Introduction
Primary liver cancer is one of the most common
malignant tumors in the world, among which hepatocellular carcinoma
(HCC) accounts for approximately 70–85% (1). Surgical operation, local ablation,
radiotherapy and chemotherapy provide opportunities to cure or
prolong the survival time of patients. However, the 5-year
recurrence rate after primary liver cancer operation is as high as
45–60%, seriously affecting the prognosis of patients with liver
cancer (2–4). Therefore, searching for the indexes for
evaluating the poor prognosis of HCC, such as proliferative
activity and early recurrence, is of significance for the tumor
staging, selection of treatment plan and prognosis.
Ki-67 is a kind of nuclear antibody Ki-67 protein,
mainly existing in the dense fibrillar components of nucleoli and
cortices, which is a reliable index of detecting the tumor cell
proliferative activity (5). The
biological manifestations of tumor are closely related to its
proliferative activity. The higher the expression of Ki-67 is, the
faster the tumor cell proliferation will be and the higher the
probability of metastasis will also be (6,7). However,
there are few studies on the correlation between
contrast-enhancement ultrasound of HCC and Ki-67
immunohistochemistry and other prognostic factors.
Compared with enhancement MRI/CT, PET and other
examinations, contrast-enhancement ultrasound (CEUS) is
characterized by the convenient and efficient application, wide
indications and no radiation, and it can show the whole process of
contrast enhancement for tumor and liver parenchyma in a real-time
and dynamic manner, which not only improves the value of diagnosis
and differential diagnosis, but also indirectly reflects some
biological manifestations of tumors (8–10). In the
field of contrast-enhancement ultrasound, there are some scholars
who use special computer software to conduct the quantization
parameter analysis for CEUS images of HCC, establish the peak
intensity curve, and study the relationship between CEUS parameters
on the curve and microvessel density (MVD), microvessel invasion
(MVI) and tumor tissue differentiation degree (11–14). These
factors are associated with the recurrence of liver cancer and the
survival time of liver cancer patients.
This study aimed to evaluate the relationship
between the enhancement mode and parameters of CEUS for HCC and the
prognostic factors and clinical recurrence, and evaluate the
biological behavior and prognosis of liver cancer from the
perspective of ultrasonography, so as to provide new non-invasive
evaluation indexes of prognosis.
Patients and methods
Subjects
One hundred and eighty patients with HCC confirmed
by operation or puncture were collected, and they all had the
background of liver cirrhosis. The tumor samples received
immunohistochemical Ki-67 and AFP-labeled staining. This study was
approved by the Ethics Committee of Qianfoshan Hospital Affiliated
to Shandong University (Jinan, China). Signed written informed
consents were obtained from all participants before the study.
Pathological examination of Ki-67
Each section was observed via 10 visual fields
randomly selected under high-power microscope (×400), and the
percentage of brown-stained cell nuclei every 100 cells was the
percentage of Ki-67. Ki-67 was divided into Ki-67-positive group
(>10%) and the Ki-67-negative group (≤10%) according to the
percentage of Ki-67.
Microvessel density, MVD
After the blood vessels in tumor tissues were
stained by antigen-antibody reaction, the slide was glanced with
low-power microscope (×40-×100) to find the area with the highest
blood vessel density. Then the stained blood vessels within the
field were counted under high-power microscope (×400). The mean MVD
of the patients included was calculated, and they were divided into
the high-MVD group (greater than the mean) and low-MVD group (lower
than the mean).
Microvessel invasion, MVI
Microvessel infiltration means that multiple or
massed cancer cells can be found in venules in para-cancer
mesenchyme, obvious endothelial cells can be found around the lumen
and red blood cells may appear within the lumen. Patients were
divided into the MVI-positive group (MVI was found) and
MVI-negative group (no MVI was found).
Contrast-enhancement ultrasound,
CEUS
Color Doppler ultrasound was used to record the
lesion site and size, internal echo and number, and Doppler blood
flow signal; and then the contrast agent was injected under the
radiography mode, and the timer and recording were started. The sum
of two maximum orthogonal radial lines of lesion on the same
section was taken as size of the tumor; according to the changes
before and after CEUS, those greater than the mean of all change
values were enrolled into the significant change group; otherwise,
they were enrolled into the non-significant change group. The image
at 10–30 sec after injection of contrast agent was taken as
arterial phase. The images with peak intensity were observed; if
there were low enhancement or no enhancement area in different
sizes in the lesion, the enhancement mode was seen as the
inhomogeneous enhancement. The irregular enhancement form of lesion
was regarded as the irregular tumor form. Whether there were
tortuous blood vessels in the enhancement process at arterial phase
were observed. The images at 90–120 sec after injection of contrast
agent were selected as the portal phase. In comparison with
surrounding liver parenchyma, the extinction time of contrast agent
was recorded, the enhancement intensity and regression degree of
lesion at portal phase were observed.
Recurrence of liver cancer
If the focal hepatic lesions showed the typical
manifestation of HCC or obvious extra hepatic metastasis in imaging
examination for the first time, it was the recurrence. According to
the first time of discovering recurrent lesion, it was divided into
early recurrence (≤1 year) and late recurrence (>1 year).
Statistical analysis
All statistics were performed using SPSS 19.0
software (SPSS Inc., Chicago, IL, USA). t-test was performed for
the comparison of mean value, Chi-square test for the significant
difference between groups. Binary logistic regression analysis was
used to evaluate the relationship between the parameters of
ultrasound contrast and prognostic factors. P<0.05 was
considered to indicate a statistically significant difference.
Results
Ki-67/AFP immunohistochemistry
In this study, the clinical characteristics between
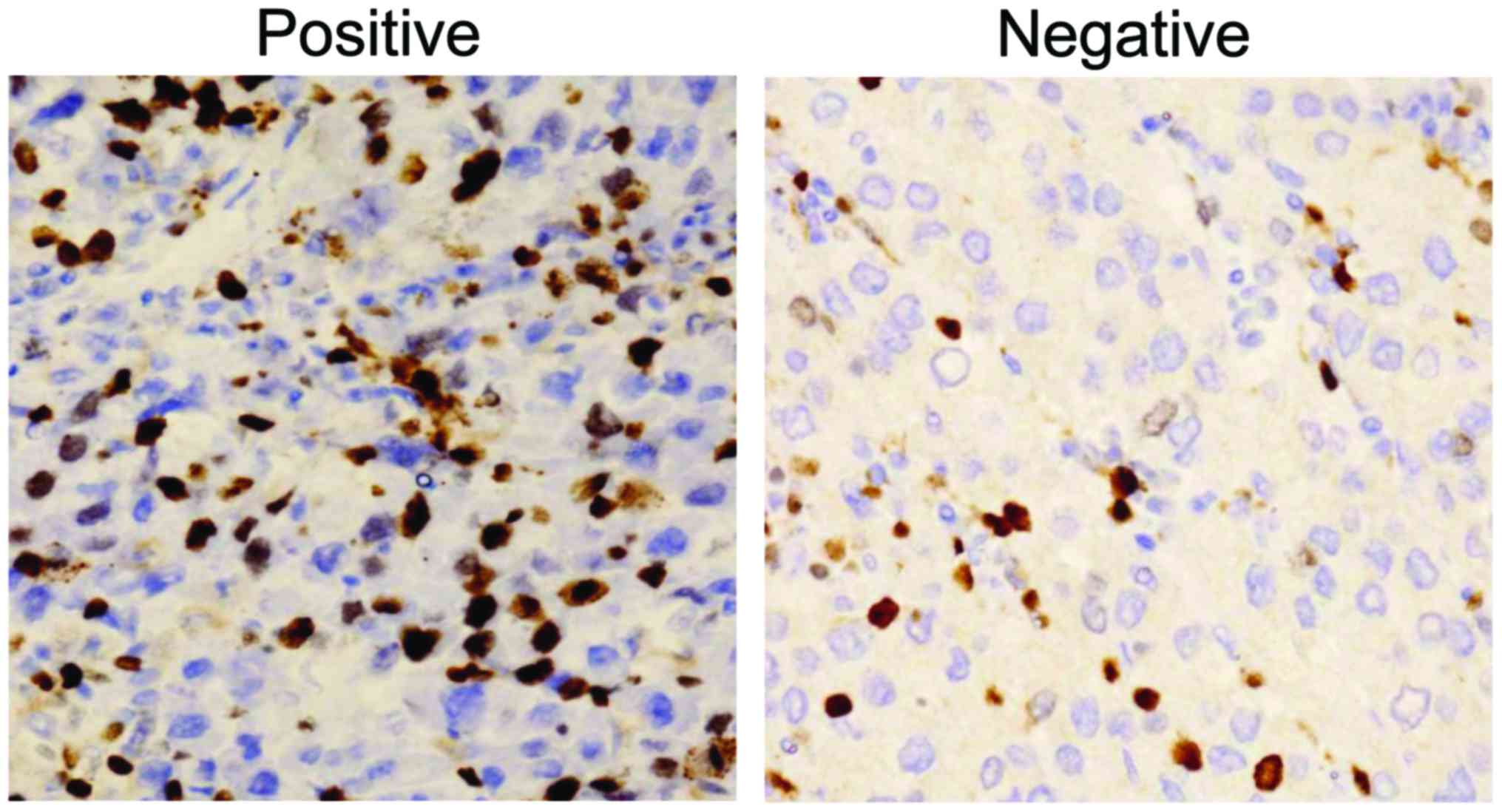
Ki-67 positive group (Fig. 1) and
Ki-67 negative group (Fig. 1), AFP
positive group and AFP negative group were compared. There were no
statistically significant differences in the age, sex, positive
rate of hepatitis B serum marker and liver function Child-Pugh
grade between the two groups (P>0.05); but the proportion of
high-differentiated liver cancer in Ki-67 negative group was higher
than that in Ki-67 positive group, and the proportion of
low-differentiated liver cancer in Ki-67 positive group was higher
than that in Ki-67 negative group (P<0.05) (Table I).
 | Table I.Basic characteristics of
Ki-67(+)/Ki-67(−) HCC subjects. |
Table I.
Basic characteristics of
Ki-67(+)/Ki-67(−) HCC subjects.
| Factors | Ki-67(+) | Ki-67(−) | Test value | P-value |
|---|
| Sex (male %) | 90.01% | 91.97% |
χ2=0.23 | 0.57 |
| Age | 45.90±8.76 | 48.86±9.34 | t=1.74 | 0.23 |
| Tumor tissue
differentiation degree |
|
|
χ2=16.28 | NS |
|
High-differentiated |
2.63% | 15.57% |
|
|
|
Medium-differentiated | 81.87% | 79.96% |
|
|
|
Low-differentiated | 15.50% | 4.47% |
|
|
| Hepatitis B serum
marker |
|
|
χ2=4.26 | 0.08 |
|
Positive | 99 | 69 |
|
|
|
Negative | 6 | 6 |
|
|
| Liver function
Child-Pugh grade |
|
|
χ2=0.02 | 0.88 |
| Grade
A | 96 | 70 |
|
|
| Grade
B | 9 | 5 |
|
|
Microvessel density (MVD)
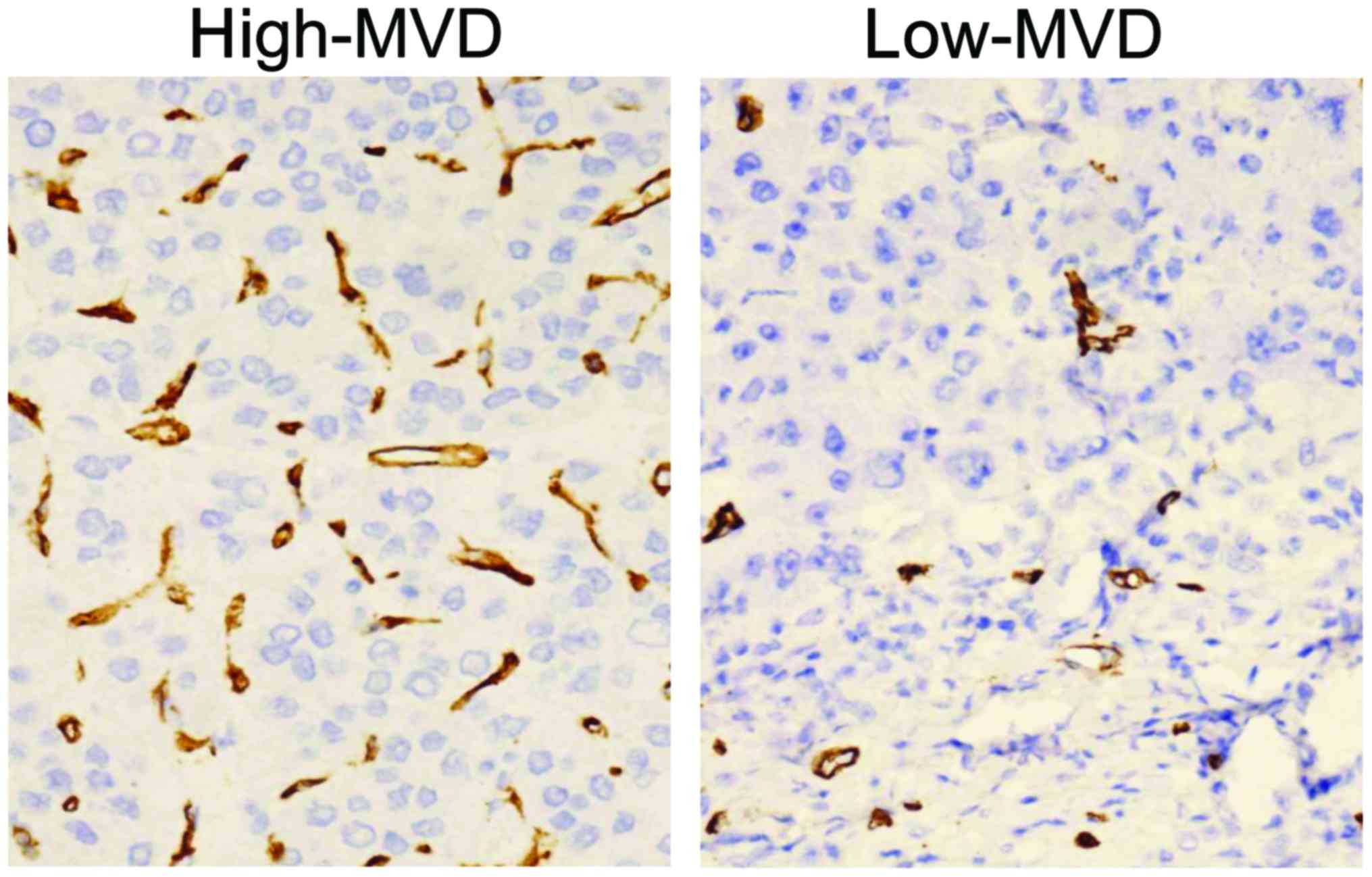
In this study, the mean MVD of patients was 63.40,
and they were divided into the high-MVD group (greater than the
mean) and low-MVD group (lower than the mean) (Fig. 2). There were no statistically
significant differences in the age, sex, hepatitis B serum marker
and liver function between high-MVD group and low-MVD group
(P>0.05).
Microvessel invasion (MVI)
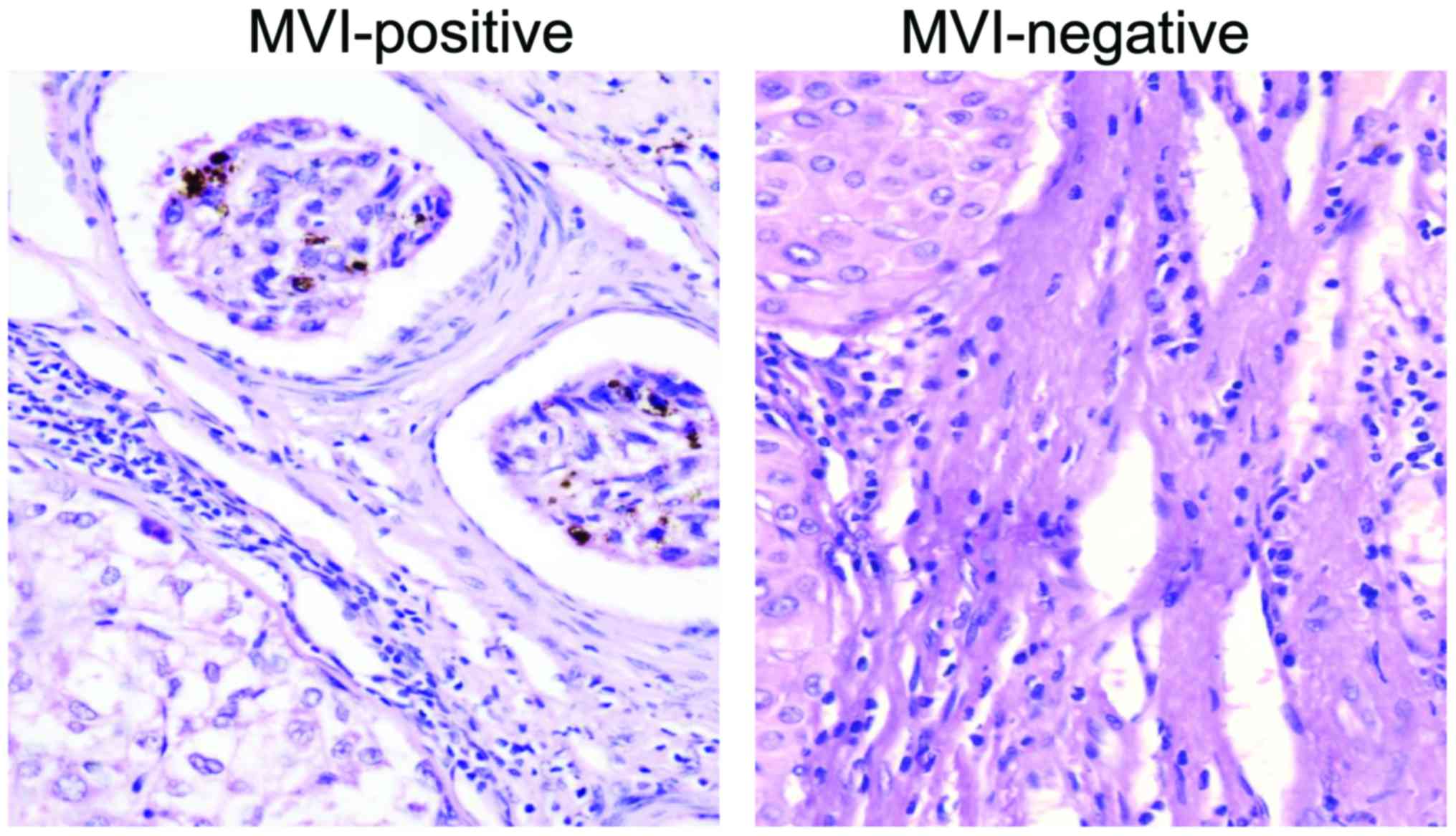
In this study, patients were divided into the
MVI-positive group (MVI was found in para-cancer mesenchyme)
(Fig. 3) and MVI-negative group (no
MVI was found in para-cancer mesenchyme) (Fig. 3). There were no statistically
significant differences in the age, sex, hepatitis B serum marker
and liver function between MVI-positive group and MVI-negative
group (P>0.05).
Relationship between CEUS parameters
vs. pathologic histology
The incidence of positive Ki-67 was high for those
cases with obvious change in tumor size, inhomogeneous enhancement
and irregular enhancement form of tumor at arterial phase, and low
enhancement and fast extinction at portal phase after CEUS
(P<0.05, Table II). There was no
significant correlation between the change in tumor size,
enhancement mode and form of tumor at arterial phase and other
parameters after CEUS and the immunohistochemical AFP (P>0.05,
Table II).
 | Table II.Comparison of CEUS parameters in
Ki-67/AFP different HCC groups. |
Table II.
Comparison of CEUS parameters in
Ki-67/AFP different HCC groups.
| Factors | Ki-67(+) | Ki-67(−) | χ2
test | P-value | AFP(+) | AFP(−) | χ2
test | P-value |
|---|
| Change of tumor
size |
|
| 6.15 | 0.01 |
|
| 2.31 | 0.10 |
|
Conspicuous | 46 | 20 |
|
| 40 | 26 |
|
|
|
Inconspicuous | 59 | 55 |
|
| 54 | 60 |
|
|
| Enhancement mode at
arterial phase |
|
| 6.57 | 0.00 |
|
| 0.13 | 1.13 |
|
Inhomogeneous | 79 | 34 |
|
| 59 | 55 |
|
|
|
Homogeneous | 26 | 41 |
|
| 35 | 31 |
|
|
| Enhancement form at
arterial phase |
|
| 7.34 | 0.02 |
|
| 0.98 | 0.56 |
|
Irregular | 49 | 21 |
|
| 40 | 31 |
|
|
|
Regular | 56 | 54 |
|
| 54 | 55 |
|
|
| Tortuous vessels |
|
| 2.39 | 0.26 |
|
| 1.13 | 0.63 |
| Yes | 44 | 24 |
|
| 33 | 36 |
|
|
| No | 61 | 51 |
|
| 61 | 50 |
|
|
| Enhancement intensity
at portal phase |
|
| 7.43 | 0.04 |
|
| 1.77 | 0.46 |
| Low
intensity | 81 | 39 |
|
| 64 | 56 |
|
|
| High or
equal intensity | 24 | 36 |
|
| 30 | 30 |
|
|
| Extinction speed at
portal phase |
|
| 8.12 | 0.05 |
|
| 3.12 | 0.50 |
| Fast | 55 | 24 |
|
| 39 | 41 |
|
|
| Low | 50 | 51 |
|
| 55 | 45 |
|
|
| Extinction extent at
portal phase |
|
| 8.64 | 0.04 |
|
| 0.85 | 0.67 |
|
Obvious | 79 | 37 |
|
| 62 | 56 |
|
|
| None or
slight | 26 | 38 |
|
| 32 | 30 |
|
|
There was no significant correlation between the
change in tumor size, enhancement mode and form of tumor at
arterial phase and other parameters after CEUS or the MVD level
(P>0.05, Table III). The
incidence of positive MVI was high for those cases with obvious
change in tumor size, irregular enhancement form of tumor at
arterial phase, tortuous vessels inside and fast extinction at
portal phase after CEUS (P<0.05, Table III).
 | Table III.Comparison of CEUS parameters in
MVD/MVI different HCC groups. |
Table III.
Comparison of CEUS parameters in
MVD/MVI different HCC groups.
| Factors | H-MVD | L-MVD | χ2
test | P-value | MVI(+) | MVI(−) | χ2
test | P-value |
|---|
| Change of tumor
size |
|
| 0.75 | 0.55 |
|
| 7.05 | 0.04 |
|
Conspicuous | 39 | 32 |
|
| 56 | 15 |
|
|
|
Inconspicuous | 53 | 56 |
|
| 74 | 35 |
|
|
| Enhancement mode at
arterial phase |
|
| 0.34 | 0.69 |
|
| 3.50 | 0.16 |
|
Inhomogeneous | 59 | 54 |
|
| 87 | 26 |
|
|
|
Homogeneous | 33 | 34 |
|
| 43 | 24 |
|
|
| Enhancement form at
arterial phase |
|
| 0.72 | 0.45 |
|
| 5.70 | 0.01 |
|
Irregular | 41 | 33 |
|
| 60 | 14 |
|
|
|
Regular | 51 | 55 |
|
| 70 | 36 |
|
|
| Tortuous
vessels |
|
| 0.13 | 0.56 |
|
| 5.70 | 0.01 |
|
Yes | 39 | 35 |
|
| 60 | 14 |
|
|
| No | 53 | 53 |
|
| 70 | 36 |
|
|
| Enhancement
intensity at portal phase |
|
| 1.55 | 0.32 |
|
| 3.12 | 0.14 |
| Low
intensity | 65 | 57 |
|
| 93 | 29 |
|
|
| High or
equal intensity | 27 | 31 |
|
| 37 | 21 |
|
|
| Extinction speed at
portal phase |
|
| 2.31 | 0.42 |
|
| 3.54 | 0.03 |
|
Fast | 40 | 45 |
|
| 67 | 17 |
|
|
|
Low | 52 | 43 |
|
| 63 | 33 |
|
|
| Extinction extent
at portal phase |
|
| 1.75 | 0.33 |
|
| 3.88 | 0.14 |
|
Obvious | 65 | 57 |
|
| 92 | 29 |
|
|
| None or
slight | 27 | 31 |
|
| 38 | 21 |
|
|
The tissue differentiation degree of tumor samples
was lower for those cases with inhomogeneous enhancement and
irregular enhancement form of tumor at arterial phase, tortuous
vessels inside, low enhancement and fast extinction at portal phase
after CEUS (P<0.05, Table
IV).
 | Table IV.Comparison of CEUS parameters in
tumor tissue differentiation HCC groups. |
Table IV.
Comparison of CEUS parameters in
tumor tissue differentiation HCC groups.
| Factors |
Low/Medium-differentiated |
Highly-differentiated | χ2
test | P-value |
|---|
| Change of tumor
size |
|
| 4.34 | 0.07 |
|
Conspicuous | 64 | 8 |
|
|
|
Inconspicuous | 96 | 22 |
|
|
| Enhancement mode at
arterial phase |
|
| 6.75 | 0.01 |
|
Inhomogeneous | 105 | 12 |
|
|
|
Homogeneous | 45 | 18 |
|
|
| Enhancement form at
arterial phase |
|
| 5.12 | 0.04 |
|
Irregular | 66 | 8 |
|
|
|
Regular | 84 | 22 |
|
|
| Tortuous
vessels |
|
| 7.13 | 0.03 |
|
Yes | 64 | 7 |
|
|
| No | 86 | 23 |
|
|
| Enhancement
intensity at portal phase |
|
| 6.45 | 0.01 |
| Low
intensity | 102 | 13 |
|
|
| High or
equal intensity | 48 | 17 |
|
|
| Extinction speed at
portal phase |
|
| 6.83 | 0.00 |
|
Fast | 106 | 12 |
|
|
|
Low | 44 | 18 |
|
|
| Extinction extent
at portal phase |
|
| 4.27 | 0.04 |
|
Obvious | 68 | 9 |
|
|
| None or
slight | 82 | 21 |
|
|
CEUS parameters vs. recurrence of
liver cancer
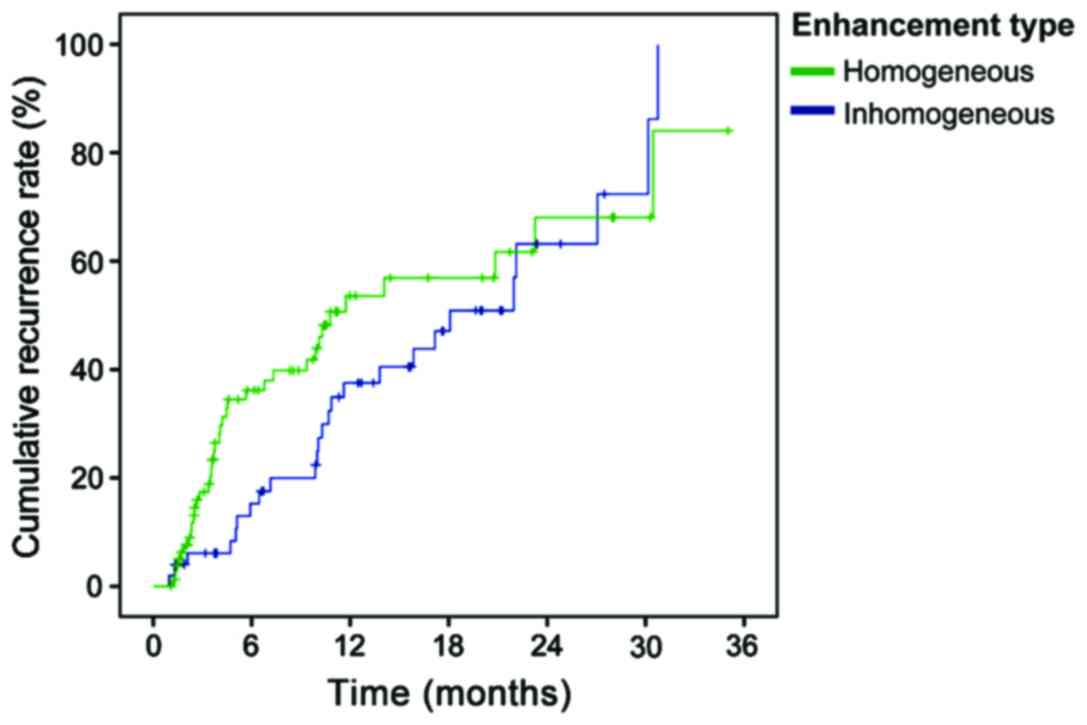
The recurrence time was shorter for those cases with
inhomogeneous enhancement of tumor at arterial phase in CEUS
(P<0.05, Table V and Fig. 4).
 | Table V.Comparison of CEUS parameters in
different recurrence conditions of HCC groups. |
Table V.
Comparison of CEUS parameters in
different recurrence conditions of HCC groups.
| Factors | Early
recurrence | Late
recurrence | No recurrence | χ2
test | P-value |
|---|
| Change of tumor
size |
|
|
| 1.90 | 0.43 |
|
Conspicuous | 26 | 8 | 38 |
|
|
|
Inconspicuous | 35 | 17 | 56 |
|
|
| Enhancement mode at
arterial phase |
|
|
| 7.84 | 0.04 |
|
Inhomogeneous | 40 | 10 | 59 |
|
|
|
Homogeneous | 21 | 15 | 35 |
|
|
| Enhancement form at
arterial phase |
|
|
| 5.87 | 0.07 |
|
Irregular | 28 | 7 | 42 |
|
|
|
Regular | 33 | 18 | 52 |
|
|
| Tortuous
vessels |
|
|
| 4.30 | 0.38 |
|
Yes | 29 | 9 | 35 |
|
|
| No | 32 | 16 | 59 |
|
|
| Enhancement
intensity at portal phase |
|
|
| 4.12 | 0.30 |
| Low
intensity | 40 | 12 | 61 |
|
|
| High or
equal intensity | 21 | 13 | 33 |
|
|
| Extinction speed at
portal phase |
|
|
| 5.13 | 0.24 |
|
Fast | 30 | 8 | 41 |
|
|
|
Low | 31 | 17 | 53 |
|
|
| Extinction extent
at portal phase |
|
|
| 3.07 | 0.36 |
|
Obvious | 40 | 12 | 59 |
|
|
| None or
slight | 21 | 13 | 35 |
|
|
Logistic regression for prognostic
factors of HCC
Logistic regression showed that the inhomogeneous
enhancement inside at the arterial phase in CEUS was more helpful
to suggest positive Ki-67 and early recurrence; irregular
enhancement form at arterial phase suggested a higher risk of MVI;
the low enhancement at portal phase might be a parameter suggesting
low tissue differentiation degree (P<0.05, Table VI).
 | Table VI.Logistic regression of HCC prognosis
factors. |
Table VI.
Logistic regression of HCC prognosis
factors.
| Factors | r-value | Wald value | P-value | OR |
|---|
| Ki-67 |
|
|
|
|
|
Inhomogeneous enhancement mode
at arterial phase | 1.15 | 26.39 | NS | 2.78 |
| MVI |
|
|
|
|
|
Irregular enhancement form at
arterial phase | 1.26 | 17.35 | NS | 3.10 |
| Tumor tissue
differentiation |
|
|
|
|
|
Inhomogeneous enhancement mode
at arterial phase | 1.20 |
7.03 | 0.02 | 3.25 |
| Low
intensity enhancement at portal phase | 1.55 | 12.39 | NS | 4.23 |
Discussion
Approximately 740,000 people die from primary HCC
around the world every year, and the patients in China account for
more than 50% of the global total (2). The high postoperative recurrence rate of
liver cancer is the main factor influencing the survival rate of
patients with liver cancer. Early prediction of related risk
factors to postoperative recurrence of liver cancer is of great
clinical significance, and examining the postoperative recurrence
as soon as possible and taking treatment measures timely can
effectively improve the prognosis of patients with liver cancer
(4).
The prognosis of HCC patients is determined by a
variety of factors. Immunohistochemical Ki-67 is a rapid, simple
and sensitive detection technique for proliferative activity of
liver cancer cells, and its overexpression (>10%) can predict
the recurrence of liver cancer after surgical treatment, which is
associated with the mortality of HCC patients (5–7). In this
study, the change in lesion size, the enhancement mode and form
inside lesion at arterial phase, and the enhancement intensity,
extinction time and degree at portal phase were associated with the
incidence of positive Ki-67; and the logistic regression analysis
showed that the inhomogeneous enhancement inside the lesion at
arterial phase might be the best parameter to predict the Ki-67
overexpression.
AFP is an important biological marker of liver
cancer, whose quantification and immunohistochemistry is associated
with the occurrence and development of liver cancer. In recent
years, the studies show that AFP lacks sufficient sensitivity and
specificity in the effective diagnosis and monitoring of liver
cancer. There are few studies about the CEUS parameters and
immunohistochemical level of AFP. The results of this study
suggested that there was no obvious relationship between the
immunohistochemistry AFP and CEUS parameters.
Tumor growth and metastasis depend on the growth of
tumor angiogenesis, and MVD, as a sign of tumor angiogenesis
degree, also suggests that it is closely related to the occurrence,
development and metastasis of tumors (11,12).
Compared with liver cancer patients with low MVD, patients with
high MVD tend to suffer from early postoperative recurrence. Some
scholars studied the correlation between time intensity curve and
MVD, and found that the peak intensity, intensity enhancement and
area under the curve were positively correlated to the MVD count
(13), but in this study, the change
in tumor size, enhancement form at arterial phase and other
parameters after CEUS had no significant relationship with MVD
count.
MVI of liver cancer means that the cancer cells have
the potential of intrahepatic metastasis and spread to the
circulatory system (14,15). In this study, it can be seen that the
change in lesion size, enhancement form at arterial phase, tortuous
vessels inside and extinction time at portal phase were related to
MVI. Logistic regression analysis showed that irregular form at
arterial phase was the most closely related to MVI. The irregular
enhancement form of lesion at arterial phase is associated with the
high incidence of MVI from both univariate and multivariate
regression analysis, which may be the most valuable independent
factor of predicting MVI.
Typical CEUS of HCC is characterized by high
enhancement of lesion at arterial phase and low enhancement at
portal phase and delayed phase, namely the so-called ‘fast in and
fast out’ of contrast agent. However, the high-differentiated HCC
may present the atypical imaging manifestations, such as the ‘fast
in and slow out’ or ‘slow in and slow out’ of contrast agent
(8–10). In this study, the enhancement mode and
form of lesions at arterial phase, tortuous vessels, and the
enhancement intensity, extinction time and degree at portal phase
were correlated with the pathological tissue differentiation
degree. Logistic regression analysis showed that the inhomogeneous
enhancement inside lesions at arterial phase and low enhancement at
portal phase were risk factors of low tissue differentiation
degree. Compared with the inhomogeneous enhancement at arterial
phase, low enhancement at portal phase is more dangerous. The study
results showed that the proportion of high or equal enhancement at
portal phase in high-differentiated HCC group was significantly
higher than that in moderate- and low-differentiated group, and the
reason may be that: i) high-differentiated HCC causes the retention
of contrast agent due to the presence of neat bone trabecula-type
cellular bundles and a large number of hepatic sinusoids; ii) with
the increase of malignancy degree, the degree of differentiation
becomes lower and the abnormal arteries are formed derived from the
increase of abnormal arterial blood supply. Normal blood supply in
hepatic artery and portal vein decreases, the duration of
enhancement at portal phase declines and the enhancement intensity
is lower; iii) there are many abnormal new vessels at the center or
around the tumor, as well as abundant arteriovenous fistulas, thus
shortening the duration of enhancement.
To sum up, this study investigated the relationship
between the enhancement pattern and parameters of CEUS for HCC and
the prognostic factors and clinical recurrence, and evaluated the
biological behavior and prognosis of liver cancer from the
perspective of pathology and ultrasonography, so as to provide a
new prognosis evaluation index.
On the background of liver cirrhosis, there were
significant differences in different enhancement modes and
quantification parameters of contrast-enhancement ultrasound for
HCC with different expression of Ki-67. The high incidence of
positive Ki-67 may suggest poor prognosis. CEUS is helpful to
predict the prognosis of HCC. The inhomogeneous enhancement at
arterial phase may predict the proliferative activity and
recurrence time of tumor cells; irregular enhancement form at
arterial phase may indicate tumor MVI; and the low enhancement of
tumor at portal phase may predict a lower degree of tissue
differentiation and poor prognosis.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Geschwind JF: Locoregional therapy for
patients with hepatocellular carcinoma. Gastroenterol Hepatol (NY).
11:698–700. 2015.
|
|
2
|
Gish RG: Hepatocellular carcinoma: Current
questions and future directions. Gastroenterol Hepatol (NY).
11:182–185. 2015.
|
|
3
|
Geschwind JF: Locoregional therapy for
patients with hepatocellular carcinoma. Clin Adv Hematol Oncol.
13:660–662. 2015.PubMed/NCBI
|
|
4
|
Janevska D, Chaloska-Ivanova V and
Janevski V: Hepatocellular carcinoma: Risk factors, diagnosis and
treatment. Open Access Maced J Med Sci. 3:732–736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tawfik O, Garimella R, Tancabelic J,
Keighley J, Pinson D, Khan Q and Templeton K: Retrospective
immunohistochemical study of VDR, RXR, Ki-67, and Bcl-2 expression
in primary and metastatic osteosarcoma. J Clin Oncol.
27:e215162009.
|
|
6
|
Li W, Zhang G, Wang HL and Wang L:
Analysis of expression of cyclin E, p27kip1 and Ki67 protein in
colorectal cancer tissues and its value for diagnosis, treatment
and prognosis of disease. Eur Rev Med Pharmacol Sci. 20:4874–4879.
2016.PubMed/NCBI
|
|
7
|
Saponara M, Di BM, Lolli C, Derenzini E,
Pantaleo MA, Santini D, Ceccarelli C, Catena F, Nannini M and
Maleddu A: Evaluation of Ki-67 in gastrointestinal stromal tumor
(GIST). J Clin Oncol. 27:e215102009.
|
|
8
|
Sirlin C: Imaging techniques for
hepatocellular carcinoma. Gastroenterol Hepatol (NY). 11:403–405.
2015.
|
|
9
|
Dong Y, Wang WP, Mao F, Fan M, Ignee A,
Serra C, Sparchez Z, Sporea I, Braden B and Dietrich CF: Contrast
enhanced ultrasound features of hepatic cystadenoma and hepatic
cystadenocarcinoma. Scand J Gastroenterol. 52:365–372. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haimerl M, Poelsterl S, Beyer LP,
Wiesinger I, Niessen C, Stroszczynski C, Wiggermann P and Jung EM:
Chronic liver disease: Quantitative MRI vs CEUS-based
microperfusion. Clin Hemorheol Microcirc. 64:435–446. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lekovic D, Gotic M, Skoda R,
Beleslin-Cokic B, Milic N, Mitrovic-Ajtic O, Nienhold R, Sefer D,
Suboticki T, Buac M, et al: Bone marrow microvessel density and
plasma angiogenic factors in myeloproliferative neoplasms:
Clinicopathological and molecular correlations. Ann Hematol.
96:393–404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang S, Zhang D, Yi S, Gong M, Lu C, Cai
Y, Tang X and Zou L: The relationship of lymphatic vessel density,
lymphovascular invasion, and lymph node metastasis in breast
cancer: A systematic review and meta-analysis. Oncotarget.
8:2863–2873. 2017.PubMed/NCBI
|
|
13
|
Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan
ST and Wong J: Tumor microvessel density as a predictor of
recurrence after resection of hepatocellular carcinoma: A
prospective study. J Clin Oncol. 20:1775–1785. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Donat M, Alonso S, Pereira F, Ferrero E,
Carrión L, Acin-Gándara D and Moreno E: Impact of histological
factors of hepatocellular carcinoma on the outcome of liver
transplantation. Transplant Proc. 48:1968–1977. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng LH, Dong H, Lau WY, Yu H, Zhu YY,
Zhao Y, Lin YX, Chen J, Wu MC and Cong WM: Novel microvascular
invasion-based prognostic nomograms to predict survival outcomes in
patients after R0 resection for hepatocellular carcinoma. J Cancer
Res Clin Oncol. 143:293–303. 2017. View Article : Google Scholar : PubMed/NCBI
|