Introduction
A statin is a drug used to treat hyperlipidemia and
functions by inhibiting 3-hydroxy-3-metylglutaryl coenzyme A
reductase. Statins have gained much recent attention due to their
anticancer effects. Previous studies have shown that statins can
prolong survival, while others have reported no benefits in cancer
patients (1). Concerning prostate
cancer, the anticancer effect of statins is controversial (2,3). We
previously reported that statins inhibit prostate cancer
progression via suppressing the expression of insulin-like growth
factor 1 receptor (IGF1R) and increasing ANXA10 (4,5). However,
the exact mechanism of their anticancer properties remains
unclear.
There has been recent interest and concerns
regarding intratumoral de novo androgen synthesis in
castration-resistant prostate cancer (CRPC). Now we are treating
CRPC patients with enzalutamide and abiraterone, which attenuate
the effects of intratumoral de novo androgens. In de novo androgen
synthesis, cholesterol is the primary material, and various enzymes
play important roles. We previously reported that intracellular
cholesterol levels are decreased in androgen-independent prostate
cancer cells after treatment with simvastatin (6); however, alterations in androgen
synthesis-related enzymes are not clear.
In this study, we determined whether simvastatin
alters the expression of enzymes involved in androgen synthesis in
CRPC cells. We also explored a new combination therapy of statins
and other drugs that inhibit the overexpression of androgen
synthesis-related enzymes.
Materials and methods
Cells and chemicals
Human prostate cancer cell lines PC-3, LNCaP, and
22RV1 were purchased from DS Pharma Biomedical (Osaka, Japan) and
cultured in RPMI 1640 (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% FBS (Moregate BioTech, Bulimba,
Australia). PC-3 is an androgen receptor-negative human prostate
cancer cell line (7). LNCaP-LA cells,
which were generated from LNCaP cells, were cultured in medium
containing 10% charcoal-stripped fetal bovine serum (FBS) for more
than 3 months.
Measurement of testosterone and
dihydrotestosterone (DHT) in culture medium
Cells were cultured on a 6-well plate and incubated
overnight in medium containing 10% FBS. Cells were then incubated
with or without simvastatin (5 µM). After 48 h, androstenedione
(100 µM) was added to the medium. After 24 h, culture medium was
collected, and testosterone and DHT concentrations were measured
using liquid chromatography-tandem mass spectrometry (LC-MS/MS)
(ASKA Pharmaceutical Medical Co., Ltd., Kawasaki, Japan). RIPA
buffer was added to wells and protein concentration was measured by
the DC Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Testosterone and DHT levels were calculated by dividing the
results of the protein assay by the total protein
concentration.
RT-qPCR
Transcript levels were quantified using the Applied
Biosystems 7300 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. Total RNA was extracted, cDNA was
synthesized (8), and polymerase chain
reaction (PCR) amplification was performed, using 2 µl cDNA and the
StAR, CYP11A1, CYP17A1, aldo-keto reductase family 1 member C3
(AKR1C3), HSD3B1, HSD3B2, SRD5A1, SRD5A2, and AKR1C2 primers (No.
Hs00986559_g1, Hs00167984_m1, Hs01124136_m1, Hs00366267_m1,
Hs00426435_m1, Hs00605123_m1, Hs00602694_mH, Hs00165843_m1, and
Hs00912742_m1, respectively; Applied Biosystems). Next, PCR was
performed for one cycle of 10 min at 95°C followed by 40 cycles of
15 sec at 95°C and 60 sec at 60°C. b-Actin (No. 4326315E, Applied
Biosystems) transcript levels were used as the internal control.
mRNA fold changes were quantified using ΔΔCq.
MTS assay
Cells were plated onto a 96-well plate in 100 µl
culture medium containing 10% FBS. After 24 h, cells were incubated
with medium containing simvastatin (5 µM) and/or meclofenamic acid
(50 µM). After incubation for 48 h, the number of living cells was
measured using the MTS assay.
Migration assay
Cells were plated onto a 12-well plate and grown to
confluence. A 1,000-µl tip was used to make a denuded area. Cells
were washed twice with PBS and incubated with medium containing
various concentrations of simvastatin for 48 h. Mitomycin C (0.5
µM) was added to the medium to inhibit cell proliferation.
Photographs were taken at 0 and 48 h, and the distance of cell
migration was determined by subtracting the values obtained at 0 h
from those obtained at 48 h. Migration distance is expressed as
fold change over the control.
siRNA transfection
Cells were transfected with ON-TARGETplus
Non-targeting Pool (no. D-001810-10-05; Dharmacon, Waltham, MA,
USA) or ON-TARGETplus AKR1C3 siRNA (No. L-008116-00-0005,
Dharmacon) using DharmaFect 2 (Dharmacon). Cells were incubated for
48 h after transfection.
Western blot analysis
Cell lysates were prepared in RIPA buffer containing
1 mM sodium orthovanadate (Sigma-Aldrich; Merck KGaA) and Halt
Protease Inhibitor Cocktail (Pierce; Thermo Fisher Scientific,
Inc.). Samples were boiled for 5 min; an equal amount of protein
(30 µg/lane) was subjected to 4–12% SDS-PAGE and transferred onto
nitrocellulose membranes. Each membrane was incubated with the
following primary polyclonal antibodies: rabbit anti-Akt (1:1,000),
rabbit anti-phospho-Akt (Ser473) (1:1,000) (Cell Signaling
Technology, Inc., Beverly, MA, USA). Blots were developed using a
1:2,000 dilution of the HRP-conjugated secondary antibody (Cell
Signaling Technology, Inc.). Proteins were visualized using
Immobilon (Merck Millipore, Darmstadt, Germany).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Differences between values were evaluated by one-way ANOVA using
Tukey's post hoc analysis and Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Simvastatin altered the expression of
genes encoding steroidogenic enzymes in androgen-independent
prostate cancer cells
We examined PC-3, LNCaP-LA and 22Rv1 cells to
determine whether simvastatin alters genes that encode
steroidogenic enzymes in androgen-independent prostate cancer
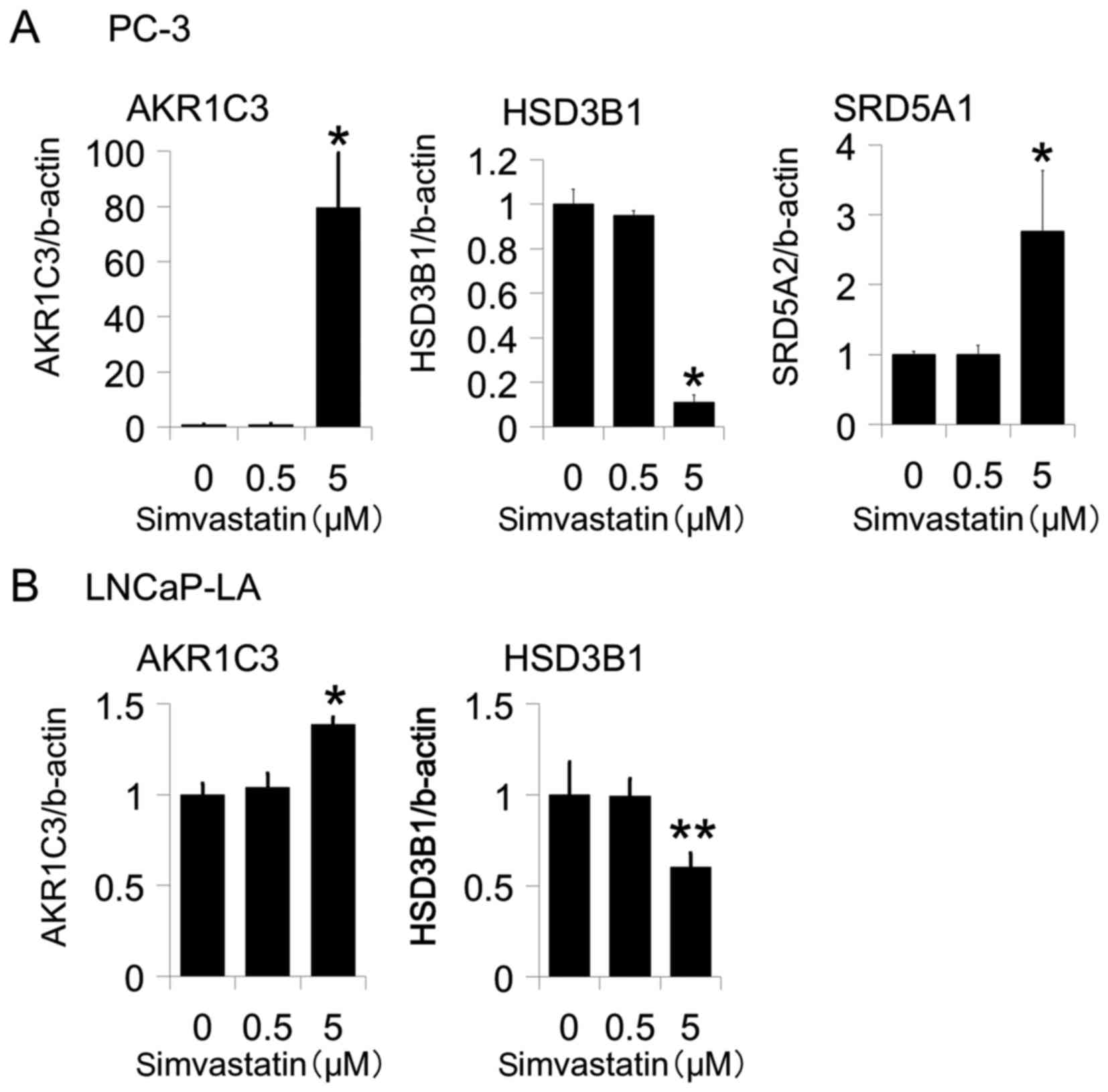
cells. After treatment with simvastatin, the expression of AKR1C3
was increased in PC-3 and LNCaP-LA cells (Figs. 1A and 2A) but not in 22Rv1 cells (data not shown).
Moreover, the fold change was more than 60 times in PC-3 cells.
Conversely, the expression of hydroxy-delta-5-steroid
dehydrogenase, 3 beta- and steroid delta-isomerase 1 (HSD3B1) was
decreased in PC-3 and LNCaP-LA cells (Figs. 1A and 2A) but not in 22Rv1 cells (data not shown).
Moreover, simvastatin increased steroid 5 alpha-reductase 1
(SRD5A1) expression in PC-3 (Fig. 1A)
but not in LNCaP-LA or 22Rv1 cells (data not shown). The expression
of steroidogenic acute regulatory protein (StAR), cytochrome P450
family 11 subfamily A member 1 (CYP11A1), cytochrome P450 family 17
subfamily A member 1 (CYP17A1), hydroxy-delta-5-steroid
dehydrogenase, 3 beta- and steroid delta-isomerase 2 (HSD3B2),
steroid 5 alpha-reductase 2 (SRD5A2), and aldo-keto reductase
family 1 member C2 (AKR1C2) did not change following treatment with
simvastatin (data not shown).
Effects of AKR1C3 expression on
testosterone and DHT levels in PC-3 cell culture medium
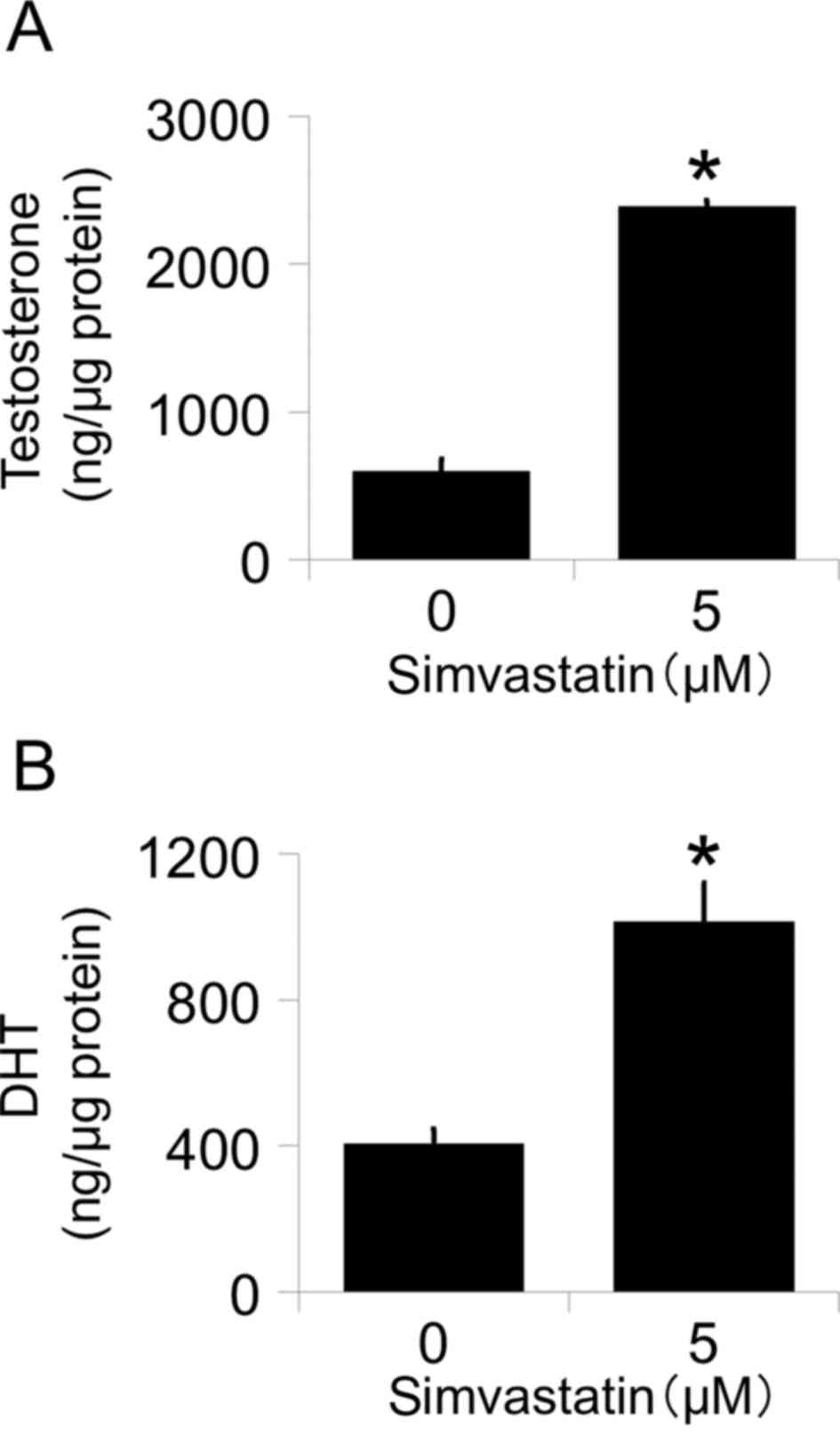
To determine whether increased levels of AKR1C3
affect the de novo synthesis of intracellular androgen, we measured
the testosterone and DHT levels in culture medium following
treatment with simvastatin by LC-MS/MS. Simvastatin significantly
increased both testosterone (Fig. 2A)
and DHT (Fig. 2B) levels after the
addition of androstenedione. These data show that the up-regulation
of AKR1C3 is functional.
AKR1C3 inhibition increased the
simvastatin-induced inhibition of cell proliferation and
migration
PC-3 is an AR-negative human prostate cancer cell
line. Therefore, there is a possibility that an increase in
testosterone and DHT levels does not affect cell viability. In
contrast, the overexpression of AKR1C3 promotes angiogenesis and
aggressiveness in PC-3 cells (9). To
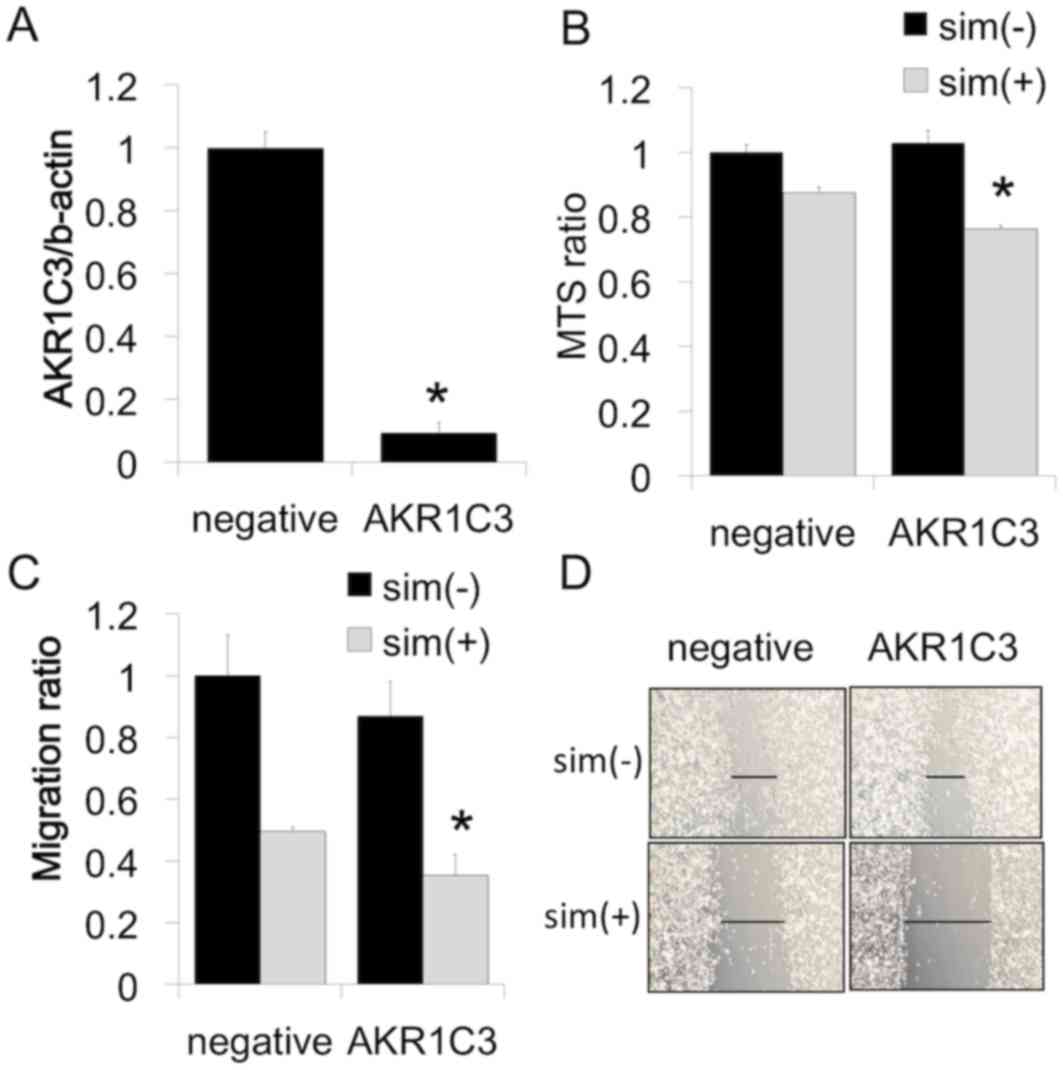
further determine whether increased AKR1C3 expression affects
simvastatin-induced cell viability, AKR1C3 expression was reduced
by transfection with siRNA against AKR1C3. siRNA treatment
inhibited the expression of AKR1C3 mRNA in PC-3 cells (Fig. 3A). The reduction in AKR1C3 expression
in PC-3 cells following siRNA transfection was not associated with
basal cell proliferation and migration; however, siRNA transfection
with simvastatin significantly decreased both cell proliferation
(Fig. 3B) and cell migration
(Fig. 3C and D) compared to
simvastatin alone.
Meclofenamic acid increased the
simvastatin-induced inhibition of cell proliferation and
migration
Some drugs are reported to inhibit AKR1C3.
Meclofenamic acid is an NSAID as well as one of the best inhibitors
of AKRs, especially AKR1C3 (10).
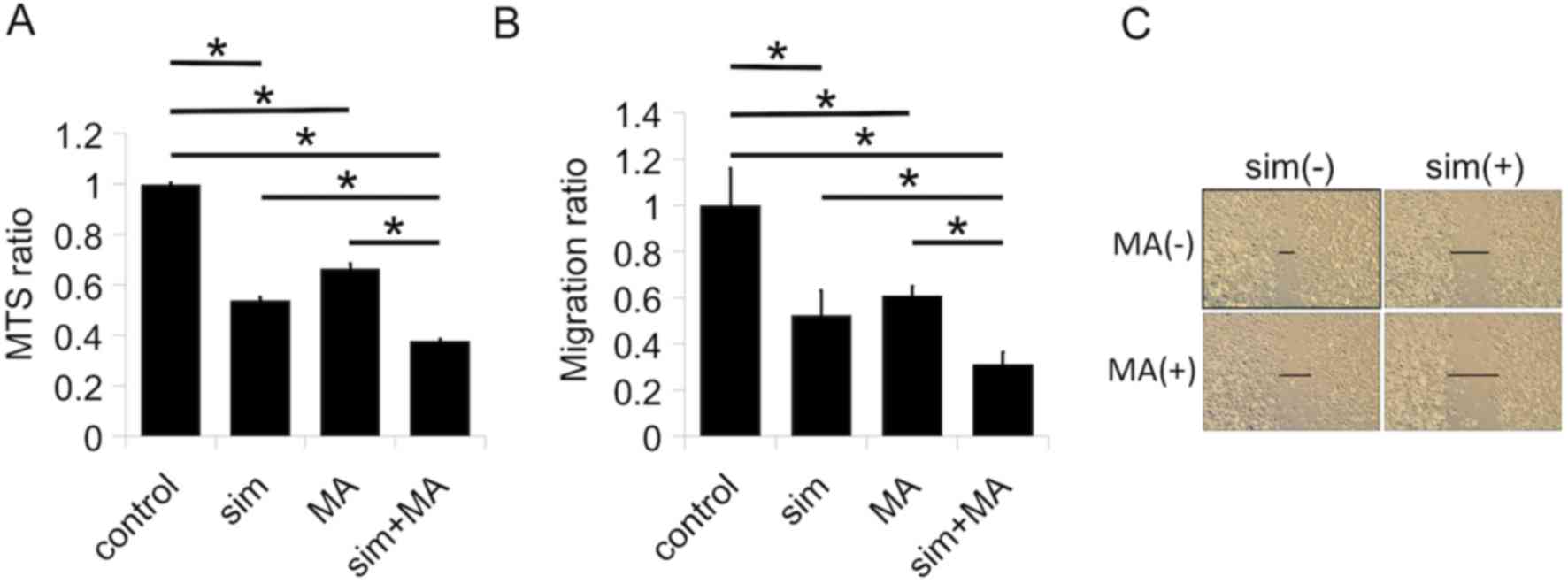
Therefore, we evaluated the combinatory effects of simvastatin and
meclofenamic acid in PC-3 cells. Treatment with either simvastatin
or meclofenamic acid alone inhibited cell proliferation (Fig. 4A) and migration (Fig. 4B and C). The combination of the two
drugs further enhanced cell proliferation (Fig. 4A) and migration (Fig. 4B and C).
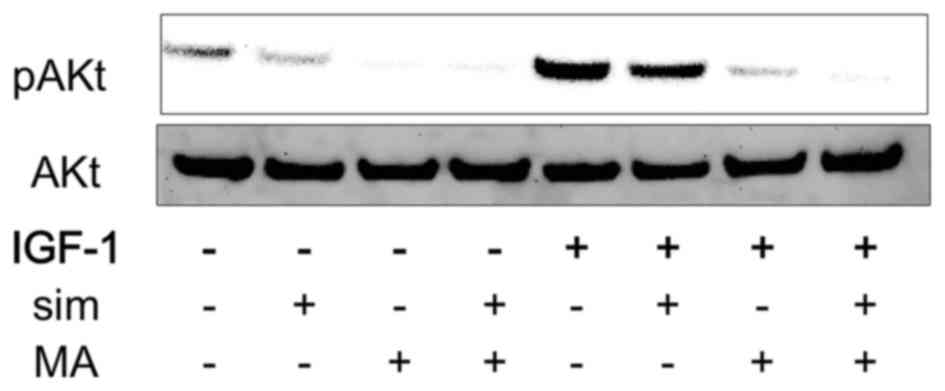
Combination of simvastatin and
meclofenamic acid inhibited IGF1-induced Akt activation
AKR1C3 overexpression induces Akt activation in PC-3
cells (9). We previously showed that
simvastatin without IGF1 decreases IGF1R expression strongly in
PC-3 cells (4). IGF1-Akt activation
is a well-known pathway in prostate cancer. We hypothesized that
inhibiting simvastatin-stimulated AKR1C3 expression with an AKR1C3
inhibitor would have a synergistic effect on simvastatin-blocked
IGF1-induced Akt activation. Therefore, the effects of the
combination of simvastatin and meclofenamic acid on IGF1-induced
Akt activation were evaluated in PC-3 cells. Treatment with either
simvastatin or meclofenamic acid alone attenuated IGF1-induced Akt
activation, whereas the combination of simvastatin and meclofenamic
acid further inhibited Akt activation (Fig. 5).
Discussion
The main finding of the present study was that
simvastatin increased AKR1C3 expression in androgen-independent
prostate cancer cells. Furthermore, the combination of simvastatin
and meclofenamic acid, an AKR1C3 inhibitor, further suppressed PC-3
cell proliferation, migration, and Akt activation compared with
simvastatin alone.
Statins have recently been studied for their
pleiotrophic effects, which may make them relevant for cancer
prevention or treatment. Clinical reports have shown that statin
use is beneficial for overall survival and cancer-specific survival
both before and after prostate cancer diagnosis (1,2). In
contrast, Platz et al reported that the use of statin drugs
was not associated with the overall risk of prostate cancer
(3). In vitro, statins exert
many biological activities that inhibit prostate cancer progression
(e.g., lowering raft cholesterol content, inhibiting
cyclin-dependent-kinase-2 activity, decreasing IGF1 receptor
expression, and increasing ANXA10 expression) (4,5,11,12). These
results indicate that statins have anticancer potential.
Statins inhibit 3-hydroxy-3-metylglutaryl coenzyme A
reductase, one of the most important players in cholesterol
biosynthesis. In androgen-dependent prostate cancer cells, statins
do not lower intracellular cholesterol levels by up-regulating the
low density lipoprotein receptor in the same manner as in normal
cells (6). Conversely, statins
decrease intracellular cholesterol levels in androgen-independent
prostate cancer cells, which cannot regulate low density
lipoprotein receptor expression (6).
In this study, we focused on the decrease in intracellular
cholesterol levels following treatment with statins in CRPC. Recent
reports have shown that de novo androgen synthesis is a therapeutic
objective in CRPC (13). In the
androgen synthesis pathway, cholesterol is the primary material.
Therefore, we examined the effects of statins on androgen
synthesis-related enzymes in CRPC cells.
Simvastatin increased the expression of AKR1C3 in
PC-3 cells. AKR1C3 exhibits 3α-, 17β- and 20α-hydroxysteroid
dehydrogenase activities (14). The
expression of AKR1C3 is increased in several human cancers,
including kidney (15) and breast
(16). Concerning prostate cancer,
localized, metastatic and recurrent prostate cancer has high levels
of AKR1C3 (17–19). In addition, elevated AKR1C3 expression
promotes the aggressiveness of PC-3 cells, which lack AR (7). These data indicate that increased levels
of AKR1C3 induce prostate cancer progression not only by
synthesizing intracellular androgen but also by
androgen-independent mechanisms.
Medical agents such as non-steroidal
anti-inflammatory drugs (NSAIDs), steroids, flavonoids,
cyclopentane derivatives, and benzodiazepines inhibit AKR1C3
(20). One example of an NSAID is
meclofenamic acid, which inhibits AKR1C3, cyclooxygenase-1, and
cyclooxygenase-2 more strongly than other NSAIDs (21,22).
Meclofenamic acid inhibits androgen-independent prostate cancer
progression both in vitro and in vivo (23). In this study, AKR1C3 siRNA did not
affect PC-3 cell proliferation and migration, whereas meclofenamic
acid inhibited these processes, suggesting that meclofenamic acid
also has anticancer effects without the AKR1C3 mechanism. The
combination of simvastatin and meclofenamic acid inhibited PC-3
cell proliferation, migration, and Akt activation to a greater
extent than simvastatin or meclofenamic acid alone. Previous
reports have described combination therapy using statins and NSAIDs
for the treatment of prostate cancer, which works by inhibiting
NF-κB (24) or IL-6 (25). These results reveal that the
inhibition of AKR1C3 is an underlying mechanism of the combination
therapy of simvastatin and meclofenamic acid.
The present study had several limitations. First,
three androgen-independent prostate cancer cell lines responded to
simvastatin with different levels of AKR1C3 expression. Prostate
cancer is very heterogenic. In particular, androgen-independent
prostate cancer cells have a different genetic background (26). Therefore, our results may not be
applicable to all CRPCs. In addition, we evaluated the effects of
simvastatin and meclofenamic acid only in PC-3 cells, and studied
the changes of gene expressions following simvastatin treatment
only in one single time rather than performing a time-course
experiment. Concerning Akt activation by IGF-1, we also checked
only in one single time and IGF1R phosphorylation status was not
evaluated. Moreover, in vivo models are required to show
whether the combination of simvastatin and meclofenamic acid may
have a curative influence on CRPC.
In summary, simvastatin increased AKR1C3 expression
in androgen-independent prostate cancer cells, and the combination
of simvastatin and meclofenamic acid further inhibited PC-3 cell
proliferation, migration and Akt activation compared with
simvastatin alone. These results suggest that the combination of
statin and NSAIDs may be an effective strategy for the treatment of
prostate cancer.
Acknowledgements
We thank Ms. Naomi Takase, Ms. Atsuko Oyama, and Ms.
Hayumi Oyama for their technical assistance. This work was
supported by JSPS KAKENHI Grant no. 25861410.
Glossary
Abbreviations
Abbreviations:
|
AKR1C3
|
aldo-keto reductase family 1 member
C3
|
|
IGF1R
|
insulin-like growth factor 1
receptor
|
|
CRPC
|
castration-resistant prostate
cancer
|
|
DHT
|
dihydrotestosterone
|
|
LC-MS/MS
|
liquid chromatography coupled with
tandem mass spectrometry
|
|
FBS
|
fetal bovine serum
|
|
IGF
|
insulin-like growth factor
|
|
NSAIDs
|
non-steroidal anti-inflammatory
drugs
|
References
|
1
|
Zhong S, Zhang X, Chen L, Ma T, Tang J and
Zhao J: Statin use and mortality in cancer patients: Systematic
review and meta-analysis of observational studies. Cancer Treat
Rev. 41:554–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bansal D, Undela K, D'Cruz S and Schifano
F: Statin use and risk of prostate cancer: A meta-analysis of
observational studies. PLoS One. 7:e466912012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Platz EA, Tangen CM, Goodman PJ, Till C,
Parnes HL, Figg WD, Albanes D, Neuhouser ML, Klein EA, Lucia MS, et
al: Statin drug use is not associated with prostate cancer risk in
men who are regularly screened. J Urol. 192:379–384. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sekine Y, Furuya Y, Nishii M, Koike H,
Matsui H and Suzuki K: Simvastatin inhibits the proliferation of
human prostate cancer PC-3 cells via down-regulation of the
insulin-like growth factor 1 receptor. Biochem Biophys Res Commun.
372:356–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyazawa Y, Sekine Y, Kato H, Furuya Y,
Koike H and Suzuki K: Simvastatin Up-regulates annexin A10 that can
inhibit the proliferation, migration, and invasion in
androgen-independent human prostate cancer cells. Prostate.
77:337–349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Furuya Y, Sekine Y, Kato H, Miyazawa Y,
Koike H and Suzuki K: Low-density lipoprotein receptors play an
important role in the inhibition of prostate cancer cell
proliferation by statins. Prostate Int. 4:56–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sobel RE and Sadar MD: Cell lines used in
prostate cancer research: A compendium of old and new lines-part 1.
J Urol. 173:342–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki K, Koike H, Matsui H, Ono Y, Hasumi
M, Nakazato H, Okugi H, Sekine Y, Oki K, Ito K, et al: Genistein, a
soy isoflavone, induces glutathione peroxidase in the human
prostate cancer cell lines LNCaP and PC-3. Int J Cancer.
99:846–852. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dozmorov MG, Azzarello JT, Wren JD, Fung
KM, Yang Q, Davis JS, Hurst RE, Culkin DJ, Penning TM and Lin HK:
Elevated AKR1C3 expression promotes prostate cancer cell survival
and prostate cell-mediated endothelial cell tube formation:
Implications for prostate cancer progression. BMC Cancer.
10:6722010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Flanagan JU, Yosaatmadja Y, Teague RM,
Chai MZ, Turnbull AP and Squire CJ: Crystal structures of three
classes of non-steroidal anti-inflammatory drugs in complex with
aldo-keto reductase 1C3. PLoS One. 8:e439652012. View Article : Google Scholar
|
|
11
|
Zhuang L, Kim J, Adam RM, Solomon KR and
Freeman MR: Cholesterol targeting alters lipid raft composition and
cell survival in prostate cancer cells and xenografts. J Clin
Invest. 115:959–968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sivaprasad U, Abbas Tand and Dutta V:
Differential efficacy of 3-hydroxy-3-methlyglutaryl CoA reductase
inhibitors on the cell cycle of prostate cancer cells. Mol Cancer
Ther. 5:2310–2316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai C, Chen S, Ng P, Bubley GJ, Nelson PS,
Mostaghel EA, Marck B, Matsumoto AM, Simon NI, Wang H, et al:
Intratumoral de novo steroid synthesis activates androgen receptor
in castration-resistant prostate cancer and is upregulated by
treatment with CYP17A1 inhibitors. Cancer Res. 71:6503–6513. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuura K, Shiraishi H, Hara A, Sato K,
Deyashiki Y, Ninomiya M and Sakai S: Identification of a principal
mRNA species for human 3alpha-hydroxysteroid dehydrogenase isoform
(AKR1C3) that exhibits high prostaglandin D2 11-ketoreductase
activity. J Biochem. 124:940–946. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Azzarello JT, Lin HK, Gherezghiher A,
Zakharov V, Yu Z, Kropp BP, Culkin DJ, Penning TM and Fung KM:
Expression of AKR1C3 in renal cell carcinoma, papillary urothelial
carcinoma, and Wilms' tumor. Int J Clin Exp Pathol. 3:147–155.
2009.PubMed/NCBI
|
|
16
|
Byrns MC, Duan L, Lee SH, Blair IA and
Penning TM: Aldo-keto reductase 1C3 expression in MCF-7 cells
reveals roles in steroid hormone and prostaglandin metabolism that
may explain its over-expression in breast cancer. J Steroid Biochem
Mol Biol. 118:177–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakamura Y, Suzuki T, Nakabayashi M, Endoh
M, Sakamoto K, Mikami Y, Moriya T, Ito A, Takahashi S, Yamada S, et
al: In situ androgen producing enzymes in human prostate cancer.
Endocr Relat Cancer. 12:101–107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stanbrough M, Bubley G, Ross K, Golub TR,
Rubin MA, Penning TM, Febbo PG and Balk SP: Increased expression of
genes converting adrenal androgens to testosterone in
androgen-independent prostate cancer. Cancer Res. 66:2815–2825.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wako K, Kawasaki T, Yamana K, Suzuki K,
Jiang S, Umezu H, Nishiyama T, Takahashi K, Hamakubo T, Kodama T
and Naito M: Expression of androgen receptor through
androgen-converting enzymes is associated with biological
aggressiveness in prostate cancer. J Clin Pathol. 61:448–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Byrns MC, Jin Y and Penning TM: Inhibitors
of type 5 17β-hydroxysteroid dehydrogenase (AKR1C3): Overview and
structural insights. J Steroid Biochem Mol Biol. 125:95–104. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kalgutkar AS, Crews BC, Rowlinson SW,
Marnett AB, Kozak KR, Remmel RP and Marnett LJ: Biochemically based
design of cyclooxygenase-2 (COX-2) inhibitors: Facile conversion of
nonsteroidal antiinflammatory drugs to potent and highly selective
COX-2 inhibitors. Proc Natl Acad Sci USA. 97:pp. 925–930. 2000;
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ouellet M and Percival MD: Effect of
inhibitor time-dependency on selectivity towards cyclooxygenase
isoforms. Biochem J. 306:247–251. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soriano-Hernández AD, Galvan-Salazar HR,
Montes-Galindo DA, Rodriguez-Hernandez A, Martinez-Martinez R,
Guzman-Esquivel J, Valdez-Velazquez LL, Baltazar-Rodriguez LM,
Espinoza-Gómez F, Rojas-Martinez A, et al: Antitumor effect of
meclofenamic acid on human androgen-independent prostate cancer: A
preclinical evaluation. Int Urol Nephrol. 44:471–477. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng X, Cui XX, Avila GE, Huang MT, Liu
Y, Patel J, Kong AN, Paulino R, Shih WJ, Lin Y, et al: Atorvastatin
and celecoxib inhibit prostate PC-3 tumors in immunodeficient mice.
Clin Cancer Res. 13:5480–5487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Cui XX, Goodin S, Ding N, Van
Doren J, Du Z, Huang MT, Liu Y, Cheng X, Dipaola RS, et al:
Inhibition of IL-6 expression in LNCaP prostate cancer cells by a
combination of atorvastatin and celecoxib. Oncol Rep. 31:835–841.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fraser M, Berlin A, Bristow RG and van der
Kwast T: Genomic, pathological, and clinical heterogeneity as
drivers of personalized medicine in prostate cancer. Urol Oncol.
33:85–94. 2015. View Article : Google Scholar : PubMed/NCBI
|