Introduction
Colorectal cancer (CRC) is a common malignant
disease, which has been intensely studied for tumor-immune
interactions in order to develop successful immunotherapies. In
particular, systemic T cell responses against tumor antigens and
tumor-infiltrating T cells have been analyzed in detail in CRC
(1–4).
A number of studies have linked a high T cell infiltration to an
improved survival in CRC (1–6). Patients with CRC as well as those with
other malignant diseases are able to mount an antigen-specific T
cell response without prior immunotherapy (7,8).
Peripheral tumor-associated antigen-directed T cell responses were
observed to have no survival benefit for patients with colorectal
cancer despite of a limited number of patients studied (9). Various components, including the immune
system, tumor stroma and tumor cells affect the induction and
modulation of tumor-directed immune responses (10). Limited antitumor activity of
spontaneous antigen-specific T cells at a clinical level in
patients with CRC may be due to multiple factors. Investigating the
profiles of infiltrating immune cells may help to understand the
interaction between innate and adaptive immune response and improve
immunotherapeutic approaches in CRC.
Traditionally, cluster of differentiation
(CD)8+ cytotoxic T cells have been considered as the key
component of effective antitumor immunity, and breast tumors with
higher levels of infiltrating CD8+ T cells have been
associated with improved patient survival (11,12).
However, studies have also shown that CD8+ T cells
frequently fail to fully function in vivo if there is a lack
of adequate assistance from CD4+ T cells (13). Therefore, heterogeneous populations of
infiltrating immune cells need to be clarified in order to
understand the antitumor immune responses within tumor.
The current consensus is that interferon
(IFN)-γ-producing CD4+ T helper (Th)1 and
CD8+ T cells, along with mature dendritic cells (DCs),
natural killer (NK) cells, M1 macrophages and type 1 NK T cells are
able to generate effective but frequently attenuated anti-tumor
responses, while CD4+ Th2 cells and type 2 NK T cells in
cooperation with CD4+ Tregs (regulatory),
myeloid-derived suppressor cells, immature DCs or M2 macrophages
suppress antitumor immunity and are able to promote tumor
progression (14–16). However, this summarized observation
comes with the caveat that variation exists among tumor types, with
the pro-tumorigenic cells, including CD4+ Th17, also
shown to produce effective antitumor responses (17,18).
The present study was undertaken to characterize the
immune cell subpopulations infiltrating human breast tumors in a
direct ex vivo analysis of fresh tumor tissue short-term
in vitro expansion. In the present study, a profile of
tumor-infiltrating T cells and macrophages in human CRC was
analyzed. A broad spectrum of markers was applied to distinguish
two subsets of macrophages. In addition, it was examined whether
tumor macrophages were prone to cytokine-driven conversion. In
addition, the expression of CXC motif chemokine (CXC) receptor 3
(CXCR3), CXC ligand (CXCL)9 and CXCL10 was analyzed. These
important molecules were associated with the intensity of
infiltration. The results provided insights into the profile of
infiltrating immune cells in human CRC and may be useful for
further study of antitumor immune responses in human CRC.
Materials and methods
Patients and specimens
Subsequent to approval from the institutional review
board of the First People's Hospital of Changzhou (Changzhou,
China) and informed consent, surgically removed tissue blocks and
peripheral blood mononuclear cells were collected from patients
with colorectal cancer from the aforementioned hospital (n=22, 12
females and 10 males; age range, 52–79 years; median age 63 years;
samples collected between April 2015 and March 2016). All analyses
were performed in compliance with the Declaration of Helsinki. The
demographic information of patients is described in Table I.
 | Table I.Demographics of surgical patients with
colorectal cancer. |
Table I.
Demographics of surgical patients with
colorectal cancer.
|
| Degree of
infiltration |
|---|
|
|
|
|---|
| Parameters | With LN
infiltration | No LN
infiltration |
|---|
| Total, n | 7 | 15 |
| Sex, n |
|
|
| Male | 2 | 8 |
|
Female | 5 | 7 |
| Mean age,
years | 62.4 | 64.7 |
| Location of tumor,
n |
|
|
|
Ascending colon | 0 | 5 |
|
Descending colon | 0 | 3 |
|
Transverse colon | 1 | 0 |
| Sigmoid
colon | 2 | 2 |
|
Rectum | 3 | 5 |
Isolation of infiltrating immune
cells
Fresh tumor and non-tumorous tissue adjacent were
harvested in sterile condition from patients during surgery and
rinsed with cold PBS to remove blood clogs, fat tissue and
surrounding necrotic tissue. The tissues were then dried with
filter papers and weighed. The tissues were cut into small pieces
(size, ~1 mm3) in cold PBS. In total, ≥5 volumes of
collagen IV (0.1 µg/ml in RPMI-1640) was added to 1 volume of
tissue suspension and then incubated at 4°C overnight. The tissue
suspension was filtered through a nylon mesh (70–100 µm) to harvest
single cells. Subsequent to washing with PBS, the mononuclear cells
were isolated by gradient centrifugation with Percoll®
Plus (GE Healthcare Life Sciences, Little Chalfont, UK) at 400 × g
at room temperature for 25 min and counted with an Axiovert 100
inverted microscope (Carl Zeiss AG, Oberkochen, Germany) at ×10
magnification. The results were expressed using a heat map for the
intensity of infiltration with HemI software (HemI Illustrator;
version 1.0.3.3; hemi.biocuckoo.org).
Isolation of macrophages and T
cells
Mononuclear cells were suspended in pH 7.4 PBS at a
density of >5×105 cells/ml and then incubated with
anti-CD14 microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany; Cat#130-050-201) for 30 min at room temperature.
Subsequent to washing, the resuspended cells passed through the MS
cell separation column to separate macrophages and other cells
according to the manufacturer's protocol. For T cell isolation, the
cells were incubated with anti-CD3 microbeads at 4°C for 30 min
(Miltenyi Biotec GmbH; Cat# 130-050-101) prior to following the
procedure as aforementioned.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells with an RNeasy
Mini kit (Qiagen, Inc., Valencia, CA, USA) according to the
manufacturer's instruction. cDNA was then synthesized with the
iScript cDNA Synthesis RT kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), according to the manufacturer's protocol. The
specific primers were designed and purchased from Sangon Biotech
Co., Ltd. (Shanghai, China). Gene expression profile was analyzed
by RT-qPCR with customized primer sets as described in Table II. Briefly, PCR was performed using
10 ng cDNA, 500 nM forward and reverse primers, and SYBR Green
master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in 20 ml reactions. Thermocycling conditions
comprised an initial holding at 50°C for 2 min, then 95°C for 10
min. This was followed by a 2-step PCR program consisting of 95°C
for 15 sec and 60°C for 60 sec for 35 cycles. Each sample was
analyzed in triplicate, and SYBR Green fluorescence was detected
using the Applied Biosystems 7900HT realtime PCR system. Data were
analyzed with 2−ΔΔCq method (19). The experiment was repeated at least
three times.
 | Table II.Primer sequences for SYBR Green
quantitative polymerase chain reaction. |
Table II.
Primer sequences for SYBR Green
quantitative polymerase chain reaction.
| Genes | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| Tbx21 |
GGTTGCGGAGACATGCTGA |
GTAGGCGTAGGCTCCAAGG |
| GATA-3 |
GCCCCTCATTAAGCCCAAG |
TTGTGGTGGTCTGACAGTTCG |
| RORC |
GTGGGGACAAGTCGTCTGG |
AGTGCTGGCATCGGTTTCG |
| Foxp3 |
GTGGCCCGGATGTGAGAAG |
GGAGCCCTTGTCGGATGATG |
| BCL-6 |
TGGTGACGCTTCAAAAGCCA |
GCTAGAATAGACGATGTTTCCCG |
| CXCR3 |
CCACCTAGCTGTAGCAGACAC |
AGGGCTCCTGCGTAGAAGTT |
| CXCL9 |
TGCAATGAACCCCAGTAGTGA |
GGTGGATAGTCCCTTGGTTGG |
| CXCL10 |
TGAAATTATTCCTGCAAGCCAA |
CAGACATCTCTTCTCACCCTTCTTT |
| GAPDH |
GAAGGTGAAGGTCGGAGTC |
GAAGATGGTGATGGGATTC |
| CD163 |
AGTCCCATCTTTCACTCTGC |
GCATCTTCTATGTCCCAGTG |
| IL-10 |
GACTTTAAGGGTTACCTGGG |
CTTGATGTCTGGGTCTTGGT |
| CD36 |
TTGCAGGTCAATCTATGCTG |
CTGGGTTTTCAACTGGAGAG |
| IL-12β |
CACAACGGAATAGACCCAAA |
TTAAATAGCATGAAGGCCCA |
| IL-1β |
CCACCCTCTATCACTGACTT |
CAAGGCTCAGTACATGCTCA |
| IL-6 |
GATGCAATAACCACCCCTGA |
TGACCAGAAGAAGGAATGCC |
| TNF-α |
TGTACCTCATCTACTCCCAG |
GAAGACCCCTCCCAGATAGA |
| β-actin |
GCATCCACGAAACTACCTTC |
GATCTCCTTCTGCATCCTGT |
Cell culture
CD14+ macrophages were prepared from
tissues and peripheral blood mononuclear cells by antibody-coated
microbeads (Miltenyi Biotec GmbH), and the purity was routinely
>90% as assessed with PE-labeled anti-CD14 antibody (cat no.,
557154; BD Biosciences, Franklin Lakes, NJ, USA) by flow cytometry
using FlowJo software (version 7.5; FlowJo LLC, Ashland, OR, USA).
Macrophages were cultured in vitro in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal calf serum (GE Healthcare Life Sciences) and granulocyte
macrophage colony-stimulating factor (GM-CSF; 50 ng/ml; R&D
Systems, Inc., Minneapolis, MN, USA). Following stimulation at 15,
30 min, 2, 4 and 24 h, the cells were washed and stimulated with
lipopolysaccharide (LPS; 100 ng/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C for 16 h, and the culture cells were
collected for the analysis of interferon responsive factor (IRF)5
expression.
Western blot analysis
Cell pellets were lysed in ice-cold buffer
containing a protease inhibitor cocktail (Roche Diagnostics, Basel,
Switzerland). The lysates (10 mg/lane) were fractionated by 8–10%
gradient SDS-PAGE. The lysates were subsequently transferred onto
polyvinylidene difluoride membranes and blocked with 10% non-fat
milk in PBS at room temperature for 1 h and analyzed by
immunoblotting with specific antibodies at room temperature for 1 h
to IRF5 (Cat#13496; dilution 1:1,000; Cell Signaling Technology,
Inc., Danvers, MA, USA) and β-actin (cat no., A1978; 1:2,000;
Sigma-Aldrich; Merck KGaA). Subsequent to washing with 0.05%
Tween-20 PBS, secondary horseradish peroxidase-conjugated
antibodies (Cat#31430; dilution 1:10,000; Pierce; Thermo Fisher
Scientific, Inc.) were added and the blots were incubated at room
temperature for 1 h. The protein bands were visualized using
enhanced chemiluminescence (Pierce; Thermo Fisher Scientific,
Inc.).
Statistical analysis
Data are presented as the mean ± standard error.
Statistical analysis was performed using two-tailed Student t-test
for unpaired data and two-way analysis of variance for multiple
comparisons with a post hoc Fisher's Least Significant Difference
test. SPSS (version 19; IBM Corp., Armonk, NY, USA) was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Infiltration profile of immune cells
in tumor and non-tumorous adjacent tissues of colorectal
cancer
The profiles of infiltrating immune cells isolated
from tumor and non-tumorous adjacent tissues obtained from patients
with colorectal cancer was analyzed by qPCR amplification of each
characteristic transcription factor of Th1, Th2, follicular T
helper (Tfh), Treg and Th17 cells. It was revealed that
significantly increased quantity of forkhead-box p3
(Foxp3)+ Treg cells, Th1 cells and Tfh cells were
present in tumor tissues compared with the adjacent tissues
(Fig. 1A-H). No statistical
difference in the number of Th2 (GATA3; Fig. 1D) and Th17 cells (RORC; Fig. 1B) was observed between tumor tissues
and the adjacent tissues. This indicated that the profile of immune
cells is distinct in the tumor tissues from the adjacent tissues.
In addition, the expression of CXCR3, CXCL9 and CXCL10 were
significantly increased in T cells isolated from tumor tissues
compared with the adjacent tissues. This indicated that high
expression of those molecules is associated with infiltration in
colorectal cancer.
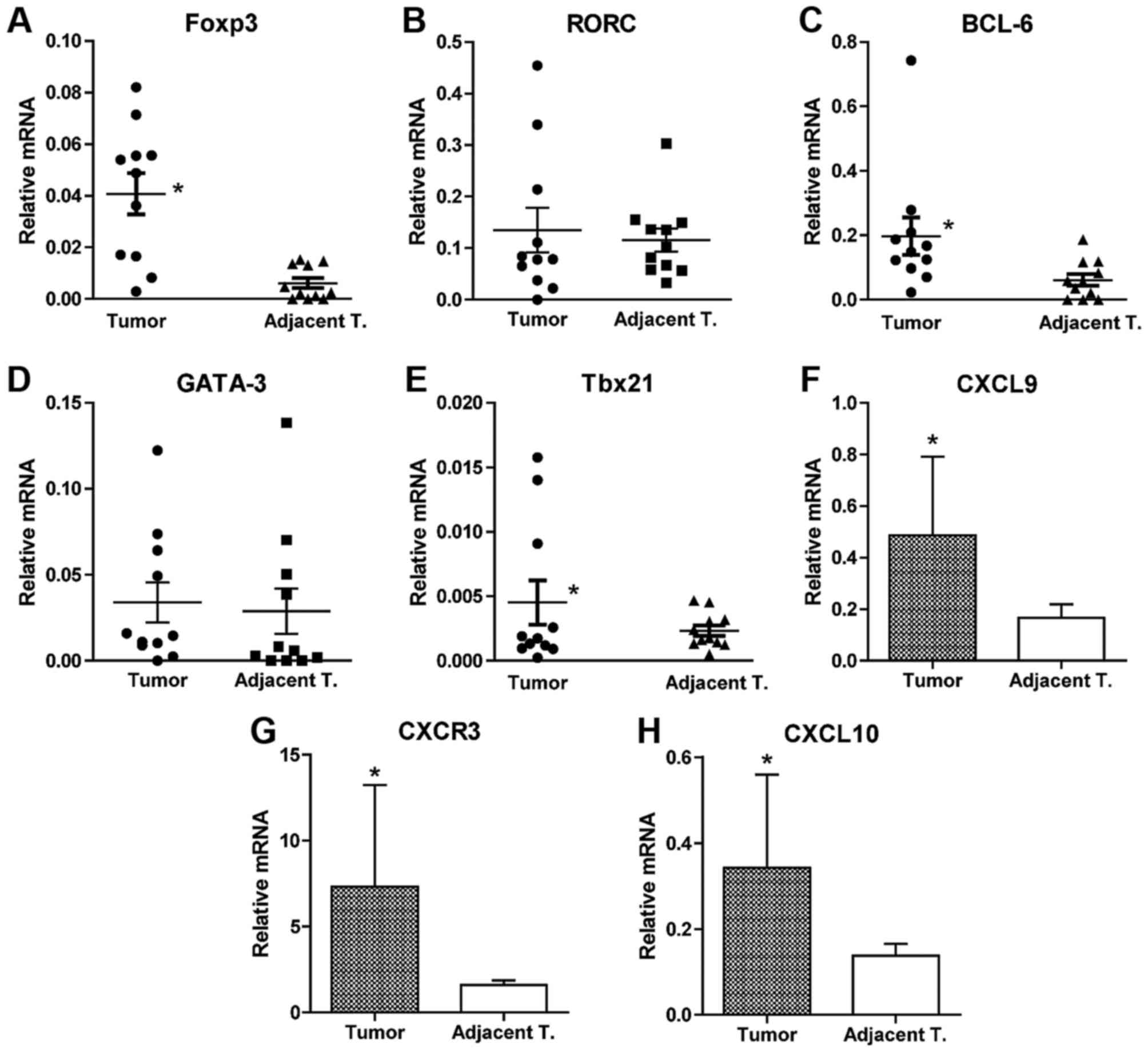 | Figure 1.Analysis of the profile of
infiltrating immune cells isolated from tumor and non-tumorous
adjacent tissue. Immune cells were isolated from tissue blocks
collected from selected patients with CRC during surgery via
collagen IV digestion and gradient density centrifugation. Total
RNA was extracted from the cells and subsequently reverse
transcribed to cDNA. Specific primer sets were designed for
transcription factors (A) Foxp3, (B) RORC, (C) BCL-6, (D) GATA-3
and (E) Tbx21 representing Treg, Th17, Tfh, Th2 and Th1 cells,
respectively. qPCR was performed using the SYBR-Green method with
specific primers to quantify the abundance of each subsets of
infiltrating immune cells. GAPDH was amplified simultaneously for
normalization. Data were analyzed using the 2−ΔΔCq
method and presented as relative values to GAPDH. T cells were
isolated from tumor and non-tumorous adjacent tissue of 6 selected
patients with CRC using T cell-specific microbeads. qPCR was
performed on RNA isolated from T cells for quantification of (F)
CXCL9, (G) CXCR3 and (H) CXCL10. Data are presented as the mean ±
standard error of mean. Statistical analysis was performed using
Student's t-test. *P<0.05, tumor tissue vs. non-tumorous
adjacent tissue. qPCR, quantitative polymerase chain reaction,
Tbx21, T-box 21; GATA3, GATA-binding protein 3; RORC, RAR-related
orphan receptor c; Foxp3, Forkhead-box p3; BCL-6, B cell lymphoma 6
protein; CXCR3, CXC motif chemokine receptor 3; CXCL9, CXC motif
chemokine ligand 9; CXCL10, CXC motif chemokine ligand 10; Treg,
regulatory T cells; Th, T helper; Tfh, follicular T helper. |
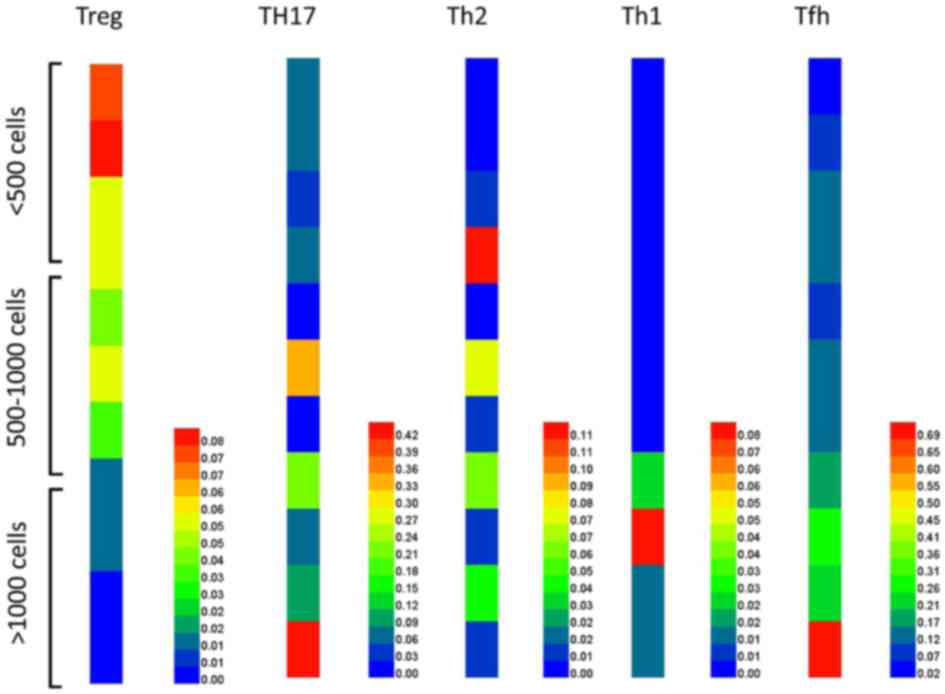
Distinctive patterns of infiltrating
immune cells in tumor tissues with low and high infiltration
The infiltrating lymphocytes in tumor tissues from
patients with colorectal cancer were counted. Furthermore, the
expression of each specific transcription factor Foxp3,
GATA-binding protein 3 (GATA3), T-box 21 (Tbx21) and RAR-related
orphan receptor C (RORc) for each different T cell population,
including Treg, Th2, Th1, Tfh and Th17 cells, were analyzed. As
shown in Fig. 2, there were
relatively a greater number of Treg cells and fewer Th1, Th17 and
Tfh cells in tumor tissues with low infiltration (<500 cells/mg
tissue) compared with tissues with medium (500–1,000 cells/mg
tissue) and high infiltration (>1,000 cells/mg tissue). By
contrast, in tissues with high infiltration (>1,000 cells/mg
tissue), there were an increased number of Th1, Th17 and Tfh cells
and fewer Treg cells compared with tissues with low (<500
cells/mg tissue) and medium (500–1,000 cells/mg tissue)
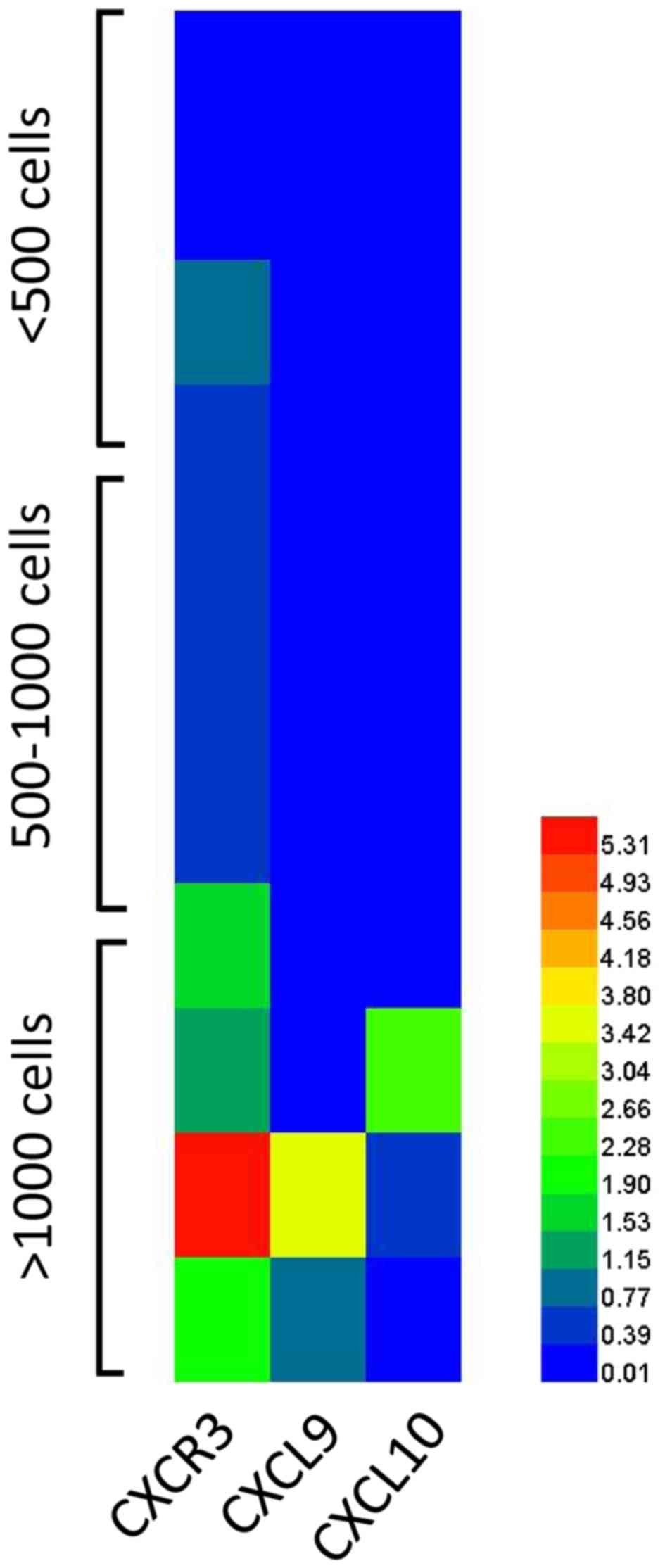
infiltration. The expression of CXCR3, CXCL9 and CXCL10 on T cells
isolated from colorectal cancer tumor tissues was examined. As
shown in Fig. 3, higher expression of
CXCR3, CXCL9 and CXCL10 was observed on T cells isolated from tumor
tissues with high infiltration compared with tumor tissues with low
infiltration.
Characterization of tumor-infiltrating
macrophages
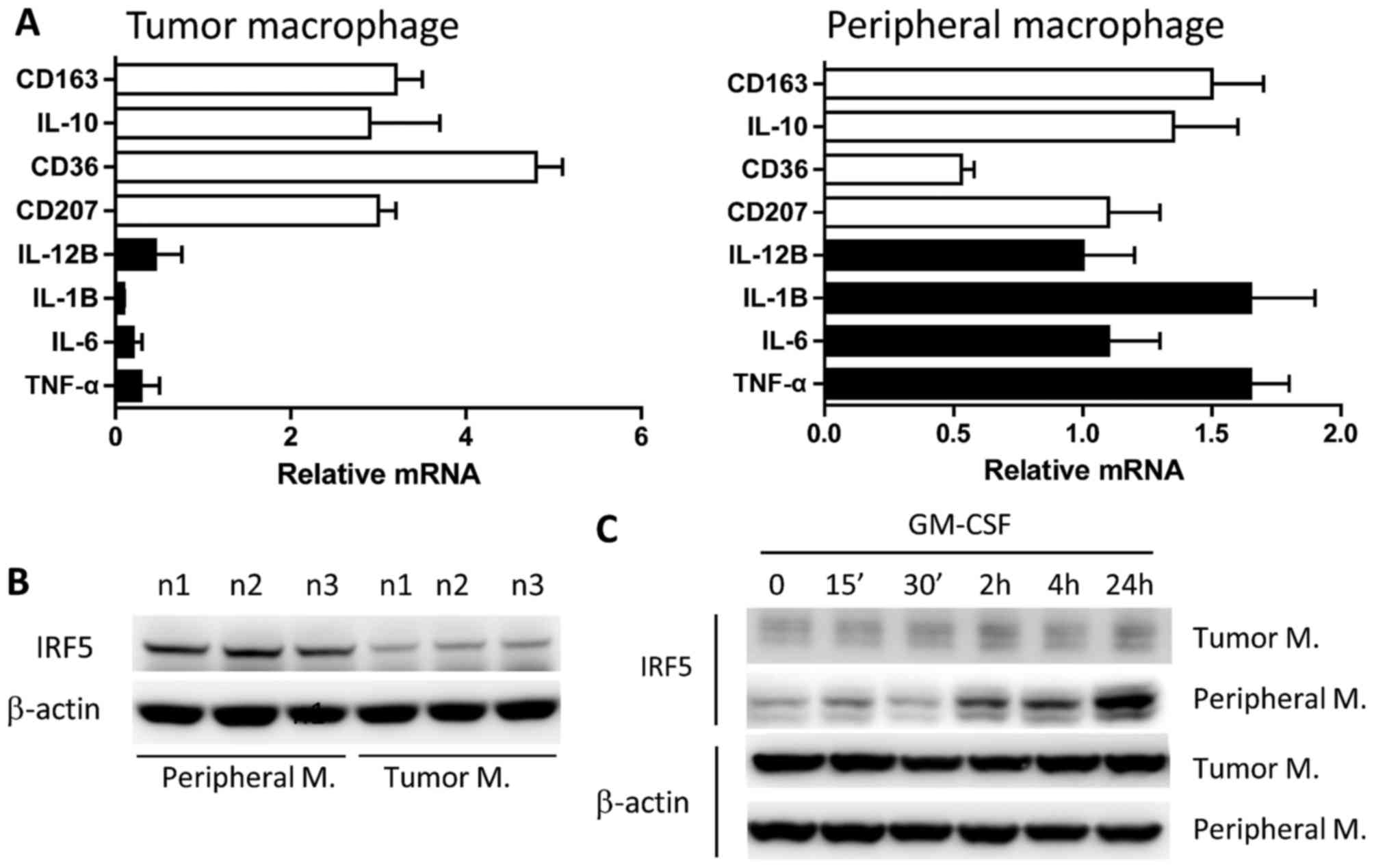
To characterize the profile of macrophages in tumor
tissue, the expression of major cytokines that are representative
of M1 and M2 cells was analyzed by qPCR due to the limited number
of isolated cells. The results revealed that tumor-infiltrating
CD14+ macrophages exhibited a dominant M2 phenotype as
characterized by elevated expression of M2 marker genes,
[interleukin (IL)-10, CD207, CD36 and CD163] compared with M1
marker genes [tumor necrosis factor (TNF)α, IL-6, IL-1β and IL-12β
(Fig. 4A).
Response of isolated CD14+ macrophages
to GM-CSF stimulation
A total of three large tumor tissue blocks (>50
mg) obtained from surgical patients with colorectal cancer were
selected for isolation of CD14+ macrophages. Purified
macrophages were stimulated with GM-CSF for different periods of
time. It was demonstrated that macrophages from tumor tissues
expressed markedly reduced IRF5, which is a characteristic
transcription factor of M1 macrophages, compared with expression in
peripheral macrophages isolated from peripheral blood of the same
patient (Fig. 4B). Furthermore, tumor
macrophages did not respond to the stimulation by GM-CSF, a driving
cytokine for M1 macrophage differentiation, as measured by the
expression of IRF5, whereas peripheral macrophages exhibited a
strong response to GM-CSF stimulation after 24 h of stimulation
(Fig. 4C). The results suggested that
tumor macrophages were less inflammatory and refractory to
conversion driven by M1 stimulating agents in colorectal cancer
compared with peripheral macrophages.
Discussion
The most common treatment for colorectal cancer is
surgery. In the case of localized tumors, surgery may completely
eliminate the cancer. When the cancer has invaded the bowel wall or
the lymph nodes, chemotherapy and/or immunotherapy are required to
achieve the best benefits. Colorectal cancer is one of the major
cancer types for which new immune-based cancer treatments are
currently in development (20). The
understandings of antitumor immune responses are crucial to the
design and implement of immunomodulation for treatment.
The evaluation of immune infiltrates is even more
complex due not only to the numerous cell types that can be found
in tumors (2,4), but also to the possibility that a given
immune cell type can vary in terms of state of maturation and/or
activation, and the fact that numerous diverse cell types can share
similar markers (5). A CD4 T cell
found in a tumor can be anergic, activated or regulatory. The same
can be said for several other immune lineages (5). Tumor-infiltrating Foxp3+
regulatory T cells have also been shown to have a strong prognostic
significance in colorectal cancer. Salama et al (21) reported that the density of regulatory
T cells in normal and tumor tissue to be independent prognostic
indicators, but not the density of CD8+ T cells.
However, it has been reported elsewhere that Foxp3+ Treg
cells were independent indicators of the prognosis of colorectal
cancer (20). Di Giorgio et al
(22) also revealed that the presence
of lymphocytic infiltration in the tumor was associated with an
improved prognosis by multivariate analysis in patients with
colorectal cancer resected between 1960 and 1978 (n=361;
P<0.001). A number of studies have also emphasized the location
of immune infiltrate in tumors; CD8+ T cell infiltrates in cancer
cell nests often were associated with improved prognosis when
compared with those in cancer stroma and marginal regions (1,4).
Therefore, it will be more informative to describe a profile rather
than emphasizing on a particular subset of immune cells in
consideration of the complexity of immune infiltrates in colorectal
cancer.
In the present study, the profiles of immune cells
were analyzed, including Treg, Th1, Th2, Tfh and macrophages, and
the profiles of low infiltration and high infiltration were
compared. Profiles of tumor-infiltrating immune cells and immune
cells in non-tumor adjacent tissues were also compared. However,
these cells were also in different stages of differentiation, which
was not addressed in the present study. The analysis of
differentiation stages may provide further important information to
define the profile of tumor-infiltrating immune cells. Notably, an
increased number of Tfh cells were observed in the tumor tissue as
compared with non-tumorous adjacent tissue, indicating significant
involvement of B cell response in tumor tissues in colorectal
cancer. The B cell response in tumor has been previously
extensively studied in a number of types of cancer, including
breast, ovarian and non-small cell lung cancer (23). B cells exhibited evidence of somatic
mutation and affinity maturation in breast cancer (23). In the present study, the increased
number of Tfh cells indicated that local B cell differentiation
occurred in tumor tissues. Consequently, it is likely the same
scenario that occurred in colorectal cancer as that in breast
cancer.
Macrophages are heterogeneous and comprise
phenotypically and functionally distinct cell populations. With an
increasing understanding of novel markers and differential roles of
macrophages in the immune response, macrophages are characterized
into different subsets. Different subsets require specific cytokine
milieu for differentiation and maintenance and exhibit specific
phenotypes and functions (24–30).
Macrophage polarization is primarily determined by cytokines and
ligands to pattern recognition receptors, including toll-like
receptors (TLRs) on macrophages. Macrophages of the M1 phenotype
are programmed to produce pro-inflammatory cytokines, including
IL-12, IL-1β, TNFα and IL-6, and perform a crucial role in the
initiation and perpetuation of inflammatory response, whereas
macrophages of the M2 phenotype exhibit anti-inflammatory
properties characterized by the production of IL-10 and IL-13 and
prominent phagocytosis (26,28,30).
Differentiation of M1 and M2 macrophages is driven by key
cytokines, such as GM-CSF for M1 differentiation and M-CSF for M2
differentiation (28). By contrast,
IFN-γ or IL-4 primes initially differentiated macrophages and
promotes their polarization (31). In
addition, activation by LPS through TLR-4 augments the production
of cytokines by macrophages (26). It
was previously reported that IRF5 and IRF4 are the putative lineage
determining transcription factors for M1 and M2 macrophages
(31,32). It has been shown that polarized M1 and
M2 macrophages exhibit high plasticity and can be rendered to shift
their phenotypes when the cytokine milieu changes. A balanced M1 to
M2 ratio is required for the immune system homeostasis (27). In the present study, it was revealed
that the macrophages of M2 phenotype isolated from tumor of
colorectal cancer were refractory to in vitro converting to
M1 phenotype, suggesting the defects of cells existed or the
anergic state of cells. Current research to develop emerging
immunotherapies that target the dysregulated M1/M2 macrophages is
considered to make significant advances in cancer immunotherapy.
Understanding the preferential accumulation of macrophages in a
specific type of cancer would greatly support the future
application of macrophage-directed immunotherapy. Although current
agents such as Coley's toxins that stimulate the growth of M1
macrophages involve great side effects (33,34), new
mediators that stimulate and maintain M1 macrophages will begin a
new chapter in cancer therapy, and in such cases colorectal cancer
may be a good candidate for macrophage-directed immunotherapy.
CXCR3, CXCL9 and CXCL10 were associated with the
intensity of infiltration of T cells to tumor microenvironment.
Zeste homologue 2 (EZH2)-mediated suppression of Th1-type
chemokines CXCL9 and CXCL10 determine effector T cell trafficking
to the tumor microenvironment (35).
Treatment with epigenetic modulators such as EZH2 inhibitor removes
the repression and increases effector T cell tumor infiltration,
slows down tumor progression, and improves the therapeutic efficacy
of programmed death-ligand 1 (PD-L1; also termed B7-H1) checkpoint
blockade and adoptive T cell transfusion in tumor-bearing mice
(35,36). In colorectal cancer, it was
demonstrated that high expression of CXCR3, CXCL9 and CXCL10 on T
cells was associated with high infiltration (>1,000 cells/mg).
By analyzing the expression of these chemokines, the present
results suggested the clinical specimens can be categorized into
different groups that may be sensitive or insensitive to PD-L1
immunotherapy.
In the present study, it was identified that Th1 and
Tfh cells, as well as M2 macrophages, are dominant cells in
colorectal cancer tumors. The results of the present study suggest
that the analysis of the profile of intratumor immune cells may
assist the prediction of prognosis.
Acknowledgements
The present study was supported by the Changzhou
Municipal Scientific Research grant (grant no. CE20125020).
References
|
1
|
Chiba T, Ohtani H, Mizoi T, Naito Y, Sato
E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y and Satomi S:
Intraepithelial CD8+ T-cell-count becomes a prognostic factor after
a longer follow-up period in human colorectal carcinoma: Possible
association with suppression of micrometastasis. Br J Cancer.
91:1711–1717. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Diederichsen AC, Hjelmborg Jv, Christensen
PB, Zeuthen J and Fenger C: Prognostic value of the CD4+/CD8+ ratio
of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR
expression on tumour cells. Cancer Immunol Immunother. 52:423–428.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Funada Y, Noguchi T, Kikuchi R, Takeno S,
Uchida Y and Gabbert HE: Prognostic significance of CD8+ T cell and
macrophage peritumoral infiltration in colorectal cancer. Oncol
Rep. 10:309–313. 2003.PubMed/NCBI
|
|
4
|
Naito Y, Saito K, Shiiba K, Ohuchi A,
Saigenji K, Nagura H and Ohtani H: CD8+ T cells infiltrated within
cancer cell nests as a prognostic factor in human colorectal
cancer. Cancer Res. 58:3491–3494. 1998.PubMed/NCBI
|
|
5
|
Oberg A, Samii S, Stenling R and Lindmark
G: Different occurrence of CD8+, CD45R0+, and CD68+ immune cells in
regional lymph node metastases from colorectal cancer as potential
prognostic predictors. Int J Colorectal Dis. 17:25–29. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ropponen KM, Eskelinen MJ, Lipponen PK,
Alhava E and Kosma VM: Prognostic value of tumour-infiltrating
lymphocytes (TILs) in colorectal cancer. J Pathol. 182:318–324.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagorsen D, Keilholz U, Rivoltini L,
Schmittel A, Letsch A, Asemissen AM, Berger G, Buhr HJ, Thiel E and
Scheibenbogen C: Natural T-cell response against MHC class I
epitopes of epithelial cell adhesion molecule, her-2/neu, and
carcinoembryonic antigen in patients with colorectal cancer. Cancer
Res. 60:4850–4854. 2000.PubMed/NCBI
|
|
8
|
Nagorsen D, Scheibenbogen C, Marincola FM,
Letsch A and Keilholz U: Natural T cell immunity against cancer.
Clin Cancer Res. 9:4296–4303. 2003.PubMed/NCBI
|
|
9
|
Nagorsen D, Scheibenbogen C, Letsch A,
Germer CT, Buhr HJ, Hegewisch-Becker S, Rivoltini L, Thiel E and
Keilholz U: T cell responses against tumor associated antigens and
prognosis in colorectal cancer patients. J Transl Med. 3:32005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blankenstein T: The role of tumor stroma
in the interaction between tumor and immune system. Curr Opin
Immunol. 17:180–186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kinnula VL, Torkkeli T, Kristo P, Sormunen
R, Soini Y, Pääkkö P, Ollikainen T, Kahlos K, Hirvonen A and
Knuutila S: Ultrastructural and chromosomal studies on manganese
superoxide dismutase in malignant mesothelioma. Am J Respir Cell
Mol Biol. 31:147–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kojima M, Morisaki T, Tsukahara Y,
Uchiyama A, Matsunari Y, Mibu R and Tanaka M: Nitric oxide synthase
expression and nitric oxide production in human colon carcinoma
tissue. J Surg Oncol. 70:222–229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura Y, Yasuoka H, Tsujimoto M,
Yoshidome K, Nakahara M, Nakao K, Nakamura M and Kakudo K: Nitric
oxide in breast cancer: Induction of vascular endothelial growth
factor-C and correlation with metastasis and poor prognosis. Clin
Cancer Res. 12:1201–1207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmed B and Van Den Oord JJ: Expression of
the inducible isoform of nitric oxide synthase in pigment cell
lesions of the skin. Br J Dermatol. 142:432–440. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Masri FA, Comhair SA, Koeck T, Xu W,
Janocha A, Ghosh S, Dweik RA, Golish J, Kinter M, Stuehr DJ, et al:
Abnormalities in nitric oxide and its derivatives in lung cancer.
Am J Respir Crit Care Med. 172:597–605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vakkala M, Kahlos K, Lakari E, Pääkkö P,
Kinnula V and Soini Y: Inducible nitric oxide synthase expression,
apoptosis, and angiogenesis in in situ and invasive breast
carcinomas. Clin Cancer Res. 6:2408–2416. 2000.PubMed/NCBI
|
|
17
|
Kono K, Salazar-Onfray F, Petersson M,
Hansson J, Masucci G, Wasserman K, Nakazawa T, Anderson P and
Kiessling R: Hydrogen peroxide secreted by tumor-derived
macrophages down-modulates signal-transducing zeta molecules and
inhibits tumor-specific T cell-and natural killer cell-mediated
cytotoxicity. Eur J Immunol. 26:1308–1313. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rotondo R, Mastracci L, Piazza T,
Barisione G, Fabbi M, Cassanello M, Costa R, Morandi B, Astigiano
S, Cesario A, et al: Arginase 2 is expressed by human lung cancer,
but it neither induces immune suppression, nor affects disease
progression. Int J Cancer. 123:1108–1116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jochems C and Schlom J: Tumor-infiltrating
immune cells and prognosis: The potential link between conventional
cancer therapy and immunity. Exp Biol Med (Maywood). 236:567–579.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salama P, Phillips M, Grieu F, Morris M,
Zeps N, Joseph D, Platell C and Iacopetta B: Tumor-infiltrating
FOXP3+ T regulatory cells show strong prognostic significance in
colorectal cancer. J Clin Oncol. 27:186–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Di Giorgio A, Botti C, Tocchi A,
Mingazzini P and Flammia M: The influence of tumor lymphocytic
infiltration on long term survival of surgically treated colorectal
cancer patients. Int Surg. 77:256–260. 1992.PubMed/NCBI
|
|
23
|
Nzula S, Going JJ and Stott DI:
Antigen-driven clonal proliferation, somatic hypermutation, and
selection of B lymphocytes infiltrating human ductal breast
carcinomas. Cancer Res. 63:3275–3280. 2003.PubMed/NCBI
|
|
24
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: Cancer as a
paradigm. Nat Immunol. 11:889–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fleetwood AJ, Lawrence T, Hamilton JA and
Cook AD: Granulocyte-macrophage colony-stimulating factor (CSF) and
macrophage CSF-dependent macrophage phenotypes display differences
in cytokine profiles and transcription factor activities:
Implications for CSF blockade in inflammation. J Immunol.
178:5245–5252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:593–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mantovani A, Sica A and Locati M:
Macrophage polarization comes of age. Immunity. 23:344–346. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martinez FO, Gordon S, Locati M and
Mantovani A: Transcriptional profiling of the human
monocyte-to-macrophage differentiation and polarization: New
molecules and patterns of gene expression. J Immunol.
177:7303–7311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martinez FO, Helming L and Gordon S:
Alternative activation of macrophages: An immunologic functional
perspective. Annu Rev Immunol. 27:451–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stein M, Keshav S, Harris N and Gordon S:
Interleukin 4 potently enhances murine macrophage mannose receptor
activity: A marker of alternative immunologic macrophage
activation. J Exp Med. 176:287–292. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krausgruber T, Blazek K, Smallie T,
Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M and Udalova
IA: IRF5 promotes inflammatory macrophage polarization and TH1-TH17
responses. Nat Immunol. 12:231–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Satoh T, Takeuchi O, Vandenbon A, Yasuda
K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh
T, et al: The Jmjd3-Irf4 axis regulates M2 macrophage polarization
and host responses against helminth infection. Nat Immunol.
11:936–944. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brunschwig A: The Efficacy of ‘Coley's
Toxin’ in the Treatment of Sarcoma: An experimental study. Ann
Surg. 109:109–113. 1939. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maletzki C, Klier U, Obst W, Kreikemeyer B
and Linnebacher M: Reevaluating the concept of treating
experimental tumors with a mixed bacterial vaccine: Coley's toxin.
Clin Dev Immunol. 2012:2306252012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao
L, Wei S, Crespo J, Wan S, Vatan L, et al: Cancer mediates effector
T cell dysfunction by targeting microRNAs and EZH2 via glycolysis
restriction. Nat Immunol. 17:95–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peng D, Kryczek I, Nagarsheth N, Zhao L,
Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, et al: Epigenetic
silencing of TH1-type chemokines shapes tumour immunity and
immunotherapy. Nature. 527:249–253. 2015. View Article : Google Scholar : PubMed/NCBI
|