Introduction
Esophageal cancer is a common primary malignant
tumor of the digestive tract, and its morbidity and mortality rank
eighth and sixth, respectively, among malignant tumors globally
(1). Esophageal cancer affects
>450,000 people worldwide and the incidence rate is increasing
(2). The overall 5-year survival rate
of patients with esophageal carcinoma ranges from 13–19%, based on
2006–2012 data (3–5). Although developments in chemotherapy
have improved the prognosis of esophageal cancer, drug resistance
remains the primary obstacle to successful treatment (6). Therefore, in order to improve treatment
efficacy and prognosis in patients, further investigation of the
genesis and underlying developmental mechanisms of esophageal
carcinoma is crucial.
MicroRNAs (miRNAs/miRs) are short (~22 nucleotides),
non-protein-coding RNAs. A growing body of evidence indicates that
miRNAs serve crucial functions in diverse cellular processes
through gene regulation (7).
Furthermore, miRNA expression profiling has revealed that certain
miRNAs are associated with tumor development, progression and
response to therapy (7). For example,
miR302a, a member of the microRNA-302 family, was initially
identified in human embryonic stem cells and human embryonic
carcinoma cells (8). Increasing
reports have indicated that miR302a participates in various cell
differentiation or tumor metastasis-associated signaling pathways
via targeting different molecules. Guo et al (9) demonstrated that miR302a is involved in
the inhibition of ovarian cancer cell proliferation, and enhances
cell apoptosis through targeting syndecan 1. In addition, miR302a
suppresses tumor cell proliferation by inhibiting protein kinase B
(AKT) in prostate cancer (10).
However, miR302a expression and its involvement in esophageal
cancer remain undetermined, and the underlying mechanisms of
miR302a in esophageal cancer cells remain unknown.
The present study revealed that miR302a expression
was decreased in esophageal cancer cell lines compared with a
healthy esophageal epithelial cell line. Furthermore, miR302a
overexpression inhibits esophageal cancer cell proliferation and
invasion. Finally, miR302a was revealed to act as a tumor
suppressor via regulation of the mitogen-activated protein kinase
(MAPK) and phosphoinositide 3-kinase (PI3K)/Akt signaling pathway
in the cell lines TE-10 and ECA109.
Materials and methods
Cell culture
The human esophageal cancer cell lines, TE-1, TE-10,
TE-11, ECA109, and the healthy human esophageal epithelial cell
line Het-1A were purchased from Shanghai Jining Industry Co., Ltd.
(Shanghai, China) TE-1 (cat. no. JN-B1846; http://www.shjning.com/plus/view.php?aid=42526),
TE-10 (cat.no. JN-B1582; http://www.shjning.com/plus/view.php?aid=42241), TE-11
(cat. no. JN-A2623; http://www.shjning.com/plus/view.php?aid=39797),
ECA109 (cat. no. JN-B1684; http://www.shjning.com/plus/view.php?aid=42362) and
Het-1A (cat. no. JN-4897; http://www.shjning.com/plus/view.php?aid=39249) cells
were grown in Dulbecco's modified Eagle's medium (DMEM) medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with 10% fetal bovine serum (FBS; Hangzhou Sijiqing
Biology Engineering Materials Co., Ltd., Hangzhou, China). Cells
were cultured at 37°C in a 5% CO2 atmosphere. Cells were
passaged with 0.25% trypsin (Sigma-Aldrich; Merck KGaA, Damstadt,
Germany) at a 1:3 ratio every 3 days.
Cell transfection
Cells (TE-10, ECA109) were maintained until mid-log
phase, and then digested with trypsin and seeded into 6-well plates
(Sigma-Aldrich; Merck KGaA) at a density of 1×105 cells
per well. Following culture for 48 h, when cells reached 80%
confluence, cells were processed for transfection. miR302a mimics
(5′-ACUUAAACGUGGAUGUACUUGCU-3′), miR302a inhibitor
(5′-UGAAUUUGCACCUACAUGAACGA-3′) and their negative control
oligonucleotides (5′-UCGUUCAUGUAGGUGCAAAUUCA-3′; all synthesized by
Guangzhou Ribobio Co., Ltd, Guangzhou, China) were transfected into
TE-10 and ECA109 cells using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The reagents, including 1 µg miR302a
mimics or inhibitors and 3 µl Lipofectamine 2000, were diluted into
50 µl Opti-Minimal Essential medium (Gibco; Thermo Fisher
Scientific, Inc.), and incubated for 20 min at room temperature.
Then, the miRNA-lipid complex was added to the cells (10 µl/well)
and gently blended. After 48 h of incubation at 37°C, the
transfected cells were collected for analysis of miRNA and protein
expression.
MTT assays
Cells at the mid-log phase were seeded in 96-well
plates (Sigma-Aldrich; Merck KGaA) at a density of 1×104
cells/well in 100 µl DMEM medium, with 5 replicates for each group.
Cells were incubated with 5 mg/ml MTT solution (10 µl) at 37°C in a
5% CO2 atmosphere for 4 h. Following incubation, the
medium was removed, 150 µl DMSO (Sigma-Aldrich; Merck KGaA). The
absorbance at 490 nm was detected using a spectrophotometer (Thermo
Fisher Scientific, Inc.), and cell viability was measured once per
day for 7 days.
Transwell assays
Cell invasion assays were performed in a 24-well
Transwell chambers with 8-mm pore size inserts according to the
manufacturer's protocol (Corning Incorporated, Corning, NY, USA).
Matrigel matrix gel (100 µl; BD Biosciences, Franklin Lakes, NJ,
USA) was diluted with DMEM without FBS overnight at a ratio of 1:6
and was added to the upper chamber of the 24-well Transwell plate.
For the assay, 1×105 cells were seeded into the upper
chamber with 100 µl DMEM without FBS. In the lower chamber, 600 µl
DMEM supplemented with 10% FBS was added. Following incubation for
24 h at 37°C in a 5% CO2 atmosphere, the non-invading
cells were removed from the plate with a cotton swab. Cells on the
lower surface of the membrane were fixed with 4% paraformaldehyde
for 10 min at room temperature and stained with 0.1% crystal violet
for 3 min at room temperature. The cells were then counted in at
least 5 random fields using an inverted microscope (Olympus
Corporation, Tokyo, Japan).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). To quantify miR302a
expression, total RNA was first polyadenylated and
reverse-transcribed using the NCode miRNA first-Strand cDNA
synthesis kit (Takara Biotechnology Co., Ltd., Dalian, China),
according to the manufacturer's protocol, followed by PCR using 1
µg cDNA via TaqMan® Universal Master Mix (Life
technology, Carlsbad, CA, USA), according to the manufacturer's
protocol. The thermocycler conditions were as follows: 95°C for 10
sec, followed by 40 amplification cycles (95°C for 5 sec, 60°C for
30 sec) and 60°C for 12 min. Primers (Sangon Biotech Co., Ltd.,
Shanghai, China) used for miR302a were as follows: Forward,
5′-CGTGGATGTACTTGCTTTGAA-3′ and reverse,
5′-TCACCAAAACATGGAAGCAC-3′. β-actin was used as an internal
control, and every sample was replicated at least five times,
β-actin forward: 5′-ACCGAGCGCGGCTACAG-3′, β-actin reverse:
5′-CTTAATGTCACGCACGATTTCC-3′. PCR products were run on a 3% agarose
gel in 1X TAE (0.04 M Tris, 1 mM EDTA, 0.02 M acetic acid, pH
8.2–8.4) buffer, and then the gels were stained with ethidium
bromide to a final concentration of 0.5 µg/ml (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) at 55°C for 1 min, observed and pictured
using Image J version 2 software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The band intensities were quantified using
Image J software. For relative quantification, miR302a expression
was normalized to β-actin expression in the corresponding
sample.
Protein extraction and western blot
analysis
After 48 h of incubation at 37°C, the transfected
cells were collected for cell lysis by treating with radio
immunoprecipitation assay (Solarbio, Beijing, China) lysis buffer
and protease inhibitor (Beyotime, Shanghai, China) on ice for 30
min. Then, it was centrifuged at 12,000 × g for 5 min at 4°C, the
supernatant was extracted and the protein concentration was
measured using a BCA kit (Beyotime), according to the
manufacturer's instructions. Extracted protein (30 µg) was added to
50 µl Laemmli sample buffer (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), and boiled for 5 min. The protein was then loaded and
separated on 10% Ready Gels, and then transferred to a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). Membranes were blocked with 5% non-fat dry milk for 1 h at
room temperature. Primary monoclonal mouse anti-human β-actin
antibodies (1:5,000; cat no. ab7813), anti-phosphorylated (p-)
extracellular signal-regulated kinase (ERK)1/2 (1:500; cat no.
ab214362), anti-ERK1/2 (1:500; cat no. ab17942), anti-p-protein
kinase B (Akt; 1:500; cat no. ab38499) and total Akt (1:500; cat
no. ab6789; all from Abcam, Cambridge, MA, USA) were added and
incubated overnight at 4°C. Following washing three times in 0.1%
Tween 20-TBS (TBST), the membranes were incubated with secondary
polyclonal goat anti-mouse horseradish peroxidase (HRP)-conjugated
antibodies (1:5,000; cat no. ab179463; Abcam, Cambridge, MA, USA)
overnight at 4°C, and then washed three times in TBST. The
immunoreactive bands were visualized using enhanced
chemiluminescence reagents, according to the manufacturer's
protocol (Western Lightning Plus-ECL; PerkinElmer, Inc., Waltham,
MA, USA), and then assessed using the Quantity One software version
4.62 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All statistical analyses were performed using SPSS
statistical software, version 19.0 (IBM Corp., Armonk, NY, USA).
Values are presented as the mean ± standard deviation. Statistical
differences among the groups were tested by one-way analysis of
variance, followed by Fisher's least significant difference tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Esophageal cancer cell lines have
reduced miR302a expression compared with healthy esophageal
cells
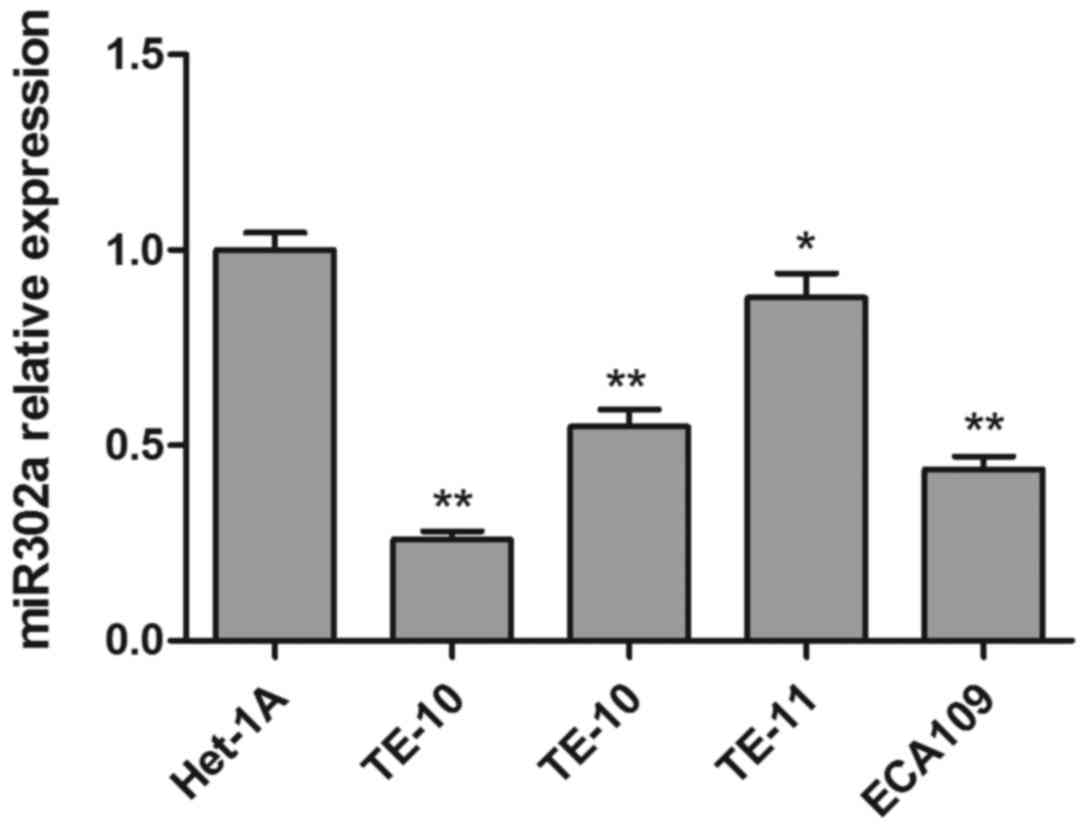
To investigate the function of miR302a in esophageal
cancer cell lines, the expression of miR302a was measured by RT-PCR
in four esophageal cancer cell lines (TE-1, TE-10, TE-11, ECA109)
and a healthy human esophageal epithelial cell (Het-1A). As
presented in Fig. 1, miR302a
expression in all four esophageal cancer cell lines was
significantly decreased compared with the healthy esophageal
epithelial cell line. Then, two esophageal cancer cell lines with
similar miR302a levels, TE-10, and ECA109, were selected for
follow-up experiments.
miR302a significantly inhibits
esophageal cancer cell proliferation
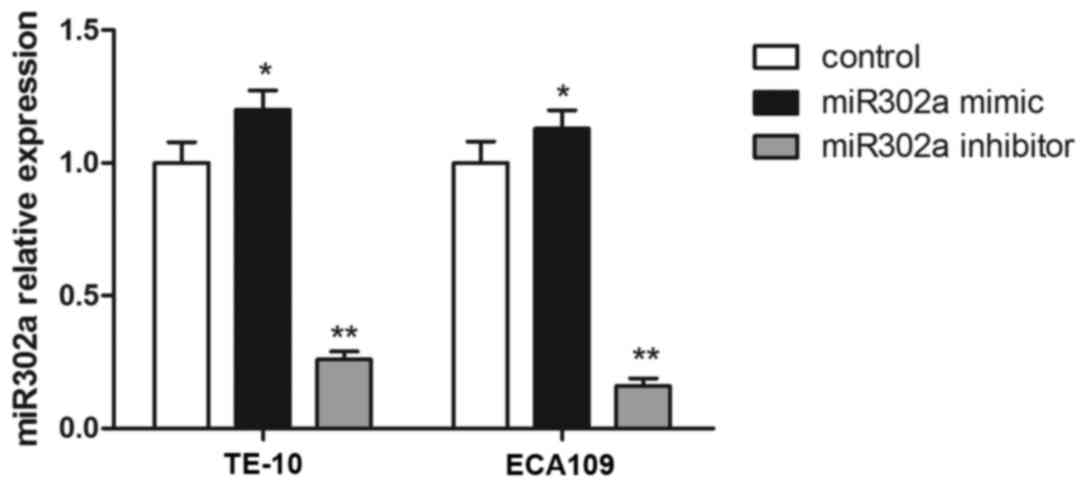
To further evaluate the effect of miR302a, miR302a
mimics or inhibitors were stably overexpressed in TE-10 and ECA109
cell lines, and the expression of miR302a was confirmed by RT-PCR.
Compared with the control group, miR302a expression was
significantly increased in the miR302a mimic group (P<0.01), and
miR302a expression was significantly decreased in the miR302a
inhibitor group in TE-10 and ECA109 cells (P<0.01; Fig. 2).
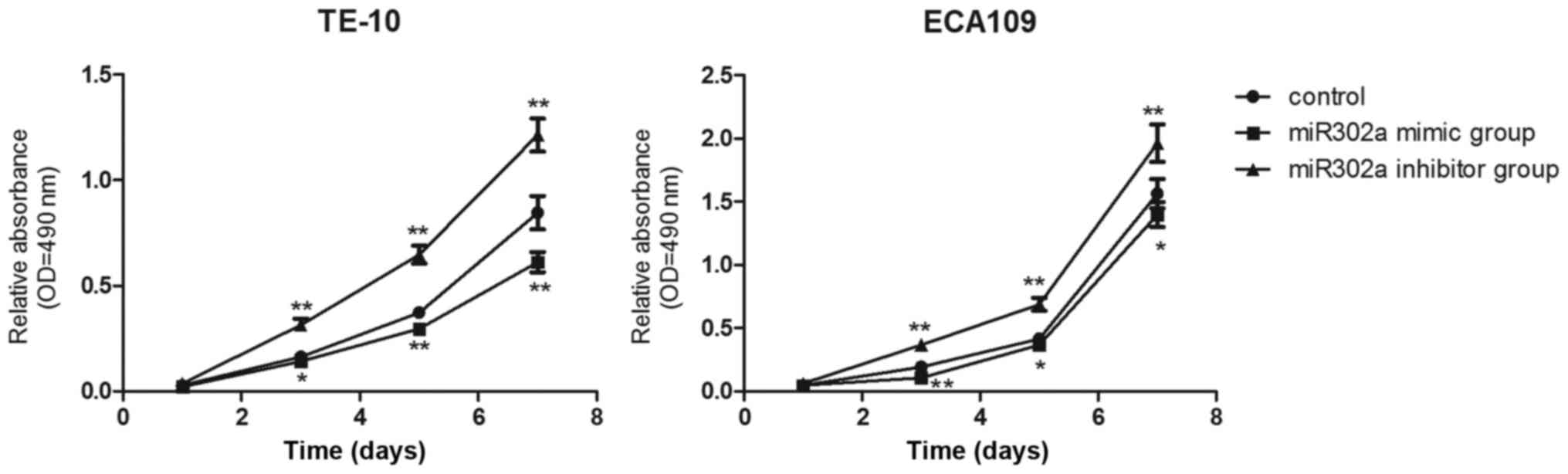
Then, MTT assays were performed to examine whether
miR302a overexpression inhibited esophageal cancer cell
proliferation in vitro. Compared with the control group,
cell proliferation in the miR302a mimic group was significantly
decreased (P<0.01), and cell proliferation in the miR302a
inhibitor group was significantly increased (P<0.01), from the
third day to the seventh day in TE-10 and ECA109 cells (Fig. 3). The results demonstrated that
miR302a significantly inhibited the viability of esophageal cancer
cells.
miR302a significantly inhibits
esophageal cancer cell invasion
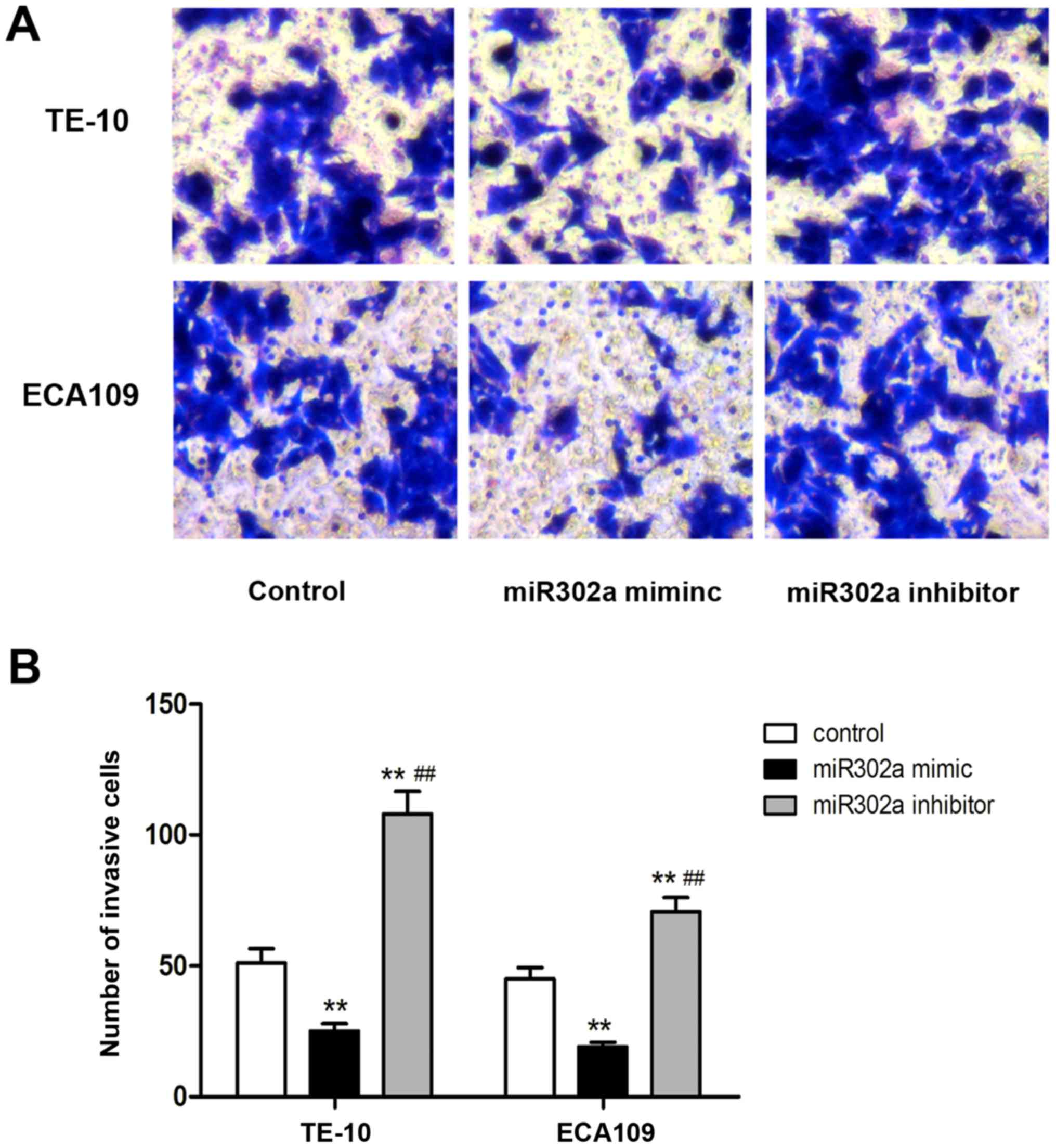
The aforementioned results revealed that miR302a
significantly inhibited the proliferation of esophageal cancer
cells. Next, invasion was assessed in esophageal cancer cells
transfected with miR302a mimics or inhibitors using Transwell
assays. As presented in Fig. 4, the
number of invasive cells in the miR302a mimics group was
significantly decreased compared with the control group in TE-10
and ECA109 cells (P<0.01), and the number of invasive cells in
the miR302a inhibitor group was significantly increased in TE-10
and ECA109 cells compared with the control group (P<0.01). These
results revealed that overexpression of miR302a inhibited the
invasion of esophageal cancer cells.
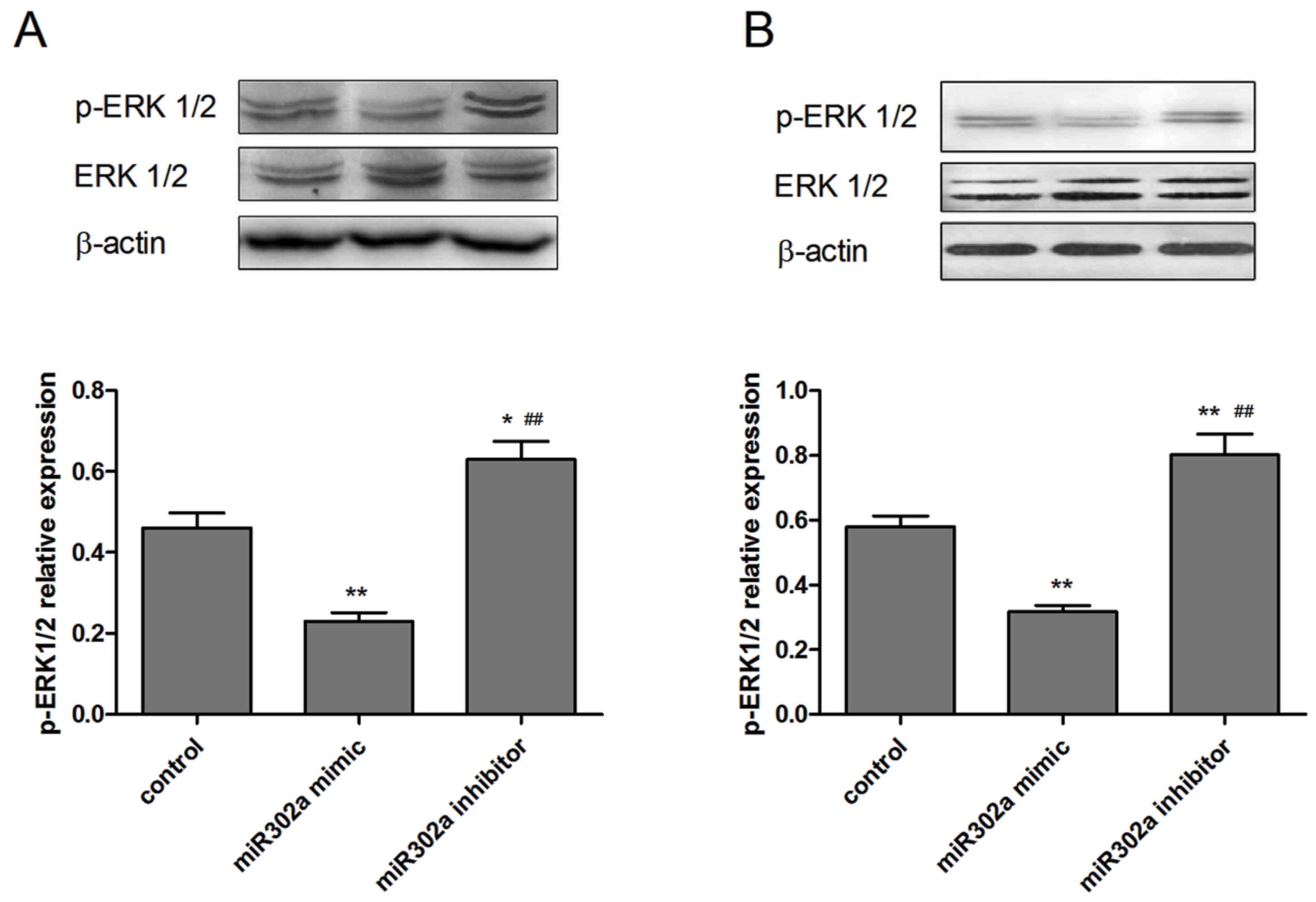
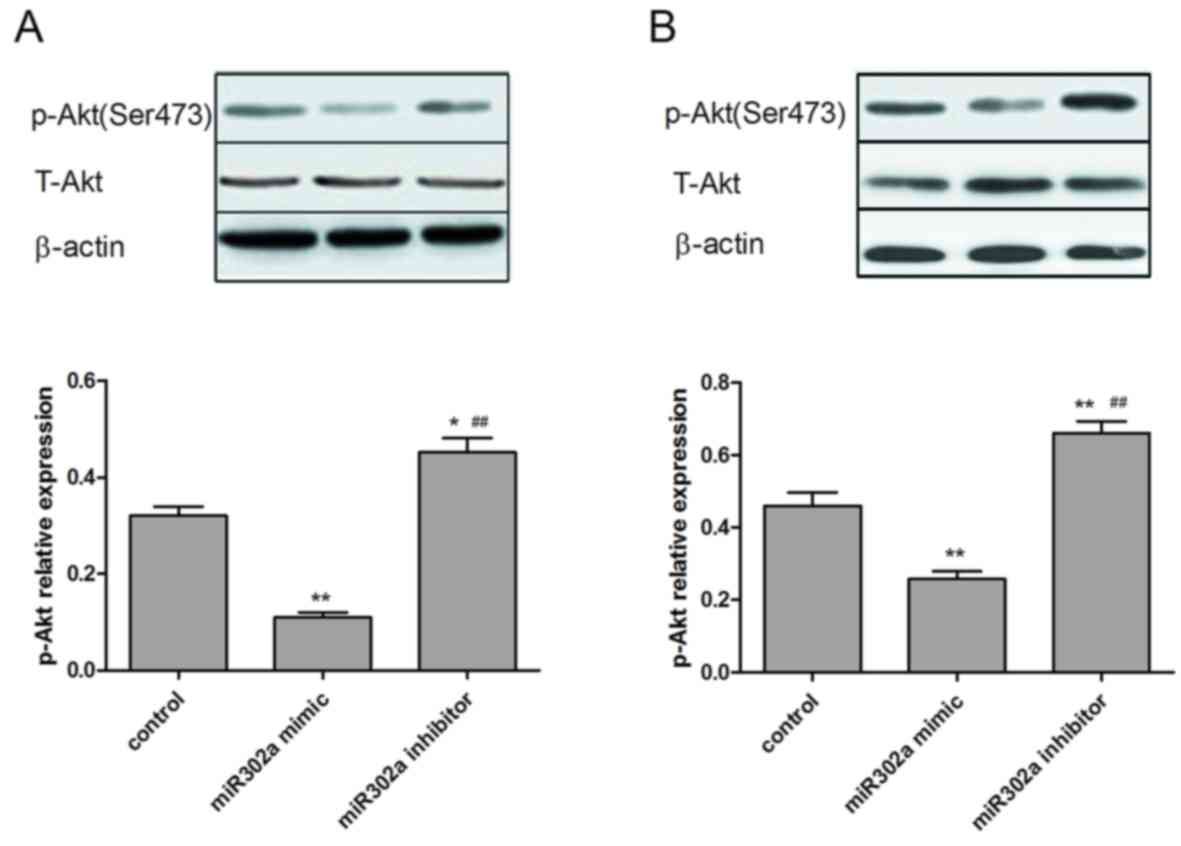
Overexpression of miR302a suppresses
the phosphorylation of proteins in the MAPK and PI3K/Akt signaling
pathways
As miR302a has been demonstrated to be associated
with cell viability and invasion in esophageal cancer cells, the
underlying mechanisms of these functions were subsequently
explored. The expression of p-Akt and p-ERK1/2, which are crucial
downstream effectors of the MAPK and PI3K/Akt signaling pathways,
were assessed in the different treatment groups. As presented in
Figs. 5 and 6, overexpression of miR302a had no
significant effect on total Akt and ERK1/2 expression levels, while
the phosphorylation level of Akt and ERK1/2 was significantly
decreased in the miR302a mimic group (P<0.05) and increased in
the miR302a inhibitor group (P<0.01), compared with the control
group in the two cell lines.
Discussion
The results of the present study revealed that
miR302a expression was significantly downregulated in several
esophageal cancer cell lines compared with a healthy esophageal
epithelial cell line. Furthermore, overexpression of miR302a
significantly inhibited the proliferation and invasion of
esophageal cancer cells. Finally, increased miR302a expression
significantly inhibited p-Akt and p-ERK1/2 protein expression
levels, indicating that miR302a may act as an upstream regulator of
MAPK and PI3K/Akt signaling in esophagus cancer.
miR302 family members have been reported to regulate
cell proliferation, invasion and apoptosis in certain types of
cancer cell. Maadi et al (11)
demonstrated that miR-302 inhibited cancer cell proliferation,
angiogenesis and invasion by reversing the epithelial-mesenchymal
transition in A-375 melanoma cells and HT-29 colorectal cancer
cells. miR302a also increases 5-fluorouracil-induced cell death and
inhibits cell viability in human colon cancer cells by inhibiting
Akt signaling (12). miRNA expression
profiles have been reported in esophageal cancer, but the function
of miR302a in esophageal cancer remains unknown (13). The present study revealed that miR302a
was significantly downregulated in esophageal cancer cell lines
(TE-1, TE-10, TE-11, ECA109) compared with the healthy esophageal
cancer cell line Het-1A and, to the best of our knowledge, its
downregulation was revealed to be associated with cell
proliferation and invasion in TE-10 and ECA109 cell lines for the
first time. Furthermore, overexpression of miR302a significantly
inhibited the viability and invasion of esophageal cancer cells,
and silencing of miR302a elicited the opposite effects. The results
of the present study may aid the development and improvement of
diagnosis and treatment of esophageal cancer in the future.
MAPK signal transduction pathways are among the most
widespread cellular regulation mechanisms (14). The Ras/ERK pathway is the prototypic
MAPK pathway, being a key signaling pathway that is involved in the
regulation of healthy cell proliferation, survival, growth and
differentiation. Ras protein is the most frequently mutated protein
in human tumors, and in its active state affects cell growth,
differentiation, cytoskeleton, protein transport and secretion
(15). Dysregulation of the ERK
pathway contributes to ~1/3 of all types of human cancer (16). Certain reports have demonstrated that
p-ERK inhibits cell proliferation and induces the apoptosis of
gastric cancer cells treated with β-elemene (17), and sustained, β-sitosterol-induced ERK
phosphorylation impedes renal tumor transformation promotion and
maintenance (18). Increased
phosphorylated ERK could promote tumor cell proliferation and
invasion, and affects cell differentiation, inhibition of
apoptosis. In addition, the PI3K/Akt signaling pathway is also
involved in the regulation of cell growth, metabolism,
proliferation, glucose homeostasis and vesicle trafficking, and
dysregulation of PI3K/Akt pathway components accounts for ~ 30% of
all cases of human cancer (19). In
particular, the Akt family of serine/threonine kinases has emerged
as a critical target of PI3K in human tumors. P-Akt increases cell
proliferation and inhibits cell apoptosis of multiple types of
cancer. For example, p-Akt promotes cell proliferation and survival
in vitro and serves an important function in prostate cancer
progression, as well as the prediction of recurrence (20), and decreased expression of p-Akt
inhibits polydatin-induced cell proliferation and induces apoptosis
in laryngeal cancer and HeLa cells (21). Furthermore, overexpression of p-Akt
promotes the proliferation and tumorigenesis of the esophageal
cancer cell line ECA-109 (22).
Therefore, miR302a may have targeted p-ERK1/2 and p-Akt through the
MAPK and PI3K signaling pathways, respectively, and this may be its
primary contribution to the inhibition of cell proliferation in
TE-10 and ECA109 cells. Consistent with previous results, in the
present study, the phosphorylation levels of Akt and ERK1/2 were
significantly decreased in the miR302a mimics group (P<0.05),
and increased in the miR302a inhibitor group (P<0.01) (23–26).
Furthermore, total Akt and ERK1/2 expression levels were also
determined by western blot analysis in TE-10 and ECA109 cells, and
overexpression of miR302a did not markedly differ between groups.
miR302a overexpression has been demonstrated to significantly
inhibit cell proliferation by inhibiting Akt in colon cancer and
prostate cancer (10,27). Furthermore, miR-302 has also been
reported to inhibit cancer cell proliferation via the Akt signaling
pathway, and the microRNA-302-367 cluster suppressed the
proliferation of cervical carcinoma cells through the novel target
Akt1 (28). Sun et al
(29) also revealed that miR302a
inhibited Akt expression by directly binding to its 3′ untranslated
region, resulting in subsequent alterations of the Akt-glycogen
synthase kinase 3β-cyclin D1 pathway. However, to date, few studies
have examined miR302a-induced inhibition of cell proliferation via
the MAPK signaling pathway in cancer cells, and to the best of our
knowledge no prior studies have been conducted concerning whether
miR302a affected the proliferation of esophageal cancer cells by
regulating the MAPK or PI3K/Akt signaling pathways. The present
study revealed that miR302a expression was decreased in esophageal
cancer cell lines compared with a healthy esophageal epithelial
cell line, and investigated the relevance of miR302a expression to
the MAPK and PI3K/Akt signaling pathways.
In conclusion, our group demonstrated that miR302a
overexpression inhibited the viability and invasion of esophageal
cancer cells via the MAPK and PI3K/Akt signaling pathways. These
results may provide a potential therapeutic target to suppress the
development, invasion and metastasis of esophageal cancer cells.
However, the effects of miR302a determined by the present study
were all based on in vitro experiments, and further in
vivo studies should be performed in the future.
References
|
1
|
Stewart BW and Wild CP: World cancer
report 2014. Lyon, France: Int Agency Res Cancer; 2014
|
|
2
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valmasoni M, Pierobon ES, Ruol A, De
Pasqual CA, Zanchettin G, Moletta L, Salvador R, Costantini M and
Merigliano S: Endoscopic tumor length should be reincluded in the
esophageal cancer staging system: Analyses of 662 consecutive
patients. PLoS One. 11:e01530682016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghidini M, Petrelli F, Hahne JC, De Giorgi
A, Toppo L, Pizzo C, Ratti M, Barni S, Passalacqua R and Tomasello
G: Clinical outcome and molecular characterization of brain
metastases from esophageal and gastric cancer: A systematic review.
Med Oncol. 34:622017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barroso-Deljesus A, Lucena-Aguilar G,
Sanchez L, Ligero G, Gutierrez-Aranda I and Menendez P: The Nodal
inhibitor Lefty is negatively modulated by the microRNA miR-302 in
human embryonic stem cells. FASEB J. 25:1497–1508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo T, Wei Y, Lv S, Zhang C and Tian Y:
MiR-302a inhibits the tumorigenicity of ovarian cancer cells by
suppression of SDC1. Int J Clin Exp Pathol. 8:4869–4880.
2015.PubMed/NCBI
|
|
10
|
Zhang GM, Bao CY, Wan FN, Cao DL, Qin XJ,
Zhang HL, Zhu Y, Dai B, Shi GH and Ye DW: MicroRNA-302a suppresses
tumor cell proliferation by inhibiting Akt in prostate cancer. PLoS
One. 10:e01244102015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maadi H, Moshtaghian A, Taha MF, Mowla SJ,
Kazeroonian A, Haass NK and Javeri A: Multimodal tumor suppression
by miR-302 cluster in melanoma and colon cancer. Int J Biochem Cell
Biol. 81:1212016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu N, Li J, Zhao Z, Han J, Jiang T, Chen
Y, Hou N and Huang C: MicroRNA-302a enhances 5-fluorouracil-induced
cell death in human colon cancer cells. Oncol Rep. 37:631–639.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feber A, Xi L, Luketich JD, Pennathur A,
Landreneau RJ, Wu M, Swanson SJ, Godfrey TE and Litle VR: MicroRNA
expression profiles of esophageal cancer. J Thorac Cardiovasc Surg.
135:255–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Turjanski AG, Vaqué JP and Gutkind JS: MAP
kinases and the control of nuclear events. Oncogene. 26:3240–3253.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schubbert S, Shannon K and Bollag G:
Hyperactive Ras in developmental disorders and cancer. Nat Rev
Cancer. 7:295–308. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li P, Zhou X, Sun W, Sheng W, Tu Y, Yu Y,
Dong J, Ye B, Zheng Z and Lu M: Elemene induces apoptosis of human
gastric cancer cell line BGC-823 via extracellular signal-regulated
kinase (ERK)1/2 signaling pathway. Med Sci Monit. 23:809–817. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharmila R and Sindhu G: Evaluate the
antigenotoxicity and anticancer role of β-sitosterol by determining
oxidative DNA damage and the expression of phosphorylated
mitogen-activated protein kinases', C-fos, C-jun, and endothelial
growth factor receptor. Pharmacogn Mag. 13:95–101. 2017.PubMed/NCBI
|
|
19
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hammerich KH, Frolov A, Li R, Ittmann M
and Ayala GE: Cellular interactions of the phosphorylated form of
AKT in prostate cancer. Hum Pathol. 63:98–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Shi B, Li Y and Yin F: Polydatin
inhibits cell proliferation and induces apoptosis in laryngeal
cancer and HeLa cells via suppression of the PDGF/AKT signaling
pathway. J Biochem Mol Toxicol. 31:2017. View Article : Google Scholar
|
|
22
|
Hong Y, Wen C, Du X, Hong Y, Chen W, Du X,
Ning H, Chen H, Shi R, Lin S, et al: Upregulation of
sex-determining region Y-box 9 (SOX9) promotes cell proliferation
and tumorigenicity in esophageal squamous cell carcinoma.
Oncotarget. 6:31241–31254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mundi PS, Sachdev J, McCourt C and
Kalinsky K: AKT in cancer: New molecular insights and advances in
drug development. Br J Clin Pharmacol. 82:943–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Briest F and Grabowski P:
PI3K-AKT-mTOR-signaling and beyond: The complex network in
gastroenteropancreatic neuroendocrine neoplasms. Theranostics.
4:336–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carpenter RL, Sirkisoon S, Zhu D, Rimkus
T, Harrison A, Anderson A, Paw I, Qasem S, Xing F, Liu Y, et al:
Combined inhibition of AKT and HSF1 suppresses breast cancer stem
cells and tumor growth. Oncotarget. 8:73947–73963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamilton G, Abraham AG, Morton J, Sampson
O, Pefani DE, Khoronenkova S, Grawenda A, Papaspyropoulos A,
Jamieson N, McKay C, et al: AKT regulates NPM dependent ARF
localization and p53mut stability in tumors. Oncotarget.
5:6142–6167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu N, Li J, Zhao Z, Han J, Jiang T, Chen
Y, Hou N and Huang C: MicroRNA-302a enhances 5-fluorouracil-induced
cell death in human colon cancer cells. Oncol Rep. 37:631–639.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai N, Wang YD and Zheng PS: The
microRNA-302-367 cluster suppresses the proliferation of cervical
carcinoma cells through the novel target AKT1. RNA. 19:85–95. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun S, Zhang G, Wu Z, Shi W, Yang B and Li
Y: MicroRNA-302a functions as a putative tumor suppressor in colon
cancer by targeting Akt. PLoS One. 9:e1159802014. View Article : Google Scholar : PubMed/NCBI
|