Introduction
Colon cancer is one of the most lethal solid tumors
of the gastrointestinal tract, and is the third most common cancer
in men and the second in women (1).
It was estimated that 134,490 new cases and 49,190 mortalities
would occur in 2016 (2). Surgical
resection is the most common treatment for all stages of colon
cancer, whereas for intermediate and advanced colon cancer,
chemotherapy and radiation therapy may be additional methods used
to protect against tumor progression (3). Despite the substantial progress that has
been made in the treatment of colon cancer, patients continue to
develop advanced cancer, resulting in a poor prognosis. Therefore,
understanding the mechanisms underlying colon cancer is essential
for an effective strategy to improve the present situation.
AMPK-related protein kinase 5 (ARK5), the fifth
member of the 5′ adenosine monophosphate-activated protein kinase
catalytic subunit family, is a tumor invasion-associated factor
regulated by Akt signaling (4).
Suzuki et al (5,6) demonstrated that ARK5 induces tumor cell
survival during nutrient starvation by inhibiting caspase-8
activation and that it resists the Fas ligand/Fas system by
negatively regulating procaspase-6 phosphorylation at
Ser257. In further studies, it was observed that ARK5
was involved in hypoxia-induced tumor cell tolerance to glucose
starvation regulated by the transforming growth factor-β signaling
pathway (7), and that ARK5 was
downstream of NDR2 during activation of insulin-like growth
factor-1 signaling (8). Previous
studies reported that ARK5 was overexpressed in numerous
malignancies, including breast cancer, colorectal carcinoma,
hepatocellular carcinoma and gliomas, and that patients
overexpressing ARK5 often had a poor prognosis (9–12). ARK5
was also a direct target of large musculoaponeurotic fibrosarcoma
proteins and this activation was mediated through MAF-recognition
element sequences (13). However, the
relationship between ARK5 and HIF1a remains unclear.
The transcription factor, hypoxia-inducible factor 1
(HIF1), has been reported to be one of the key prognostic tumor
factors that is accumulated and detectable under hypoxic conditions
(14). HIF1 is a heterodimer
consisting of two components; HIF1-α and HIF1-β. HIF1-α is
oxygen-regulated and determines the activity of HIF-1 (15). As an important regulator in hypoxic
conditions, HIF-1α regulates tumor metabolism, proliferation,
apoptosis, metastasis, inflammation and angiogenesis (16). In a study by Wang et al
(17), HIF1-α was overexpressed in
colon cancer and was associated with a poor prognosis.
In the present study, the relationship between ARK5
and HIF1a in colon cancer was investigated in order to identify
novel molecular targets for the treatment of colon cancer.
Materials and methods
Patients and samples
Tissue samples were obtained from 60 patients with
colon cancer (32 men and 28 women; aged 30–80 years) who had
undergone surgery at the Department of Endoscopic Surgery, Renmin
Hospital of Wuhan University (Hubei, China). All patients had
received an intraoperative pathological diagnosis and none of the
patients had previously undergone radiotherapy or chemotherapy.
Patient charts were reviewed to obtain clinical data regarding age,
sex, Tumor-Node-Metastasis (TNM) staging (18), tumor histologic grade (18) and lymph node metastasis. Written
informed consent was obtained from all patients, and all
experiments were approved by the medical ethics committee of Renmin
Hospital of Wuhan University (Wuhan, China).
Cell lines and cell culture
An SW480 cell line was purchased from the Shanghai
Cell Bank at the Chinese Academy of Sciences (Shanghai, China).
This cell line was cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) at 37°C in a humidified incubator containing 5%
CO2. A 3-chamber air incubator flushed with a gas
mixture of 5% CO2 and 94% N2 at 37°C was used
to create the hypoxic conditions. The final O2 pressure
of the medium was measured at 1%.
Total RNA extraction and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
A TRIzol Total RNA Extraction kit (Invitrogen;
Thermo Fisher Scientific, Inc.) was used for total RNA extraction
from the SW480 cell line; cDNA was synthesized using a ReverTra
Ace-α reverse transcription kit (Invitrogen; Thermo Fisher
Scientific, Inc.). For RT-qPCR analysis, the Roche LightCycler
(Roche Applied Science, Penzberg, Germany) was used with the Takara
SYBR Premix Extaq system (Takara Biotechnology Co., Ltd., Dalian,
China). All procedures were performed according to the
manufacturer's protocols. The thermocycling conditions were as
follows: Cycle 1 at 95°C for 30 sec, cycle 2, 40 cycles at 95°C for
5 sec and at 60°C for 30–60 sec, followed by dissociation. Primers
were synthesized by Shanghai Sangon Biological Engineering
Technology Services Co., Ltd. (Shanghai, China). The nucleotide
sequences of the primers were as follows: β-actin forward, 5-ATG
GGG AAG GTG AAG GTC G-3′ and reverse, 5-ATG GGG AAG GTG AAG GTC
G-3′; ARK5 forward, 5′-GGGAAGGTGAAGGTCG-3′ and reverse,
5′-GGGAAGGTGAAGGTCG-3′; and HIF1-α forward, 5-ATC CAT GTG ACC ATG
AGG AAA TG-3′ and reverse, 5′-ATCCATGTGACCATGAGGAAATG-3′. Each
sample was taken in triplicate, β-actin was used as an internal
reference and the 2−∆∆Cq method (19) was used to analyze the PCR results.
Western blot analysis
Total protein was extracted from harvested SW480
cells using a protein extraction kit (Beyotime Institute of
Biotechnology, Haimen, China). Total protein was quantified
according to the protocol of the Pierce BCA-200 Protein Assay kit
(Thermo Scientific™, Waltham, USA) and denatured by
boiling (ARK5=74 kD, HIF1-α=120 kD, β-actin=43 kD), separated by
10% SDS-PAGE Precast Gel and transferred onto polyvinylidene
fluoride membranes. The membranes were then blocked by 5% milk (at
37°C for 1 h) prior to being incubated with primary antibodies
overnight at 4°C. The membranes were then washed 3 times with TBST
(Tris-buffered saline with 0.1% Tween-20), prior to being incubated
with a secondary horseradish peroxidase goat anti-rabbit/anti-mouse
immunoglobulin G (H+L) antibody (dilution 1:2,000; cat no.
ab97051/ab97023; Abcam, Cambridge, UK) for 2 h at 37°C. Finally,
the membranes were visualized using a chemiluminescence kit
(Beyotime Institute of Biotechnology) on a Bio-Rad imaging system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The primary
antibodies used in the present study were as follows: HIF1-α (cat
no. ab114977), ARK5 (cat no. ab37641) and β-actin (cat no. ab8227;
all at a dilution of 1:1,000; Abcam).
Immunohistochemical analysis
Tissues of 60 patients with colon cancer from our
hospital were sent to Shanghai OUTDO Biotech Co., Ltd and converted
into a microarray, and immunostaining was performed as per the
following protocol. Tissue microarrays (thickness of 4 µm) were
deparaffinized and placed in a solution of absolute methanol and
0.3% hydrogen peroxide for 30 min. Antigen retrieval was then
performed by heating the slides in citrate buffer (pH 6.2) in a
microwave oven at 95°C for 20 min and washing them in PBS 3 times
prior to staining with immunoperoxidase. Slides were incubated in a
humidified chamber with blocking agent (5% FBS) for 30 min at room
temperature. The slides were then incubated overnight with
anti-ARK5 antibody (dilution, 1:100; cat no. ab37641; Abcam) and
anti-HIF1-α antibody (dilution, 1:100; cat no. ab114977; Abcam) in
5% FBS at 4°C. Following being washed with phosphate-buffered
saline, the slides were incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin for 40 min at
room temperature. Results were visualized by reaction with
diaminobenzidine and counterstained with hematoxylin (at room
temperature for 3 min). For each sample, images of 5
randomly-selected areas were visualized using a light microscope
(magnification, ×100) and captured using a high-power objective
lens camera (Axiocam ERc 5s; Zeiss GmbH, Jena, Germany) under the
same conditions. Evaluation of immunohistochemistry results was
performed by 2 independent observers to determine the percentage of
positive cells following inspection of all fields in the sections,
as described previously (20).
Transfection of the SW480 cell line
with small interfering (si)RNA
Human-specific HIF1-α and ARK5 siRNA were designed,
constructed and purified by Jima Biotech Co. (Shanghai, China).
Their respective sequences were as follows: HIF1-α,
5′-GGAAATGAGAGAAATGCTTAC-3′; and ARK5, 5-GAA GTT ATG CTT TAT TCA
C-3. After determining the best effect of interference and the best
transfer multiplicity of infection (60) through the fluorescence
camera, and performing RT-qPCR and western blot analysis, SW480
cells were seeded onto 6-well plates at a concentration of
0.3×105 per well (20–30% confluence) 1 day prior to
siRNA transfection. The 5 µl of 50 nM HIF1-α siRNA, ARK5 siRNA and
negative control were transfected into different cells with
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Following incubation at room temperature for 6 h, medium was
replaced with fresh RPMI-1640. According to the indicated times,
the cells were harvested for subsequent studies.
Cell counting kit (CCK)-8 and
Transwell assay
ARK5 siRNA-transfected cells were collected and then
seeded at a density of 1.0×104 cells/well onto 96-well
plates. Cell viability was subsequently measured using CCK-8
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) at 8, 24,
48 and 72 h. Other procedures were according to the manufacturer's
protocols. Next, 1×105 cells transfected with ARK5 siRNA
were seeded into a serum-free minimum essential medium (MEM) in the
upper chamber on an insert coated without Matrigel (cat. no.
PSET010R5; EMD Millipore, Billerica, MA, USA). In the lower
chamber, medium was swapped for MEM with 10% FBS. The cells were
incubated for 24 h. Following fixation in room temperature with
100% methanol for 10 min and staining by crystal violet for 5 min
at room temperature, cells on the bottom surface that had invaded
across the membranes were counted and images were captured under a
light microscope (magnification, ×100) using a camera (Axiocam ERc
5s; Zeiss GmbH). All experiments were performed in triplicate.
Cell apoptosis
SW480 cells transfected with ARK5 siRNA or control
were trypsinized and washed 3 times with pre-chilled PBS buffer and
resuspended in 100 µl PBS at 1×106 cells/ml after 24 h
of treatment in hypoxic conditions. Apoptotic SW480 cells were
measured by staining with fluorescein isothiocyanate and a
propidium iodide (PI) kit (Annexin V-FITC/PI Apoptosis Assay kit,
70-AP101-100; MultiSciences Biotech Co. Ltd., Hangzhou, China)
according to the manufacturer's protocol (at room temperature for
30 min in the dark), and were analyzed using a flow cytometer (BD
FACSCalibur; BD Biosciences) and FlowJo software 7.6 (FlowJo LLC,
Ashland, OR, USA).
Statistical analysis
Statistical analysis was performed using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as
the mean ± standard deviation and Student's t-test and
paired-samples t-test were performed as appropriate. The
χ2 test or Fisher's exact test were used to evaluate any
potential association between ARK5/HIF1-α expression and the
clinicopathological parameters. Associations between expression of
ARK5 and HIF1-α were analyzed using Spearman's correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Immunohistochemical staining of HIF1-α
and ARK5, and correlation with clinicopathological features in
human colon cancer
Immunohistochemical staining was used to determine
the expression of HIF1-α and ARK5 in 60 patients with colon cancer,
and HIF1-α and ARK5 were revealed to be overexpressed in colon
cancer. Among these 60 colon cancer tissues, 76.7% (46/60) and
71.7% (43/60) were positive for HIF1-α and ARK5 expression,
respectively. Additionally, these two proteins were predominantly
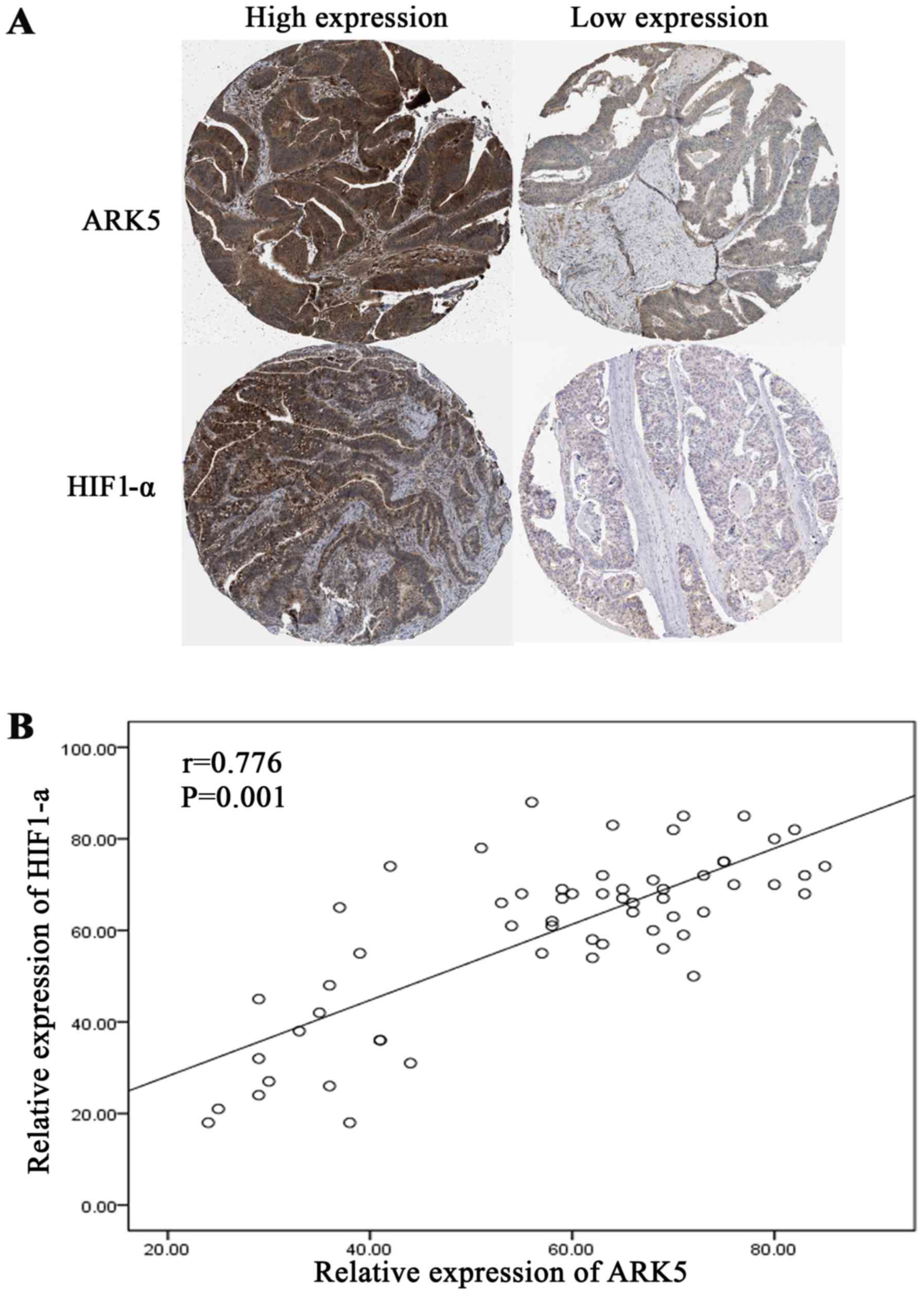
expressed in the cytoplasm and the nucleus (Fig. 1A). Single factor analysis demonstrated
that expression of HIF1-α and ARK5 was associated with tumor stage
(73.9 and 74.4%, respectively), tumor grade (78.3 and 81.4%,
respectively), lymph node metastasis (82.6 and 79.1%, respectively)
and liver metastasis (32.6 and 30.2%, respectively) (Table I). These results suggest that an
association between HIF1-α and ARK5 may exist. Linear correlation
analysis was then used for assessing the expression of HIF1-α and
ARK5, and it was revealed that a correlation existed between the
expression of these two proteins (r=0.776, P=0.001) (Fig. 1B).
 | Table I.Association between HIF1-α and ARK5
expression and clinicopathological features of colon cancer. |
Table I.
Association between HIF1-α and ARK5
expression and clinicopathological features of colon cancer.
|
| HIF1-α
expression | ARK5 expression |
|---|
|
|
|
|
|---|
| Variables | High | Low | P-value | High | Low | P-value |
|---|
| Age, years |
|
|
|
|
|
|
| ≤60 | 26 | 10 | 0.304 | 24 | 12 | 0.282 |
|
>60 | 20 | 4 |
| 19 | 5 |
|
| Sex |
|
|
|
|
|
|
| Male | 28 | 4 | 0.143 | 25 | 8 | 0.263 |
|
Female | 18 | 10 |
| 18 | 9 |
|
| TNM staging |
|
|
|
|
|
|
| I–II | 12 | 14 | 0.001a | 11 | 15 | 0.008a |
|
III–IV | 34 | 0 |
| 32 | 2 |
|
| Grade |
|
|
|
|
|
|
|
Well/moderate | 10 | 12 | 0.005a | 8 | 14 | 0.009a |
| Poor | 36 | 2 |
| 35 | 3 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
| Yes | 38 | 2 | 0.000a | 34 | 2 | 0.005a |
| No | 8 | 12 |
| 9 | 15 |
|
| Liver metastasis |
|
|
|
|
|
|
| Yes | 15 | 0 | 0.014a | 13 | 0 | 0.010a |
| No | 31 | 14 |
| 30 | 17 |
|
ARK5 is upregulated by HIF1-α under
hypoxic conditions in the SW480 cell line
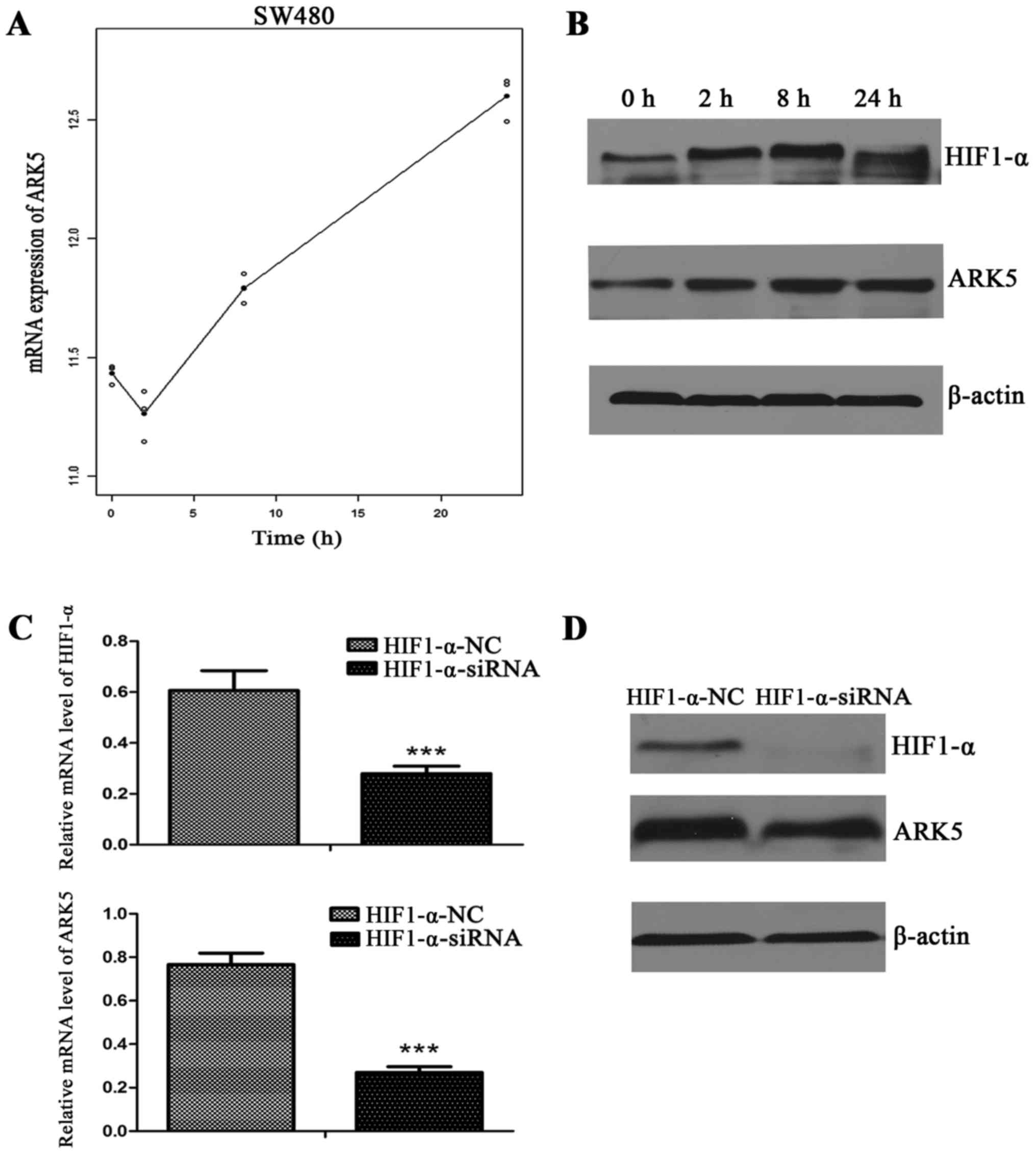
To study the association between ARK5 and HIF1-α,
the change in expression of ARK5 and HIF1-α was observed in the
SW480 cell line under hypoxic conditions. During the period of
hypoxia (0, 2, 8 and 24 h), the mRNA level of ARK5 was markedly
increased in the SW480 cell line, and reached its highest level at
24 h (Fig. 2A). Using western blot
analysis to detect the change of protein expression in the SW480
cell line under the hypoxic conditions, the expression of HIF1-α
was found to be increased and reached its highest level at 8 h,
while the expression of ARK5 was increased throughout the period of
hypoxia (Fig. 2B). Such results
demonstrate that ARK5 was upregulated under the hypoxic conditions
in the SW480 cell line. To verify that ARK5 was regulated by HIF1-α
under hypoxia, siRNA was used to suppress the expression of HIF1-α
under the hypoxia condition, and both the mRNA and protein levels
of ARK5 were markedly decreased (Fig. 2C
and D).
HIF1-α promotes tumor proliferation
and migration under hypoxic conditions by upregulating expression
of ARK5
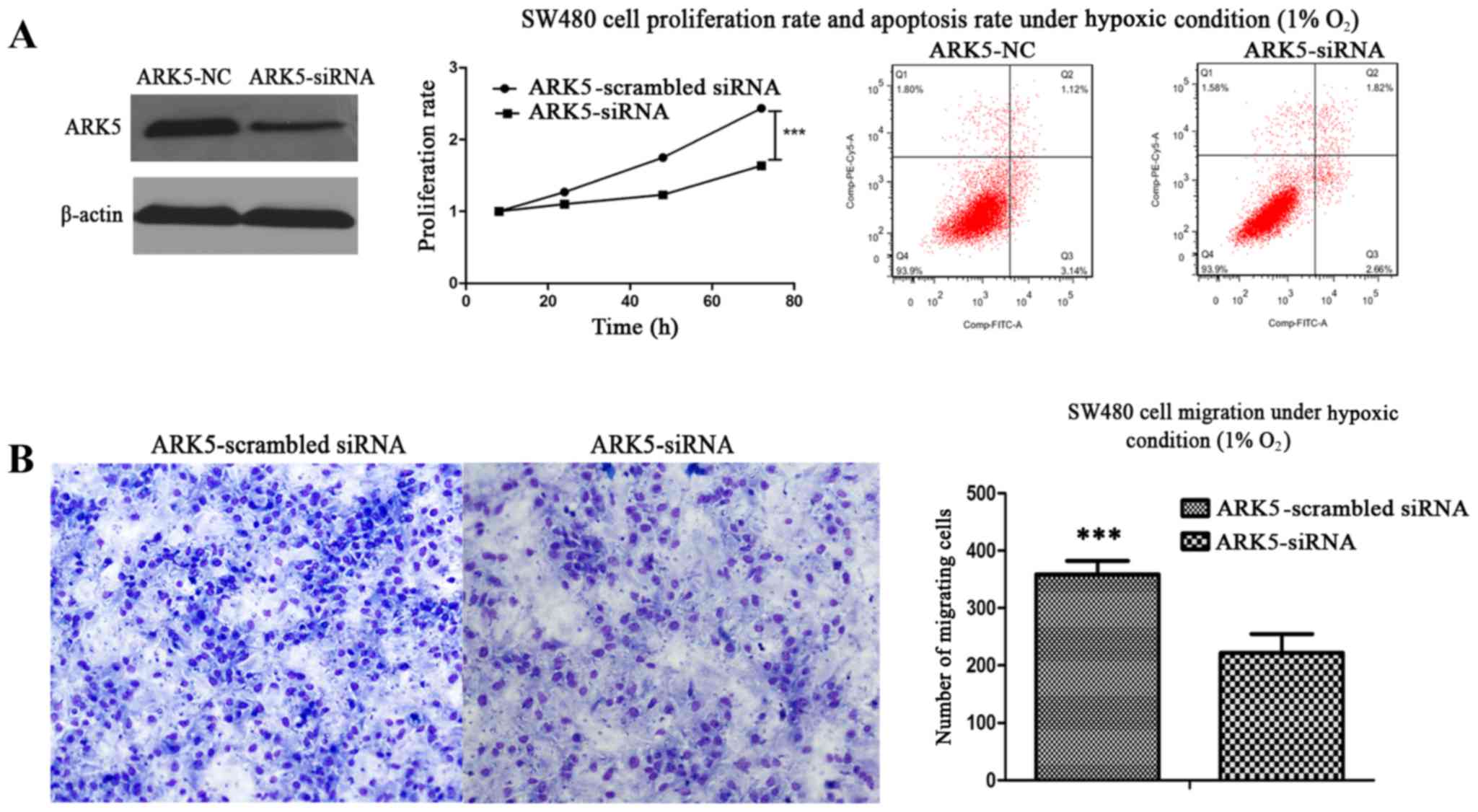
As ARK5 was upregulated by HIF1-α under hypoxic
conditions and as HIF1-α may promote tumor proliferation and
migration, it was suggested that there may be an association
between ARK5 and tumor proliferation and migration under hypoxic
conditions. To verify this hypothesis, siRNA was used to suppress
the expression of ARK5, and cell proliferation, apoptosis and
migration were observed by CCK-8, flow cytometry and Transwell
assays under hypoxic conditions. These results demonstrated that
the proliferation and migration abilities of the SW480 cell line
were significantly decreased under the hypoxic conditions, while
apoptosis did not differ (Fig. 3A and
B). Therefore, ARK5 was the gene downstream of HIF1-α for
proliferation and migration of the SW480 cell line under hypoxic
conditions.
Discussion
Previous studies have suggested that ARK5 serves an
important role in cancer proliferation, migration, invasion,
starvation and drug resistance in a number of cancer types,
including breast cancer, hepatocellular carcinoma, colorectal
cancer and glioma, with ARK5 overexpression being associated with a
poor prognosis (5,9–12,21). However, the expression of ARK5 was not
static but dynamic in response to conditions such as hypoxic
stress. The role served by ARK5 in hypoxia remains unclear, as does
the association between the expression of ARK5 and that of
HIF1-α.
As Kusakai et al (22) had demonstrated that ARK5
overexpression is involved in the progression of colon cancer, the
present study only assessed the expression of ARK5 and HIF1-α in
cancer tissues. Based upon this, the present study demonstrated
that ARK5 and HIF1-α were overexpressed in colon cancer, with
linear correlations being identified. Furthermore, ARK5 was
upregulated under hypoxic conditions over time in the SW480 cell
line. Such findings suggest that ARK5 may be regulated by HIF1-α
and may promote tumor cell survival during hypoxic conditions. To
verify this hypothesis, we used siRNA to knockdown the expression
of HIF1-α in the SW480 cell line under conditions of hypoxia.
Notably, the expression level of ARK5 was also markedly decreased.
These results demonstrated that ARK5 was upregulated by HIF1-α
under conditions of hypoxia.
Hypoxia is a condition existing in all types of
tumor and is involved in cancer proliferation, drug resistance,
migration, invasion and immune escape. HIF1-α is serving a critical
regulatory role under conditions of hypoxia (23–25).
Analysis of the clinical data for 60 colon cancer patients revealed
that the expression of ARK5 and HIF1-α was associated with tumor
stage, tumor grade, lymph node metastasis and liver metastasis, and
that ARK5 was downstream of HIF1-α under hypoxic conditions. We
hypothesized that a partial function of HIF1-α under hypoxic
conditions was conducted by ARK5 in colon cancer cells. SiRNA was
used to knockdown expression of ARK5 in the SW480 cell line and,
when the SW480 cell line was placed under hypoxic conditions, the
proliferation and migration ability of the cells were significantly
decreased compared with that of the negative control and such
proliferation changes were not a result of apoptosis. Therefore, it
can be concluded that HIF1-α promotes cell proliferation and
migration under hypoxia in the SW480 cell line by upregulating
expression of ARK5.
However, as the survival data of the aforementioned
60 patients was not available, it was not possible to analyze the
tumor-free survival and overall survival associated with the
co-expression of ARK5 and HIF1-α. Therefore, further studies are
required to address this issue. Additionally, the neighboring
tissue was not analyzed as a control, as Kusakai et al
(22) had previously demonstrated
that ARK5 was overexpressed in colon tissue. To conclude, to the
best of our knowledge, the present study was the first to identify
an association between ARK5 and HIF1-α in colon cancer tissues,
which may provide novel concepts for the clinical treatment of
colon cancer.
References
|
1
|
WILD BWSCP: World Cancer Report:
International Agency for Research on Cancer. 978-992-832-0443-5.
2014.
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Board., P.A.T.E., PDQ Adult Treatment
Editorial Board, . Colon Cancer Treatment (PDQ®):
Patient Version. PDQ Cancer Information Summaries [Internet].
Bethesda (MD): National Cancer Institute (US); 2016
|
|
4
|
Suzuki A, Lu J, Kusakai G, Kishimoto A,
Ogura T and Esumi H: ARK5 is a tumor invasion-associated factor
downstream of Akt signaling. Mol Cell Biol. 24:3526–3535. 2004.
View Article : Google Scholar
|
|
5
|
Suzuki A, Kusakai G, Kishimoto A, Lu J,
Ogura T and Esumi H: ARK5 suppresses the cell death induced by
nutrient starvation and death receptors via inhibition of caspase 8
activation, but not by chemotherapeutic agents or UV irradiation.
Oncogene. 22:6177–6182. 2003. View Article : Google Scholar
|
|
6
|
Suzuki A, Kusakai G, Kishimoto A, Shimojo
Y, Miyamoto S, Ogura T, Ochiai A and Esumi H: Regulation of
caspase-6 and FLIP by the AMPK family member ARK5. Oncogene.
23:7067–7075. 2004. View Article : Google Scholar
|
|
7
|
Suzuki A, Kusakai G, Shimojo Y, Chen J,
Ogura T, Kobayashi M and Esumi H: Involvement of transforming
growth factor-beta 1 signaling in hypoxia-induced tolerance to
glucose starvation. J Biol Chem. 280:31557–31563. 2005. View Article : Google Scholar
|
|
8
|
Suzuki A, Ogura T and Esumi H: NDR2 acts
as the upstream kinase of ARK5 during insulin-like growth factor-1
signaling. J Biol Chem. 281:13915–13921. 2006. View Article : Google Scholar
|
|
9
|
Chang XZ, Yu J, Liu HY, Dong RH and Cao
XC: ARK5 is associated with the invasive and metastatic potential
of human breast cancer cells. J Cancer Res Clin Oncol. 138:247–254.
2012. View Article : Google Scholar
|
|
10
|
Cui J, Yu Y, Lu GF, Liu C, Liu X, Xu YX
and Zheng PY: Overexpression of ARK5 is associated with poor
prognosis in hepatocellular carcinoma. Tumor Biol. 34:1913–1918.
2013. View Article : Google Scholar
|
|
11
|
Sun X, Gao L, Chien HY, Li WC and Zhao J:
The regulation and function of the NUAK family. J Mol Endocrinol.
51:R15–R22. 2013. View Article : Google Scholar
|
|
12
|
Lu S, Niu N, Guo H, Tang J, Guo W, Liu Z,
Shi L, Sun T, Zhou F, Li H, et al: ARK5 promotes glioma cell
invasion and its elevated expression is correlated with poor
clinical outcome. Eur J Cancer. 49:752–763. 2013. View Article : Google Scholar
|
|
13
|
Suzuki A, Iida S, Kato-Uranishi M, Tajima
E, Zhan F, Hanamura I, Huang Y, Ogura T, Takahashi S, Ueda R, et
al: ARK5 is transcriptionally regulated by the Large-MAF family and
mediates IGF-1-induced cell invasion in multiple myeloma: ARK5 as a
new molecular determinant of malignant multiple myeloma. Oncogene.
24:6936–6944. 2005. View Article : Google Scholar
|
|
14
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
15
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:pp. 5510–5514. 1995; View Article : Google Scholar
|
|
16
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar
|
|
17
|
Wang JS, Jing CQ, Shan KS, Chen YZ, Guo
XB, Cao ZX, Mu LJ, Peng LP, Zhou ML and Li LP: Semaphorin 4D and
hypoxia-inducible factor-1α overexpression is related to prognosis
in colorectal carcinoma. World J Gastroenterol. 21:2191–2198. 2015.
View Article : Google Scholar
|
|
18
|
Amin MB, Greene FL, Edge S, et al: AJCC
cancer staging manual. 8th. New York: Springer; pp. 1–274. 2017
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Kong D, Su G, Zha L, Zhang H, Xiang J, Xu
W, Tang Y and Wang Z: Coexpression of HMGA2 and Oct4 predicts an
unfavorable prognosis in human gastric cancer. Med Oncol.
31:1302014. View Article : Google Scholar
|
|
21
|
Xu T, Zhang J, Chen W, Pan S, Zhi X, Wen
L, Zhou Y, Chen BW, Qiu J, Zhang Y, et al: ARK5 promotes
doxorubicin resistance in hepatocellular carcinoma via
epithelial-mesenchymal transition. Cancer Lett. 377:140–148. 2016.
View Article : Google Scholar
|
|
22
|
Kusakai G, Suzuki A, Ogura T, Miyamoto S,
Ochiai A, Kaminishi M and Esumi H: ARK5 expression in colorectal
cancer and its implications for tumor progression. Am J Pathol.
164:987–995. 2004. View Article : Google Scholar
|
|
23
|
Shin DH, Choi YJ and Park JW: SIRT1 and
AMPK mediate hypoxia-induced resistance of non-small cell lung
cancers to cisplatin and doxorubicin. Cancer Res. 74:298–308. 2014.
View Article : Google Scholar
|
|
24
|
Mitani T, Ito Y, Harada N, Nakano Y, Inui
H, Ashida H and Yamaji R: Resveratrol reduces the hypoxia-induced
resistance to doxorubicin in breast cancer cells. J Nutr Sci
Vitaminol (Tokyo). 60:122–128. 2014. View Article : Google Scholar
|
|
25
|
Casazza A, Di Conza G, Wenes M,
Finisguerra V, Deschoemaeker S and Mazzone M: Tumor stroma: A
complexity dictated by the hypoxic tumor microenvironment.
Oncogene. 33:1743–1754. 2014. View Article : Google Scholar
|