Introduction
Ovarian cancer is a major cause of gynecological
malignancy-associated mortality worldwide. The majority of patients
are diagnosed with epithelial ovarian cancer at the advanced stage
and the 5-year survival rate is 20–40% (1). Platinum-based adjuvant chemotherapy
following cytoreductive surgery is commonly used for treatment of
ovarian cancer, which is initially effective in a high percentage
of cases (2). However, an increasing
number of patients eventually relapse due to the decreased
sensitivity to cisplatin (3,4). Therefore, novel therapeutic strategies
are required to increase the sensitivity of cancer cells to
cisplatin.
Chemotherapeutic drug-induced apoptosis is usually
disturbed by the anti-apoptotic proteins, including B cell
lymphoma-2 (Bcl-2), X-linked inhibitor of apoptosis protein (XIAP)
and Survivin (5). Bcl-2 regulates
programmed cell death in various cancer cell lines. It exerts a
survival function in response to a wide range of apoptotic stimuli
and inhibits the release of mitochondrial cytochrome c
(6). XIAP is well known as a direct
inhibitor of apoptosis, which binds to caspase-3, −7 and −9 and
prevents their activities (7).
Survivin interferes with apoptosis by inhibiting the activity of
caspases or via interaction with other regulators. It has been
demonstrated that Survivin binds to XIAP and inhibits the
activation of caspase-9 (8). The
overexpression of these proteins is involved in the insensitivity
of epithelial ovarian cancer cells to cisplatin (9–11).
Mammalian target of rapamycin (mTOR) activation is
essential in the development of cisplatin insensitivity in ovarian
cancer cells. It was reported that the phosphorylation level of
mTOR was higher in cisplatin-insensitive clear-cell ovarian
carcinoma cells compared with in cisplatin-sensitive cells
(12,13). mTOR also performs a key role in the
modulation of cisplatin-induced apoptotic cell death. Cisplatin
activates the mTOR survival pathway in epithelial and clear-cell
carcinoma ovarian cancer cells, which may counteract
cisplatin-induced apoptosis and in turn lead to cisplatin
resistance (14,15). The mTOR inhibitors, rapamycin and its
analogs, including everolimus, efficiently enhance the therapeutic
efficacy of platinum in clear-cell ovarian carcinoma cells
(16,17). Therefore, mTOR is a promising
therapeutic target for the treatment of ovarian cancer when
combined with cisplatin.
Cardamonin is a natural chalcone compound, which
increases cell apoptosis in nasopharyngeal carcinoma, prostate
cancer and triple negative breast cancer cells and induces
autophagy in colorectal carcinoma cells (18–21).
Cardamonin inhibits a number of signaling pathways, including c-Jun
N-terminal kinase, nuclear factor-κB, and signal transducer and
activator of transcription 3 (21,22).
Combined with doxorubicin, cardamonin sufficiently prevents the
enrichment of drug-resistant triple negative breast cancer stem
cells and retards tumor growth (23).
It may also increase the anti-proliferative effect of cisplatin in
cervical carcinoma, hepatocellular carcinoma, prostate cancer and
colon cancer cells (24). However,
the underlying mechanism has not been previously studied. Our
previous studies demonstrated that cardamonin inhibits the
proliferation, angiogenesis and metastasis of non-small cell lung
carcinoma cells, epithelial ovarian cancer cells and Lewis lung
carcinoma cells by inhibition of mTOR activation (25–27). In
the current study, the therapeutic potency of cardamonin combined
with cisplatin in ovarian cancer was evaluated and the underlying
mechanism was investigated.
Materials and methods
Chemical reagents
Solutions and supplements for cell culture were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Cardamonin, cisplatin, dimethyl sulfoxide (DMSO) and MTT were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Antibodies against mTOR (cat. no. 2972; dilution, 1:1,000),
phospho-mTOR (Ser 2448; cat. no. 2971; dilution, 1:1,000), 70 kDa
ribosomal protein S6 kinase (p70S6K; cat. no. 9202; dilution,
1:1,000), phospho-p70S6K (Thr 389; cat. no. 9205; dilution,
1:1,000), Bcl-2 (cat. no. 2872; dilution, 1:1,000), Survivin (cat.
no. 2803; dilution, 1:1,000), XIAP (cat. no. 2042; dilution,
1:1,000) and actin (cat. no. 4967; dilution, 1:1,000), and the
secondary antibody (anti-rabbit IgG, horseradish peroxidase-linked
antibody; cat. no. 7074; dilution, 1:2,000) were purchased from
Cell Signaling Technologies, Inc. (Danvers, MA, USA). The cell
cycle assay kit was from BD Biosciences (San Jose, CA, USA).
Cell culture
Epithelial ovarian cancer SKOV3 and A2780 cells were
obtained from Wuhan Boster Bio-Engineering Ltd. Co. (Wuhan, China),
and cultured, at 37°C in a saturated humidity incubator with an
atmosphere containing 5% CO2, in McCoy's 5A and high
glucose Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc.), respectively. The media was supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), penicillin (100 U/ml) and streptomycin (100 µg/ml) (HyClone;
GE Healthcare Life Sciences, Little Chalfont, UK).
Cell viability assay
MTT assay was used to analyze the effect of
cardamonin on cell viability as previously described (28). Cells were cultured overnight at 37°C
in a saturated humidity incubator with an atmosphere containing 5%
CO2 in 96-well plates (5×103 cells/well).
Cell viability was assessed following the addition of cardamonin
and cisplatin at the indicated concentrations for 48 h in medium
containing 10% FBS. Surviving cells were assessed using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
for the determination of the absorbance at 570 nm of the
DMSO-dissolved formazan product following the addition of MTT (5
mg/ml) for 4 h. Five independent experiments were performed.
Clonogenic survival assay
The clonogenic survival assay was performed as
previously described (28). Cells
(1×103/well) were seeded on 6-well tissue culture plates
in medium containing 10% FBS. The cells were cultured overnight at
37°C in a saturated humidity incubator with an atmosphere
containing 5% CO2, and then treated with cisplatin in
the presence or absence of cardamonin for 48 h, followed by two
washes in McCoy's 5A and DMEM, respectively. The cells were then
cultured in medium containing 10% FBS and allowed to proliferate
for 7 days. Cells were fixed with ethanol (75%) at room temperature
for 15 min and stained by 1% crystal violet at room temperature for
60 min. Colonies (>30 cells/colony) were counted with a Leica
DMIL LED microscope (magnification, ×200; Leica Microsystems GmbH,
Wetzlar, Germany) for each group to generate survival rate in
triplicate wells. Five independent experiments were performed.
Cell cycle assay
Cell cycle distribution was determined by flow
cytometry based on DNA content measurement of nuclei, stained with
propidium iodide (PI). Following treatment with cisplatin in the
presence or absence of cardamonin for 48 h, cells were the washed
with cold PBS and harvested by centrifugation at 400 × g at room
temperature for 5 min. Cells were then fixed in cold ethanol (70%)
overnight at 4°C. Next, the cells were stained with 50 µg/ml PI and
10 µg/ml RNase A (BD Biosciences, San Jose, CA, USA) in the dark at
room temperature for 30 min in the dark. Cell cycle distribution
was analyzed by flow cytometry (BD Biosciences). Results were
calculated by the Mod Fit LT software version 2.0 (BD Biosciences).
Five independent experiments were performed.
Western blot analysis
Treated with indicated drugs for 48 h, cells were
washed with ice-cold PBS twice and lysed in
radioimmunoprecipitation assay lysis buffer (20 mM Tris-HCl (pH
7.5), 150 mM NaCl, 1 mM Na2 EDTA, 1 mM EGTA, 1% NP-40,
1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM
beta-glycerophosphate, 1 mM Na3VO4 and 1
µg/ml leupeptin) with the addition of 1 mM phenylmethylsulfonyl
fluoride for 20 min at 4°C. Lysates were centrifuged at 15,000 × g
at 4°C for 20 min, and protein concentrations of the supernatants
were determined using a bicinchoninic acid protein assay reagent.
Proteins (40 µg) were separated by 6–12% SDS-PAGE and transferred
to polyvinylidene difluoride membranes. The membranes were blocked
with 5% bovine serum albumin (Cell Signaling Technologies, Inc.) in
1X TBS-Tween 20 for 1 h at room temperature and then incubated
overnight at 4°C with the following primary antibodies: mTOR (cat.
no. 2972; dilution, 1:1,000), Ser 2448 (cat. no. 2971; dilution,
1:1,000), p70S6K (cat. no. 9202; dilution, 1:1,000), Thr 389 (cat.
no. 9205; dilution, 1:1,000), Bcl-2 (cat. no. 2872; dilution,
1:1,000), Survivin (cat. no. 2803; dilution, 1:1,000), XIAP (cat.
no. 2042; dilution, 1:1,000) and actin (cat. no. 4967; dilution,
1:1,000) all purchased from Cell Signaling Technologies, Inc.
Immunoblots were visualized with horseradish peroxidase-conjugated
goat anti-rabbit immunoglobulin (Cell Signaling Technologies, Inc.;
cat. no. 7074; dilution, 1:2,000) at room temperature for 1 h using
enhanced chemiluminescence (GE Healthcare Life Sciences) and
exposure to X-ray film to produce bands within the linear range.
The protein purity was evaluated by densitometry analysis using the
Quantity One software version 4.6.2 (Bio-Rad Laboratories, Inc.).
Three independent experiments were performed.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed with SPSS version
16.0 software (SPSS, Inc., Chicago, IL, USA). Data were analyzed by
one-way analysis of variance and Tukey-Kramer multiple comparison
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
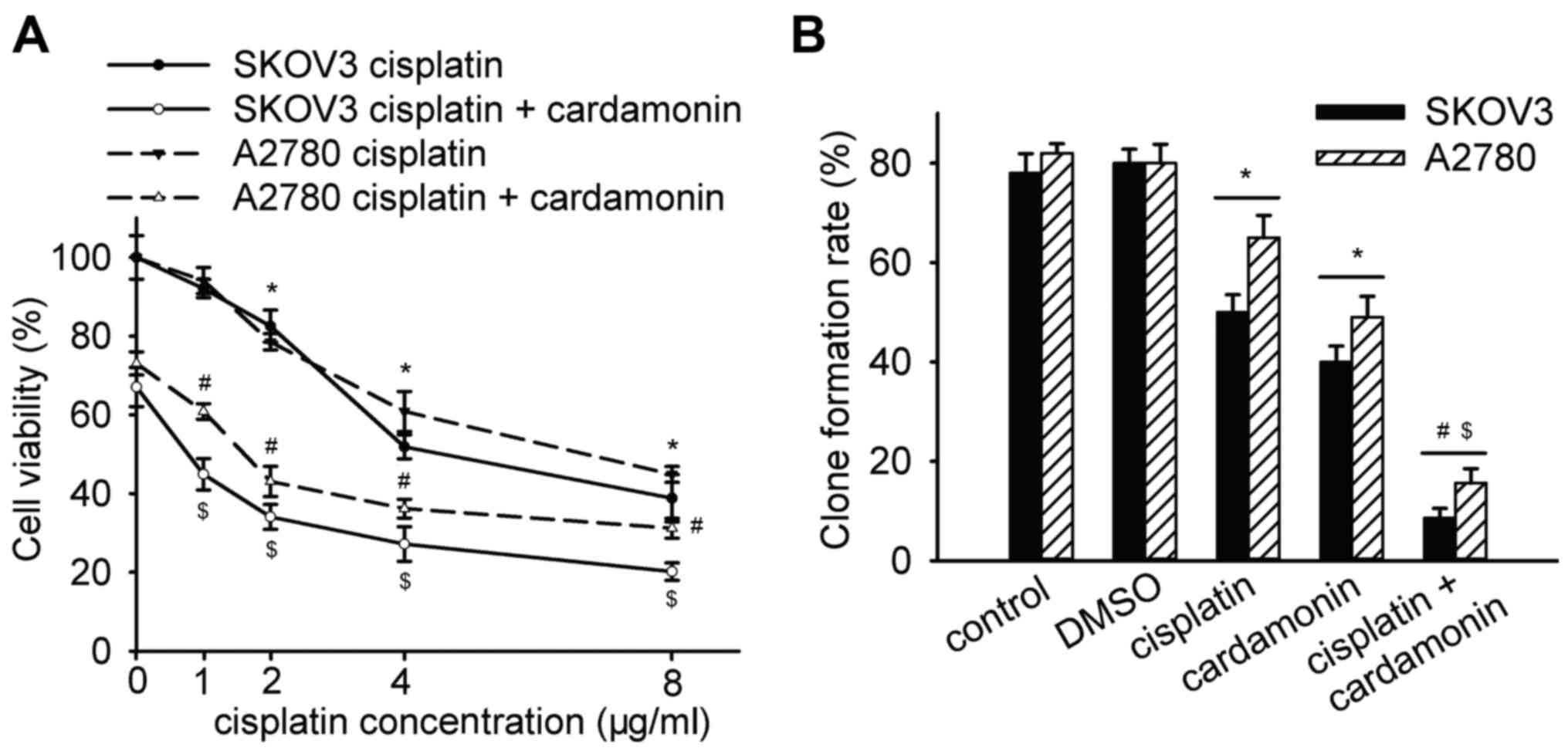
Anti-proliferation efficacy of
cisplatin is enhanced by cardamonin
Our previous study demonstrated that 20 µM
cardamonin effectively inhibited the activation of mTOR in SKOV3
cells (unpublished data). In the present study, whether 20 µM
cardamonin enhanced the efficacy of cisplatin was determined. The
anti-proliferation effect of cisplatin (1, 2, 4 and 8 µg/ml) was
enhanced by cardamonin in a dose-dependent manner in SKOV3 and
A2780 cells (Fig. 1A). Cisplatin at a
concentration of 2 µg/ml was selected for the following experiments
according to its nephrotoxicity in clinical use. Furthermore, the
effect of cardamonin combined with cisplatin (2 µg/ml) on
anti-proliferation was more potent in SKOV3 cells compared with
that in A2780 cells (cell viability, 34.8±3.14 vs. 43.1±3.82%;
P<0.05). In addition, the clone formation was significantly
inhibited by cardamonin combined with cisplatin in SKOV3 and A2780
cells (Fig. 1B).
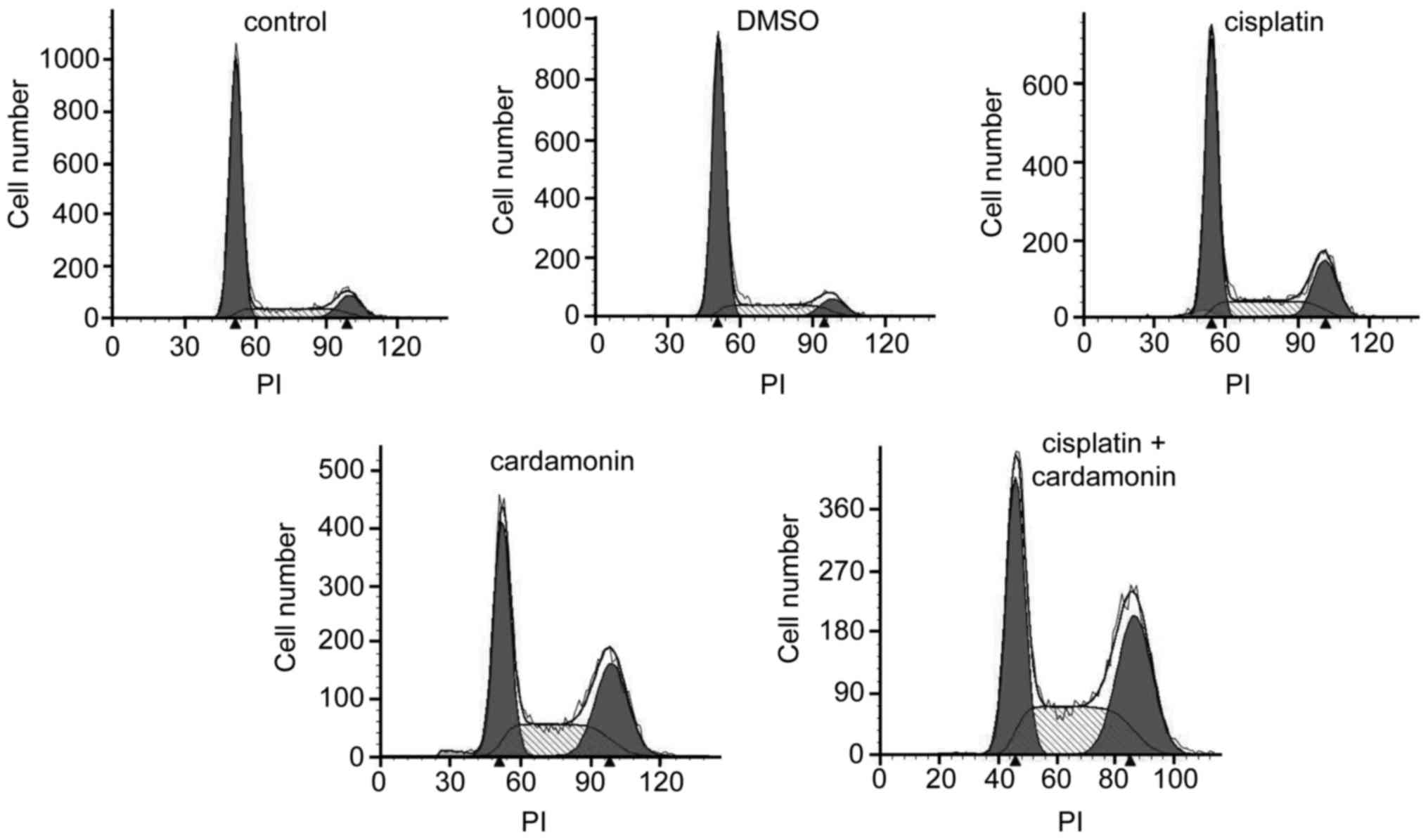
Cell cycle is arrested by cardamonin
and cisplatin at the G2/M phase
It was then determined whether cardamonin was able
to enhance cisplatin-induced cell cycle arrest. As presented in
Fig. 2, cardamonin and cisplatin
induced the accumulation of cells in the S, and G2/M phase. As
expected, cardamonin significantly increased the cisplatin-induced
accumulation of cells in the G2/M phase, and the accumulation of
cells in G0/G1 phases was significantly reduced (Table I).
 | Table I.Effect of cardamonin combined with
cisplatin on cell cycle distribution. |
Table I.
Effect of cardamonin combined with
cisplatin on cell cycle distribution.
|
|
| Cell cycle, % |
|---|
|
|
|
|
|---|
| Drug | n |
G0/G1 | S |
G2/M |
|---|
| Control | 5 |
69.96±3.25 |
18.50±2.52 |
11.54±3.82 |
| DMSO | 5 |
70.38±2.87 |
19.01±4.28 |
10.61±1.60 |
| Cisplatin | 5 |
55.99±1.58a |
22.67±1.29a |
21.34±0.68a |
| Cardamonin | 5 |
41.05±2.14a |
28.54±2.74a |
30.42±4.27a |
| Cisplatin +
cardamonin | 5 |
35.33±2.86a, b |
31.48±3.54a, b |
33.19±1.16a, b |
Anti-apoptotic proteins are decreased
by cardamonin and cisplatin co-administration
Cisplatin-induced apoptosis may be disturbed by the
anti-apoptotic proteins. The effect of cardamonin on anti-apoptotic
proteins was examined. Cisplatin and cardamonin decreased the
protein expression of XIAP and Survivin. Notably, it was
demonstrated that combination of the two agents significantly
reduced the protein expression of Bcl-2, XIAP and Survivin in SKOV3
cells (Fig. 3).
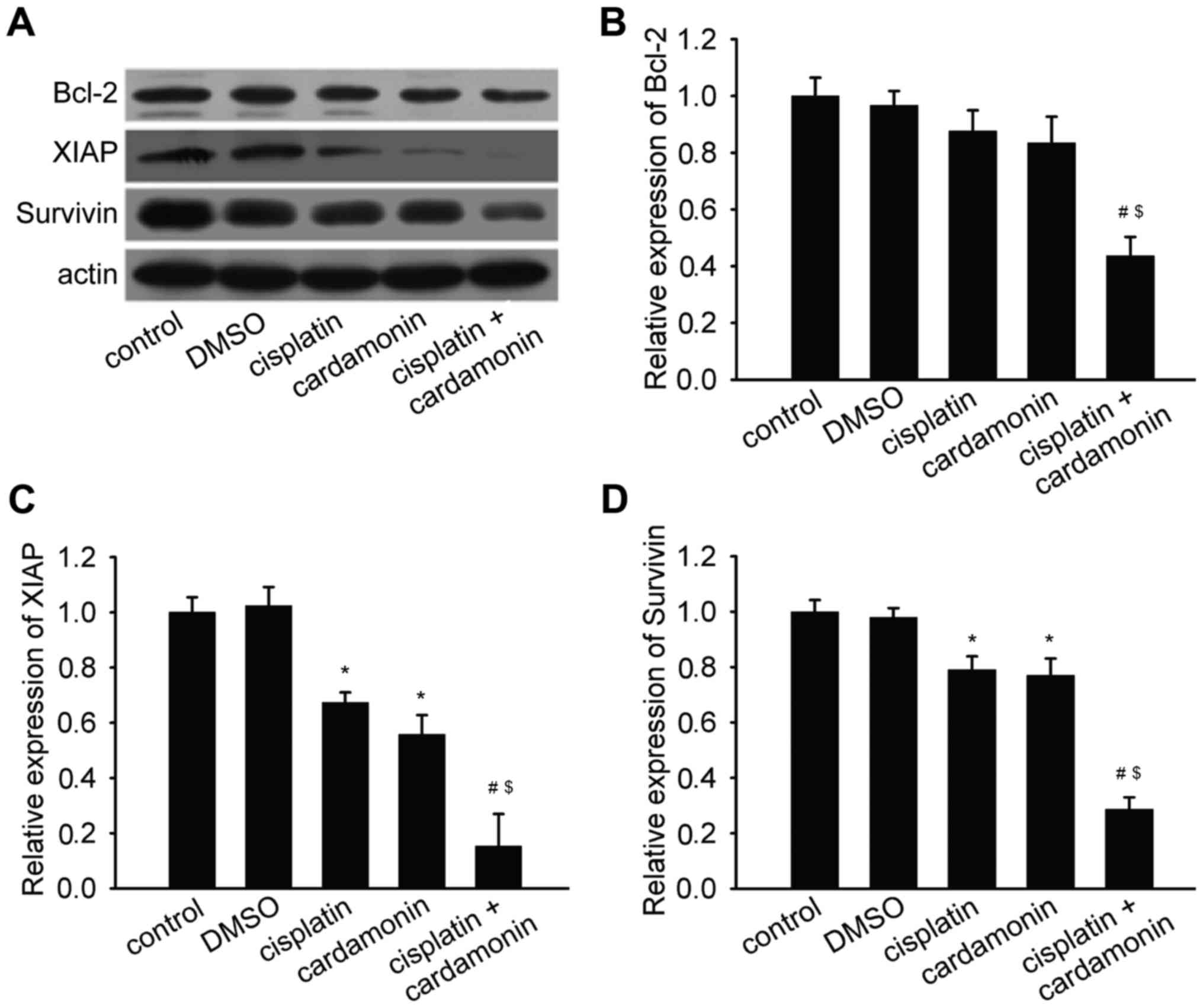 | Figure 3.Cardamonin combined with cisplatin
decreased the expression of anti-apoptotic proteins. SKOV3 cells
were treated with cardamonin (20 µM), cisplatin (2 µg/ml) or a
combination of the two agents for 48 h. The expression of Bcl-2,
XIAP and Survivin was determined by western blot analysis. Actin
was used as the loading control (n=3). (A) Representative western
blotting protein bands for Bcl-2, XIAP, Survivin and actin.
Quantification of (B) Bcl-2, (C) XIAP and (D) Survivin protein
expression levels normalized to actin, and compared with control,
cisplatin or cardamonin. Data are presented as the mean ± standard
deviation. *P<0.05 vs. control; #P<0.01 vs.
cisplatin; $P<0.01 vs. cardamonin. DMSO, dimethyl
sulfoxide; Bcl-2, B cell lymphoma-2; XIAP, X-linked inhibitor of
apoptosis protein. |
Activation of mTOR and p70S6K is
attenuated by cardamonin
Elevated phosphorylation of mTOR and p70S6K was
detected in SKOV3 cells. To examine the effect of mTOR pathway
inhibition by cardamonin on ovarian cancer cells, the effect of
cardamonin and cisplatin on the phosphorylation of mTOR and p70S6K
was examined. No significant effects were observed on the
phosphorylation of mTOR and its downstream target (p70S6K)
following cisplatin treatment; whereas the activation of mTOR
signaling was significantly inhibited by cardamonin, or by the
combination of cardamonin and cisplatin (Fig. 4).
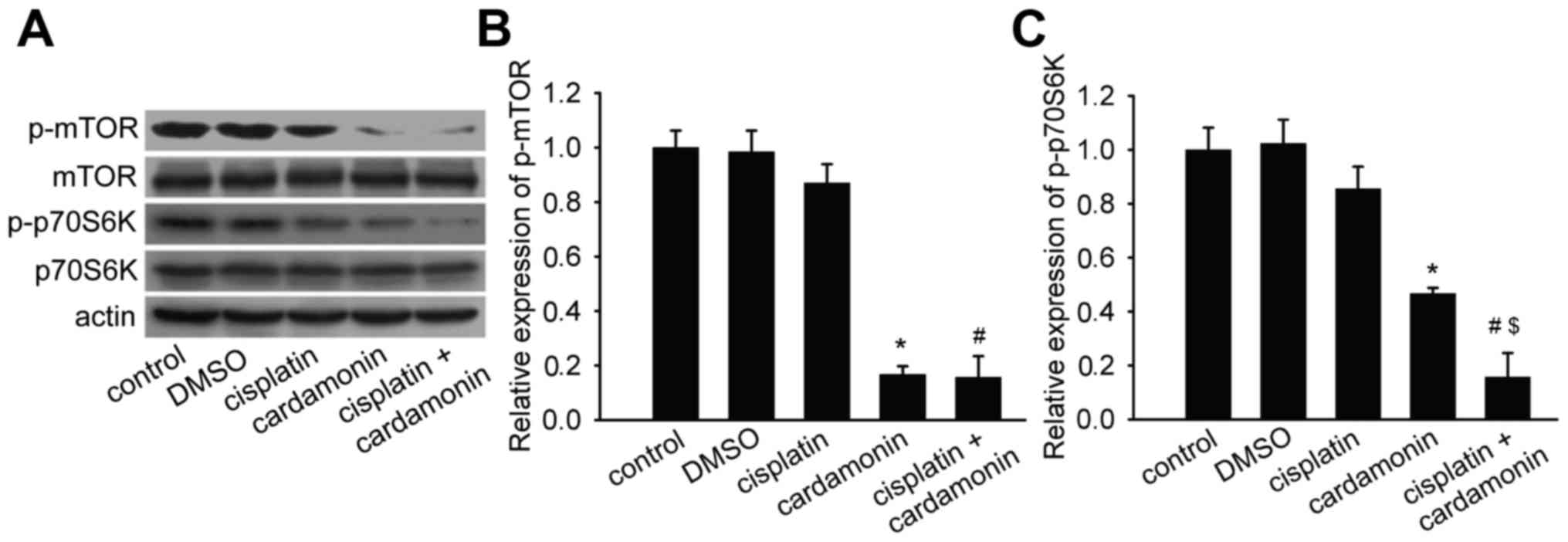 | Figure 4.Cardamonin inhibited the activation
of mTOR and p70S6K. SKOV3 cells were treated with cardamonin (20
µM), cisplatin (2 µg/ml) or the combination of both agents for 48
h. The expression of mTOR, phospho-mTOR (Ser2448), p70S6K and
phospho-p70S6K (Thr389) was determined by western blot analysis.
Actin was used as the loading control (n=3). (A) Representative
western blotting protein bands for mTOR, p-mTOR, p70S6K, p-p70S6K
and actin. Quantification of (B) p-mTOR and (C) p-p70S6K protein
expression. The relative density ratio of protein was normalized to
actin and compared with control, cisplatin or cardamonin. Data are
presented as the mean ± standard deviation. *P<0.01 vs. control;
#P<0.01 vs. cisplatin; $P<0.01 vs.
cardamonin. DMSO, dimethyl sulfoxide; mTOR, mammalian target of
rapamycin; p, phosphorylated; p70S6K, 70 kDa ribosomal protein S6
kinase. |
Discussion
Platinum-based chemotherapy is the most commonly
used therapeutic approach for ovarian cancer following initial
cytoreductive surgery. Although ovarian cancer is highly responsive
to cisplatin, there is a substantial risk of emergence of drug
resistance, which results in a poorer clinical outcome (29,30). Thus,
improving the antitumor efficiency of cisplatin is an essential way
for patients with ovarian cancer. A number of flavonoid compounds,
including baicalein, apigenin and fisetin may enhance the
cytotoxicity of cisplatin in head and neck squamous cell carcinoma,
embryonal carcinoma and cervical carcinoma cells (31–33).
Epithelial ovarian cancer accounts for almost 80% of all ovarian
cancer cases and is the leading cause of cancer-associated
mortality among women (34).
Therefore, it was investigated whether cardamonin was able to
potentiate the anticancer effect of cisplatin in epithelial ovarian
cancer cells, including SKOV3 and A2780 cell lines. The results
demonstrated that cardamonin significantly enhanced the
anti-proliferative effect of cisplatin in SKOV3 and A2780 cells.
The combinatorial effect on anti-proliferation using low
concentrations of cisplatin was similar to that of cisplatin in the
high concentration, which indicated that cardamonin may decrease
the dose of cisplatin required to inhibit the proliferation of
ovarian cancer cells, which may be beneficial in gaining clinical
efficacy with less adverse effects.
Numerous chemotherapeutic agents, including
cisplatin, paclitaxel and fluorouracil, disrupt the cell cycle,
which ultimately inhibits cell proliferation (35,36). Cell
cycle arrest is a major cellular response to DNA damage. Cisplatin
was able to induce G2/M arrest in ovarian cancer cells (37,38). A
previous study demonstrated that cardamonin inhibits cell growth
and induces cell cycle arrest at the G2/M phase in colon cancer
SW480 cells by suppressing the expression of cyclin D1, and c-myc
(39). The results of the present
study revealed that cardamonin increased the accumulation of cells
in the S and G2/M phase, and the combination of cardamonin and
cisplatin significantly increased the cisplatin-induced G2/M
arrest, which indicated that the potential anti-proliferative
effect of cardamonin was associated with cell cycle arrest.
The sensitivity of cancer cells to chemotherapeutic
drug-induced apoptosis depends on the balance between pro-apoptotic
and anti-apoptotic signals, and the inhibition of anti-apoptotic
signals has been proposed as a promising strategy to enhance the
efficacy of conventional chemotherapeutic agents (40). Several studies have reported that
cardamonin is able to inhibit the expression of Bcl-2, Survivin and
induce apoptosis (22,41). Furthermore, the mTOR inhibitor CCI-779
decreases the expression of anti-apoptotic proteins and restores
the sensitivity of cisplatin-resistant small cell lung cancer cells
to cisplatin (42). Therefore,
additional study is required to clarify whether cardamonin
potentiates the anti-proliferative effect of cisplatin through the
anti-apoptotic proteins. The results of the present study revealed
that cardamonin significantly enhanced the efficacy of cisplatin by
inhibiting the anti-apoptotic signals (Bcl-2, XIAP and
Survivin).
mTOR activation is associated with tumor malignancy
and poor prognosis in numerous types of human cancer. In addition,
mTOR is frequently hyper-activated in cisplatin-insensitive ovarian
cancer cells (43). In the present
study, it was demonstrated that the activation of mTOR and p70S6K
was significantly inhibited by cardamonin, and cardamonin combined
with cisplatin. This indicated that cardamonin may prevent the
occurrence of cisplatin resistance and reduce the incidence of poor
prognosis of patients with ovarian cancer induced by mTOR
activation. mTOR inhibition enhances the sensitivity of cisplatin
in numerous types of human cancers, including esophageal squamous
cell carcinoma, lung cancer and ovarian cancer (42,44,45).
RAD001, the analog of rapamycin, in combination with cisplatin was
demonstrated to induce a distinct increase in the number of
apoptotic cells by downregulating the pro-survival molecules,
Bcl-2, Survivin and Cyclin D1 in hepatocellular carcinoma (46), which was consistent with the present
results. Therefore, mTOR inhibition by cardamonin provides a
strategy for increasing the efficacy of platinum-based treatment
for ovarian cancer.
The clinical application of cisplatin is reduced by
its dose-dependent toxicity to normal organs, including the liver
and kidney. Cardamonin significantly enhanced the
anti-proliferative effect of low concentration of cisplatin in
ovarian cancer SKOV3 and A2780 cells, which may be useful in
reducing the toxicity of cisplatin on normal tissues. In addition,
compared with rapamycin, cardamonin has a number of advantages
in vivo, it attenuated the oxidative stress and inflammation
to protect against cisplatin-induced nephrotoxicity in rats
(24).
In conclusion, these results indicated that the
combination of cardamonin and cisplatin is more effective in
anti-proliferation compared with the agent used alone in ovarian
cancer cells. Cardamonin combined with cisplatin is a promising
therapeutic strategy, particularly for patients with mTOR
hyperactivation, which is beneficial for increasing the sensitivity
to cisplatin in ovarian cancer.
Acknowledgements
The present study was supported by the Youth
Scientific Foundation of Fujian Provincial Health and Family
Planning Commission (grant no. 2013-1-11) and the Natural Science
Foundation of Fujian Province (grant nos. 2015J01368 and
2016J01492), China.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qian X, Qin J, Pan S, Li X, Pan Y and Ma
S: Maintenance therapy in ovarian cancer with targeted agents
improves PFS and OS: A systematic review and meta-analysis. PLoS
One. 10:e01390262015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen J, Solomides C, Parekh H, Simpkins F
and Simpkins H: Cisplatin resistance in human cervical, ovarian and
lung cancer cells. Cancer Chemother Pharmacol. 75:1217–1227. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J and Wu GS: Role of autophagy in
cisplatin resistance in ovarian cancer cells. J Biol Chem.
289:17163–17173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Makin G and Dive C: Apoptosis and cancer
chemotherapy. Trends Cell Biol. 11:S22–S26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shaltouki A, Freer M, Mei Y and Weyman CM:
Increased expression of the pro-apoptotic Bcl2 family member PUMA
is required for mitochondrial release of cytochrome C and the
apoptosis associated with skeletal myoblast differentiation.
Apoptosis. 12:2143–2154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scott FL, Denault JB, Riedl SJ, Shin H,
Renatus M and Salvesen GS: XIAP inhibits caspase-3 and −7 using two
binding sites: Evolutionarily conserved mechanism of IAPs. EMBO J.
24:645–655. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zumbrägel FK, Machtens DA, Curth U, Lüder
CG, Reubold TF and Eschenburg S: Survivin does not influence the
anti-apoptotic action of XIAP on caspase-9. Biochem Biophys Res
Commun. 482:530–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Q, Wang H, Chen S, Lan X, Xiao H, Shi
H and Ma Y: Fiber-optic-based micro-probe using hexagonal 1-in-6
fiber configuration for intracellular single-cell pH measurement.
Anal Chem. 87:7171–7179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Z, Xie Z, Sun G, Yang P, Li J, Yang
H, Xiao S, Liu Y, Qiu H, Qin L, et al: Reversing drug resistance of
cisplatin by hsp90 inhibitors in human ovarian cancer cells. Int J
Clin Exp Med. 8:6687–6701. 2015.PubMed/NCBI
|
|
11
|
Zhao WJ, Deng BY, Wang XM, Miao Y and Wang
JN: XIAP associated factor 1 (XAF1) represses expression of
X-linked inhibitor of apoptosis protein (XIAP) and regulates
invasion, cell cycle, apoptosis, and cisplatin sensitivity of
ovarian carcinoma cells. Asian Pac J Cancer Prev. 16:2453–2458.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mabuchi S, Kawase C, Altomare DA,
Morishige K, Sawada K, Hayashi M, Tsujimoto M, Yamoto M,
Klein-Szanto AJ, Schilder RJ, et al: mTOR is a promising
therapeutic target both in cisplatin-sensitive and
cisplatin-resistant clear cell carcinoma of the ovary. Clin Cancer
Res. 15:5404–5413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu S, Fu GB, Tao Z, OuYang J, Kong F,
Jiang BH, Wan X and Chen K: MiR-497 decreases cisplatin resistance
in ovarian cancer cells by targeting mTOR/P70S6K1. Oncotarget.
6:26457–26471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng X, Li L, Jiang H, Jiang K, Jin Y and
Zheng J: Dihydroartemisinin potentiates the anticancer effect of
cisplatin via mTOR inhibition in cisplatin-resistant ovarian cancer
cells: Involvement of apoptosis and autophagy. Biochem Biophys Res
Commun. 444:376–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng DJ, Wang J, Zhou JY and Wu GS: Role
of the Akt/mTOR survival pathway in cisplatin resistance in ovarian
cancer cells. Biochem Biophys Res Commun. 394:600–605. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu H, Zhao Y, Mu X, Wu H, Chen L, Liu W,
Mu Y, Liu J and Wei X: A silica-polymer composite nano system for
tumor-targeted imaging and p53 gene therapy of lung cancer. J
Biomater Sci Polym Ed. 26:384–400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mabuchi S, Hisamatsu T, Kawase C, Hayashi
M, Sawada K, Mimura K, Takahashi K, Takahashi T, Kurachi H and
Kimura T: The activity of trabectedin as a single agent or in
combination with everolimus for clear cell carcinoma of the ovary.
Clin Cancer Res. 17:4462–4473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Qin Y, Yang C, Zhang H, Li Y, Wu B,
Huang J, Zhou X, Huang B, Yang K and Wu G: Cardamonin induces
ROS-mediated G2/M phase arrest and apoptosis through inhibition of
NF-κB pathway in nasopharyngeal carcinoma. Cell Death Dis.
8:e30242017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Sikka S, Siveen KS, Lee JH, Um
JY, Kumar AP, Chinnathambi A, Alharbi SA, Basappa, Rangappa KS, et
al: Cardamonin represses proliferation, invasion, and causes
apoptosis through the modulation of signal transducer and activator
of transcription 3 pathway in prostate cancer. Apoptosis.
22:158–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shrivastava S, Jeengar MK, Thummuri D,
Koval A, Katanaev VL, Marepally S and Naidu VGM: Cardamonin, a
chalcone, inhibits human triple negative breast cancer cell
invasiveness by downregulation of Wnt/β-catenin signaling cascades
and reversal of epithelial-mesenchymal transition. Biofactors.
43:152–169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim YJ, Kang KS, Choi KC and Ko H:
Cardamonin induces autophagy and an antiproliferative effect
through JNK activation in human colorectal carcinoma HCT116 cells.
Bioorg Med Chem Lett. 25:2559–2564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu N, Liu J, Zhao X, Yan Z, Jiang B, Wang
L, Cao S, Shi D and Lin X: Cardamonin induces apoptosis by
suppressing STAT3 signaling pathway in glioblastoma stem cells.
Tumour Biol. 36:9667–9676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia D, Tan Y, Liu H, Ooi S, Li L, Wright
K, Bennett S, Addison CL and Wang L: Cardamonin reduces
chemotherapy-enriched breast cancer stem-like cells in vitro and in
vivo. Oncotarget. 7:771–785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El-Naga RN: Pre-treatment with cardamonin
protects against cisplatin-induced nephrotoxicity in rats: Impact
on NOX-1, inflammation and apoptosis. Toxicol Appl Pharmacol.
274:87–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang Y, Fang Q, Shi D, Niu P, Chen Y and
Deng J: mTOR inhibition of cardamonin on antiproliferation of A549
cells is involved in a FKBP12 independent fashion. Life Sci.
99:44–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niu PG, Zhang YX, Shi DH, Liu Y, Chen YY
and Deng J: Cardamonin inhibits metastasis of lewis lung carcinoma
cells by decreasing mTOR activity. PLoS One. 10:e01277782015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue ZG, Niu PG, Shi DH, Liu Y, Deng J and
Chen YY: Cardamonin inhibits angiogenesis by mTOR downregulation in
SKOV3 cells. Planta Med. 82:70–75. 2016.PubMed/NCBI
|
|
28
|
Mabuchi S, Altomare DA, Cheung M, Zhang L,
Poulikakos PI, Hensley HH, Schilder RJ, Ozols RF and Testa JR:
RAD001 inhibits human ovarian cancer cell proliferation, enhances
cisplatin-induced apoptosis, and prolongs survival in an ovarian
cancer model. Clin Cancer Res. 13:4261–4270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
González-Martín A, Sánchez-Lorenzo L,
Bratos R, Márquez R and Chiva L: First-line and maintenance therapy
for ovarian cancer: Current status and future directions. Drugs.
74:879–889. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mould T: An overview of current diagnosis
and treatment in ovarian cancer. Int J Gynecol Cancer. 22 Suppl
1:S2–S4. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan LP, Chou TH, Ding HY, Chen PR, Chiang
FY, Kuo PL and Liang CH: Apigenin induces apoptosis via tumor
necrosis factor receptor- and Bcl-2-mediated pathway and enhances
susceptibility of head and neck squamous cell carcinoma to
5-fluorouracil and cisplatin. Biochim Biophys Acta. 1820:1081–1091.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tripathi R, Samadder T, Gupta S, Surolia A
and Shaha C: Anticancer activity of a combination of cisplatin and
fisetin in embryonal carcinoma cells and xenograft tumors. Mol
Cancer Ther. 10:255–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Wang Q, Zhang S, Zhang Y and Tao
L: Baicalein increases the cytotoxicity of cisplatin by enhancing
gap junction intercellular communication. Mol Med Rep. 10:515–521.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sankaranarayanan R and Ferlay J: Worldwide
burden of gynaecological cancer: The size of the problem. Best
Pract Res Clin Obstet Gynaecol. 20:207–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Elias ST, Borges GA, Rêgo DF, E Silva LF,
Avelino S, DE Matos Neto JN, Simeoni LA and Guerra EN: Combined
paclitaxel, cisplatin and fluorouracil therapy enhances ionizing
radiation effects, inhibits migration and induces G0/G1 cell cycle
arrest and apoptosis in oral carcinoma cell lines. Oncol Lett.
10:1721–1727. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Li W, Deng W, Gan Y, Wu K and Sun J:
Synergistic anti-proliferative and pro-apoptotic activities of 5F
and cisplatin in human non-small cell lung cancer NCI-H23 cells.
Oncol Lett. 14:5347–5353. 2017.PubMed/NCBI
|
|
37
|
Chen X, Gong L, Ou R, Zheng Z, Chen J, Xie
F, Huang X, Qiu J, Zhang W, Jiang Q, et al: Sequential combination
therapy of ovarian cancer with cisplatin and γ-secretase inhibitor
MK-0752. Gynecol Oncol. 140:537–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taylor-Harding B, Orsulic S, Karlan BY and
Li AJ: Fluvastatin and cisplatin demonstrate synergistic
cytotoxicity in epithelial ovarian cancer cells. Gynecol Oncol.
119:549–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park S, Gwak J, Han SJ and Oh S:
Cardamonin suppresses the proliferation of colon cancer cells by
promoting β-catenin degradation. Biol Pharm Bull. 36:1040–1044.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang H, Zhang Z, Wei X and Dai R:
Small-molecule inhibitor of Bcl-2 (TW-37) suppresses growth and
enhances cisplatin-induced apoptosis in ovarian cancer cells. J
Ovarian Res. 8:32015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yadav VR, Prasad S and Aggarwal BB:
Cardamonin sensitizes tumour cells to TRAIL through ROS- and
CHOP-mediated up-regulation of death receptors and down-regulation
of survival proteins. Br J Pharmacol. 165:741–753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wangpaichitr M, Wu C, You M, Kuo MT, Feun
L, Lampidis T and Savaraj N: Inhibition of mTOR restores cisplatin
sensitivity through down-regulation of growth and anti-apoptotic
proteins. Eur J Pharmacol. 591:124–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Aide N, Kinross K, Cullinane C, Roselt P,
Waldeck K, Neels O, Dorow D, McArthur G and Hicks RJ: 18F-FLT PET
as a surrogate marker of drug efficacy during mTOR inhibition by
everolimus in a preclinical cisplatin-resistant ovarian tumor
model. J Nucl Med. 51:1559–1564. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hou G, Yang S, Zhou Y, Wang C, Zhao W and
Lu Z: Targeted inhibition of mTOR signaling improves sensitivity of
esophageal squamous cell carcinoma cells to cisplatin. J Immunol
Res. 2014:8457632014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cai Y, Tan X, Liu J, Shen Y, Wu D, Ren M,
Huang P and Yu D: Inhibition of PI3K/Akt/mTOR signaling pathway
enhances the sensitivity of the SKOV3/DDP ovarian cancer cell line
to cisplatin in vitro. Chin J Cancer Res. 26:564–572.
2014.PubMed/NCBI
|
|
46
|
Tam KH, Yang ZF, Lau CK, Lam CT, Pang RW
and Poon RT: Inhibition of mTOR enhances chemosensitivity in
hepatocellular carcinoma. Cancer Lett. 273:201–209. 2009.
View Article : Google Scholar : PubMed/NCBI
|