Introduction
Four general therapeutic approaches have been
developed during the long fight with cancer: Surgery, chemotherapy,
radiotherapy and immunotherapy (1).
Unlike the first three, which target the tumor directly,
immunotherapy works indirectly through the immune system (2). The initial evidence demonstrating that
immunotherapy could be effective in targeting cancer was from a
study published by Coley in 1893 (3).
Coley injected streptococcal organisms (Coley's toxin) into
patients with cancer, and, in a number of cases, tumor regression
was observed, presumably due to the host's immune system (3). Since then, attitudes towards cancer
immunotherapy have fluctuated, although efforts by oncologists and
immunologists to develop treatments have never ceased (4,5). Recently,
immunotherapy has received positive results as a cancer treatment,
with clinical successes with blocking antibodies at two immune
checkpoints, cytotoxic T-lymphocyte-associated protein 4 and
programmed cell death protein 1, and with chimeric antigen
receptor-transduced T cells (6).
Cancer immunotherapy has adopted two basic
strategies: Passive transfer of anticancer monoclonal antibodies
and immune-active T cells, without activating endogenous immunity;
and the active stimulation of specific antitumor immunity in
patients with cancer (7). The initial
step in the second strategy is to develop an efficient cancer
vaccine that specifically triggers antitumor T cell responses
(8). Although many tumor-specific
antigens have been identified, when activated alone, the majority
lack a strong immunogenic activity and fail to initiate effective
antitumor immunity (9). The key
limiting factor is the lack of an optimal adjuvant therapy that
could enhance, in terms of the magnitude, quality and duration,
specific immune responses to antigens, while exerting minimal
toxicity or immune effects of its own (7). Although novel adjuvant therapies,
including saponin (10), bacterial
DNA (11), and cytokines (12), have been reported, aluminum salts
(alum) remain the sole adjuvant therapy for the majority of
therapeutic vaccines (13). Alum
effectively induces the antibody-producing type 2 T-helper (Th2)
response, but has no effect on cellular immunity, including the
type 1 Th (Th1) response, and is associated with toxicity from
aluminum accumulation (14).
Therefore, in cancer immunotherapy research there is a great demand
for a non-toxic adjuvant therapy that stimulates Th1 responses.
Successful anticancer immunity requires the
activation of CD8+ and CD4+ T cells (15). CD8+ cytotoxic T cells
directly target and destroy tumor cells expressing major
histocompatibility complex class I molecules. CD4+ T
cells, specifically the Th1 cells, orchestrate multiple cell types
to eradicate tumor cells through the secretion of Th1 cytokines,
including interferon (IFN)-γ and interleukin (IL)-2 (15). Cell types affected by CD4+
T cells include CD8+ T cells, macrophages and natural
killer cells (16); therefore, an
immunoadjuvant therapy that promotes Th1 responses may benefit the
development of antigen-specific antitumor immunity.
Polysaccharides are natural, low-toxicity
macromolecules with various biological functions, including
immune-modulation (17). In the
laboratory, the mechanisms underlying polysaccharides isolated from
fungi and plants and their functions, in regulating immune
responses, were investigated. The previous study demonstrated that
the low-molecular-weight polysaccharides of the fungus Agaricus
blazei Murrill, ABP-AW1, function as a potent
Th1-immunity-stimulating adjuvant therapy in wild-type Institute
for Cancer Research mice (18). In
the present study, the functional significance and underlying
mechanisms of ABP-AW1 in cancer immunity were examined, using
ovalbumin (OVA) as the model tumor antigen.
Materials and methods
Experimental animals and cells
This study was approved by the Institutional Animal
Care and Use Committee of Qiqihar Medical University (Heilongjiang,
China). A total of 50 female C57BL/6 mice (6–8 weeks old; body
weight, 20±2 g) were purchased from Yisi Experimental Animal
Technology Co., Ltd. (Jilin, China). The mice were housed in a
specific pathogen-free facility at 24±1°C and 50±10% relative
humidity under a 12/12 h light/dark cycle, with food and water
provided ad libitum.
The E.G7-OVA T-lymphoma cell line, stably expressing
full-length OVA, was provided by Xuetao Cao (Second Military
Medical University, Shanghai, China). The E.G7-OVA cells were
cultured in RPMI-1640 medium containing 10% fetal calf serum (FCS;
both from HyClone; GE Healthcare Life Sciences, Logan, UT, USA) in
a 37°C incubator with 5% CO2.
Extraction and isolation of
ABP-AW1
The ABP-AW1 polysaccharide was prepared and
characterized as previously described (19). Briefly, Agaricus blazei were
treated under reflux 3 times with 95% ethanol at 75°C for 6 h to
remove lipids. The residue was extracted 3 times with distilled
water at 75°C for 3 h. The precipitate was washed, dried and
extracted twice with 0.5 M NaOH solution containing 0.3% (w/w)
NaBH4 at room temperature overnight. After the debris
was removed through filtering, the suspension was neutralized with
0.1 M hydrochloric acid and filtered again. The supernatant
containing water-soluble polysaccharide was dialyzed, concentrated,
ethanol-precipitated and dried to generate crude polysaccharide
(CABP-AW).
The CABP-AW was re-dissolved in distilled water and
centrifuged at 4,000 × g and 4°C for 10 min. The supernatant was
applied to a diethylaminoethanol Sepharose Fast Flow column
(Amersham Biosciences; GE Healthcare Life Sciences, Pittsburgh, PA,
USA) that had been equilibrated with ultrapure water. After loading
the sample, the column was eluted stepwise with NaCl aqueous
solution (0, 0.2, 0.4 and 0.6 M) at a flow rate of 4 ml/min. The
fractions (8 ml) were collected using a Frac-950 (GE Healthcare
Sciences). The polysaccharide was further purified by
gel-permeation chromatography on a Sepharose 6 Fast Flow column
(2.6×100 cm; GE Healthcare Life Sciences) eluted with 0.15 M NaCl
at a flow rate of 1 ml/min.
Three polysaccharide fractions, ABP-AW1, ABP-AWA1
and ABP-AWB1, were obtained. The eluted ABP-AW1 was applied to a
Sephadex® G-25 column (2.6×40 cm; GE Healthcare Life
Sciences) to remove any salts. Subsequently, ABP-AW1 was collected,
dialyzed and lyophilized to obtain purified polysaccharide for use
in subsequent experiments.
Analysis by gas chromatography-mass spectrometry
revealed that the isolated ABP-AW1 had a backbone consisting of
(1→6)-linked-β-d-galactopyranosyl, (1→6)-linked-β-d-glucopyranosyl
and (1→3, 6)-linked-β-d-glucopyranosyl. The backbone terminated
with (1→)-linked fucose, arabinose and mannose residues at the O-3
position of (1→3, 6)-linked-β-d-glucopyranosyl. These six types of
residues were present in the ratio of 29:10:10:6:2:2 (19).
Establishment of xenograft tumors and
antitumor immunization
To establish xenograft tumors, E.G7-OVA cells in the
log phase of growth were washed once with serum-free RPMI-1640
medium and resuspended in 10 ml fresh serum-free RPMI-1640 medium
at 5×106 cells/ml. A total of 5×105 cells
were then injected subcutaneously into the right lateral thighs of
the mice.
After inoculation with the E.G7-OVA cells, all mice
were randomly divided into 5 groups of 10 mice each. On days 3, 10
and 17 post-inoculation, the mice received a subcutaneous injection
of 100 µl PBS (for PBS group), 100 µl PBS containing 100 µg OVA
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; for OVA-alone
group), or 100 µg OVA together with 50, 100 or 200 µg ABP-AW1 (for
low-, intermediate-, or high-dose OVA and ABP-AW1 groups,
respectively).
From day 7 after tumor cell inoculation, tumor
growth in all groups was monitored every two days by measuring the
length (L) and width (W) of the growing nodule, and the volume (V)
was calculated as V = (L × W2)/2.
On day 28 after tumor inoculation, all mice were
euthanized and no mice presented signs of distress, cachexia or
ulceration on the tumors. The tumor was isolated and weighed, and
other tissues including the spleen and the peripheral blood were
collected for further experiments.
MTT assay of splenocyte
viability/proliferation
The whole mouse spleen was isolated and dissociated
into single cells by grinding against a 70-µm nylon mesh. The
dissociated cells were resuspended in 3 ml serum-free RPMI-1640
medium and centrifuged at 800 × g and 4°C for 30 min in order to
remove erythrocytes and cellular debris. The splenocytes were
washed in serum-free RPMI-1640 medium. They were then resuspended
in RPMI-1640 with 10% FCS, and seeded into 96-well plates (Corning
Inc., Corning, NY, USA) in triplicate, at 5×105
cells/100 µl/well, and incubated at 37°C in a humidified 5%
CO2 incubator, together with concanavalin A (ConA; 5
µg/ml; Sigma-Aldrich; Merck KGaA) or OVA (10 µg/ml).
After 60 h, 20 µl MTT agent (5 mg/ml) was added into
each well and incubated at 37°C for a further 4 h. After
centrifugation at 250 × g and room temperature for 5 min, the
supernatant was discarded from each well. Dimethylsulfoxide (150
µl/well) was added into each well to dissolve the formazan
crystals. The absorbance was measured using a microplate reader at
570 nm with a 630 nm reference. The stimulation index (SI) was
calculated based on the following formula: SI = absorbance value
for treated cultures/absorbance value for non-treated cultures.
Measurement of peripheral blood
CD4+ and CD8α+ T cells
The peripheral blood CD4+ and
CD8+ T cells were measured viaflow cytometry, as
previously described (20). Briefly,
peripheral blood from the retro-orbital sinus was collected into
anti-coagulant tubes. After the lysis of erythrocytes with red
blood cell lysis buffer (BD Pharmingen; BD Biosciences, San Jose,
CA, USA), the remaining peripheral blood cells were stained with 20
µl anti-mouse CD4-fluorescein isothiocyanate antibody (undiluted;
cat. no. 553032; BD Pharmingen; BD Biosciences) and 20 µl CD8
α-phycoerythrin antibody (undiluted; cat. no. 557307; BD
Pharmingen; BD Biosciences) diluted in fluorescence-activated cell
sorting (FACS) buffer (0.5% bovine serum albumin in PBS) for 30 min
at room temperature. The cells were then washed with FACS buffer,
resuspended in 400 µl PBS and analyzed usinga BD
FACSCalibur™ flow cytometer (BD Biosciences).
Measurement of OVA-specific IgG1 and
IgG2b in serum
The serum levels of OVA-specific IgG1 and IgG2b were
measured using an in-house indirect ELISA as previously described
(21). In brief, peripheral blood was
collected from the retro-orbital sinus and stored at room
temperature for 2 h. Following blood coagulation, the sample was
centrifuged at 800 × g and 4°C for 15 min, and the serum
supernatant was transferred into a clean Eppendorf tube and stored
at −80°C for future use.
For ELISA analysis, 96-well microtiter plates were
coated with 100 µl OVA solution (50 µg/ml in 50 mM
carbonate-bicarbonate buffer; pH 9.6) overnight at 4°C. After 3
washes in PBS with 0.05% Tween (PBST), the plates were blocked with
200 µl of 5% FCS/PBS at 37°C for 2 h. After 3 washes with PBST, 100
µl each diluted serum sample (1:500 dilution) was added into the
wells, in triplicate, and incubated for 1 h at 37°C. Following
another 3 washes with PBST, IgG1 and IgG2b (contained in the serum
sample) were detected using horseradish peroxidase-conjugated goat
anti-mouse IgG1 (1:5,000 dilution, cat. no. 1070–05) or IgG2b
(1:5,000 dilution, cat no. 1090-05) (both from Southern Biotech,
Birmingham, AL, USA) at 37°C for 1 h. The unbound antibodies were
removed by washing 6 times with PBST.
The 3,3′,5,5′-tetramethylbenzidine substrate (cat.
no. T2885; Sigma-Aldrich; Merck KGaA) was added and incubated for
10 min at 37°C in the dark, following which the reaction was
stopped by adding 2M H2SO4 (50 µl/well). The
optical density was measured at 450 nm using a microplate
reader.
Evaluation of the cytokines present
within cultured splenocyte supernatants
The splenocytes were seeded into 96-well tissue
culture plates at 1×106 cells/100 µl/well in triplicate.
Cells were treated with OVA (final concentration, 10 µg/ml) at 37°C
in a humidified 5% CO2 incubator. After 48 h, the plates
were centrifuged at 250 × g and 4°C for 10 min and the supernatant
was collected for the measurement of secreted IFN-γ or IL-2 using
mouse ELISA kits (DKW12-2000-096 for IFN-g ELISA kit and
DKW12-2020-096 for IL-2 ELISA kit; Dakewe Biotech Co., Ltd.,
Shenzhen, Guangdong, China), in accordance with the manufacturer's
instructions. To measure the production of IFN-γ from cultured
splenocytes on the single-cell level, a mouse IFN-γ ELISPOT kit
(DKW22-2000-096s; Dakewe Biotech Co., Ltd.) was used as instructed
by the manufacturer.
Statistical analysis
Quantitative data are expressed as the mean ±
standard deviation. Multiple comparisons were performed using ANOVA
with Student-Newman-Keuls test as a post hoc test in order to
analyze the differences among the groups using SPSS version 17.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
OVA-induced tumor growth
To examine the in vivo efficacy of ABP-AW1 as
an adjuvant therapy to boost the tumor response to a cancer
vaccine, OVA was used as the tumor antigen and a subcutaneous
xenograft mouse model was established, using OVA-expressing E.G7
T-lymphoma cells.
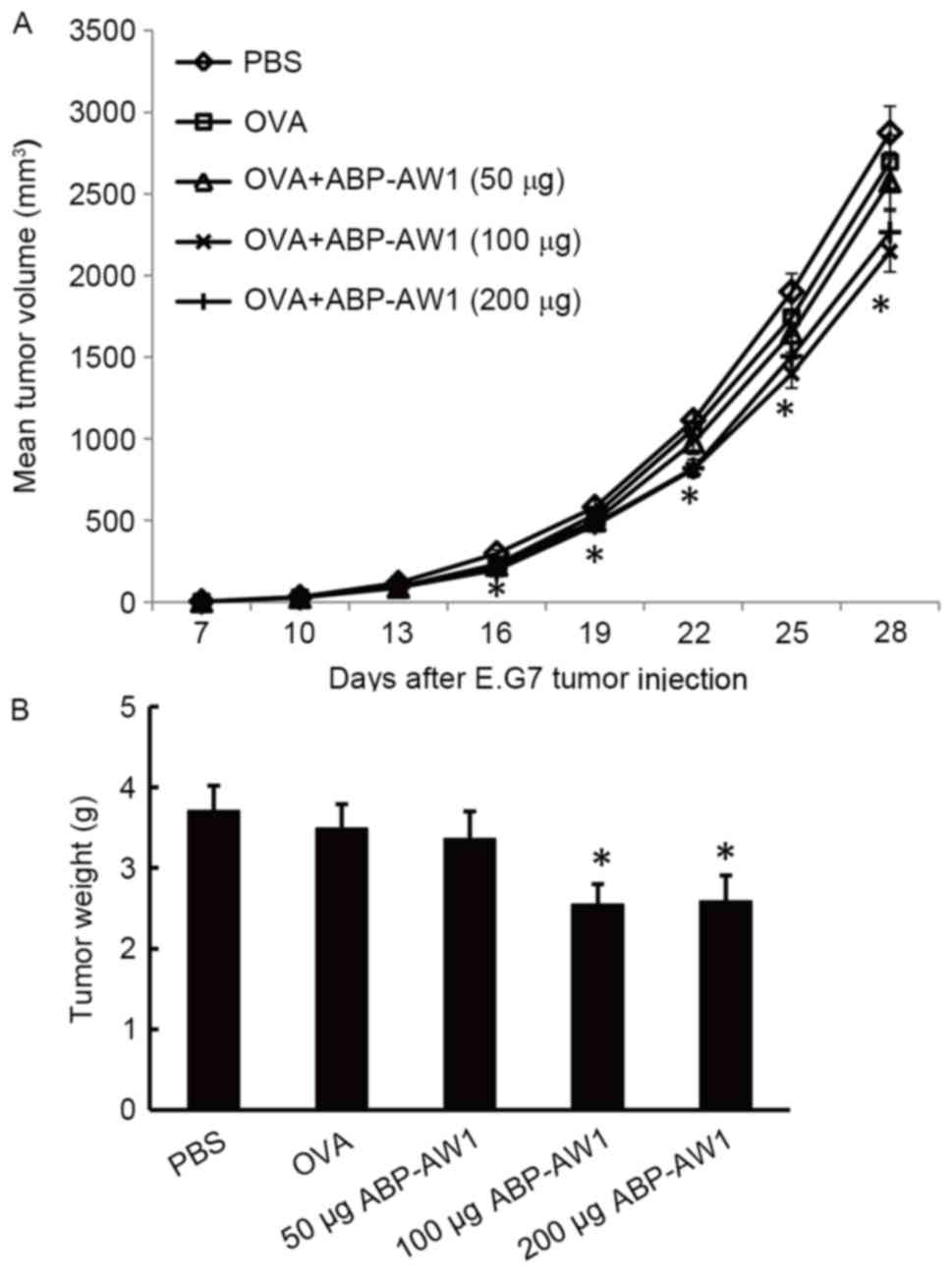
By monitoring tumor growth after immunization with
PBS (control), OVA alone or OVA with various doses of ABP-AW1, it
was determined that the effects of OVA by itself, or together with
low-dose ABP-AW1 (50 µg), were similar to those of the PBS control
group (P>0.05; Fig. 1A). However,
OVA combined with intermediate-(100 µg) or high-dose (200 µg)
ABP-AW1 was associated with significantly smaller tumor volumes,
relative to the control, starting from day 16 after tumor cell
inoculation (P<0.05; Fig. 1A).
Consistently, by day 28, the tumor weights of the OVA with
intermediate- or high-dose ABP-AW1 (100 or 200 µg) groups were
significantly lower than those of the other three groups [PBS, OVA
alone, or OVA with low-dose ABP-AW1 (50 µg) group; P<0.05]. The
tumor weights of the PBS, OVA-alone, and OVA with low-dose ABP-AW1
(50 µg) groups were comparable with each other by day 28 following
the inoculation of lymphoma cells (P>0.05; Fig. 1B).
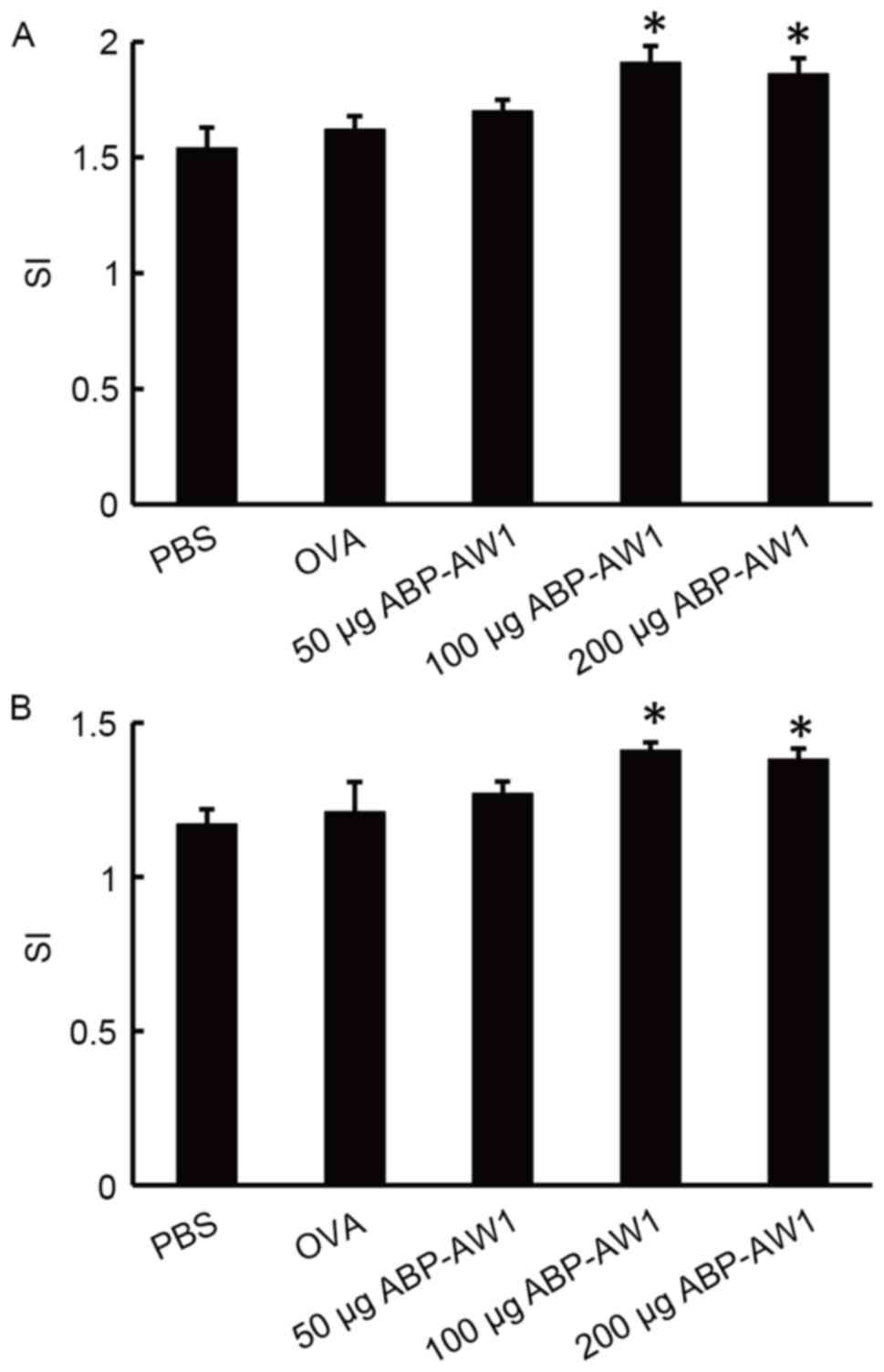
Splenocyte
viability/proliferation
To investigate the effect of ABP-AW1 on the overall
immune activity, the viability/proliferation of splenocytes
isolated from the different groups of mice in response to a
non-specific lymphocyte mitogen, ConA, or the specific tumor
antigen, OVA was examined (Fig. 2).
ConA and OVA treatments resulted in a greater number of splenocytes
in mice of the OVA and ABP-AW1 (100 and 200 µg) groups, compared
with the mice of the PBS, OVA-alone, or OVA and ABP-AW1 (50 µg)
groups (P<0.05); however, no significant differences were
detected among the latter three groups (P>0.05).
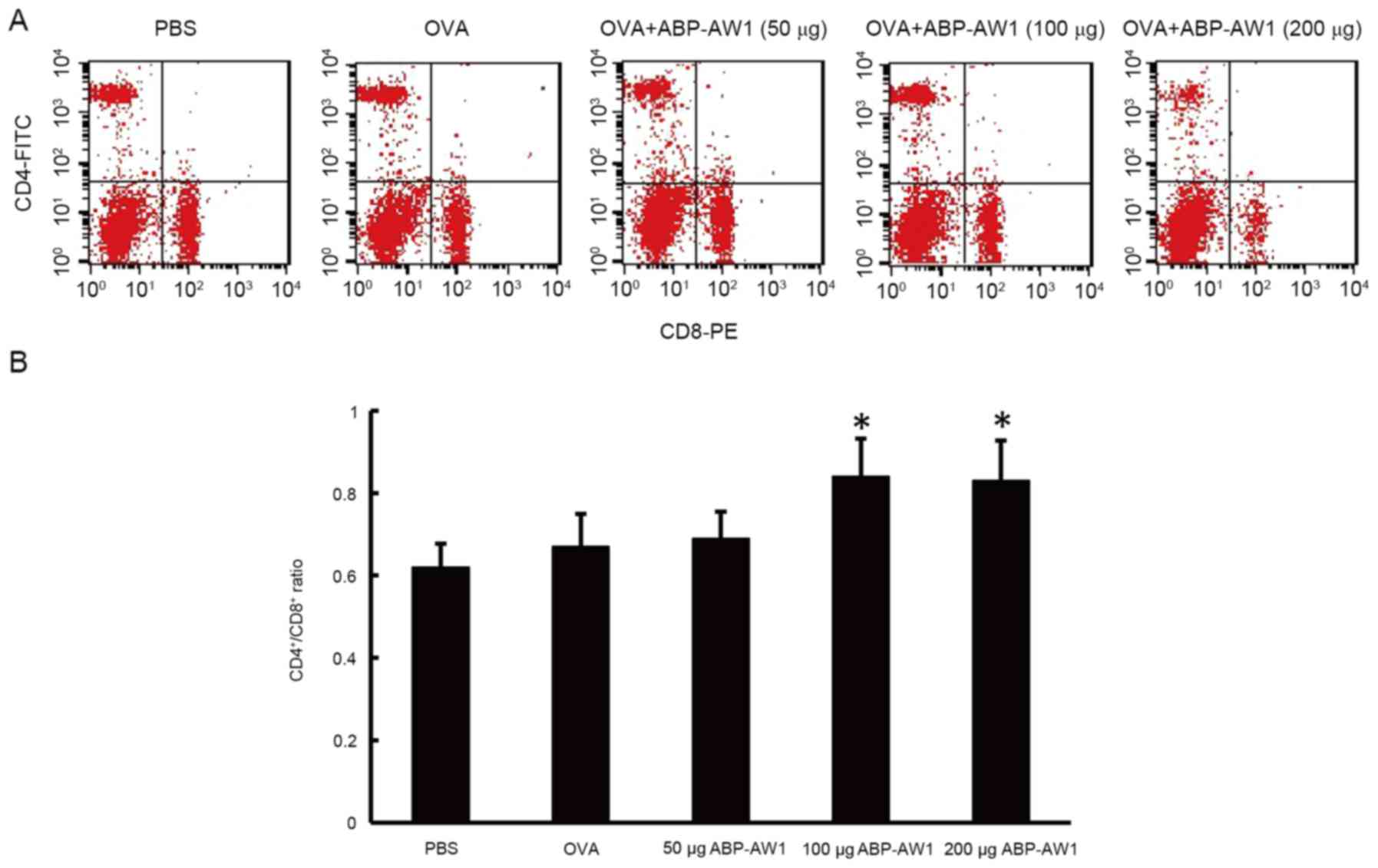
CD4+/CD8+ T-cell
ratio in peripheral blood
The ratio of CD4+ and CD8+ T
cells is closely associated with host immune activity. Through flow
cytometry analysis, it was determined that the peripheral blood
CD4+/CD8+ ratio of the OVA-alone and OVA and
ABP-AW1 (50 µg) groups was not significantly different from that of
the PBS group (P>0.05, Fig. 3);
however, the CD4+/CD8+ ratios of the OVA and
ABP-AW1 (100 and 200 µg) mice were significantly higher than those
of the other three groups (P<0.05). This suggests that
intermediate- or high-dose ABP-AW1 notably enhanced the anticancer
immune activity of OVA.
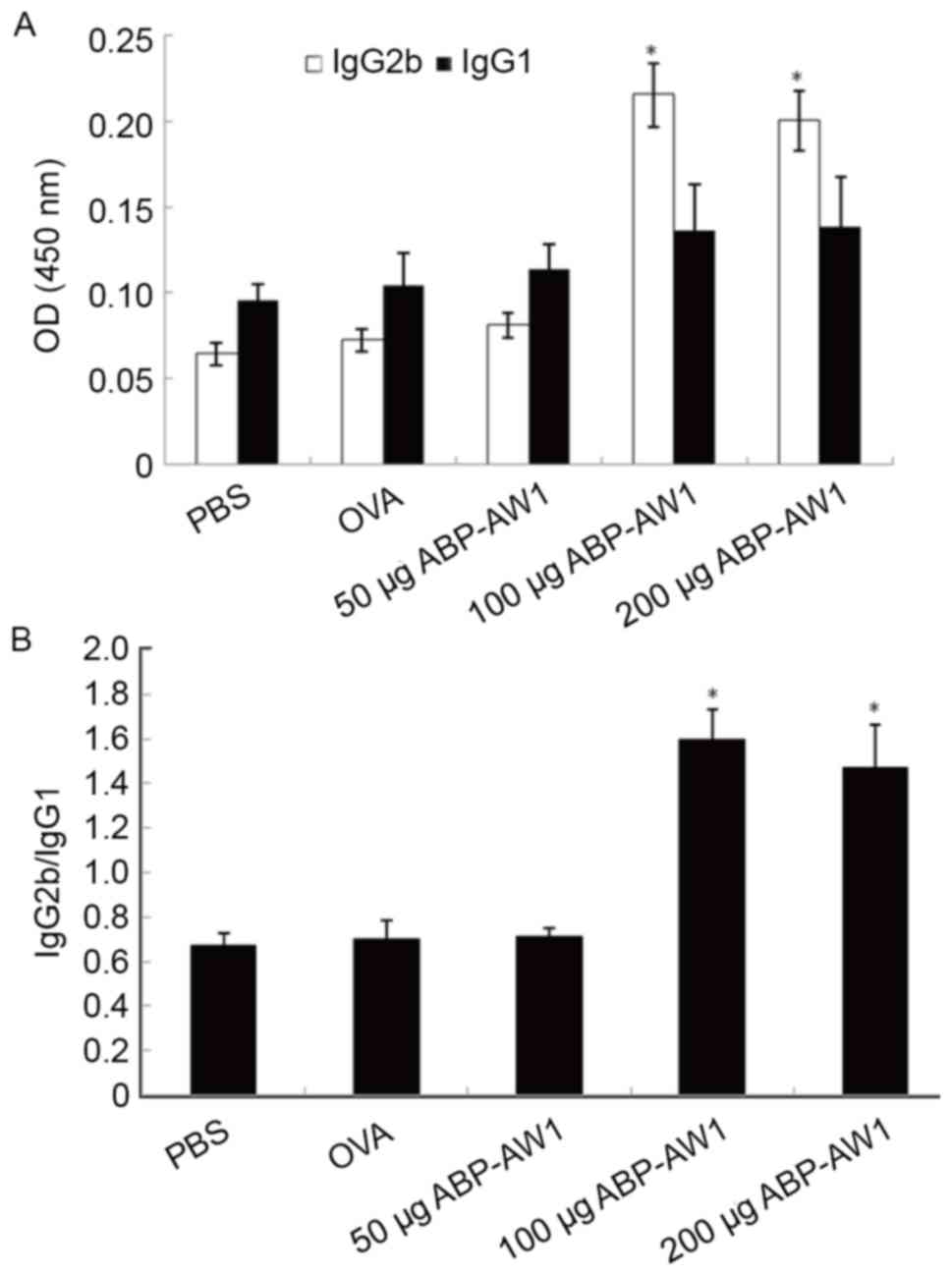
Serum levels of OVA-specific IgG2b and
IgG1 antibody
Among the serum antibody IgG subtypes, the IgG1 is a
marker dominated by Th2-type immune responses, whereas IgG2b is
associated with Th1-type immunity (22,23). To
analyze which T cell response is preferentially boosted by ABP-AW1,
the serum levels of OVA-specific IgG1 and IgG2b were measured using
an ELISA (Fig. 4A). Between all five
groups, OVA-specific IgG1 level was not significantly different
(P>0.05; Fig. 4A). The serum IgG2b
level, however, was lower than the IgG1 level in the PBS, OVA alone
or OVA with low-dose ABP-AW1 (50 µg) group, resulting in an
IgG2b/IgG1 ratio of <1 (Fig. 4B).
In contrast, the OVA-specific IgG2b level in the OVA with
intermediate or high-dose ABP-AW1 (100 or 200 µg) group was
significantly increased, and thus there was a notably higher
IgG2b/IgG1 ratio (>1) compared with the other three groups [the
PBS, OVA alone, or OVA with low-dose ABP-AW1 (50 µg) group;
P<0.05]. This indicated that intermediate- and high-dose ABP-AW1
is sufficient to preferentially induce a Th1 response over a Th2
response.
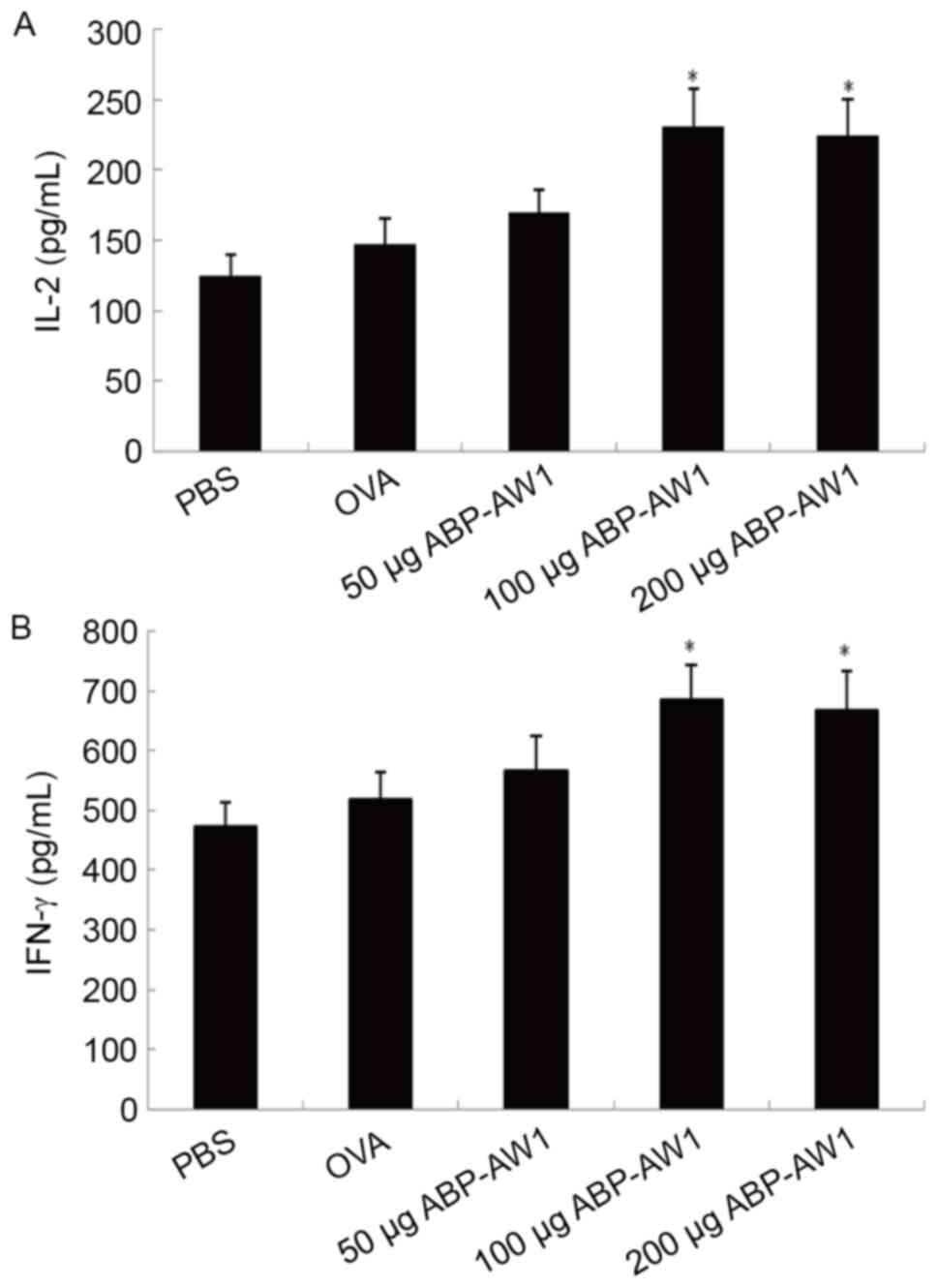
Cytokine production by
splenocytes
To further characterize the Th1 responses augmented
by ABP-AW1, ELISAs were performed to evaluate the cytokines
secreted by cultured splenocytes in response to the specific
antigen OVA (Fig. 5). In vivo
immunization with OVA and ABP-AW1 (100 or 200 µg) was associated
with significantly higher levels of the Th1 cytokines IL-2
(Fig. 5A) and IFN-γ compared with
those of the other three groups (Fig.
5B; P<0.05). The levels of these cytokines in the OVA and
ABP-AW1 (50 µg) groups were not significantly higher than those in
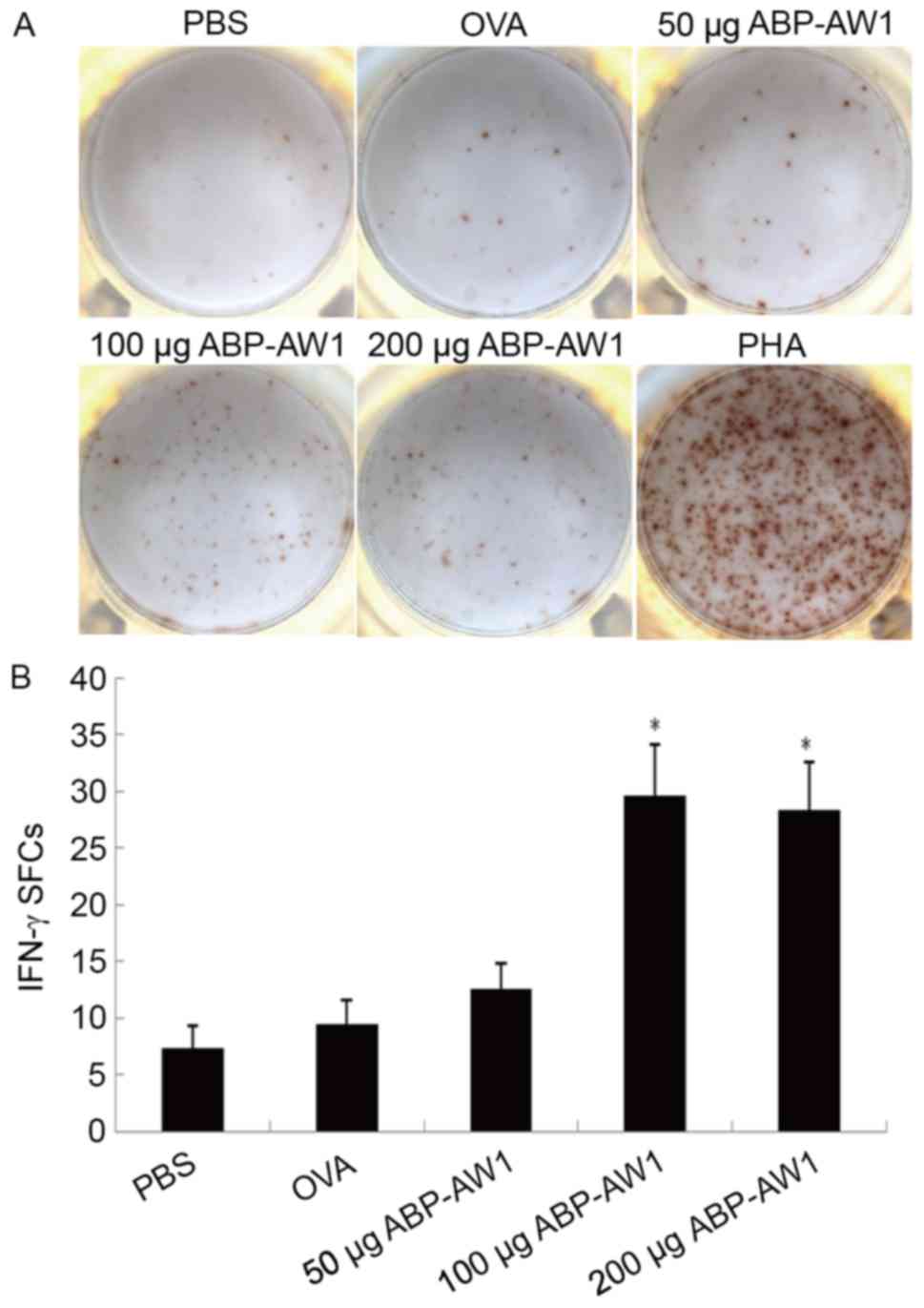
the PBS or OVA-alone group (P>0.05). The IFN-γ-producing
splenocytes were quantified by ELISPOT analysis, which consistently
indicated a significantly higher number of IFN-γ-positive
splenocytes in the OVA and ABP-AW1 (100 and 200 µg) groups,
compared with in the other three groups (P<0.05; Fig. 6); therefore, the data indicate that
intermediate-andhigh-dose ABP-AW1 enhances Th1 polarization in the
context of cancer development.
Discussion
The present study evaluated the in vivo
efficacy of ABP-AW1, isolated from Agaricus blazei, as an
immunoadjuvant therapy to boost the anticancer activity of a cancer
vaccine, using OVA as the antigen in a mouse model of T-cell
lymphoma. The model was created via inoculation with E.G7-OVA
cells, and the mice were subsequently treated with PBS, OVA-alone,
or OVA together with ABP-AW1 (OVA and ABP-AW1) at low (50 µg),
intermediate (100 µg) or high doses (200 µg). By all measures
(tumor growth, splenocyte viability/proliferation, peripheral blood
CD4+/CD8+ T-lymphocytes, serum OVA-specific
IgG2b, splenocyte secretions of IL-2 and IFN-γ), it was observed
that ABP-AW1 at intermediate and high doses was an efficacious
immunoadjuvant therapy, relative to the other treatment groups.
The development of cancer has generally been
associated with six biological traits: Self-sufficiency in growth
signals, insensitivity to anti-growth signals, evading apoptosis,
limitless replicative potential, sustained angiogenesis, and tissue
invasion and metastasis (24). In the
last decade, two hallmarks were added to the list: Reprogramming
energy metabolism; and evading immune destruction (25). The ability of a tumor to suppress the
immune system responses and evade immune destruction supports the
importance of cancer immunotherapy. The identification of
tumor-associated antigens and of epitopes specifically recognized
by distinct T cell subtypes has greatly stimulated the development
of cancer vaccines. However, tumor-associated antigens or epitopes
are predominantly antigenic and not immunogenic, with minimal
induction of anticancer immunity (26). This justifies the incorporation of an
immunoadjuvant therapy as a key component in cancer vaccines
(27).
The host's immune system utilizes multiple
mechanisms to attempt to control or eradicate cancer, mainly
through T-lymphocytes, natural killer cells and macrophages. By
contrast, humoral immunity involving antibody production is not a
key factor in anticancer immunity, and may paradoxically promote
tumor growth. Among the distinct T cell subtypes, the predominant
types with antitumor activities are CD8+ cytotoxic
T-lymphocytes and CD4+ T helper (Th) cells (15). Activated CT8+ cytotoxic
T-lymphocytes mainly serve as immune effectors to induce tumor cell
apoptosis, while Th cells, through the production of cytokines,
regulate the activation of cytotoxic T-lymphocytes and recruit
cells of the innate immune system, including macrophages and mast
cells (28). CD4+ Th cells
consist of two predominant subtypes: Th1 cells characterized by the
production of IL-2, IFN-γ, tumor necrosis factor and Th2 cells
characterized by the production of IL-4, IL-5, IL6 and IL-10
(29). Functionally, Th1 cells
stimulate cellular immunity, target intracellular pathogens,
activate phagocytosis, induce IgG2a, IgG2b and IgG3 antibodies and
promote delayed-type hypersensitivity responses (30). By contrast, Th2 cells mainly promote
humoral responses, which particularly involve IgG1, IgE and IgA
(31).
In the paradigm of cancer, myriad studies suggest
that Th1-dominant responses are a reflection of anticancer
immunity, whereas Th2-dominant responses favor the progression of
cancer (32,33). In a mouse model of B cell
leukemia/lymphoma, susceptible mice developed a Th2-dominant
response, while those developing a Th1-dominant response were more
resistant to the disease (34).
Similar observations were also noted in mouse models of melanoma,
renal cell carcinoma and colon cancer (35,36).
Consistent with the mouse models, Th2-predominant phenotypes are
also observed in patients with lung cancer, breast cancer, gastric
cancer, glioblastoma, renal cell carcinoma, prostate cancer and
other types of cancer (32).
Collectively, these studies indicate that tilting the balance
between the Th1 and Th2 responses towards the former stimulates
anticancer immunity, due to the latter promoting the development
and progression of cancer; therefore, an ideal immunoadjuvant
therapy for cancer vaccine should stimulate the Th1 response, in
addition to being non-immunogenic, offering excellent stability,
safety, and tolerability, and being cost-effective to produce
(14).
Polysaccharides are natural, have low toxicity and
are biodegradable (37). They have
long been appreciated for their immunomodulating activities and are
under intensive investigation for their potential as immunoadjuvant
therapies (37). A recent example is
the adjuvant therapy Advax™, a natural plant-derived
polysaccharide that has broad-spectrum boosting effects on B cell,
CD4 and CD8 T cell responses, and demonstrated initial success when
applied with an influenza (38) or
hepatitis B vaccine (39).
In the laboratory, the in vivo immune
activity of ABP-AW1 was examined, using OVA as a model antigen.
ABP-AW1 was determined to potently induce Th1 responses, as
identified by the significantly higher production and secretion of
IFN-γ from the splenocytes of immunized mice (18). This observation positions ABP-AW1 as
an ideal candidate adjuvant therapy for cancer vaccines, and
prompted testing of its in vivo efficacy on cancer
development. For this purpose, OVA was used as the model antigen
and a xenograft tumor model was established via subcutaneous
injection of the mouse lymphoma cell line E.G7, which stably
expresses OVA. By immunizing the mice with PBS, OVA alone or OVA
together with various doses of ABP-AW1, the in vivo efficacy
of ABP-AW1 was assessed as an immunoadjuvant therapy. The data
demonstrated that OVA by itself was not sufficient to affect tumor
growth over time. This is consistent with previous findings that
the tumor is antigenic, but not immunogenic (40). Similar results were also observed for
low-dose (50 µg) ABP-AW1 in the present study; however, at
intermediate (100 µg) and high (200 µg) doses, ABP-AW1 was
associated with markedly less tumor growth over time and a
reduction in the tumor size or weight. This indicated that ABP-AW1
is capable of boosting the anticancer activity of cancer
vaccines.
To understand the immune-modulatory mechanisms
underlying ABP-AW1, the overall immune activities in mice were
assessed. Lymphocytes are key cellular components of the immune
system, and their proliferation in response to lymphocyte mitogen
or specific antigen is an important measure for host immune
activity (41). In the present study,
splenocytes were isolated from mice bearing xenograft tumors that
had been treated with PBS, OVA alone, or OVA with different doses
of ABP-AW1, and the isolated splenocytes were treated in
vitro with either ConA (a lymphocyte mitogen) or OVA (a
specific tumor antigen). Splenocytes from mice treated with OVA
with intermediate- or high-dose ABP-AW1 grew to a much higher
number when compared with those from mice treated with PBS, OVA
alone, or OVA plus low-dose ABP-AW1. This suggests that
co-immunization of the mouse model of lymphoma with OVA and the
higher doses of ABP-AW1 not only promotes non-specific lymphocyte
proliferation in response to ConA, but also stimulates
antigen-specific lymphocyte viability/proliferation.
The second parameter used to measure host immune
activity following treatment with OVA and ABP-AW1 was the
peripheral CD4+/CD8+ T cell ratio, a marker
for immune system status, in which a high
CD4+/CD8+ ratio indicates strong immune
activity, and a low ratio suggests immune suppression (42). It was determined that OVA combined
with intermediate- or high-dose ABP-AW1 was associated with a
notably higher peripheral blood CD4+/CD8+
ratio compared with the other groups, also indicating a more active
host immune status.
Given that Th1 responses have a crucial role in
anticancer immunity, and ABP-AW1 significantly boosts the
anticancer activity of a specific tumor antigen, the status of Th1
responses in mice treated with OVA and ABP-AW1 were followed.
Specifically, the serum levels of an OVA-specific Th1 antibody
(IgG2b) and a Th2 antibody (IgG1) were measured, as well as the
production and secretion of Th1 cytokines from splenocytes in
response to the specific antigen OVA. In the groups treated with
PBS, OVA alone, or OVA and low-dose ABP-AW1, the serum IgG1 levels
were higher than the IgG2b levels; that is, the IgG2b/IgG1 ratio
was <1, indicating that Th2 was predominant in these mice. In
groups treated with OVA and intermediate- or high-dose ABP-AW1, the
serum IgG2b levels were markedly higher compared with in the other
groups, although the serum IgG1 levels were not significantly
affected, resulting in IgG2b/IgG1 ratios of >1. These data
indicate that ABP-AW1 at higher doses boosts Th1 responses, with
minimal effect on Th2 responses, and thus shifts the balance from a
Th2-predominant phenotype, as observed during cancer development,
to a Th1-predominant phenotype, to stimulate anticancer immunity
toward the tumor-specific antigen. Consistent with the significant
promotion of Th1 responses, significantly higher production of the
Th1 cytokine IFN-γ, and higher levels of IFN-γ or IL-2 derived from
splenocytes treated with OVA and intermediate- or high-dose ABP-AW1
after in vitro stimulation with OVA were detected, compared
with in the other groups.
In conclusion, the evidence demonstrated that
ABP-AW1, when incorporated with the tumor-specific antigen OVA into
mice bearing OVA-expressing tumors, significantly boosted host
immune activity, with specific stimulation of Th1 responses, and
notably inhibited tumor growth. It was concluded that ABP-AW1 is a
promising adjuvant therapy for cancer immunotherapy.
Acknowledgements
This study was supported by Qiqihar Municipal Bureau
of Science and Technology (grant. no. SFGG-201551) and the Natural
Science Foundation of China (grant no. 81173609).
References
|
1
|
Arruebo M, Vilaboa N, Sáez-Gutierrez B,
Lambea J, Tres A, Valladares M and González-Fernández A: Assessment
of the evolution of cancer treatment therapies. Cancers.
3:3279–3330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farkona S, Diamandis EP and Blasutig IM:
Cancer immunotherapy: The beginning of the end of cancer? BMC Med.
14:732016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coley WB: The treatment of malignant
tumors by repeated inoculations of erysipelas. With a report of ten
original cases. 1893. Clin Orthop Relat Res. 3–11. 1991.PubMed/NCBI
|
|
4
|
Parish CR: Cancer immunotherapy: The past,
the present and the future. Immunol Cell Biol. 81:106–113. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wiemann B and Starnes CO: Coley's toxins,
tumor necrosis factor and cancer research: A historical
perspective. Pharmacol Ther. 64:529–564. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Drake CG, Lipson EJ and Brahmer JR:
Breathing new life into immunotherapy: Review of melanoma, lung and
kidney cancer. Nat Rev Clin Oncol. 11:24–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo C, Manjili MH, Subjeck JR, Sarkar D,
Fisher PB and Wang XY: Therapeutic cancer vaccines: Past, present,
and future. Adv Cancer Res. 119:421–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buonaguro L, Petrizzo A, Tornesello ML and
Buonaguro FM: Translating tumor antigens into cancer vaccines. Clin
Vaccine Immunol. 18:23–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rajput ZI, Hu SH, Xiao CW and Arijo AG:
Adjuvant effects of saponins on animal immune responses. J Zhejiang
Univ Sci B. 8:153–161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jones DE, Palmer JM, Burt AD, Walker C,
Robe AJ and Kirby JA: Bacterial motif DNA as an adjuvant for the
breakdown of immune self-tolerance to pyruvate dehydrogenase
complex. Hepatology. 36:679–686. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tovey MG and Lallemand C: Adjuvant
activity of cytokines. Methods Mol Biol. 626:287–309. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tefit JN and Serra V: Outlining novel
cellular adjuvant products for therapeutic vaccines against cancer.
Expert Rev Vaccines. 10:1207–1220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petrovsky N and Aguilar JC: Vaccine
adjuvants: Current state and future trends. Immunol Cell Biol.
82:488–496. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schietinger A, Philip M, Liu RB, Schreiber
K and Schreiber H: Bystander killing of cancer requires the
cooperation of CD4(+) and CD8(+) T cells during the effector phase.
J Exp Med. 207:2469–2477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HJ and Cantor H: CD4 T-cell subsets
and tumor immunity: The helpful and the not-so-helpful. Cancer
Immunol Res. 2:91–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramberg JE, Nelson ED and Sinnott RA:
Immunomodulatory dietary polysaccharides: A systematic review of
the literature. Nutr J. 9:542010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui L, Sun Y, Xu H, Cong H and Liu J: A
polysaccharide isolated from Agaricus blazei Murill (ABP-AW1) as a
potential Th1 immunity-stimulating adjuvant. Oncol Lett.
6:1039–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J and Sun Y: Structural analysis of an
alkali-extractable and water-soluble polysaccharide (ABP-AW1) from
the fruiting bodies of Agaricus blazei Murill. Carbohydrate
Polymers. 86:429–432. 2011. View Article : Google Scholar
|
|
20
|
Hartmann G, Marschner A, Viveros PR,
Stahl-Hennig C, Eisenblatter M, Suh YS, Endres S, Tenner-Racz K,
Uberla K, Racz P, et al: CpG oligonucleotides induce strong humoral
but only weak CD4+ T cell responses to protein antigens
in rhesus macaques in vivo. Vaccine. 23:3310–3317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sjölander A, van't Land B and Bengtsson
Lövgren K: Iscoms containing purified Quillaja saponins upregulate
both Th1-like and Th2-like immune responses. Cell Immunol.
177:69–76. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawase O, Goo YK, Jujo H, Nishikawa Y and
Xuan X: Starfish, Asterias amurensis and Asterina pectinifera, as
potential sources of Th1 immunity-stimulating adjuvants. J Vet Med
Sci. 73:227–229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mountford AP, Fisher A and Wilson RA: The
profile of IgG1 and IgG2a antibody responses in mice exposed to
Schistosoma mansoni. Parasite Immunol. 16:521–527. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Escors D: Tumour immunogenicity, antigen
presentation and immunological barriers in cancer immunotherapy.
New J Sci. 2014:7345152014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dubensky TW Jr and Reed SG: Adjuvants for
cancer vaccines. Semin Immunol. 22:155–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Knutson KL and Disis ML: Tumor
antigen-specific T helper cells in cancer immunity and
immunotherapy. Cancer Immunol Immunother. 54:721–728. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Romagnani S: T-cell subsets (Th1 versus
Th2). Ann Allergy Asthma Immunol. 85:9–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X and Meng D: Innate endogenous
adjuvants prime to desirable immune responses via mucosal routes.
Protein Cell. 6:170–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Liu J, Yu H and Gong C: Isolation
and evaluation of immunological adjuvant activities of saponins
from the roots of Pulsatilla chinensis with less adverse
reactions. Int Immunopharmacol. 10:584–590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shurin MR, Lu L, Kalinski P, Stewart-Akers
AM and Lotze MT: Th1/Th2 balance in cancer, transplantation and
pregnancy. Springer Semin Immunopathol. 21:339–359. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zamarron BF and Chen W: Dual roles of
immune cells and their factors in cancer development and
progression. Int J Biol Sci. 7:651–658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee PP, Zeng D, McCaulay AE, Chen YF,
Geiler C, Umetsu DT and Chao NJ: T helper 2-dominant antilymphoma
immune response is associated with fatal outcome. Blood.
90:1611–1617. 1997.PubMed/NCBI
|
|
35
|
Hu HM, Urba WJ and Fox BA: Gene-modified
tumor vaccine with therapeutic potential shifts tumor-specific T
cell response from a type 2 to a type 1 cytokine profile. J
Immunol. 161:3033–3041. 1998.PubMed/NCBI
|
|
36
|
Ghosh P, Komschlies KL, Cippitelli M,
Longo DL, Subleski J, Ye J, Sica A, Young HA, Wiltrout RH and Ochoa
AC: Gradual loss of T-helper 1 populations in spleen of mice during
progressive tumor growth. J Natl Cancer Inst. 87:1478–1483. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tzianabos AO: Polysaccharide
immunomodulators as therapeutic agents: Structural aspects and
biologic function. Clin Microbiol Rev. 13:523–533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Honda-Okubo Y, Saade F and Petrovsky N:
Advax™, a polysaccharide adjuvant derived from delta
inulin, provides improved influenza vaccine protection through
broad-based enhancement of adaptive immune responses. Vaccine.
30:5373–5381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Saade F, Honda-Okubo Y, Trec S and
Petrovsky N: A novel hepatitis B vaccine containing
Advax™, a polysaccharide adjuvant derived from delta
inulin, induces robust humoral and cellular immunity with minimal
reactogenicity in preclinical testing. Vaccine. 31:1999–2007. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Blankenstein T, Coulie PG, Gilboa E and
Jaffee EM: The determinants of tumour immunogenicity. Nat Rev
Cancer. 12:307–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chaplin DD: Overview of the immune
response. J Allergy Clin Immunol. 125 Suppl 2:S3–S23. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Serrano-Villar S, Sainz T, Lee SA, Hunt
PW, Sinclair E, Shacklett BL, Ferre AL, Hayes TL, Somsouk M, Hsue
PY, et al: HIV-infected individuals with low CD4/CD8 ratio despite
effective antiretroviral therapy exhibit altered T cell subsets,
heightened CD8+ T cell activation, and increased risk of
non-AIDS morbidity and mortality. PLoS Pathog. 10:e10040782014.
View Article : Google Scholar : PubMed/NCBI
|