Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide, with ~1,590,000 mortalities
in 2012 (1). Although there have been
considerable advances in the treatment for lung cancer in previous
decades, this disease still remains incurable. Dexamethasone (DEX)
is widely used in the clinic, however its pharmacological effects
are mainly anti-inflammatory and anti-allergic (2,3). There is
a growing body of literature, which reports on the beneficial
effects of DEX in tumors. DEX exerts inhibitory effects on cell
migration and invasion of colon cancer (4); promotes cell proliferation via
inhibiting apoptosis of bladder cancer cells (5); induces apoptosis in a leukemia cell line
(6) and pre-treatment of lung cancer
patients with DEX reduced hematological toxicity and enhanced
efficacy of chemotherapy drugs (7).
Previously, it has been reported that DEX effectively inhibits the
growth of Lewis lung carcinoma, which indicated that DEX has an
antitumor effect in lung cancer (8).
In addition, another study reported that DEX contributed another
function by inhibiting transforming growth factor (TGF)-β1
signaling by downregulating the expression and secretion of TGF-β1
(9,10).
The TGF-β family is comprised of multifunctional
cytokines that function as tumor suppressors by inhibiting cell
proliferation and inducing apoptosis in normal epithelial cells and
precancerous tissues. However, TGF-β also accelerates the
progression of established cancers by promoting cell proliferation,
invasion, and metastasis (11,12) TGF-β1
is a member of the TGF-β family, and functions in the regulation of
cell growth and differentiation, and contributing to the apoptotic
pathway (13–16). A previous study reported that treating
A549 cells with TGF-β1 may enhance the apoptosis of A549 cells but
prolonged exposure to TGF-β1 inhibited the apoptosis induced by
Fas/Fasl (17). SB-431542 is a small
molecule inhibitor that was identified as an inhibitor of TGF-β,
with the capacity to inhibit phosphorylation of Smad family member
2 (Smad2) (18). Smad2 is involved in
critical role in TGF-β induced apoptosis of prostate epithelial
cells, which is activated by TGF-β1 (19). The aim of the present study was to
investigate the involvement of Smad2 in TGF-β1 induced apoptosis of
A549 cells.
In the present study, it was observed that the
proliferation of A549 cells decreased and the apoptosis rate
significantly increased following exposure to DEX. Furthermore, the
expression of TGF-β1 and Smad2 were significantly increased
following DEX stimulation, and this effect was partially abrogated
by SB431542. The results of the present study concluded that the
TGF-β1/Smad2 pathway may be involved in DEX induced apoptosis of
A549 cells.
Materials and methods
Cell culture
The A549 cells were provided by the Drug Engineering
Research Center of Chongqing Medical University (Chongqing, China).
Cells were cultured in Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) at
37°C in a humidified incubator containing 5% CO2 in air.
The medium was changed every 2 days. A549 cells in the logarithmic
growth phase treated with DEX (Tianjin Jinyao Amino Acid Co., Ltd.,
Tianjin, China) and SB431542 (Med Chem Express Co., Monmouth
Junction, NJ, USA) were used for the following experiments. All the
cells were harvested using pancreatin (Thermo Fisher Scientific,
Inc.), washed with PBS and collected following centrifugation at
200 × g for 5 min at room temperature.
3-(4,5-diethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt (MTS) proliferation assay
The MTS cell proliferation assay was used to
quantify viable A549 cells. In brief, A549 cells were seeded onto
96-well microplates (5×104 cells/well) in 100 µl culture
medium (DMEM with 10% FBS) and cultured until the cells reached 70%
confluency. Following this, cells were further cultured for 0, 12,
24 or 48 h, with medium containing DEX at a range of concentrations
(0, 0.1, 1.0 and 10 mmol/l). The cell proliferation assay was
performed using the MTS reagent kit (MTS; Promega Corporation,
Madison, WI, USA) according to the manufacturer's protocol. In
brief, 10 µl MTS was added, and cells were incubated at 37°C in a
humidified incubator for 1–2 h. The absorbance values for each well
were detected at 490 nM using a micro plate reader (Omega Bio-Tek
Inc., Norcross, GA, USA).
Hoechst 33342 and Annexin V/propidium
iodide (PI) staining
The DEX- or SB431542-treated A549 cells were fixed
in 0.5 ml formalin (4%) at room temperature for 10 min, washed
twice in PBS, and stained with 0.5 ml Hoechst 33342 at room
temperature for 5 min. Cells were washed twice with PBS, and cell
nuclei in five random fields were observed using a fluorescence
microscope (Olympus Corporation, Tokyo, Japan; magnification,
×200). The apoptosis rate of A549 cells was measured using flow
cytometry with Annexin V-fluorescein isothiocyanate (FITC)/PI
Apoptosis Detection kit, which was purchased from the Beyotime
Institute of Biotechnology (Shanghai, China). In brief, all cells
were washed with PBS to remove the medium. A minimum of
1×105 cells were resuspended in 100 µl binding buffer
containing Annexin V-FITC and PI (Annexin V-FITC/PI Apoptosis
Detection kit; Beyotime Institute of Biotechnology). A FACScan flow
cytometer was used to quantify Annexin V-FITC and PI binding using
channels FL-1 (Annexin V-FITC) and FL-3 (PI). Quadrant analysis was
performed using the Cellquest Pro software (version 5.1; BD
Biosciences, Franklin Lanes, NJ, USA).
Western blot analysis
The pre-cooled cells from each treatment group were
harvested for total protein extraction and treated with a lysis
buffer containing 20 mmol/l Tris (PH 7.5), 150 mmol/l NaCl, 1%
Triton X-100 and inhibitors of protease and phosphates on ice for
30 min. The cell lysis products were centrifuged for 10 min at
2,000 × g in a 4°C refrigerated centrifuge and the supernatants
were collected. The final protein concentration was measured using
a BCA protein kit (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and supernatants were boiled for 5 min. An aliquot of 40 µg of
cellular protein was electrophoresed on an 8% gel using SDS-PAGE
and transferred onto a polyvinylidene fluoride membrane. The
membrane was then blocked for 2 h with 5% bovine serum albumin
(Beyotime Institute of Biotechnology) at room temperature, and
incubated overnight with primary antibodies against TGF-β1 (cat.
no. ab92486), phosphorylated (p-)Smad2 (cat. no. ab53100), cleaved
caspase-3 (cat. no. ab136812) and β-actin (cat. no. ab8226;
1:1,000; Abcam, Cambridge, MA, USA) at 4°C. Membranes were washed
with Tris-buffered saline containing 0.1% Tween-20 and incubated
for 2 h with horseradish peroxidase-conjugated goat anti-mouse
immunoglobulin G secondary antibody (cat. no. ab97035) at room
temperature (1:1,000; Abcam). The immunoreactivity of each protein
was visualized using the Millipore western blot chemiluminescence
horseradish peroxidase substrate ECL Chemiluminescence reagent kit
(EMD Millipore, Billerica, MA, USA). The results were analyzed
using Quantity One software (version 4.4.02; Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
All the experiments were performed in triplicate and
data were expressed as the mean ± standard deviation. Statistical
significance was analyzed using a one-way analysis of variance
followed by Dunnett's post hoc test to analyze the difference
between DEX groups. A student's t-test was performed to compare the
differences among medicine groups (GraphPad Prism, version 5.01;
GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
DEX treatment decreased the
proliferation and increased the apoptosis rate of A549 cells
In order to investigate whether DEX decreases the
proliferation of A549 cells in a dose-time-dependent manner, cells
were treated with DEX at concentrations of 0.1, 1.0 and 10.0 mmol
for 0, 12, 24 and 48 h (Fig. 1A). A
significant time- and dose-dependent decrease of proliferation in
A549 cells was observed from 1.0–10.0 mmol DEX, following 24 and 48
h of culture. Hoechst 33342 staining was performed to observe the
nuclei change in A549 cells. The nuclei of the DEX-treated group
emitted white blue fluorescence, whereas the control group emitted
blue fluorescence, which indicated a dose-dependent increase in
apoptosis in the DEX-treated group (Fig.
1B). Flow cytometry was conducted to test the apoptotic rate of
cells. The results demonstrated that the rate of early apoptotic
death in DEX-treated groups was significantly higher compared with
that of the control. In addition, DEX induced apoptosis in a time
and dose-dependent manner (Fig.
1C-D).
 | Figure 1.The anticancer effect of DEX in A549
cells. (A) Cell proliferation rate following DEX treatment (0, 0.1,
1.0 or 10.0 mmol/l) for 0, 12, 24 and 48 h, as assessed by
3-(4,5-diethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt assay. (B) Nuclear morphological changes of apoptotic
cells following Hoechst staining (magnification, ×200). Arrows
indicate pathologic changes of apoptosis. (C) A549 cells were
treated with DEX (0, 0.1, 1.0 or 10.0 mmol/l) for 12, 24 and 48 h,
and the apoptosis rate was tested by flow cytometry, with (D)
quantification. *P<0.05, **P<0.01 and ***P<0.001 vs.
control. DEX, dexamethasone. |
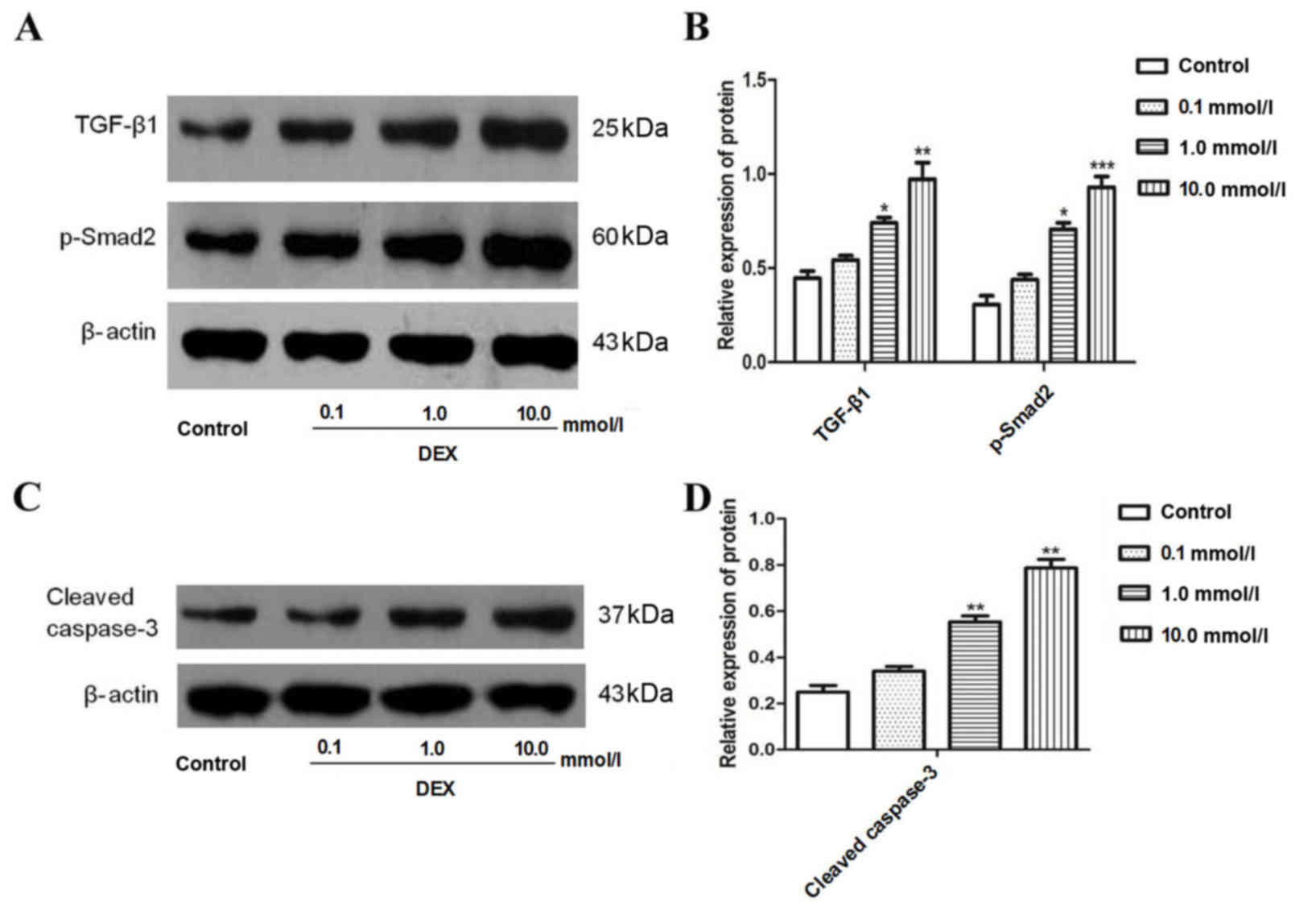
Protein expression levels of TGF-β1,
Smad2 and caspase-3 in A549 cells were significantly increased
following DEX exposure
To investigate whether DEX induced the expression of
TGF-β1 and Smad2 in A549 cells, cells were treated with 0.1–10.0
mmol/l DEX for 48 h. The results demonstrated that DEX
significantly increased the expression of TGF-β1 and Smad2 when
compared with the control (Fig. 2A and
B). Cleaved caspase-3, an indicator of apoptosis, was also
measured. The results from the present study revealed that DEX
significantly increased the expression of cleaved caspase-3
(Fig. 2C and D).
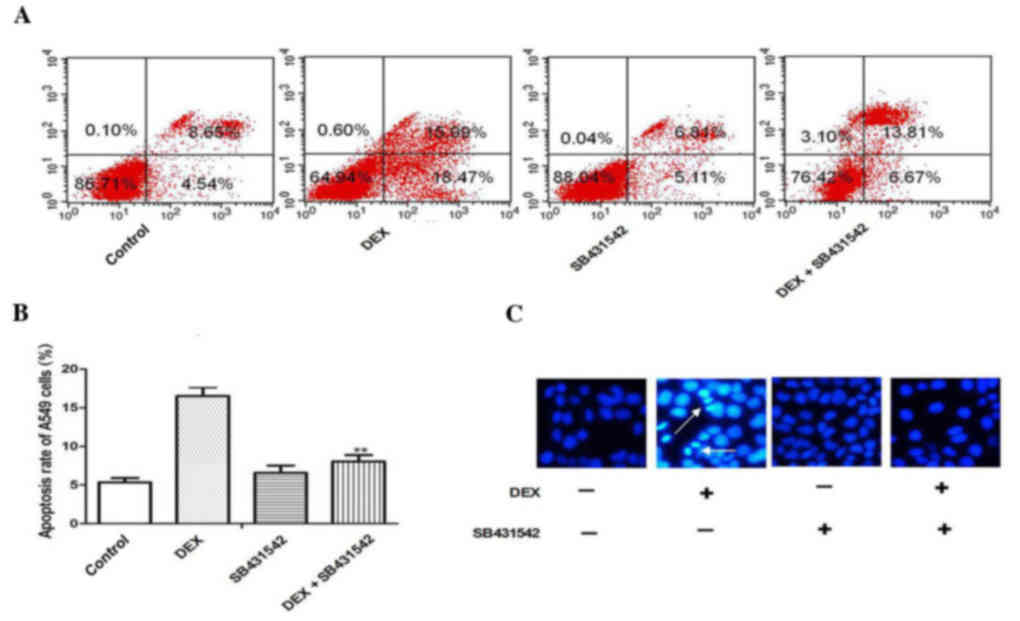
Cell apoptosis induced by DEX exposure
is inhibited by SB431542 treatment
To explore whether TGF-β1/Smad2 signaling was
involved in DEX-induced apoptosis of A549 cells, the TGF-β1
receptor was blocked with SB431542 at a concentration of 10 mmol/l,
which was previously verified in a preliminary experiment (data not
shown). The results of flow cytometry demonstrated that DEX
increased the apoptosis rate of A549 cells, and in response to
SB431542, the apoptosis of A549 cells induced by DEX was
significantly inhibited (Fig. 3A and
B; P<0.05). Hoechst staining revealed that SB431542
treatment also protected A549 cells from DEX induced apoptosis
(Fig. 3C).
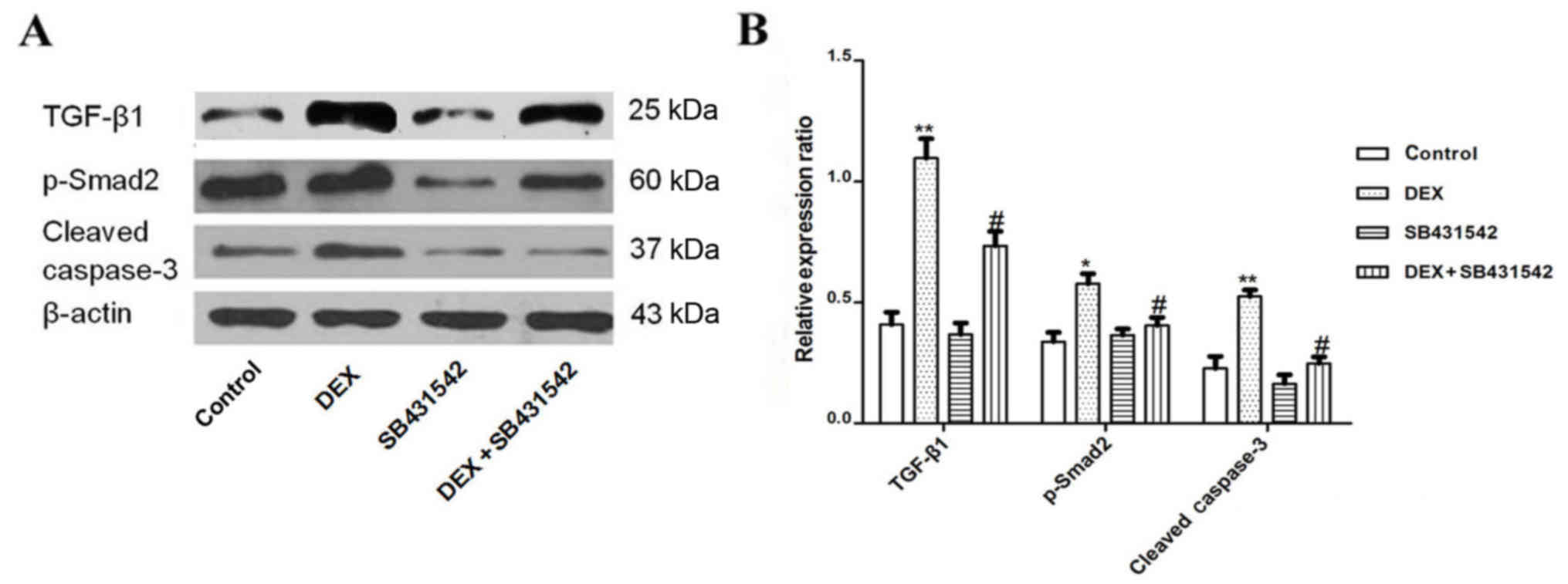
DEX-induced protein expression of
TGF-β1, Smad2 and caspase-3 was significantly inhibited by
SB431542
The present study demonstrated that SB431542
inhibited apoptosis of A549 cells, however the mechanism involved
remains unknown. Therefore, in the present study, the TGF-β1
receptor was blocked with SB431542, and the expression of TGF-β1,
Smad2 and caspase-3 were analyzed by western blot. The expression
of TGF-β1, Smad2 and caspase-3 were significantly decreased in the
SB431542 group when compared with the DEX group alone (Fig. 4A and B).
Discussion
In the present study, a mechanism by which DEX
induced apoptosis in A549 cells was demonstrated. The results
revealed that DEX exposure significantly increased apoptotic cell
accumulation, caspase-3 production and TGF-β1/Smad2 pathway
activity. The results also revealed that SB431542 inhibited A549
cell apoptosis, which may act via the TGF-β1/Smad2 pathway.
Glucocorticoids are commonly used anti-inflammatory
drugs in the clinic, and inhibit TGF-β1 activity (9,20). TGF-β
is a multifunctional protein, which influences a variety of
cellular functions including cell growth, differentiation and
immune regulation. Previous studies have demonstrated that TGF-β1
is involved in the process of apoptosis (21). Smad family proteins are pivotal TGF-β
signal transmission carriers and are activated in the cytoplasm
prior to transferring into the nucleus, wherein they activate or
inhibit the transcription of target genes (22). Miyazaki et al (23) reported that TGF-β1 stimulates or
decreases cell proliferation via the down or upregulation of
cyclin-dependent kinase inhibitor 1A respectively, which is a
direct target of Smad proteins (24,25). Zhang
et al (21) reported that
inhibition of Smad2/3 gene expression partially decreased
the apoptosis rate of gliomas. Yang et al (19) demonstrated that Smad2 is involved in
TGF-β induced prostate epithelial cell apoptosis. Taken together,
these results suggest that TGF-β1/Smad2 is involved in the
regulation of the cell apoptosis process; however, this mechanism
has not been previously reported in A549 cells. To the best of our
knowledge, the present study was the first to demonstrate a
decrease in cell proliferation and an increase in the apoptosis
rate of A549 cells following DEX treatment (Fig. 1A-E). Furthermore, the expression of
TGF-β1, Smad2 and caspase-3 were significantly increased following
DEX exposure, which indicated that the TGF-β1/Smad2 pathway may be
involved in DEX-induced apoptosis of A549 cells (Fig. 2A-D).
It has previously been reported that TGF-β1 has a
dual role in apoptosis (26).
Multiple types of tumor cells may at times secrete TGF-β1, which
induces growth factor secretion from stromal cells, which in turn
may enhance the proliferation of cancer cells (27,28). This
is thought to be one mechanism by which TGF-β1 activity increases
the malignancy of cancer (29).
However, it was further reported that TGF-β1 enhances apoptosis in
A549 cells (18,30). Thus, inhibition of growth factor
induction may counteract the effects of TGF-β1 on the suppression
of lung tumor growth. Therefore, in the present study, the TGF-β1
receptor was antagonized with SB431542 with or without DEX
treatment, and SB431542 was observed to inhibit apoptosis and the
expression of TGF-β1, Smad2 and caspase-3 in DEX treated A549 cells
(Figs. 3 and 4).
To conclude, DEX-induced apoptosis of A549 cells may
function via the induction of the TGF-β1/Smad2 signaling pathway,
and DEX may be a potential anti-lung cancer treatment. However, it
is suggested for future studies, that the aforementioned
experiments in the present study be conducted in an in vivo
model, to confirm these results and the therapeutic potential of
dexamethasone.
References
|
1
|
Islami F, Torre LA and Jemal A: Global
trends of lung cancer mortality and smoking prevalence. Transl Lung
Cancer Res. 4:327–338. 2015.PubMed/NCBI
|
|
2
|
Ingawale DK, Mandlik SK and Patel SS: An
emphasis on molecular mechanisms of anti-inflammatory effects and
glucocorticoid resistance. J Complement Integr Med. 12:1–13. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choksi A, Sarojini KV, Vadnal P, Dias C,
Suresh PK and Khandare J: Comparative anti-inflammatory activity of
poly (amidoamine) (PAMAM) dendrimer-dexamethasone conjugates with
dexamethasone-liposomes. Int J Pharm. 449:28–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim JH, Hwang YJ, Han SH, Lee YE, Kim S,
Kim YJ, Cho JH, Kwon KA, Kim JH and Kim SH: Dexamethasone inhibits
hypoxia-induced epithelial-mesenchymal transition in colon cancer.
World J Gastroenterol. 21:9887–9899. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng Y, Izumi K, Li Y, Ishiguro H and
Miyamoto H: Contrary regulation of bladder cancer cell
proliferation and invasion by dexamethasone-mediated glucocorticoid
receptor signals. Mol Cancer Ther. 11:2621–2632. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chauhan D, Li G, Podar K, Hideshima T,
Neri P, He D, Mitsiades N, Richardson P, Chang Y, Schindler J, et
al: A novel carbohydrate-based therapeutic GCS-100 overcomes
bortezomib resistance and enhances dexamethasone-induced apoptosis
in multiple myeloma cells. Cancer Res. 65:8350–8358. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rinehart J, Arnold S, Kloecker G, Lim A,
Zaydan MA, Baeker T, Maheshwari JG, Carloss H, Slone S, Shelton B,
et al: Phase II randomized trial of carboplatin and gemcitabine
with or without dexamethasone pre-treatment in patients with Stage
IV non-small cell lung cancer. Cancer Chemother Pharmacol.
71:1375–1383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geng Y, Wang J, Jing H, Wang HW and Bao
YX: Inhibitory effect of dexamethasone on Lewis mice lung cancer
cells. Genet Mol Res. 13:6827–6836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang YH, Shin HS, Sun Choi H, Ryu ES, Jin
Kim M, Ki Min S, Lee JH, Kook Lee H, Kim KH and Kang DH: Effects of
dexamethasone on the TGF-β1-induced epithelial-to-mesenchymal
transition in human peritoneal mesothelial cells. Lab Invest.
93:194–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bolkenius U, Hahn D, Gressner AM,
Breitkopf K, Dooley S and Wickert L: Glucocorticoids decrease the
bioavailability of TGF-beta which leads to a reduced TGF-beta
signaling in hepatic stellate cells. Biochem Biophys Res Commun.
325:1264–1270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Javelaud D, Alexaki VI, Dennler S,
Mohammad KS, Guise TA and Mauviel A: TGF-β/SMAD/GLI2 signaling axis
in cancer progression and metastasis. Cancer Res. 71:5606–5610.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johansson J, Berg T, Kurzejamska E, Pang
MF, Tabor V, Jansson M, Roswall P, Pietras K, Sund M, Religa P and
Fuxe J: MiR-155-mediated loss of C/EBPβ shifts the TGF-β response
from growth inhibition to epithelial-mesenchymal transition,
invasion and metastasis in breast cancer. Oncogene. 32:5614–5624.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kakudo N, Kushida S, Suzuki K, Ogura T,
Notodihardjo PV, Hara T and Kusumoto K: Effects of transforming
growth factor-beta1 on cell motility, collagen gel contraction,
myofibroblastic differentiation, and extracellular matrix
expression of human adipose-derived stem cell. Hum Cell. 25:87–95.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu Y, Yang S, Huang J, Ruan S, Zheng Z and
Lin J: Tgf-β1 induces autophagy and promotes apoptosis in renal
tubular epithelial cells. Int J Mol Med. 29:781–790.
2012.PubMed/NCBI
|
|
15
|
Miao ZF, Li WY, Wang ZN, Zhao TT, Xu YY,
Song YX, Huang JY and Xu HM: Lung cancer cells induce senescence
and apoptosis of pleural mesothelial cells via transforming growth
factor-beta1. Tumour Biol. 36:2657–2665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng RP, Bai T, Zhou XG, Xu CG, Wang W,
Xu MW and Zhang J: Lefty A protein inhibits TGF-β1-mediated
apoptosis in human renal tubular epithelial cells. Mol Med Rep.
8:621–625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bai L, Yu Z, Wang C, Qian G and Wang G:
Dual role of TGF-β1 on Fas-induced apoptosis in lung epithelial
cells. Respiratory Physiol Neurobiol. 177:241–246. 2011. View Article : Google Scholar
|
|
18
|
Liu Z, Xue L, Liu Z, Huang J, Wen J, Hu J,
Bo L and Yang R: Tumor necrosis factor-like weak inducer of
apoptosis accelerates the progression of renal fibrosis in lupus
nephritis by activating SMAD and p38 MAPK in TGF-β1 signaling
pathway. Mediators Inflamm. 2016:89864512016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Wahdan-Alaswad R and Danielpour D:
Critical role of Smad2 in tumor suppression and transforming growth
factor-beta-induced apoptosis of prostate epithelial cells. Cancer
Res. 69:2185–2190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Lei W, Wang X, Tang Y and Song J:
Glucocorticoid induces mesenchymal-to-epithelial transition and
inhibits TGF-β1-induced epithelial-to-mesenchymal transition and
cell migration. FEBS Lett. 584:4646–4654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Wu L, Wang J, Li G, Feng D, Zhang
B, Li L, Yang J, Ma L and Qin H: Opposing effects of PI3K/Akt and
Smad-dependent signaling pathways in NAG-1-induced glioblastoma
cell apoptosis. PLoS One. 9:e962832014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koćwin M, Jonakowski M, Przemęcka M, Zioło
J, Panek M and Kuna P: The role of the TGF-SMAD signalling pathway
in the etiopathogenesis of severe asthma. Pneumonol Alergol Pol.
84:290–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyazaki M, Ohashi R, Tsuji T, Mihara K,
Gohda E and Namba M: Transforming growth factor-beta 1 stimulates
or inhibits cell growth via down- or up-regulation of p21/Waf1.
Biochem Biophys Res Commun. 246:873–880. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang N, Zhao B, Rasul A, Qin H, Li J and
Li X: PIAS1-modulated Smad2/4 complex activation is involved in
zinc-induced cancer cell apoptosis. Cell Death Dis. 4:e8112013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kidd M, Schimmack S, Lawrence B, Alaimo D
and Modlin IM: EGFR/TGFα and TGFβ/CTGF signaling in neuroendocrine
neoplasia: Theoretical therapeutic targets. Neuroendocrinology.
97:35–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sánchez-Capelo A: Dual role for TGF-beta1
in apoptosis. Cytokine Growth Factor Rev. 16:15–34. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Penafuerte C and Galipeau J: TGF beta
secreted by B16 melanoma antagonizes cancer gene immunotherapy
bystander effect. Cancer Immunol Immunother. 57:1197–1206. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Yu Z, Muranski P, Palmer DC,
Restifo NP, Rosenberg SA and Morgan RA: Inhibition of TGF-β
signaling in genetically engineered tumor antigen-reactive T cells
significantly enhances tumor treatment efficacy. Gene Ther.
20:575–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Glick AB: TGFbeta1, back to the future:
Revisiting its role as a transforming growth factor. Cancer Biol
Ther. 3:276–283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Gao W and Zhang D: Effects of
cigarette smoke extract on A549 cells and human lung fibroblasts
treated with transforming growth factor-beta1 in a coculture
system. Clin Exp Med. 10:159–167. 2010. View Article : Google Scholar : PubMed/NCBI
|