Introduction
Quantum dots (QD), also known as semiconductor
nanocrystals, mainly consist of elements including II–IV group
elements (Cdse and Cds) or III–V group elements (InP and InAs). The
particle is smaller than 100 nm in diameter. Due to the presence of
crystal core, QD can emit specific fluorescence (1,2). In order
to combine with other biological protein molecules when applying in
medical field, amino groups or carboxyl groups can often be seen on
the surface of QD (3). QD
modification mainly uses static adsorption, covalent coupling,
thiol exchange and other methods to modify antibodies, peptides and
oligonucleotides onto the surface of QD, where covalent coupling is
the most common and stable method. Compared to the traditional
organic molecular fluorescent materials, QD has the following
advantages: i) wide and continuous excitation spectrum, with large
absorption coefficient and high quantum yield; ii) narrow and
symmetric emission spectrum without trailing; iii) strong
fluorescence intensity and high signal to noise ratio; iv) good
light stability and strong ability to resist photobleaching; v)
emission wavelength changes with particle size. Owing to its unique
chemical and optical properties, QD has gradually been applied in
tumor biological medicine, such as prostate cancer (4), pancreatic cancer (5), breast cancer (5), liver cancer (6), colorectal cancer (7) and nasopharyngeal carcinoma (NPC)
(8). This study aimed to explore the
605 nm carboxyl of water-soluble quantum dots to assess the
practicability of the NPC marker EBNA1 antibody.
NPC is a common malignant tumor in the Southern
Coast, Western Hunan and other places of China (9). Its incidence is up to 30/100,000. It is
the most frequent malignant tumor of otolaryngology head and neck
surgery. Due to the complex nasopharyngeal anatomy, NPC is
difficult to detect in early stage and only 10–20% of the patients
are diagnosed at the early stage. Once there appear obvious
symptoms, the patients are mainly at middle-to-advanced stage, so
it is crucial to early diagnose NPC (10). The main causes of diagnostic
difficulty in NPC are the atypical symptoms and no proper biopsy.
There is a certain correlation between NPC and EB virus infection
(11), so it is a main measurement
for early NPC diagnosis in clinic to detect serum EB virus
infection (such as EB-VCA IgA, EBNA1, Zta-IgA, EA/IgA and EBV-DNA)
of NPC patients (12–14). EBNA1 is expressed in all of the NPC
patients. It is the only virus core protein in NPC, so its change
may be one of the cancer-promotion factors (15). Studies have found that EBNA1 may block
NPC cell cycle at stage G1 through in vitro induction, thus
blocking the growth of NPC cells. Currently, enzyme-linked
immunosorbent assay (ELISA) qualitative analysis is the major
method to detect EBNA1 antibody in clinic (16), but it still has the following
disadvantages: i) ELISA kits are produced by different
manufacturers and there is no unified standard; ii) ELISA method is
complicated in use, so the results are affected by the operators,
which is not likely to be carried out in grass-roots hospitals;
iii) ELISA method is unable to realize rapid detection and moreover
it is qualitative analysis.
Therefore, water-soluble carboxylated QD was used as
the luminescent material in this study. Coupled with EBNA1 by
covalent cross-linking method, the feasibility of 605 nm
water-soluble carboxylated QD to label EBNA1 was explored and
reported.
Materials and methods
Materials and reagents
Water-soluble (605 nm) carboxylated QD was purchased
from Jiayuan Quantum Dots Co., Ltd. (Wuhan, Hubei, China).
Crosslinking agent, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (EDC) was purchased from Sigma (Jiayuan, China). EBV
EBNA1 antigen and positive standard of EBV EBNA1 antibody were
purchased from Abcam (Cambridge, UK). Other common reagents were
purchased from the Otolaryngology Laboratory of Xiangya Hospital of
Central South University (Hunan, China).
All healthy control blood samples were obtained from
volunteers, who were the permanent residents in Hunan province,
China. All NPC samples were taken from NPC patients hospitalized in
Xiangya Hospital of Central South University from May, 2012 to
December, 2012. The patients were recorded for personal basic
information and clinical data (age, sex, hospital, department,
admission number, pathological diagnosis, clinical stage, main
findings, ID number, native place, home address and phone number),
as shown in the spreadsheet. All the samples were verified by the
patients' consent and informed consent was signed. The study was
approved by the Ethics Committee of Xiangya Hospital of Central
South University.
Serum collection
i) A 5 ml serum collection tube was used to collect
fasting venous blood. The tube was filled with the blood as full as
possible, for ~3–4 ml, and then slightly turned upside down 3
times; ii) the tube was placed at room temperature for 1 h; iii)
the tube was centrifuged (1,600 × g) for 10 min at 4°C; iv) the
upper layer of the serum was carefully removed to i). The 5 ml
cryogenic tube was placed on ice, ~600 µl ×2 tubes. The tubes were
numbered and labeled, and preserved at −80°C.
Instruments and equipments
UV–VIS spectrophotometer (UV-2550; Shimadzu, Kyoto,
Japan); LS-55 fluorescence spectrophotometer (Perkin Elmer,
Waltham, MA, USA); ultrafiltration tube (Millipore, Billerica, MA,
USA); desalting column (GE Healthcare, Piscataway, NJ, USA); immune
chromatography test paper (Wuhan Jiayuan Quantum Dots Co., Ltd.,
Wuhan, China); gel imaging system (Alpha Imager HP).
Sample pretreatment
EBV EBNA1 antigen (Abcam) at −20°C, was thawed at
room temperature. It was dissolved in the buffer solution (20 mM
borate, pH 7.4). Ultrafiltration tube with molecular cut off of 10
kDa was used to remove impurities, and final sample concentration
was 2.2 mg/ml (Table I).
 | Table I.Sample amount. |
Table I.
Sample amount.
| Antigen | Antigen/QD | Maximum emission
peak | Full width at half
maximum |
|---|
| Solution | 10:1 | 605 nm | 24 nm |
| Solution | 40:1 | 605 nm | 24 nm |
Labeling method
Covalent cross-linking was carried out on
water-soluble carboxylated QD (Q3605) and EBV EBNA1 antigen under
EDC to form QD-labeled antigen. Ultrafiltration tube with molecular
cut off of 100 kDa was used to remove unreacted antigen and other
impurities, and final product was stored in 50 mM borate at pH 8.4
with 0.05% NaN3.
Experimental procedure
i) Assemble of immune chromatography test paper:
chromatography pad, nitrocellulose membrane (NC membrane) and
absorbent filter paper were pasted on the viscous base in turn.
Biodot strip cutting machine was used to cut the blank test paper
into strips of 3 mm width. ii) Envelope of antigen: 0.5 µl EB NA1
antigen was removed from NC membrane. Until the humidity was
<20%, the membrane was placed in an oven at 25°C for 2 h. iii)
Detection buffer preparation: Antibody dilution was used to dilute
the QD-labeled antigen as the detection buffer, and 75 µl was
removed to the microplate. iv) Sample loading: 5 µl serum from each
of the 60 NPC patients and 30 healthy people and positive samples
of different concentrations, were mixed with detection buffer. Test
paper in (ii) was inserted into microplate of (iii). The liquid
will go upwards along the strip due to capillary action (5). It was observed under UV after 6 min. If
there was a positive reaction, orange fluorescence was observed
under UV. The higher the EBNA1 antibody content was, the stronger
the fluorescence was. However, if there was no corresponding
antibody, then there was no fluorescence or just a weaker one. The
findings were observed and imaged by gel imaging system with UV
light. ELISA absorbency value was measured at the same time. SPSS
13.0 (SPSS Inc., Chicago, IL, USA) was used to obtain ROC of
absorbance to calculate the specificity and sensitivity.
Results
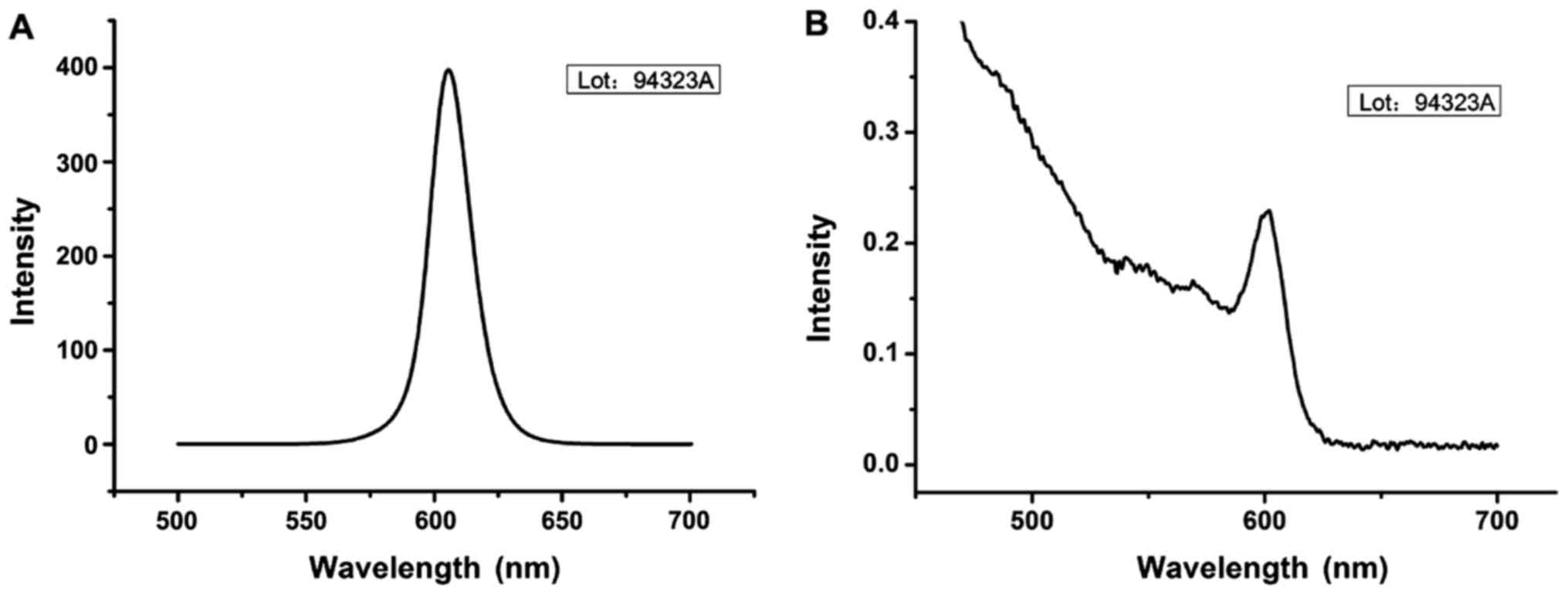
QD fluorescence emission spectrum showed that
fluorescence emission peak reached the peak around 605 nm with the
peak height of 380 (Fig. 1). Agarose
gel electrophoresis showed that the stable covalent bond was formed
between water-soluble carboxyl QD and EBV EBNA1 antigen by amino
and carboxyl condensation on the surface. Water-soluble carboxyl QD
and EBV EBNA1 antigen were successfully linked suggesting a
successful labeling.
There were significant differences between NPC
patients and normal controls in QD-labeled fluorescence intensity
(Table II).
 | Table II.ELISA absorption results of NPC
patients and normal people. |
Table II.
ELISA absorption results of NPC
patients and normal people.
| Group | Minimum | Maximum | Mean (X) | Standard deviation
(S) |
|---|
| Serum absorption of
NPC patients | 0.032044 | 3.217905 | 0.97469163 | 0.667895186 |
| Serum absorption of
normal people | 0.085533 | 0.690848 | 0.17310760 | 0.159114266 |
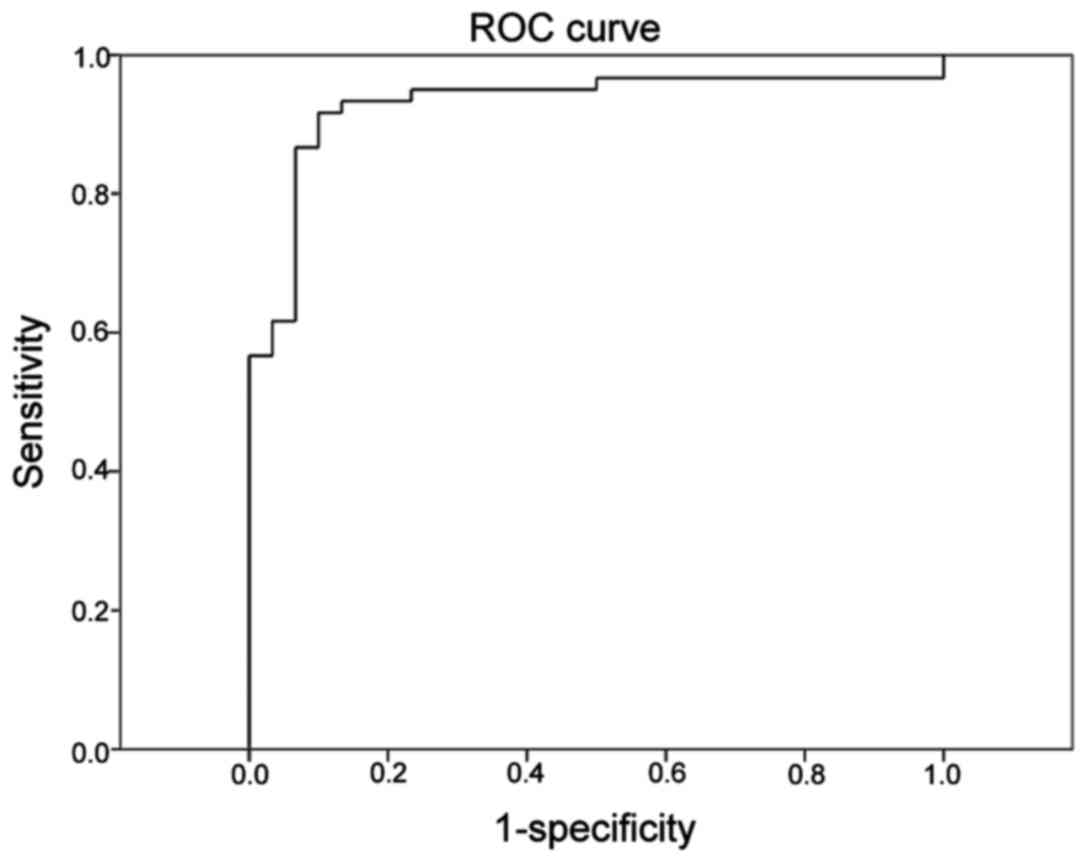
Area under the curve (AUC) was 0.929 (95% CI,
0.871–0.987), which had statistically significant difference
compared to A=0.5 (P<0.001). The most upper and left point on
curve in Fig. 2 was selected and
Youden index was calculated according to coordinates of each point
in SPSS analysis. With the maximum tangent point of Youden index as
the critical point, the optimal threshold of FLISA fluorescence
value in the diagnosis of NPC was 0.680817, with sensitivity of
0.917, specificity of 0.900 and Youden index of 0.817.
A smooth curve was drawn by hand above ROC (fold
line) obtained from SPSS, which was tried to overlap the original
line as far as possible. Then it was easy to see the general trend
of ROC, which was steep in the bottom, slightly flat in the middle
and flat in the upper part. In the lower part of ROC, the true
positive rate increased quickly while the false positive rate more
slowly. After turning point (0.867 and 0.867), the true positive
rate slowed down from the vertical jump, while the false positive
rate accelerated, suggesting the two groups of people had large
overlap; Again, after turning point (0.933 and 0.233), true
positive rate slowed down again, while false positive rate
fastened, suggesting the overlapping of the two groups of people
was decreased. Therefore, dubious value should be selected between
the two turning points, where there was considerable overlapping.
According to statistical results, the corresponding cut-off points
were 1.000988 and 1.091343, the doubtful range was 1.000988 and
1.091343). Sensitivity and specificity of the two points are shown
in Table III.
 | Table III.Sensitivity and specificity of minimum
and maximum values of doubtful range. |
Table III.
Sensitivity and specificity of minimum
and maximum values of doubtful range.
| Cut-off point | Sensitivity | Specificity |
|---|
| 1.000988 | 0.867 | 0.933 |
| 1.091343 | 0.933 | 0.767 |
Discussion
Based on an association between NPC and EB virus
infection, detection of serum EB virus antibodies (e.g., EB-VCA
IgA, EBNA1, Zta-IgA and EA/IgA) can be used for early NPC diagnosis
in the clinics (17–21). With the application of nanotechnology
in biomedical filed, quantum dot emission, especially, with its
unique physical and chemical advantages, small QD, high
fluorescence yield, simple connection method and good stability, is
used for detection on biological molecules. Moreover, QD can be
used as a kind of new light-emitting marker when combined with
tumor markers, which are expected to replace traditional colloidal
gold for immune chromatography technology.
In this study, water-soluble carboxylated QD was
combined with a crosslinking agent, EDC. The reaction principle is
that -COOH on water-soluble carboxylated QD was activated by EDC to
form a medium product, which was reacted with -NH2 on EBV EBNA1
antigen to finally form a stable covalent bond between EBV EBNA1
antigen and QD. Agarose gel electrophoresis showed that the stable
covalent bond was formed between water-soluble carboxyl QD and EBV
EBNA1 antigen by amino and carboxyl condensation on the surface.
Water-soluble carboxyl QD and EBV EBNA1 antigen were successfully
linked suggesting a successful labeling. QD fluorescence emission
spectrum showed that emission peak of QD-EBV EBNA1 was around 380
nm with good fluorescence properties.
This experiment used QD immune chromatography to
detect ELISA absorption value of standard NPC marker EB NA1
antibody of different concentrations. By using the ROC, the
sensitivity and specificity of this technique were calculated.
However, the experiment sample size is too small to determine the
optimal concentration of positive standard, so whether serum sample
EBV EBNA1 is negative or positive cannot be reasonably judged, and
further research is needed to solve this question. Independent
sample test was performed on statistical results by using Levene
test. With F test <0.1, we can obtain the results that variance
is not equal and P<0.001, namely there is statistically
significant difference between NPC group and normal control group
in absorption value of serum. Therefore, it is confirmed that dual
antigen sandwich method combined with QD immune chromatography is
feasible, thus laying a solid foundation for further EBV EBNA1 QD
immune chromatography.
Early diagnosis on malignant tumor depends on the
discovery of new biomarkers, while the progress of immune detection
also relies on the improvement of detection method. With the
application of nanotechnology in biomedical filed, quantum dot
emission, especially, with its unique physical and chemical
advantages, such as small QD, high fluorescence yield, simple
connection method and good stability, is used for detection on
biological molecules. Moreover, it can be used as a kind of new
light-emitting marker when combined with tumor markers, which are
expected to replace traditional colloidal gold for immune
chromatography technology. In this study, the application of QD to
label NPC marker EBV EBNA1 antigen was studied among NPC patients.
Combined with ELISA and immune chromatography, it is confirmed that
dual antigen sandwich method combined with QD immune chromatography
is feasible to detect EBV EBNA1 antibody in NPC patients. However,
due to sample size and optimization of antibody standard
concentration, further research is still needed to determine the
detection threshold. In future studies, we are expected to develop
agent kits for detection of EBV EBNA1 antibody by QD technology and
develop a small-size, convenient and simple instrument. More
detailed work is needed for application of QD in rapid immune
detection in order to achieve QD detection with good repeatability,
high credibility, and simple operation.
References
|
1
|
Yoo G, Park J, Lee SS and Sim HS:
Anisotropic charge Kondo effect in a triple quantum dot. Phys Rev
Lett. 113:2366012014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karki KJ, Widom JR, Seibt J, Moody I,
Lonergan MC, Pullerits T and Marcus AH: Coherent two-dimensional
photocurrent spectroscopy in a PbS quantum dot photocell. Nat
Commun. 5:58692014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blum AS, Moore MH and Ratna BR: Quantum
dot fluorescence as a function of alkyl chain length in aqueous
environments. Langmuir. 24:9194–9197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee H, Kim C, Lee D, Park JH, Searson PC
and Lee KH: Optical coding of fusion genes using multicolor quantum
dots for prostate cancer diagnosis. Int J Nanomed. 12:4397–4407.
2017. View Article : Google Scholar
|
|
5
|
Nigam Joshi P, Agawane S, Athalye MC,
Jadhav V, Sarkar D and Prakash R: Multifunctional inulin tethered
silver-graphene quantum dots nanotheranostic module for pancreatic
cancer therapy. Mater Sci Eng C. 78:1203–1211. 2017. View Article : Google Scholar
|
|
6
|
Li K, Xia C, Wang B, Chen H, Wang T, He Q,
Cao H and Wang Y: Effects of quantum dots on the ROS amount of
liver cancer stem cells. Colloids Surf B Biointerfaces.
155:193–199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Li Y, Chen Z, Wang T, Gu J, Wu X,
Yin Y, Wang M and Pan Z: The evaluation of colorectal cancer risk
in serum by anti-DESMIN-conjugated CdTe/CdS quantum dots. Clin Lab.
63:579–586. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Zhao Y, Luo H, Liu F and Wu Y:
Construction of EGFR peptide gefitinib/quantum dots long
circulating polymeric liposomes for treatment and detection of
nasopharyngeal carcinoma. Biochem Biophys Res Commun. 490:141–146.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li LN, Xiao T, Yi HM, Zheng Z, Qu JQ,
Huang W, Ye X, Yi H, Lu SS, Li XH, et al: MiR-125b increases
nasopharyngeal carcinoma radioresistance by targetingA20/NF-kappaB
signaling pathway. Mol Cancer Ther. 16:2094–2106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su Z, Li G, Liu C, Ren S, Deng T, Zhang S,
Tian Y, Liu Y and Qiu Y: Autophagy inhibition impairs the
epithelial-mesenchymal transition and enhances cisplatin
sensitivity in nasopharyngeal carcinoma. Oncol Lett. 13:4147–4154.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith C, Tsang J, Beagley L, Chua D, Lee
V, Li V, Moss DJ, Coman W, Chan KH, Nicholls J, et al: Effective
treatment of metastatic forms of Epstein-Barr virus-associated
nasopharyngeal carcinoma with a novel adenovirus-based adoptive
immunotherapy. Cancer Res. 72:1116–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen QY, Tang QN, Tang LQ, Chen WH, Guo
SS, Liu LT, Li CF, Li Y, Liang YJ, Sun XS, et al: Pretreatment
serum amyloid a and c - reactive protein comparing with
Epstein-Barr virus DNA as prognostic indicators in patients with
nasopharyngeal carcinoma: A prospective study. Cancer Res Treat.
Jul 14–2017.(Epub ahead of print). View Article : Google Scholar
|
|
13
|
Wang C, Wang H, Zhang Y, Guo W, Long C,
Wang J, Liu L and Sun X: Berberine inhibits the proliferation of
human nasopharyngeal carcinoma cells via an Epstein-Barr virus
nuclear antigen 1-dependent mechanism. Oncol Rep. 37:2109–2120.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ramayanti O, Juwana H, Verkuijlen SA,
Adham M, Pegtel MD, Greijer AE and Middeldorp JM: Epstein-Barr
virus mRNA profiles and viral DNA methylation status in
nasopharyngeal brushings from nasopharyngeal carcinoma patients
reflect tumor origin. Int J Cancer. 140:149–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao R, Wang L, Liu Q, Zhang LF, Ye YF, Xie
SH, Du JL, Chen SH, Guo J, Yang MJ, et al: Evaluation of seven
recombinant VCA-IgA ELISA kits for the diagnosis of nasopharyngeal
carcinoma in China: A case-control trial. BMJ Open. 7:e0132112017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dobson R, Topping J and Giovannoni G:
Comparison of two commercial ELISA systems for evaluating
anti-EBNA1 IgG titers. J Med Virol. 85:128–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai YL, Li J, Lu AY, Zheng YM, Zhong WM,
Wang W, Gao JQ, Zeng H, Cheng JR and Tang MZ: Diagnostic
significance of combined detection of Epstein-Barr virus
antibodies, VCA/IgA, EA/IgA, Rta/IgG and EBNA1/IgA for
nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 15:2001–2006.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang F, Liu Q and Hu CM: Epstein-Barr
virus-encoded LMP1 increases miR-155 expression, which promotes
radioresistance of nasopharyngeal carcinoma via suppressing UBQLN1.
Eur Rev Med Pharmacol Sci. 19:4507–4515. 2015.PubMed/NCBI
|
|
19
|
Luo YL, Chen H, Peng SG, Lin JH and Huang
PY: Assessment of detection assays of Epstein-Barr viral Rta-IgG,
VCA-IgA, EA-IgA and Epstein-Barr viral DNA at different clinical
stages in the diagnosis of nasopharyngeal carcinoma. Zhonghua Yi
Xue Za Zhi. 93:3516–3519. 2013.(In Chinese). PubMed/NCBI
|
|
20
|
Zhang XM, Zhong JM, Tang MZ, Zhang XG,
Liao J, Zheng YM, Deng H and Zeng Y: Comparison of IgA/VCA, IgA/EA,
IgG/EA in immunoenzyme methods and ZEBRA ELISA in early diagnosis
of nasopharyngeal carcinoma. Zhonghua Shi Yan He Lin Chuang Bing Du
Xue Za Zhi. 20:263–265. 2006.(In Chinese). PubMed/NCBI
|
|
21
|
Cai YL, Zheng YM, Cheng JR, Wang W, Zhang
YN, Wang WH, Wu YS, Zhong WM, Li J and Mo YK: Relationship between
clinical stages of nasopharyngeal carcinoma and Epstein-Barr virus
antibodies Rta/IgG, EBNA1/IgA, VCA/IgA and EA/IgA. Nan Fang Yi Ke
Da Xue Xue Bao. 30:509–511. 2010.(In Chinese). PubMed/NCBI
|