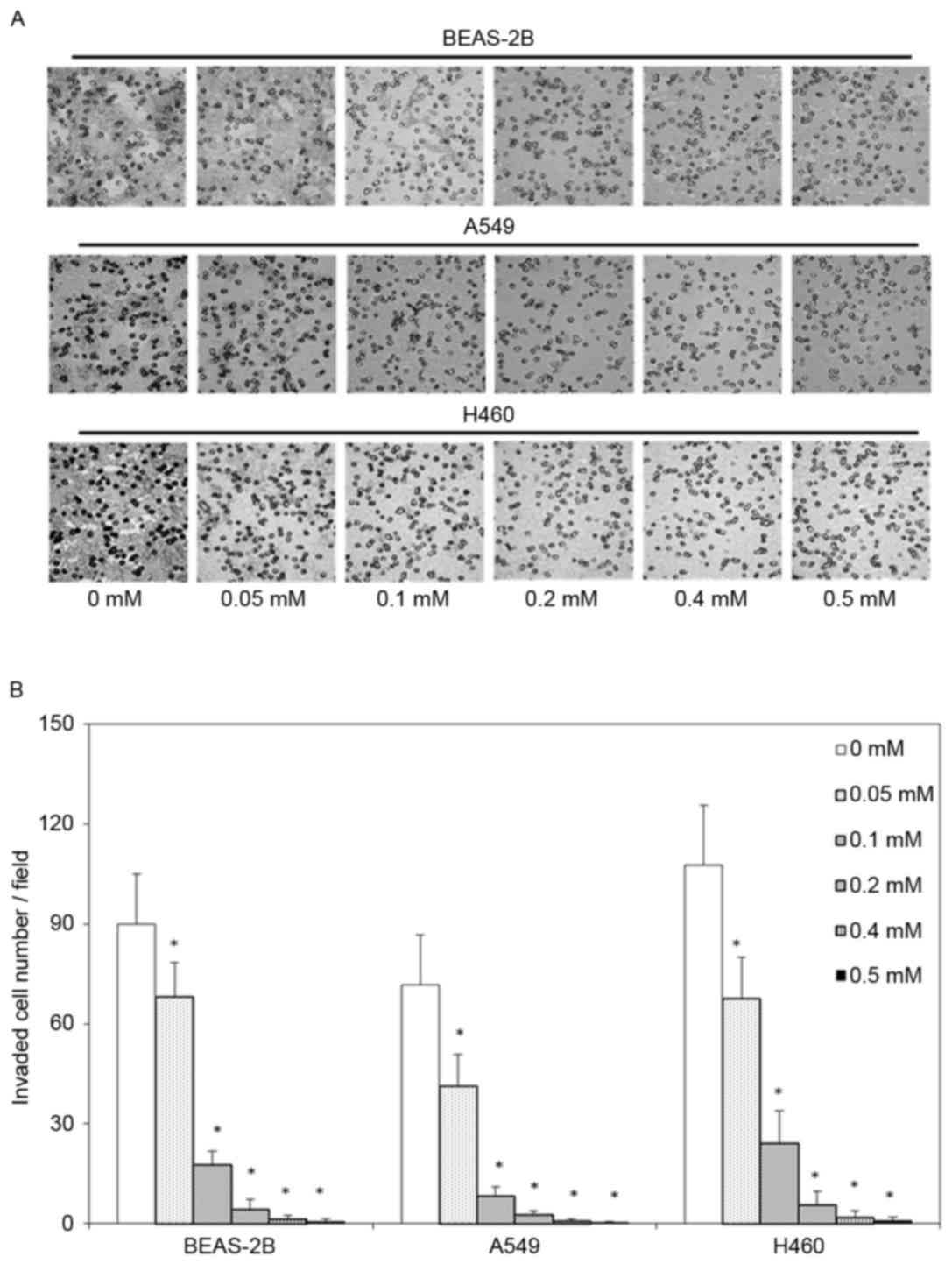
Moreover, the invasive ability of BEAS-2B, A549 and H460 cells was
evaluated as the number of cells that passed through a
polycarbonate filter with 12 µm pores, the number of invaded in
BEAS-2B, A549 and H460 cells that were treated with 0.05, 0.1, 0.2,
0.4 or 0.5 mM escitalopram oxalate for 24 h cells was significantly
smaller than the corresponding number in the control group
(Fig. 2).
Escitalopram oxalate increases sub-G1
population and induces apoptosis in A549 and H460 cells
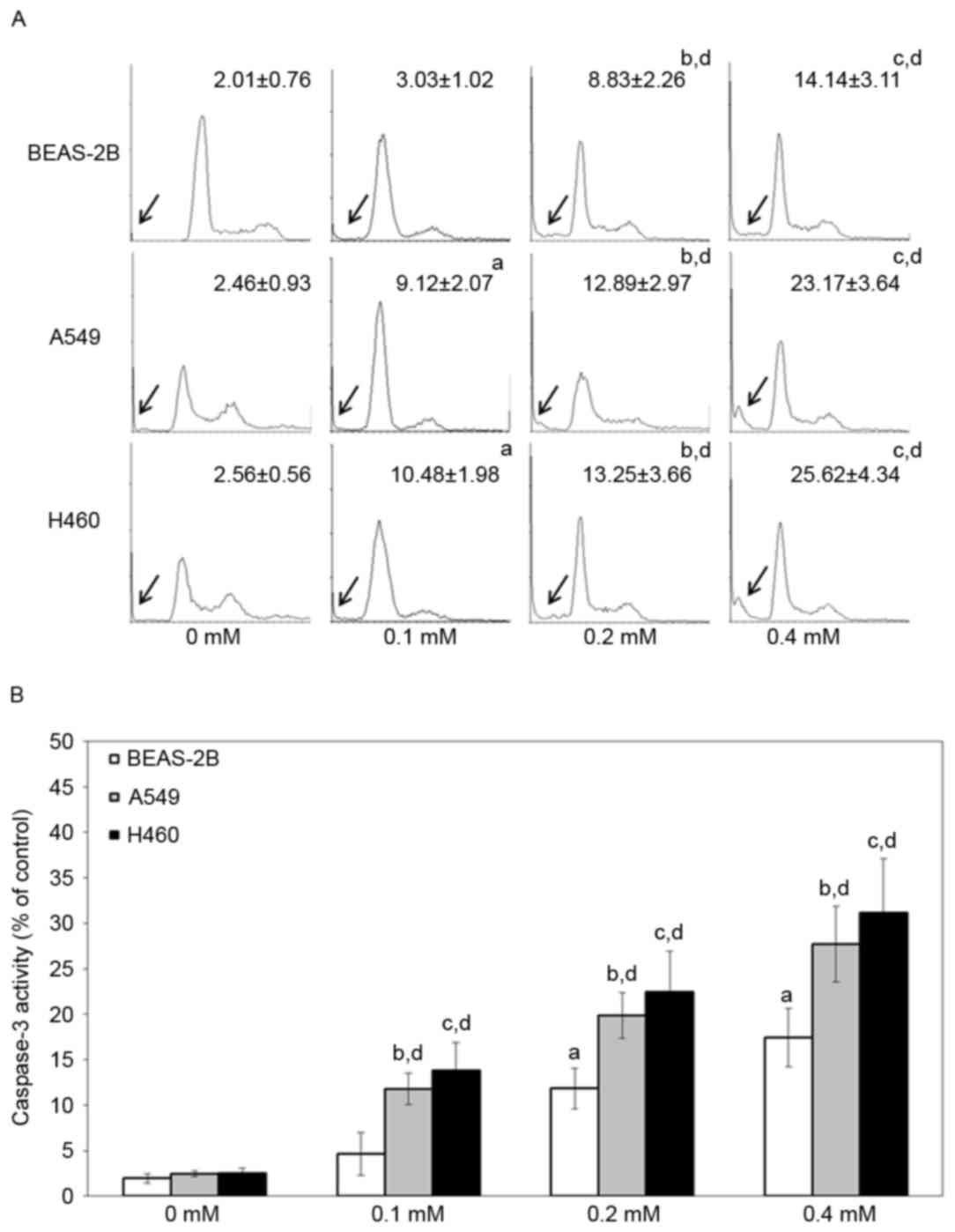
To study the cell death pathway that is involved,
BEAS-2B, A549 and H460 cells were treated with various
concentrations of escitalopram oxalate for 24 h and examined by
flow cytometry with PI staining and caspase-3 activity assay.
Significantly increased sub-G1 proportions were detected in both
A549 and H460 cells that had been treated with 0.1, 0.2 and 0.4 mM
escitalopram oxalate, relative to those in the control group or the
control cell line, BEAS-2B (Fig. 3A).
Significantly increased caspase-3 activities were detected in both
A549 and H460 cells that had been treated with 0.1, 0.2 and 0.4 mM
escitalopram oxalate, relative to cells in the control group or the
control cell line, BEAS-2B (Fig. 3B).
Since significantly increased sub-G1 and caspase-3 activity were
detected in A549 and H460 cells, relative to BEAS-2B cells, the
following experiments were performed on A549 and H460 cells.
Escitalopram oxalate-induced
mitochondrial dependent apoptosis in A549 and H460 cells
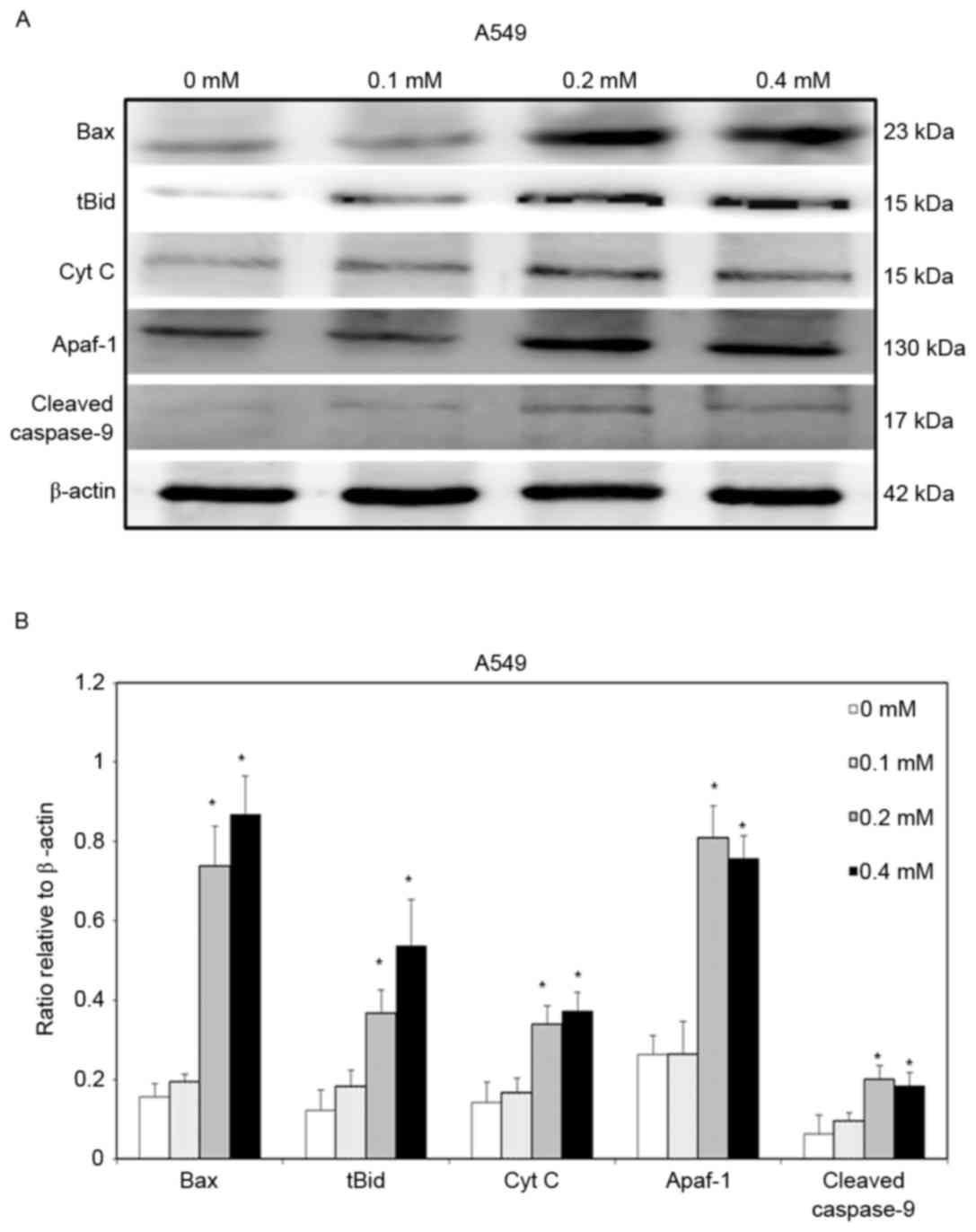
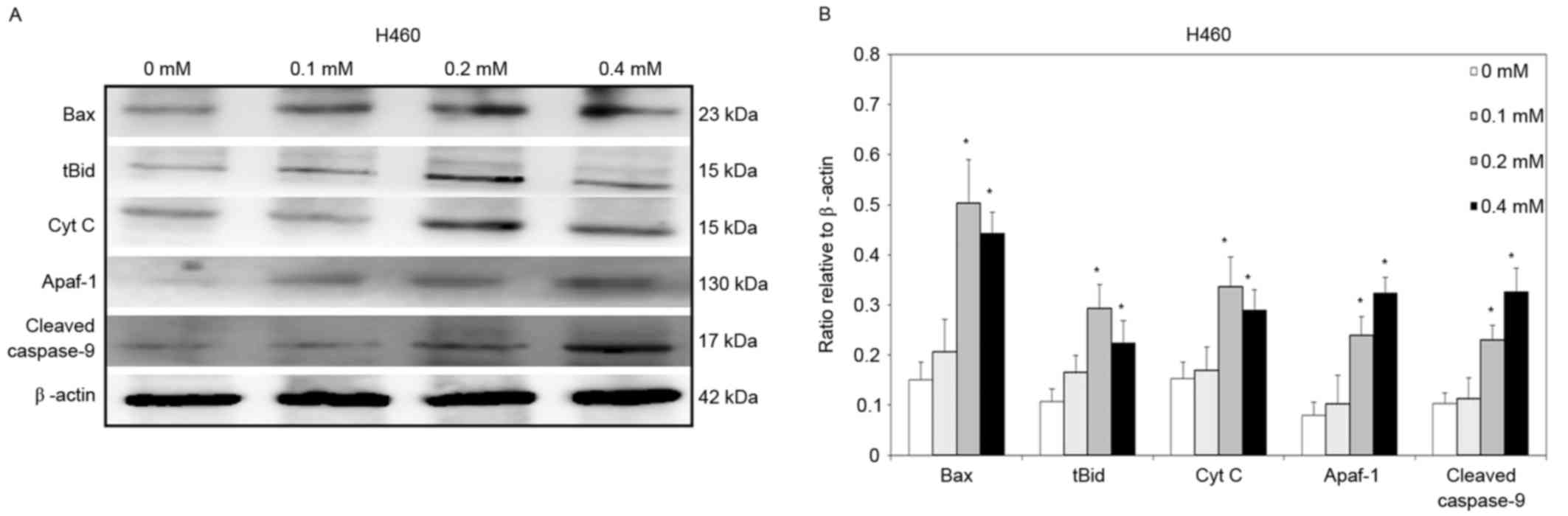
To clarify further the signaling that is involved in
escitalopram oxalate-induced apoptosis in both A549 and H460 cells,
the expressions of Bax, tBid, cytochrome c, Apaf-1 and
cleaved caspase-9 proteins were detected. Significant increases in
levels of Bax and tBid proteins were detected in A549 cells that
were treated with 0.2 and 0.4 mM escitalopram oxalate for 24 h
relative to those in the control group (Fig. 4). Thersefore, significant increases in
cytochrome c and Apaf-1 were detected in A549 cells that were
treated with 0.2 and 0.4 mM escitalopram oxalate for 24 h, relative
to those in the control group (Fig.
4). Furthermore, cleaved caspase-9, the level of a downstream
molecule of Apaf-1, was significantly increased in A549 cells that
were treated with 0.2 and 0.4 mM escitalopram oxalate for 24 h,
relative to those in the control group (Fig. 4). Similar results were observed for
H460 cells. Significantly increased Bax, tBid, cytochrome c,
Apaf-1 and cleaved caspase-9 protein levels were detected in H460
cells that had been treated with 0.2 and 0.4 mM escitalopram
oxalate for 24 h, relative to those in the control group (Fig. 5).
Signaling molecules involved in the
escitalopram oxalate induced-apoptosis in A549 and H460 cells
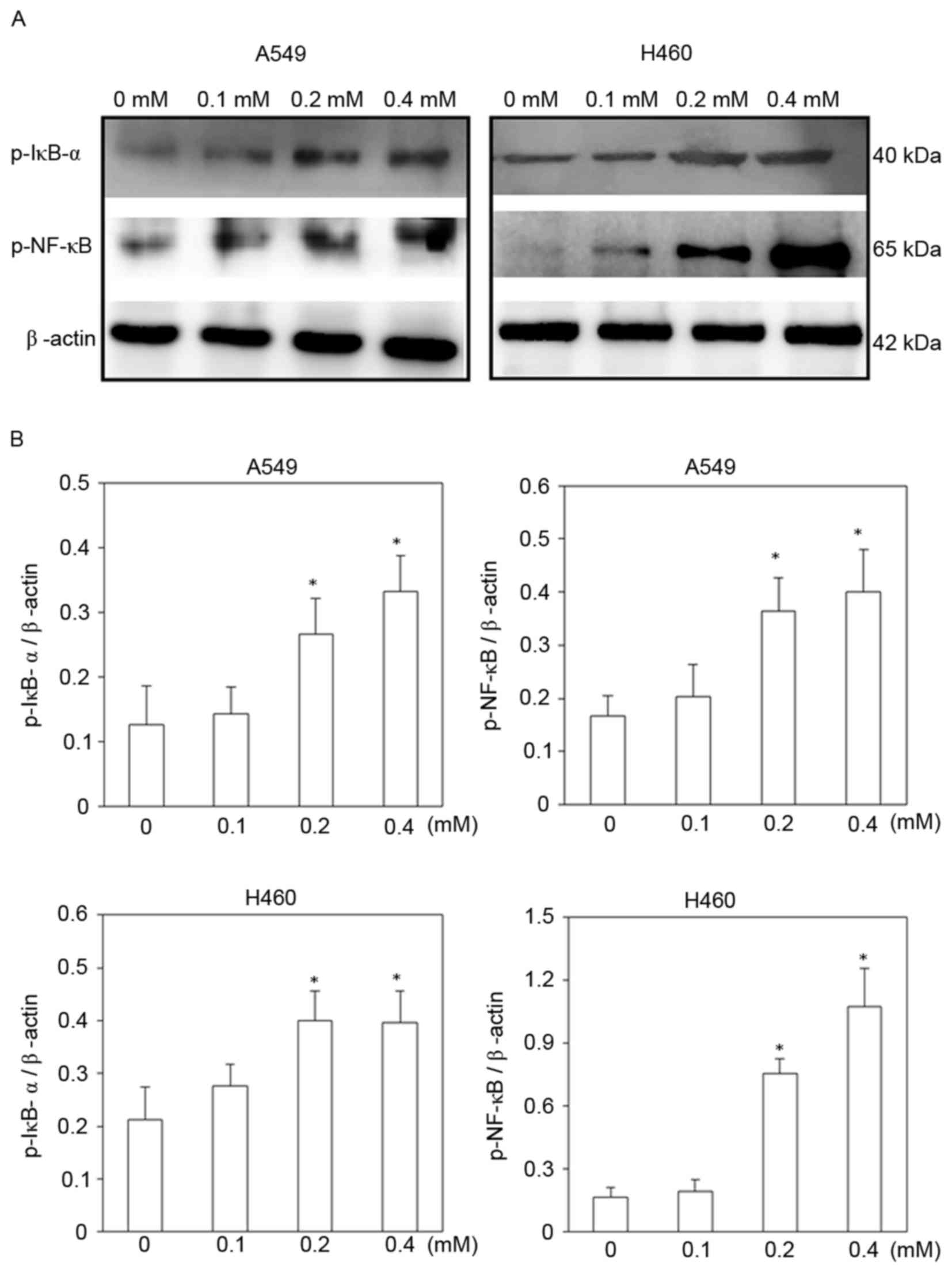
To identify signaling pathways that may be involved
in escitalopram oxalate-induced apoptosis in A549 and H460 cells,
the expressions of p-IκB-α and NF-κB (p65-p) proteins were
examined. The expressions of both p-IκB-α and NF-κB (p65-p)
proteins were significantly increased in both A549 and H460 cells
following treatment with 0.2 and 0.4 mM escitalopram oxalate for 24
h, relative to those in the control group (Fig. 6). Significantly increased p-IκB-α and
NF-κB (p65-p) protein levels were also detected in H460 cells that
had been treated with at 0.2 and 0.4 mM escitalopram oxalate for 24
h, relative to those in the control group (Fig. 6).
Discussion
Neuroleptic medications are reportedly associated
with a reduced risk of certain cancers. However, relatively little
is known about the mechanism by which SSRI causes NSCLC cell death.
This study demonstrated that escitalopram oxalate, a more effective
SSRI, has significantly inhibitory effects on the proliferation and
invasive ability of A549 and H460 cells, relative to the controls.
Escitalopram oxalate also caused significant apoptosis by inducing
mitochondria-associated cascades in both A549 and H460 cells. These
findings suggest that escitalopram oxalate may have therapeutic
potential against NSCLC.
Cell migration is known to involve a complex
mechanism. It is required for many biological activities, including
embryogenesis, wound healing, immune response and tissue repair
(13). Errors in the cell migration
process cause serious pathologic episodes, including cancer
invasion and metastasis (14,15), which are important characteristics of
malignant tumor cells (16). Cancer
metastasis comprises four essential steps, including detachment,
migration, invasion and adhesion, which are different but
interrelated (17,18). Once cancer cells have spread beyond
their initial primary site, the cancer is typically incurable and
fatal (19). Cancer metastasis is the
leading cause of morbidity and mortality, and is responsible for
almost 90% of all cancer mortality (20). Therefore, constraining cancer
metastasis is important in cancer therapy. In this study,
escitalopram oxalate significantly reduced the motility and
invasive abilities of A549 and H460 cells, exhibiting a potential
to inhibit the migration of NSCLC cells.
Unlimited proliferation is known to be a critical
process in cell carcinogenesis. Therefore, compounds that suppress
tumor growth by inducing cell cycle arrest and apoptosis are
favored for cancer therapy (21,22). The
induction of apoptosis is one of the main mechanisms for impeding
cancer growth and is regarded as necessary for screening novel
anti-cancer agents (23). The
mechanisms of apoptosis are highly complex and sophisticated. The
two main pathways of apoptosis are extrinsic and intrinsic, which
are linked with each other (24). The
extrinsic signaling pathways that initiate apoptosis involve
transmembrane receptor-mediated interactions, such as the
engagement of tumor necrosis factor (TNF) and TNF receptor
(25). The intrinsic signaling
pathways that induce apoptosis involve intracellular signals that
act directly on targets in the cell and are mitochondria-initiated
events. The events involve cytochrome c, which activates the
caspase-dependent mitochondrial pathway. Cytochrome c binds
and activates Apaf-1 and procaspase-9, forming an ‘apoptosome’
(26). The clustering of procaspase-9
in this manner eventually results in caspase-9 and caspase-3
activation (27). In this study, the
cytotoxic activity of escitalopram oxalate was triggered by
releasing Bax, tBid, cytochrome c and Apaf-1, resulting in
the proteolytic cleavage of caspase-9 and caspase-3 in A549 and
H460 cells. These findings suggest that the therapeutic efficacy of
escitalopram oxalate against NSCLC cells involves inducing
mitochondria-dependent apoptosis. However, other possible
mechanisms and interactive targets that are involved in
escitalopram oxalate-induced cell death in NSCLC cells warrant
further investigation.
Evidence reveals that neuroleptic medications are
associated with a reduced cancer risk (6,28). Various
SSRIs, especially fluoxetine, are known to reduce the risk of
cancer (29–31), including lung cancer (9). However, the side-effects of fluoxetine
remain problematic (32).
Escitalopram oxalate is a superior SSRI that has been demonstrated
to have favorable tolerability and to cause generally milder and
more temporary adverse events than other SSRIs (11). The present study firstly demonstrated
that escitalopram oxalate significantly inhibits the proliferation
and invasion of A549 and H460 cells and induces
mitochondria-dependent apoptosis therein. These findings suggest
that escitalopram oxalate is more effective than other SSRIs and
probably effective for reducing the risk of NSCLC development.
In summary, this study firstly revealed that
escitalopram oxalate significantly inhibits the viability and
mobility in A549 and H460 cells, resulting in subsequent
mitochondria-dependent apoptosis through p-IκB-α/NF-κB (p65-p)
signaling. Accordingly, escitalopram oxalate also reduces cell
viability and mobility and induces apoptosis in the control cell
lines, BEAS-2B; however, these phenomena are milder than those
detected in A549 and H460 cells. Although further animal study may
be required, this study reveals that escitalopram oxalate exhibits
significant cytotoxic effects on A549 and H460 cells and suggests a
therapeutic potential of escitalopram oxalate in the treatment of
NSCLC patients.
Acknowledgements
This work was supported by clinical research grant
from Kaohsiung Armed Force General Hospital, Kaohsiung, Taiwan (no.
105-30) and partially supported by Chang Gung Medical Foundation,
Chiayi Chang Gung Memorial Hospital and Chang Gung University,
Chiayi, Taiwan-Taiwan Ministry of Health and Welfare Clinical Trial
and Research Center of Excellence (CMRPG6E0272). Ted Knoy is
appreciated for his editorial assistance.
References
|
1
|
Islami F, Torre LA and Jemal A: Global
trends of lung cancer mortality and smoking prevalence. Transl Lung
Cancer Res. 4:327–338. 2015.PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petersen I and Warth A: Lung cancer:
Developments, concepts, and specific aspects of the new WHO
classification. J Cancer Res Clin Oncol. 142:895–904. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
American Cancer Society: Lung Cancer
(Non-Small Cell), . American Joint Committee on Cancer Staging
(AJCC-2010). Atlanta, GA: 2013
|
|
6
|
Jones GR: Cancer therapy: Phenothiazines
in an unexpected role. Tumori. 71:563–569. 1985.PubMed/NCBI
|
|
7
|
Toh S, Rodríguez LA and Hernández-Díaz S:
Use of antidepressants and risk of lung cancer. Cancer Causes
Control. 18:1055–1064. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dodd S, Berk M, Kelin K, Zhang Q, Eriksson
E, Deberdt W and Craig Nelson J: Application of the Gradient
Boosted method in randomised clinical trials: Participant variables
that contribute to depression treatment efficacy of duloxetine,
SSRIs or placebo. J Affect Disord. 168:284–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stepulak A, Rzeski W, Sifringer M, Brocke
K, Gratopp A, Kupisz K, Turski L and Ikonomidou C: Fluoxetine
inhibits the extracellular signal regulated kinase pathway and
suppresses growth of cancer cells. Cancer Biol Ther. 7:1685–1693.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burke WJ: Escitalopram. Expert Opin
Investig Drugs. 11:1477–1486. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kirino E: Escitalopram for the management
of major depressive disorder: A review of its efficacy, safety and
patient acceptability. Patient Prefer Adherence. 6:853–861. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiu CC, Shi YF, Yang JJ, Hsiao YC, Tzang
BS and Hsu TC: Effects of human parvovirus B19 and bocavirus VP1
unique region on tight junction of human airway epithelial A549
cells. PLoS One. 9:e1079702014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Binamé F, Pawlak G, Roux P and Hibner U:
What makes cells move: Requirements and obstacles for spontaneous
cell motility. Mol Biosyst. 6:648–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sahai E: Mechanisms of cancer cell
invasion. Curr Opin Genet Dev. 15:87–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liotta LA, Rao CN and Wewer UM:
Biochemical interactions of tumor cells with the basement membrane.
Ann Rev Biochem. 55:1037–1057. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gabbert H: Mechanisms of tumor invasion:
Evidence from in vivo observations. Cancer Metastasis Rev.
4:293–309. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guan X: Cancer metastases: Challenges and
opportunities. Acta Pharm Sin B. 5:402–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wells A, Grahovac J, Wheeler S, Ma B and
Lauffenburger D: Targeting tumor cell motility as a strategy
against invasion and metastasis. Trends Pharmacol Sci. 34:283–289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seyfried TN and Huysentruyt LC: On the
origin of cancer metastasis. Crit Rev Oncog. 18:43–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Los M, Burek CJ, Stroh C, Benedyk K, Hug H
and Mackiewicz A: Anticancer drugs of tomorrow: Apoptotic pathways
as target for drug design. Drug Discov Today. 8:67–77. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiu J, Zhao BB, Shen Y, Chen W, Ma YD and
Shen YM: A novel p-terphenyl derivative inducing cell-cycle arrest
and apoptosis in MDA-MB-435 cells through topoisomerase inhibition.
Eur J Med Chem. 68:192–202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Igney FH and Krammer PH: Death and
anti-death: Tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Locksley RM, Killeen N and Lenardo MJ: The
TNF and TNF receptor superfamilies: Integrating mammalian biology.
Cell. 104:487–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hill MM, Adrain C, Duriez PJ, Creagh EM
and Martin SJ: Analysis of the composition, assembly kinetics and
activity of native Apaf-1 apoptosomes. EMBO J. 23:2134–2145. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dalton SO, Johansen C, Poulsen AH,
Nørgaard M, Sørensen HT, McLaughlin JK, Mortensen PB and Friis S:
Cancer risk among users of neuroleptic medication: A
population-based cohort study. Br J Cancer. 95:934–939. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mun AR, Lee SJ, Kim GB, Kang HS, Kim JS
and Kim SJ: Fluoxetine-induced apoptosis in hepatocellular
carcinoma cells. Anticancer Res. 33:3691–3697. 2013.PubMed/NCBI
|
|
30
|
Kraft SL, Baker NM, Carpenter J and
Bostwick JR: Procarbazine and antidepressants: A retrospective
review of the risk of serotonin toxicity. Psychooncology.
23:108–113. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kannen V, Garcia SB, Silva WA Jr, Gasser
M, Mönch R, Alho EJ, Heinsen H, Scholz CJ, Friedrich M, Heinze KG,
et al: Oncostatic effects of fluoxetine in experimental colon
cancer models. Cell Signal. 27:1781–1788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Andersen J, Kristensen AS, Bang-Andersen B
and Strømgaard K: Recent advances in the understanding of the
interaction of antidepressant drugs with serotonin and
norepinephrine transporters. Chem Commun (Camb). 25:3677–3692.
2009. View
Article : Google Scholar
|