Lung cancer is one of the most common types of
cancer worldwide; high morbidity and mortality rates are observed
for all types of malignant lung tumor (1). Despite the extensive understanding of
tumor biology, and advances in cancer diagnosis and treatment, the
five-year survival rate of lung cancer improved by just 5% during
the past two decades (2). Non-small
cell lung cancer (NSCLC) accounts for 80–85% of lung cancer cases
(3). More than half of the patients
(55%) with NSCLC are diagnosed at an advanced stage of the disease
(4), and the response rate to
combination chemotherapy is only 20% (5). The lack of a system for early diagnosis
or an effective treatment, tumor relapse and resistance to
chemotherapy are the main problems in the diagnosis and treatment
of NSCLC. Therefore, further understanding lung cancer biology,
establishing an early detection system, searching for novel
therapeutic targets and overcoming chemotherapy resistance are key
to improving the prognosis of patients with NSCLC.
Notch signaling is a highly conserved evolutionary
pathway. In mammals, the Notch signaling pathway consists of four
Notch receptor isoforms (Notch1-4) and five ligands [Jagged1 and 2,
delta-like ligand (DLL) 1, 3 and 4 (DLL4)] (6). Upon binding, the Notch receptor
undergoes a series of proteolytic cleavages, resulting in the
release of the Notch intracellular domain (NICD), which
translocates to the nucleus and drives the expression of target
genes, including Hes family BHLH transcription factor 1 (Hes 1)
(7). Notch target genes serve
important functions in cell fate determination, cell proliferation,
differentiation, and apoptosis (8).
Overwhelming data indicate that the abnormal expression of Notch
has been identified in many types of malignant tumor, including
NSCLC (9–12). The function of Notch as an oncogene or
tumor suppressor depends on the cellular context (13). In this review, the expression and
clinical value of Notch signaling in patients with NSCLC will be
examined and the biological roles of Notch signaling in NSCLC cells
will be detailed.
The abnormal expression of Notch signaling pathway
members, including Notch receptors, ligands and downstream genes,
is a relatively frequently identified in NSCLC studies. Dang et
al (10) indicated that the
overexpression of Notch3 was identified in 40% of patients with
NSCLC, and that this overexpression was associated with a
translocation involving 19p. Another previous study revealed that
Notch3 was highly expressed in 51.1% of patients with NSCLC, which
was significantly higher than its expression in adjacent
noncancerous lung tissue (14).
Gain-of-function mutations of Notch1 were present in 10% of
patients with NSCLC, whereas the downregulation of Numb, a negative
regulator of Notch, was observed in 30% of patients with NSCLC,
resulting in increased Notch activity (15). One study indicated that the percentage
of expression of Notch1 and Delta-like ligand 4 (DLL4) in lung
squamous cell carcinoma (SCC) cells was significantly lower when
compared with the other subtypes (16). In contrast, Li et al (17) identified that the percentage of
expression of Notch1 protein in SCC was significantly higher than
in adenocarcinomas (AC). These studies indicate that the expression
of Notch signaling pathway members diverges in different
histopathological types. However, the clinicopathological and
prognostic roles of Notch signaling in NSCLC remain controversial.
A number of studies have indicated that the overexpression of
Notch1 is associated with tumor progression and poor prognosis in
NSCLC (14,16,18,19).
However, other studies suggest that Notch1 and 3 may function as
tumor suppressors (17,20). In summary of these different studies,
Yuan et al (21) performed a
meta-analysis to evaluate the association of Notch signaling
pathway members with the clinicopathological parameters and
prognosis of patients with NSCLC (Table
I). The study indicated that the overexpression of Notch1 and 3
was associated with a greater possibility of lymph node metastasis
and reduced overall survival time in patients with NSCLC.
Furthermore, the expression of Notch signaling ligand DLL4 and its
target gene, Hes family bHLH transcription factor 1 (Hes1), were
inversely associated with the overall survival rate in patients
with NSCLC. The meta-analysis suggested that Notch signaling is a
potential biomarker to predict the progression and prognosis of
patients with NSCLC (20).
It is established that stromal cells within tumors
serve important roles in the biology of cancer cells, including
proliferation, apoptosis, migration and drug resistance (22,23). The
influence of the members of the Notch signaling pathway in stroma
on tumor progression and prognosis has also been investigated
(16) (Table I). The prognostic impact of measuring
Notch signaling in stroma was evident in the study: The high
expression of the members of the Notch signaling pathway predicted
a better outcome for patients with NSCLC. It was therefore
concluded to be likely that stromal Notch signaling inhibits cancer
progression.
Although the results of the meta-analysis are
relatively persuasive, certain questions remain to be answered.
Firstly, only a limited number of patients were enrolled in the
included studies, and the majority of the studies were
retrospective. Multicenter prospective studies based on large,
homogeneous patient populations are required to confirm the
observations. Secondly, either immunohistochemistry or reverse
transcription polymerase chain reaction were used to measure the
expression of Notch signaling pathway members in the studies.
Furthermore, the scoring system and cut-off values varied between
studies, which may have subjectively influenced the final results.
Also, tumor heterogeneity may have contributed to the inconsistent
results of clinical studies.
The expression of Notch signaling and its clinical
value differ in different histopathological types of NSCLC. A study
has investigated the expression of Notch1 and its clinical
significance in patients with different histological subtypes of
lung AC (24). The study demonstrated
that the overexpression of Notch1 was often observed in papillary
predominant AC and micropapillary predominant AC tissues, whereas
the negative expression of Notch1 was often presented in solid
predominant AC tissues. This suggests that the expression and
function of Notch1 are distinct in different phases of tumor
development.
It is possible that the expression of Notch
receptors and ligands fails to precisely reflect the activity of
Notch signaling. Expression of the NICD and Notch target genes may
more representatively reflect the activity of Notch signaling. The
studies with this focus are limited in number. However, Westhoff
et al (15) demonstrated that
intermediate/high levels of activated Notch1 were observed in 26%
of patients with NSCLC. There was a significant association between
Notch activation and a relatively poor prognosis in patients with
p53 mutations (Table I). A recent
clinical study demonstrated that Notch1 serves distinct roles
depending on its activation status in patients with NSCLC (25). Detectable tumor Notch1 was observed in
50% of cases, and was negatively associated with an advanced stage
and nodal status. In contrast, activated Notch1 was identified in
12% of NSCLC patients, and was not significantly associated with
stage or nodal status. These results suggest that future studies
should also focus on the activation status of Notch signaling and
its function in patients with NSCLC.
Several studies have reported that Notch signaling
is involved in the tumorigenesis of NSCLC cells. Using an inducible
LSL-KRASG12D in vivo model of lung cancer,
Osanyingbemi-Obidi et al (26)
observed a transient upregulation of Notch pathway activity (Hes1)
in early tumor precursor lesions. Further study revealed that the
inhibition of Notch signaling by dominant-negative Mastermind-like
(DNMAML) expression in vivo did not suppress cellular
transformation or tumor growth in this model. It is possible that
individual Notch isoforms have opposing effects on lung
oncogenesis, whereas DNMAML expression inhibits Notch signaling
(26). Therefore, more rigorous
experiments are necessary.
In conclusion, these studies provide strong evidence
that Notch signaling serves a critical role in tumorigenesis of
NSCLC. However, individual Notch isoforms may have distinct or even
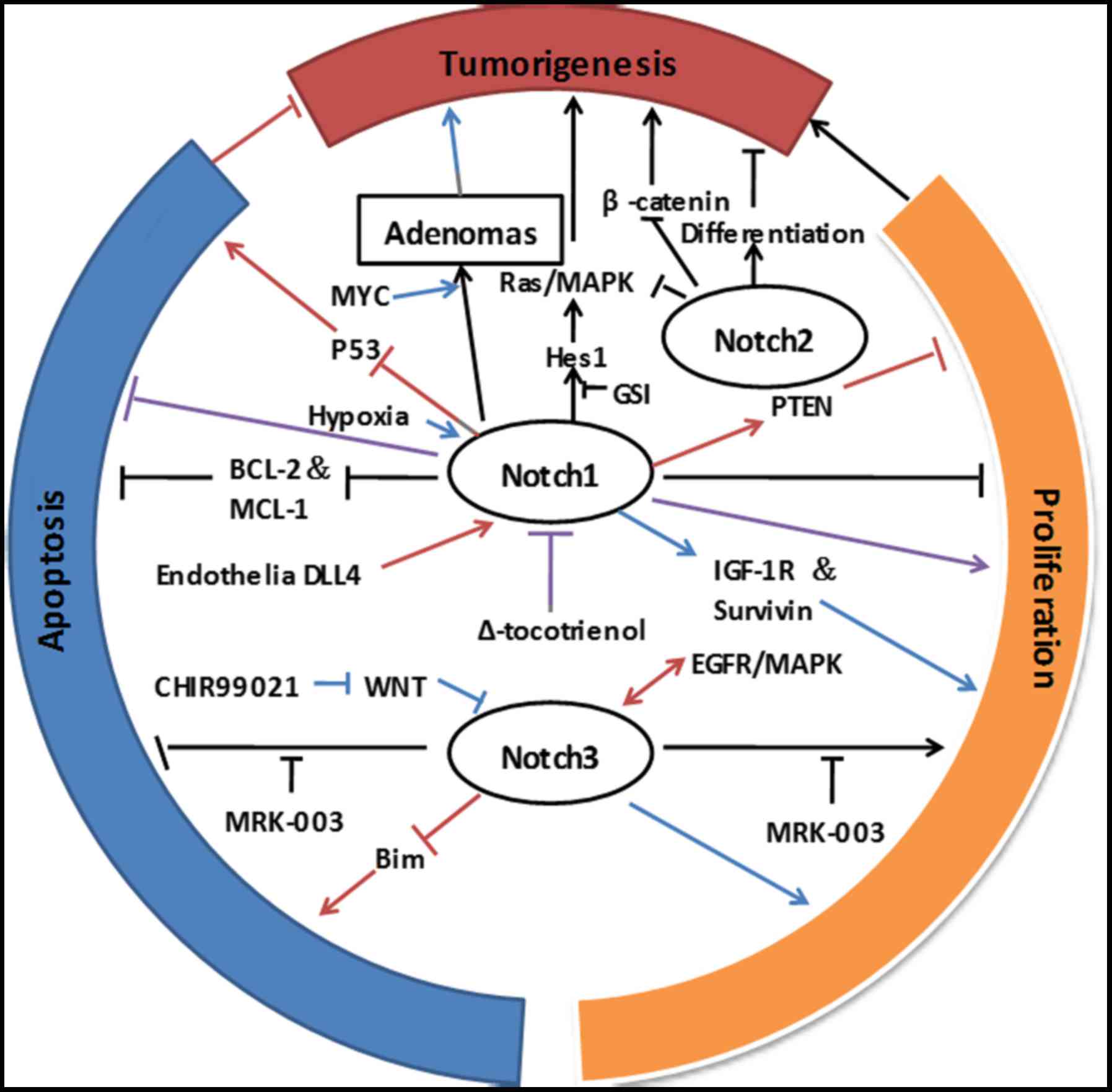
opposite effects on NSCLC tumorigenesis (Fig. 1). This suggests that the specific
inhibition of different Notch molecules should be considered when
devising a therapeutic approach, at least in the context of NSCLC.
The tumorigenic role of other components of Notch signaling and
their mechanisms also require elucidation.
A number of studies have explored the function of
Notch signaling in the proliferation and apoptosis of NSCLC cells.
Chen et al (31) demonstrated
that the overexpression of Notch1 Intracellular Domain (N1ICD) in
AC cells inhibited cell growth and induced apoptosis. A recent
study evaluated these effects of Notch signaling in different lung
cancer cell lines; it was observed that the influence of Notch1 on
proliferation and apoptosis depended on the cell type (32). The knockdown of Notch1 resulted in
increased cell proliferation and enhanced apoptosis, whereas its
induction inhibited the growth of small cell lung cancer cells.
Although Notch1 knockdown increased cell proliferation and
inhibited apoptosis in A549 lung AC cells, it had no effect in SCC
cells (31).
A number of studies have reported that the indirect
inhibition of Notch1 in NSCLC cells resulted in decreased cell
proliferation and the induction of apoptosis. For instance,
δ-tocotrienol inhibits cancer cell proliferation, induces apoptosis
and reduces cancer cell invasion at least in part through the
downregulation of Notch1 (33,34). In
hypoxic conditions, Notch1 was markedly elevated in lung AC cell
lines, and the inhibition of Notch signaling via γ-secretase
inhibitor induced apoptosis, which could be rescued by the
reintroduction of active Notch1 (31). These data indicate that Notch1 may be
essential for cell survival in hypoxia. Further studies have
demonstrated that Notch1 stimulated NSCLC tumor growth and survival
through the direct upregulation of insulin-like growth factor 1
receptor (IGF-1R) (35) and survivin
(36) in hypoxia. One study revealed
that the proliferation of NSCLC cells was significantly suppressed
in vitro and in vivo when co-cultured with
DLL4-expressing endothelial cells (37). Furthermore, silencing endothelial DLL4
or Notch1 significantly attenuated the effects of DLL4 on NSCLC
cells. Phosphate and tensin homolog (PTEN) expression was increased
by the introduction of endothelial cell-DLL4 or recombinant
human-DLL4 protein, and was attenuated by Notch1 interference.
These results suggest that the inhibitory effect of endothelial
cells on NSCLC cells was via the DLL4/Notch1/PTEN signaling pathway
(36). Taken together, these studies
indicate that the biological outcome of Notch signaling in NSCLC
cells was highly dependent on the cell type and context (Fig. 1). Therefore, the tumor
microenvironment should also be considered when developing a
therapeutic strategy. Organoid culture in vitro and
xenografts in vivo may be helpful in understanding the
definite roles of Notch signaling in the proliferation and
apoptosis of NSCLC cells.
The inhibition of Notch3 using dominant negative
Notch3, γ-secretase inhibitor MRK-003 or specific Notch3 peptides
results in growth suppression and the induction of apoptosis of
lung cancer cells in vitro and in vivo (38–40). These
results provide indirect evidence that Notch3 is involved in the
proliferation and apoptosis of lung cancer cells. Further study
demonstrated that Notch3 co-operated with the epidermal growth
factor receptor (EGFR)-mitogen-activated protein kinase pathway to
inhibit apoptosis through the inhibition of the pro-apoptotic
protein, Bcl-2 interacting mediator of cell death (Bim), suggesting
there is significant cross-talk between the two pathways (41). A recent study revealed the cross-talk
between Wnt and Notch3 signaling pathways in the regulation of
proliferation and apoptosis in NSCLC cell lines (42). The inhibition of Wnt signaling by
CHIR99021 resulted in the upregulation of Notch3 protein and its
downstream genes, Hes1 and Hes related family BHLH transcription
factor with YRPW motif 1. CHIR99021 and Notch3 synergistically
promoted the proliferation of NSCLC cells, and Notch3-short hairpin
RNA (shRNA) significantly attenuated the positive effects of
CHIR99021 on cell proliferation. Furthermore, Notch3-shRNA induced
apoptosis and significantly weakened the inhibitory effect of
CHIR99021 on apoptosis in H460 cells. These results support the
hypothesis that the inhibition of Notcth3 activation represents a
potential new approach for the targeted therapy of NSCLC (Fig. 1).
Chemotherapy and radiation therapy are typically
used to treat NSCLC tumors via the inhibition of cancer cell
proliferation or the induction of apoptosis. Notch signaling may
influence the response of tumor cells to chemotherapy and radiation
therapy. The acquired resistance to conventional chemotherapy or
targeted therapy is common in patients with NSCLC who initially
respond to treatment. Low-dose cisplatin treatment can induce
doxorubicin and paclitaxel tolerance, and the enrichment of
CD133(+) cells, in lung AC cell lines (43). Another study demonstrated an evident
weakening of this effect by treatment with a γ-secretase inhibitor
(GSI) or Notch1 shRNAs (44). These
results suggest that Notch signaling was implicated in the
cisplatin-induced enrichment of CD133(+) cells and multidrug
resistance.
One study explored the mechanism of acquired
resistance to EGFR tyrosine kinase inhibitors in NSCLC tumors
harboring an EGFR exon19 deletion (45). It was observed that the expression of
Notch1 was highly upregulated in gefitinib-resistant PC9 lung
cancer cells and that the activation of Notch1 resulted in an
epithelial-mesenchymal transition (EMT) phenotype. Additionally,
Notch1 knockdown reversed the EMT phenotype and restored
sensitivity to gefitinib in gefitinib-resistant PC9 lung cancer
cells. Further in vivo experimentation demonstrated that the
inhibition of Notch signaling resulted in tumor growth retardation
in Balb/cathymic (nu+/nu+) mice with acquired resistant lung cancer
xenografts. These results provide evidence that gefitinib-acquired
resistance in lung cancer cells undergoing EMT depends on the
activation of Notch1 signaling. The molecular mechanism of
Notch-induced EMT in NSCLC was comprehensively analyzed by Yuan
et al (46). In addition to
its association with the acquired resistance to chemotherapy or
targeted therapy, Notch signaling may be involved in primary
resistance. A clinical study has indicated that Notch3 expression
was negatively associated with the sensitivity to platinum-based
chemotherapy in patients with NSCLC. The clinical evidence suggests
that Notch3 was involved in chemotherapy resistance and could act
as a biomarker to predict the chemotherapy response and prognosis
of patients with NSCLC (47).
Previous research provides evidence that Notch
signaling may also be involved in radiation resistance (48). In this study, Notch1 or 3 was
activated subsequent to radiation treatment, depending on the cell
type. Radiation followed by GSI treatment conferred a greater
suppressive effect on lung cancer cells in vitro and in
vivo compared with other treatments. The activation of Notch
signaling by radiation contributed to radiation resistance and the
inhibition of Notch signaling restored the sensitivity of cancer
cells to radiation. Radiation treatment induced Notch1 expression
and promoted EMT in NSCLC cells. This could be inhibited by
rhamnetin and cirsiliol, resulting in the recovery of
radiosensitization (49). Theys et
al (50) demonstrated that Notch
activity had no effect on the radiation sensitivity of H460 cells
in vitro, although high Notch1 activity promoted tumor
growth and induced radiation resistance in vivo. It is
possible that the role of Notch signaling in vitro does not
represent the behaviors of cancer cells in vivo. Taken
together, these results suggest that Notch signaling serves
important roles in radiation resistance and that the inhibition of
Notch signaling may be a promising approach to improve the outcome
of patients with NSCLC undergoing radiotherapy. These findings
further support the hypothesis that the biological outcome of Notch
signaling in NSCLC cells is highly dependent on the cell type and
context.
Notch signaling appears to be essential for cancer
cell survival, particularly as a response to specific
microenvironment conditions or stress. Therefore, Notch inhibition
in combination with chemotherapy or radiation may achieve an
improved therapeutic outcome compared with that with a single
treatment. Notch inhibitors are typically GSIs, short interfering
RNA or monoclonal antibodies against Notch receptors or ligands
(51). GSIs are the most extensively
explored in clinical trials and were reviewed by Yuan et al
(52). It was concluded that the
combination of chemotherapy or radiotherapy with GSIs had a
synergistic effect, and holds promise for cancer control.
The source, maintenance and molecular markers of
CSCs remain incompletely characterized. A previous study supports
the hypothesis that cancer stem cells are derived from normal stem
cells following oncogenic transformation (66). Another hypothesis states that
malignant progenitor cells or differentiated cells can be induced
to acquire stem cell properties through additional gene activation
or mutation (67,68). The role of Notch signaling in lung
CSCs has been explored by a number of studies. Aldehyde
dehydrogenase (ALDH) is an established marker for lung CSCs
(61). ALDH+ lung cancer
cells are capable of self-renewal and possess a highly tumorigenic
and clonogenic ability compared with their ALDH−
counterparts. Higher activity of Notch signaling was observed in
ALDH+ lung cancer cells than in the ALDH−
subpopulation, and the inhibition of the Notch pathway resulted in
a significant decrease in the proportion of ALDH+ lung
cancer cells. These results suggest that Notch signaling serves a
role in the maintenance of lung CSCs (69). Using a lentiviral Notch reporter
vector and flow cytometry, lung AC cells can be divided into
subpopulations of green fluorescent protein (GFP)-bright and
GFP-dim cells (70). GFP-bright cells
exhibit the stem cell properties of self-renewal, multi-potency,
continuous tumorigenic ability and chemotherapy resistance in
vivo. Zheng et al (71)
identified CD24+ITGB4+Notchhi
cells as tumor-propagating cells, which have an increased ability
to self-renew and propagate in vitro and in vivo. GSI
treatment or Notch3 knockdown decreased self-renewal and tumor
propagation in NSCLC cell lines and patient primary tumors. This
suggests an important role of Notch3 in the self-renewal and
propagation of NSCLC cells. These studies provide strong evidence
that Notch signaling serves an important role in lung CSCs and that
the inhibition of Notch signaling may be a promising way to target
lung CSCs.
The abnormal expression of Notch signaling pathway
members is a relatively frequent event in patients with NSCLC
(9,13–16).
Although the clinicopathological and prognostic value of Notch
signaling in NSCLC remains controversial, the meta-analysis by Yuan
et al (21) revealed that a
number of Notch molecules and their ligands were associated with
lymph node metastasis and could act as biomarkers to predict the
prognosis of patients. Further large-scale analyses are required to
clarify the clinical value of members of the Notch signaling
pathway in different populations.
The present study was supported in part by grants
from the National Natural Science Foundation of China (grant no.
81260024), the Natural Science Foundation of China (grant no.
81460065) and the Graduate Innovation Fund of Jiangxi Province
(grant no. YC2015-B009).
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson DH, Schiller JH and Bunn PA Jr:
Recent clinical advances in lung cancer management. J Clin Oncol.
32:973–982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Howlader NNA, Krapcho M, Garshell J,
Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
Mariotto A, et al: SEER cancer statistics review, 1975–2012.
National Cancer Institute; Bethesda MD: 2015
|
|
5
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fleming RJ: Structural conservation of
Notch receptors and ligands. Semin Cell Dev Biol. 9:599–607. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kageyama R and Ohtsuka T: The Notch-Hes
pathway in mammalian neural development. Cell Res. 9:179–188. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hori K, Sen A and Artavanis-Tsakonas S:
Notch signaling at a glance. J Cell Sci. 126:2135–2140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zagouras P, Stifani S, Blaumueller CM,
Carcangiu ML and Artavanis-Tsakonas S: Alterations in Notch
signaling in neoplastic lesions of the human cervix. Proc Natl Acad
Sci USA. 92:pp. 6414–6418. 1995; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dang TP, Gazdar AF, Virmani AK, Sepetavec
T, Hande KR, Minna JD, Roberts JR and Carbone DP: Chromosome 19
translocation, overexpression of Notch3, and human lung cancer. J
Natl Cancer Inst. 92:1355–1357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santagata S, Demichelis F, Riva A,
Varambally S, Hofer MD, Kutok JL, Kim R, Tang J, Montie JE,
Chinnaiyan AM, et al: JAGGED1 expression is associated with
prostate cancer metastasis and recurrence. Cancer Res.
64:6854–6857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grabher C, von Boehmer H and Look AT:
Notch 1 activation in the molecular pathogenesis of T-cell acute
lymphoblastic leukaemia. Nat Rev Cancer. 6:347–359. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roy M, Pear WS and Aster JC: The
multifaceted role of Notch in cancer. Curr Opin Genet Dev.
17:52–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye YZ, Zhang ZH, Fan XY, Xu XL, Chen ML,
Chang BW and Zhang YB: Notch3 overexpression associates with poor
prognosis in human non-small-cell lung cancer. Med Oncol.
30:5952013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Westhoff B, Colaluca IN, D'Ario G,
Donzelli M, Tosoni D, Volorio S, Pelosi G, Spaggiari L, Mazzarol G,
Viale G, et al: Alterations of the Notch pathway in lung cancer.
Proc Natl Acad Sci USA. 106:pp. 22293–22298. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Donnem T, Andersen S, Al-Shibli K, Al-Saad
S, Busund LT and Bremnes RM: Prognostic impact of Notch ligands and
receptors in nonsmall cell lung cancer: Coexpression of Notch-1 and
vascular endothelial growth factor-A predicts poor survival.
Cancer. 116:5676–5685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Burns JA, Cheney CA, Zhang N,
Vitelli S, Wang F, Bett A, Chastain M, Audoly LP and Zhang ZQ:
Distinct expression profiles of Notch-1 protein in human solid
tumors: Implications for development of targeted therapeutic
monoclonal antibodies. Biologics. 4:163–171. 2010.PubMed/NCBI
|
|
18
|
Andersen S, Donnem T, Al-Saad S, Al-Shibli
K, Stenvold H, Busund LT and Bremnes RM: Correlation and
coexpression of HIFs and NOTCH markers in NSCLC. Anticancer Res.
31:1603–1606. 2011.PubMed/NCBI
|
|
19
|
Okayama H, Kohno T, Ishii Y, Shimada Y,
Shiraishi K, Iwakawa R, Furuta K, Tsuta K, Shibata T, Yamamoto S,
et al: Identification of genes upregulated in ALK-positive and
EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res.
72:100–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SM, Jung CK, Ko YH, Choi JY, Lee KY
and Kang CS: Expression of Notch 1 and 3 is related to inhibition
of lymph node metastasis and progression in non-small cell lung
carcinomas. Basic Appl Pathol. 1:93–97. 2008. View Article : Google Scholar
|
|
21
|
Yuan X, Wu H, Xu H, Han N, Chu Q, Yu S,
Chen Y and Wu K: Meta-analysis reveals the correlation of Notch
signaling with non-small cell lung cancer progression and
prognosis. Sci Rep. 5:103382015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu R, Wei S, Chen J and Xu S: Mesenchymal
stem cells in lung cancer tumor microenvironment: Their biological
properties, influence on tumor growth and therapeutic implications.
Cancer Lett. 353:145–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang J, Song H, Liu B, Yu B, Wang R and
Chen L: Expression of Notch-1 and its clinical significance in
different histological subtypes of human lung adenocarcinoma. J Exp
Clin Cancer Res. 32:842013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nguyen D, Rubinstein L, Takebe N, Miele L,
Tomaszewski JE, Ivy P, Doroshow JH and Yang SX: Notch1 phenotype
and clinical stage progression in non-small cell lung cancer. J
Hematol Oncol. 8:92015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Osanyingbemi-Obidi J, Dobromilskaya I,
Illei PB, Hann CL and Rudin CM: Notch signaling contributes to lung
cancer clonogenic capacity in vitro but may be circumvented in
tumorigenesis in vivo. Mol Cancer Res. 9:1746–1754. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maraver A, Fernández-Marcos PJ, Herranz D,
Muñoz-Martin M, Gomez-Lopez G, Cañamero M, Mulero F, Megías D,
Sanchez-Carbayo M, Shen J, et al: Therapeutic effect of γ-secretase
inhibition in KrasG12V-driven non-small cell lung carcinoma by
derepression of DUSP1 and inhibition of ERK. Cancer Cell.
22:222–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Allen TD, Rodriguez EM, Jones KD and
Bishop JM: Activated Notch1 induces lung adenomas in mice and
cooperates with Myc in the generation of lung adenocarcinoma.
Cancer Res. 71:6010–6018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Licciulli S, Avila JL, Hanlon L, Troutman
S, Cesaroni M, Kota S, Keith B, Simon MC, Puré E, Radtke F, et al:
Notch1 is required for Kras-induced lung adenocarcinoma and
controls tumor cell survival via p53. Cancer Res. 73:5974–5984.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baumgart A, Mazur PK, Anton M, Rudelius M,
Schwamborn K, Feuchtinger A, Behnke K, Walch A, Braren R, Peschel
C, et al: Opposing role of Notch1 and Notch2 in a Kras(G12D)-driven
murine non-small cell lung cancer model. Oncogene. 34:578–588.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Y, De Marco MA, Graziani I, Gazdar
AF, Strack PR, Miele L and Bocchetta M: Oxygen concentration
determines the biological effects of NOTCH-1 signaling in
adenocarcinoma of the lung. Cancer Res. 67:7954–7959. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wael H, Yoshida R, Kudoh S, Hasegawa K,
Niimori-Kita K and Ito T: Notch1 signaling controls cell
proliferation, apoptosis and differentiation in lung carcinoma.
Lung Cancer. 85:131–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji X, Wang Z, Geamanu A, Sarkar FH and
Gupta SV: Inhibition of cell growth and induction of apoptosis in
non-small cell lung cancer cells by delta-tocotrienol is associated
with notch-1 down-regulation. J Cell Biochem. 112:2773–2783. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji X, Wang Z, Geamanu A, Goja A, Sarkar FH
and Gupta SV: Delta-tocotrienol suppresses Notch-1 pathway by
upregulating miR-34a in nonsmall cell lung cancer cells. Int J
Cancer. 131:2668–2677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eliasz S, Liang SY, De Marco MA, Machek O,
Skucha S, Miele L and Bocchetta M: Notch1 stimulates survival of
lung adenocarcinoma cells during hypoxia by activating the IGF-1R
pathway. Oncogene. 29:2488–2498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Y, Li D, Liu H, Xu H, Zheng H, Qian
F, Li W, Zhao C, Wang Z and Wang X: Notch-1 signaling facilitates
survivin expression in human non-small cell lung cancer cells.
Cancer Biol Ther. 11:14–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding XY, Ding J, Wu K, Wen W, Liu C, Yan
HX, Chen C, Wang S, Tang H, Gao CK, et al: Cross-talk between
endothelial cells and tumor via delta-like ligand 4/Notch/PTEN
signaling inhibits lung cancer growth. Oncogene. 31:2899–2906.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haruki N, Kawaguchi KS, Eichenberger S,
Massion PP, Olson S, Gonzalez A, Carbone DP and Dang TP:
Dominant-negative Notch3 receptor inhibits mitogen-activated
protein kinase pathway and the growth of human lung cancers. Cancer
Res. 65:3555–3561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Konishi J, Kawaguchi KS, Vo H, Haruki N,
Gonzalez A, Carbone DP and Dang TP: Gamma-secretase inhibitor
prevents Notch3 activation and reduces proliferation in human lung
cancers. Cancer Res. 67:8051–8057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin L, Mernaugh R, Yi F, Blum D, Carbone
DP and Dang TP: Targeting specific regions of the Notch3
ligand-binding domain induces apoptosis and inhibits tumor growth
in lung cancer. Cancer Res. 70:632–638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Konishi J, Yi F, Chen X, Vo H, Carbone DP
and Dang TP: Notch3 cooperates with the EGFR pathway to modulate
apoptosis through the induction of bim. Oncogene. 29:589–596. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li C, Zhang S, Lu Y, Zhang Y, Wang E and
Cui Z: The roles of Notch3 on the cell proliferation and apoptosis
induced by CHIR99021 in NSCLC cell lines: A functional link between
Wnt and Notch signaling pathways. PLoS One. 8:e846592013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Salnikov AV, Gladkich J, Moldenhauer G,
Volm M, Mattern J and Herr I: CD133 is indicative for a resistance
phenotype but does not represent a prognostic marker for survival
of non-small cell lung cancer patients. Int J Cancer. 126:950–958.
2010.PubMed/NCBI
|
|
44
|
Liu YP, Yang CJ, Huang MS, Yeh CT, Wu AT,
Lee YC, Lai TC, Lee CH, Hsiao YW, Lu J, et al: Cisplatin selects
for multidrug-resistant CD133+ cells in lung adenocarcinoma by
activating Notch signaling. Cancer Res. 73:406–416. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie M, He CS, Wei SH and Zhang L: Notch-1
contributes to epidermal growth factor receptor tyrosine kinase
inhibitor acquired resistance in non-small cell lung cancer in
vitro and in vivo. Eur J Cancer. 49:3559–3572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yuan X, Wu H, Han N, Xu H, Chu Q, Yu S,
Chen Y and Wu K: Notch signaling and EMT in non-small cell lung
cancer: Biological significance and therapeutic application. J
Hematol Oncol. 7:872014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shi C, Qian J, Ma M, Zhang Y and Han B:
Notch 3 Protein, not its gene polymorphism, is associated with the
chemotherapy response and prognosis of advanced NSCLC patients.
Cell Physiol Biochem. 34:743–752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mizugaki H, Sakakibara-Konishi J, Ikezawa
Y, Kikuchi J, Kikuchi E, Oizumi S, Dang TP and Nishimura M:
γ-Secretase inhibitor enhances antitumour effect of radiation in
Notch-expressing lung cancer. Br J Cancer. 106:1953–1959. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kang J, Kim E, Kim W, Seong KM, Youn H,
Kim JW, Kim J and Youn B: Rhamnetin and cirsiliol induce
radiosensitization and inhibition of epithelial-mesenchymal
transition (EMT) by miR-34a-mediated suppression of Notch-1
expression in non-small cell lung cancer cell lines. J Biol Chem.
288:27343–27357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Theys J, Yahyanejad S, Habets R, Span P,
Dubois L, Paesmans K, Cleutjens J, Groot AJ, Schuurbiers OCJ,
Lambin P, et al: High NOTCH activity induces radiation resistance
in non small cell lung cancer. Radiother Oncol. 108:440–445. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Takebe N, Nguyen D and Yang SX: Targeting
notch signaling pathway in cancer: Clinical development advances
and challenges. Pharmacol Ther. 141:140–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S,
Wu GS and Wu K: Notch signaling: An emerging therapeutic target for
cancer treatment. Cancer Lett. 369:20–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rahman M, Deleyrolle L, Vedam-Mai V, Azari
H, Abd-El-Barr M and Reynolds BA: The cancer stem cell hypothesis:
Failures and pitfalls. Neurosurgery. 68:531–545. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 100:pp.
3983–3988. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
58
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra
D, Zhou J, Claypool K, et al: Highly purified CD44+
prostate cancer cells from xenograft human tumors are enriched in
tumorigenic and metastatic progenitor cells. Oncogene.
25:1696–1708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jiang F, Qiu Q, Khanna A, Todd NW, Xing L,
Wang H, Liu Z, Su Y, Stass S and Katz RL: Aldehyde dehydrogenase 1
is a tumor stem cell-associated marker in lung cancer. Mol Cancer
Res. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Eyler CE and Rich JN: Survival of the
fittest: Cancer stem cells in therapeutic resistance and
angiogenesis. J Clin Oncol. 26:2839–2845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Berns A: Stem cells for lung cancer? Cell.
121:811–813. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kim CF, Jackson EL, Woolfenden AE,
Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT and Jacks T:
Identification of bronchioalveolar stem cells in normal lung and
lung cancer. Cell. 121:823–835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Huntly BJ and Gilliland DG: Leukaemia stem
cells and the evolution of cancer-stem-cell research. Nat Rev
Cancer. 5:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Krivtsov AV, Twomey D, Feng Z, Stubbs MC,
Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al:
Transformation from committed progenitor to leukaemia stem cell
initiated by MLL-AF9. Nature. 442:818–822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mathieu J, Zhang Z, Zhou W, Wang AJ,
Heddleston JM, Pinna CM, Hubaud A, Stadler B, Choi M, Bar M, et al:
HIF induces human embryonic stem cell markers in cancer cells.
Cancer Res. 71:4640–4652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sullivan JP, Spinola M, Dodge M, Raso MG,
Behrens C, Gao B, Schuster K, Shao C, Larsen JE, Sullivan LA, et
al: Aldehyde dehydrogenase activity selects for lung adenocarcinoma
stem cells dependent on notch signaling. Cancer Res. 70:9937–9948.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hassan KA, Wang L, Korkaya H, Chen G,
Maillard I, Beer DG, Kalemkerian GP and Wicha MS: Notch pathway
activity identifies cells with cancer stem cell-like properties and
correlates with worse survival in lung adenocarcinoma. Clin Cancer
Res. 19:1972–1980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zheng Y, de la Cruz CC, Sayles LC,
Alleyne-Chin C, Vaka D, Knaak TD, Bigos M, Xu Y, Hoang CD, Shrager
JB, et al: A rare population of CD24(+)ITGB4(+)Notch(hi) cells
drives tumor propagation in NSCLC and requires Notch3 for
self-renewal. Cancer Cell. 24:59–74. 2013. View Article : Google Scholar : PubMed/NCBI
|