Introduction
Adherens junctions and tight junctions are major
components involved in the maintenance of epithelial cell stability
(1–3).
Adherens junctions are composed of epithelial (E-)cadherin and
p120-ctn, α, β and γ-catenin, which are its associated proteins
(4–6).
Tight junctions involve claudin, occludin and additional
components, including the peripheral protein zonula occludens-1
(ZO-1) (7,8). Decreased expression of adherens junction
and tight junction proteins is one of the leading causes of tumor
invasion and metastasis.
Zinc finger protein 668 (ZNF668) belongs to the
kruppel C2H2-type zinc finger protein family and contains 16
C2H2-type zinc fingers. ZNF668 has been characterized as a breast
tumor suppressor gene that regulates p53 stability by binding to
the mouse double minute 2 homolog (MDM2) (9). ZNF668 has also been demonstrated to
mediate Tip60 acetylation and ionizing radiation-induced
hyperacetylation of H2A histone family member X following DNA
damage (10). Nevertheless, to the
best of our knowledge, the effect of ZNF668 on invasion and
metastasis of human tumors has not been determined to date.
In the present study, the expression of ZNF668 was
investigated in 167 cases of non-small cell lung cancer (NSCLC) and
62 cases of paired normal lung tissues using immunohistochemistry.
Associations with clinicopathological features were also examined.
Furthermore, the effect of ZNF668 on invasion and migration and the
associated downstream effectors were investigated.
Materials and methods
Patients
The present study was approved by the local
institutional review board of China Medical University (Shenyang,
China). Tissue samples were obtained from 167 patients (median age,
60 years; age range, 29–79 years; 107 males and 60 females), who
underwent complete surgical excision at the First Affiliated
Hospital of China Medical University between April 2010 and August
2012 with a diagnosis of lung squamous cell carcinoma or lung
adenocarcinoma. No neoadjuvant radiotherapy or chemotherapy was
provided prior to surgery and all patients were given standard
chemotherapy following surgery. Of the 167 patients, 55 (32.9%)
were treated with platinum-based adjuvant chemotherapy, 18 (10.8%)
underwent platinum-based adjuvant chemoradiotherapy and the other
94 patients were treated outside (no information of treatment).
Histological diagnosis and grading were evaluated according to the
2015 World Health Organization classification of tumors of the lung
(11). Tumor staging was performed
according to the seventh edition of the International Union against
Cancer tumor-node-metastasis (TNM) Staging System for Lung Cancer
(12). A total of 73 squamous cell
lung carcinoma and 94 lung adenocarcinoma cases, were included. The
use of specimens and data was approved by the Ethics Committee of
China Medical University and informed consent was written and
obtained from the patients prior to participation in the present
study. A total of 20 freshly isolated specimens, including tumor
and paired normal tissues, were stored at −80°C immediately
following resection and used for protein extraction.
Western blotting
Total protein was extracted using a lysis buffer at
4°C for 20 min (Pierce; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and quantified using the Bradford protein assay. A total
of 50 µg protein/lane was separated via SDS-PAGE on a 10%
polyacrylamide gel. The separated proteins were transferred onto a
polyvinylidene fluoride membrane (Merck KGaA, Darmstadt, Germany).
The membranes were blocked by 5% bovine serum albumin (Merck KGaA)
at 37°C for 2 h. The membranes were then incubated overnight at 4°C
with the following primary antibodies against ZNF668 (1:100; cat.
no. HPA043048; Sigma-Aldrich; Merck KGaA), GAPDH (1:5,000; cat. no.
G5262; Sigma-Aldrich; Merck KGaA), snail family transcriptional
repressor 1 (Snail; 1:1,000; cat. no. 3895; Cell Signaling
Technology, Inc., Danvers, MA, USA), snail family transcriptional
repressor 2 (Slug; 1:1,000; cat. no. 9585; Cell Signaling
Technology, Inc.), Myc-tag (1:1,000; cat. no. 2276; Cell Signaling
Technology, Inc.), Vimentin (1:1,000; cat. no. 5741; Cell Signaling
Technology, Inc.), E-cadherin (1:1,000; cat. no. 610181; BD
Biosciences, Franklin Lakes, NJ, USA), neural (N-)cadherin
(1:1,000; cat. no. 610921; BD Biosciences), Fibronectin (1:1,000;
cat. no. 610077; BD Biosciences), β-catenin (1:1,000; cat. no.
610153; BD Biosciences), α-catenin (1:1,000; cat. no. 610193; BD
Biosciences), ZO-1 (1:500; cat. no. 21773-1-AP; ProteinTech Group,
Inc., Chicago, IL, USA) and Occludin (1:500; cat. no. 13409-1-AP;
ProteinTech Group, Inc.). Membranes were washed six times with
TBS-Tween-20 and subsequently incubated with horseradish
peroxidase-conjugated anti-mouse (1:1,000; cat. no. sc-2954; Santa
Cruz Biotechnology Inc., Dallas, TX, USA) or anti-rabbit
immunoglobulin G (IgG; cat. no. sc-516087, Santa Cruz
Biotechnology) at 37°C for 2 h. Protein bands were visualized using
electrochemiluminescence (Pierce; Thermo Fisher Scientific, Inc.)
and quantified using a bio-imaging system (DNR Bio-Imaging Systems,
Ltd., Neve Yamin, Israel).
Immunohistochemistry
Samples were fixed using 10% neutral buffered
formalin at room temperature for 30 min, embedded in paraffin and
cut into 4-µm-thick sections. Immunostaining was performed using
the streptavidin-peroxidase method. The antigen retrieval was
performed by heating to a temperature of 100°C with Citrate buffer
(Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China). The sections were
then blocked with goat serum (Fuzhou Maixin Biotech Co., Ltd.) at
37°C for 1 h. The sections were incubated with a polyclonal rabbit
anti-ZNF668 antibodies (1:100; cat. no. HPA043048; Sigma-Aldrich;
Merck KGaA) at 4°C overnight, followed by biotinylated goat
anti-rabbit IgG secondary antibodies at 37°C for 30 min (1:100;
cat. no. KIT-9710; Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China).
Subsequent to washing three times with PBS, the sections were
incubated with horseradish peroxidase-conjugated
streptavidin-biotin at 37°C for 30 min (cat. no. KIT-9710; Fuzhou
Maixin Biotech Co., Ltd.) and subsequently stained with
3,3-diaminobenzidine tetra-hydrochloride for 1 min at 25°C (cat.
no. KIT-0014; Fuzhou Maixin Biotech Co., Ltd.). Finally, samples
were counterstained with hematoxylin at room temperature for 5 min,
dehydrated in alcohol (the concentration of alcohol ranged between
100 and 70%), and mounted. Two investigators, blinded to the
clinical data, scored the slides semi-quantitatively by light
microscopy at ×200magnification by evaluating the staining
intensity and the percentage of stained cells in representative
areas. The staining intensity was scored as 0, no signal; 1, weak;
2, moderate or 3, high. The percentage of cells stained was scored
as 1, 1–25%; 2, 26–50%; 3, 51–75%; or 4, 76–100%. A final score of
0–12 was obtained by multiplying the intensity and percentage
scores. Tumors with a score ≥4 were considered ZNF668 positive,
whereas a score <4 was considered to indicate negative or low
ZNF668 expression.
Cell culture
The HBE cell line was obtained from the American
Type Culture Collection (Manassas, VA, USA). The A549, H460, H292
and SPC-A-1 (SPC) cell lines were obtained from the Shanghai Cell
Bank of the Chinese Academy of Sciences (Shanghai, China). Cells
were maintained in RPMI 1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin
(Sigma-Aldrich; Merck KGaA) and 100 µg/ml streptomycin
(Sigma-Aldrich; Merck KGaA). Cells were passaged every two days
using a 0.25% trypsin solution (Invitrogen; Thermo Fisher
Scientific, Inc.).
Immunofluorescence analysis
Cells were fixed with 4% paraformaldehyde at 25°C
for 30 min, blocked with 1% bovine serum albumin (Sigma-Aldrich;
Merck KGaA) at 25°C for 30 min and incubated with a polyclonal
anti-ZNF668 antibody (1:50; cat. no. HPA043048; Sigma-Aldrich;
Merck KGaA) overnight at 4°C, followed by a tetramethylrhodamine
isothiocyanate-conjugated secondary goat anti-rabbit IgG antibody
(1:100; cat. no. ab6718; Abcam, Cambridge, UK) at room temperature
for 1 h. Cells were subsequently counterstained with DAPI at 25°C
for 5 min. Epifluorescence microscopy was performed using an
inverted microscope (TE300; Nikon Corporation, Tokyo, Japan) and
confocal microscopy was performed using a laser scanning confocal
microscope at ×600 magnification in three fields of view (Radiance
2000; Carl Zeiss AG, Oberkochen, Germany).
Plasmid transfection and small
interfering RNA (siRNA) treatment
The plasmid concentration was 1 µg/µl The
pCMV6-ddk-myc and pCMV6-ddk-myc-ZNF668 plasmids were purchased from
OriGene Technologies, Inc. (Rockville, MD, USA). ZNF668-siRNA (cat.
no. sc-63255) and negative control-siRNA (cat. no. sc-37007) were
purchased from Santa Cruz Biotechnology, Inc. Transfection was
performed using the Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The plasmid transfection was harvested at
48 h after transfection, and the siRNA treatment was harvested at
72 h after transfection
Matrigel invasion assay
Cell invasion assays were performed using a 24-well
Transwell chamber with 8 µm pores (Costar; Sigma-Aldrich; Merck
KGaA). The inserts were coated with 20 µl Matrigel (1:3 dilution;
BD Biosciences). Following a 48 h transfection period, cells were
trypsinized and a total of 3×105 cells in 100 µl
serum-free medium (RPMI-1640 medium; Invitrogen; Thermo Fisher
Scientific, Inc.) were plated in the upper Matrigel chamber for 18
h. Media supplemented with 10% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.) was added in the lower chamber as a
chemoattractant. Following incubation at 37°C for 18 h, cells that
passed through the filter were fixed with 4% paraformaldehyde at
25°C for 30 min and stained with hematoxylin at 25°C for 5 min.
Invasive cells were observed by light microscopy at ×200
magnification, counted in ten randomly-selected fields.
Wound healing assay
In cultures with cell density <90%, following a
48 h transfection period, wounds were created in confluent areas
using a 200-µl pipette tip. Wound healing within the scrape line
was observed at different time points (0 and 24 h) at 37°C and
representative pictures for each cell line were captured. Duplicate
wells were examined for each condition, and each experiment was
performed 3 times. Data were analyzed using Image J 1.48 u Software
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
SPSS version 17.0 for windows (SPSS, Inc., Chicago,
IL, USA) was used for statistical analyses. Pearson's χ2
test was used to analyze the associations between ZNF668 expression
and several clinicopathological features. Student's t tests were
used for the analysis of western blotting data. P<0.05 was
considered to indicate a statistically significant difference.
Results
ZNF668 protein expression in
NSCLC
The expression of ZNF668 in clinical NSCLC samples
was initially investigated using western blotting. In lung cancer
tissues ZNF668 protein expression was lower compared with the
corresponding adjacent noncancerous tissues (17/20; 85%; Fig. 1A). Analysis of western blotting data
indicated a normalized value of 0.7935±0.1120 for ZNF668 protein
expression in NSCLC specimens, which was significantly lower
compared with that of corresponding noncancerous tissues
(0.3057±0.05185; P<0.001; Fig.
1B). The subcellular localization of ZNF668 expression in NSCLC
and adjacent noncancerous tissues was investigated using
immunohistochemistry. A representative paired sample, demonstrating
lower ZNF668 expression in the tumor sample, was selected for this
analysis. Weak ZNF668 expression was observed in the NSCLC samples
(Fig. 1C), in agreement with the
western blotting data.
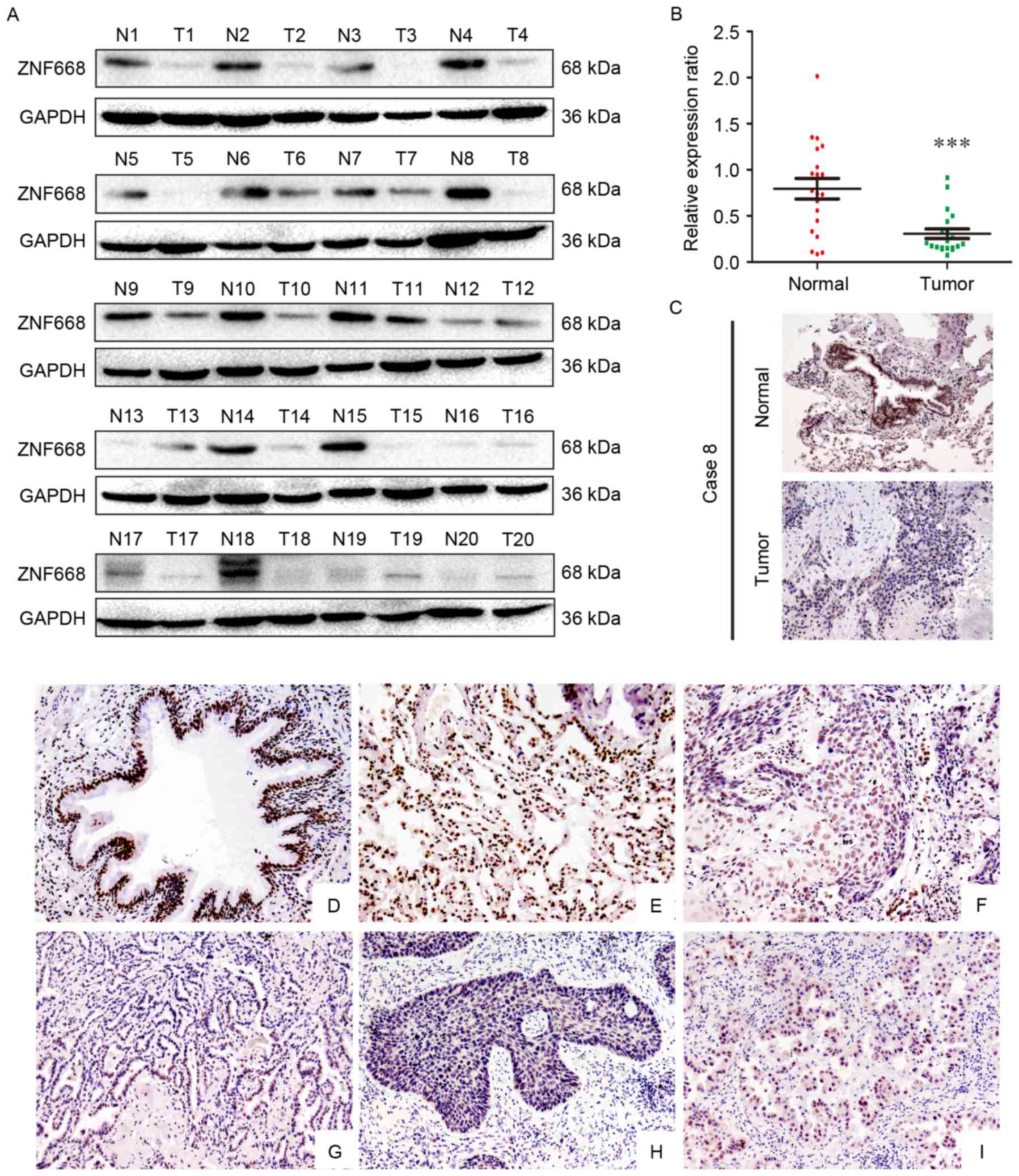 | Figure 1.Western blotting and
immunohistochemical analysis of ZNF668 protein expression in NSCLC
specimens. (A) Among the 20 examined specimens, cases 1, 2, 3, 4,
5, 6, 7, 8, 9, 10, 11, 14, 15, 16, 17,18 and 19 demonstrated
decreased ZNF668 expression in NSCLC compared with adjacent
noncancerous tissues. (B) Quantification of western blotting data
revealed significantly decreased ZNF668 expression in NSCLC
specimens compared with the corresponding noncancerous tissues. (C)
Immunohistochemical analysis of ZNF668 protein expression in paired
primary NSCLC and normal lung tissue from a single patient (case
8). ZNF668 immunostaining was positive in the adjacent normal
bronchial epithelia, whereas weak ZNF668 expression was observed in
the NSCLC sample. Magnification, ×200. (D; magnification, ×200)
Normal bronchial epithelium and (E; magnification, ×200) alveoli
were positive for ZNF668. Negative or weak nuclear ZNF668
immunoreactivity was observed in (F; magnification, ×200) lung
squamous cell carcinoma and (G; magnification, ×200) adenocarcinoma
samples. In certain (H; magnification, ×200) squamous cell
carcinoma and (I; magnification, ×200) adenocarcinoma cases, ZNF668
was localized in the cytoplasm and nuclei. ***P<0.001. NSCLC,
non-small cell lung carcinoma; ZNF668, zinc finger protein 668; N,
normal; T, tumor. |
The expression of ZNF668 in NSCLC and adjacent
noncancerous tissues was also investigated using
immunohistochemistry. ZNF668 was strongly expressed in the nuclei
of normal tissues adjacent to carcinomas (Fig. 1D and E); whereas nuclear ZNF668
expression was decreased in lung cancer samples (Fig. 1F and G). Notably, cytoplasmic and
nuclear expression was observed in a number of cases (Fig. 1H and I). ZNF668 expression in cancer
specimens (51/167, 30.5%) was significantly decreased compared with
normal lung tissues (43/62, 69.4%; P<0.001). As presented in
Table I, ZNF668 expression was
negatively associated with lymph node metastasis (P=0.002) and
advanced TNM stage (P=0.019) in NSCLC. No statistically significant
associations were identified between ZNF668 expression and sex,
age, histological type and differentiation in NSCLC. The
association between ZNF668 expression and a number of
clinicopathological features in different histological types of
NSCLC was also evaluated. As presented in Table I, no statistically significant
association between ZNF668 expression and any clinicopathological
feature was identified in 73 lung squamous cell carcinoma cases. In
94 lung adenocarcinoma cases, ZNF668 negative expression was
significantly associated with lymph node metastasis (P=0.001).
However, no statistically significant association was detected
between ZNF668 expression and other factors in NSCLC.
 | Table I.Associations between zinc finger
protein 668 expression with clinicopathological features in 167
cases of non-small cell lung carcinoma. |
Table I.
Associations between zinc finger
protein 668 expression with clinicopathological features in 167
cases of non-small cell lung carcinoma.
|
| Overall | Squamous cell
carcinoma | Adenocarcinoma |
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | N | (+) | (−) | χ2 | P-value | N | (+) | (−) | χ2 | P-value | N | (+) | (−) | χ2 | P-value |
|---|
| Age, years |
|
|
| 0.021 | 0.885 |
|
|
|
| 0.631 |
|
|
| 0.128 | 0.817 |
|
<60 | 80 | 24 | 56 |
|
| 34 | 11 | 23 | 0.296 |
| 46 | 13 | 33 |
|
|
| ≥60 | 87 | 27 | 60 |
|
| 39 | 15 | 24 |
|
| 48 | 12 | 36 |
|
|
| Sex |
|
|
| 1.354 | 0.295 |
|
|
| 1.536 | 0.409 |
|
|
| 0.002 | 0.964 |
| Male | 107 | 36 | 71 |
|
| 66 | 25 | 41 |
|
| 41 | 11 | 30 |
|
|
|
Female | 60 | 15 | 45 |
|
| 7 | 1 | 6 |
|
| 53 | 14 | 39 |
|
|
| Histological
type |
|
|
| 1.576 | 0.238 |
|
|
|
|
|
|
|
|
|
|
| Squamous
cell carcinoma | 73 | 26 | 47 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Adenocarcinoma | 94 | 25 | 69 |
|
|
|
|
|
|
|
|
|
|
|
|
| Differentiation |
|
|
| 0.102 | 0.859 |
|
|
| 0.000 |
|
|
|
| 0.738 | 0.483 |
| Well | 56 | 18 | 38 |
|
| 14 | 5 | 9 |
| 0.993 | 42 | 13 | 29 |
|
|
|
Moderate +Poor | 111 | 33 | 78 |
|
| 59 | 21 | 38 |
|
| 52 | 12 | 40 |
|
|
| TNM
classification |
|
|
| 5.750 | 0.019 |
|
|
| 2.321 | 0.148 |
|
|
| 3.438 | 0.101 |
|
I+II | 88 | 34 | 54 |
|
| 39 | 17 | 22 |
|
| 49 | 17 | 32 |
|
|
|
III | 79 | 17 | 62 |
|
| 34 | 9 | 25 |
|
| 54 | 8 | 37 |
|
|
| Lymph node
metastasis |
|
|
| 9.909 | 0.002 |
|
|
| 1.828 | 0.223 |
|
|
| 9.823 | 0.002 |
|
Positive | 73 | 13 | 60 |
|
| 33 | 9 | 24 |
|
| 40 | 4 | 36 |
|
|
|
Negative | 94 | 38 | 56 |
|
| 40 | 17 | 23 |
|
| 54 | 21 | 33 |
|
|
Expression of ZNF668 in lung cancer
cell lines
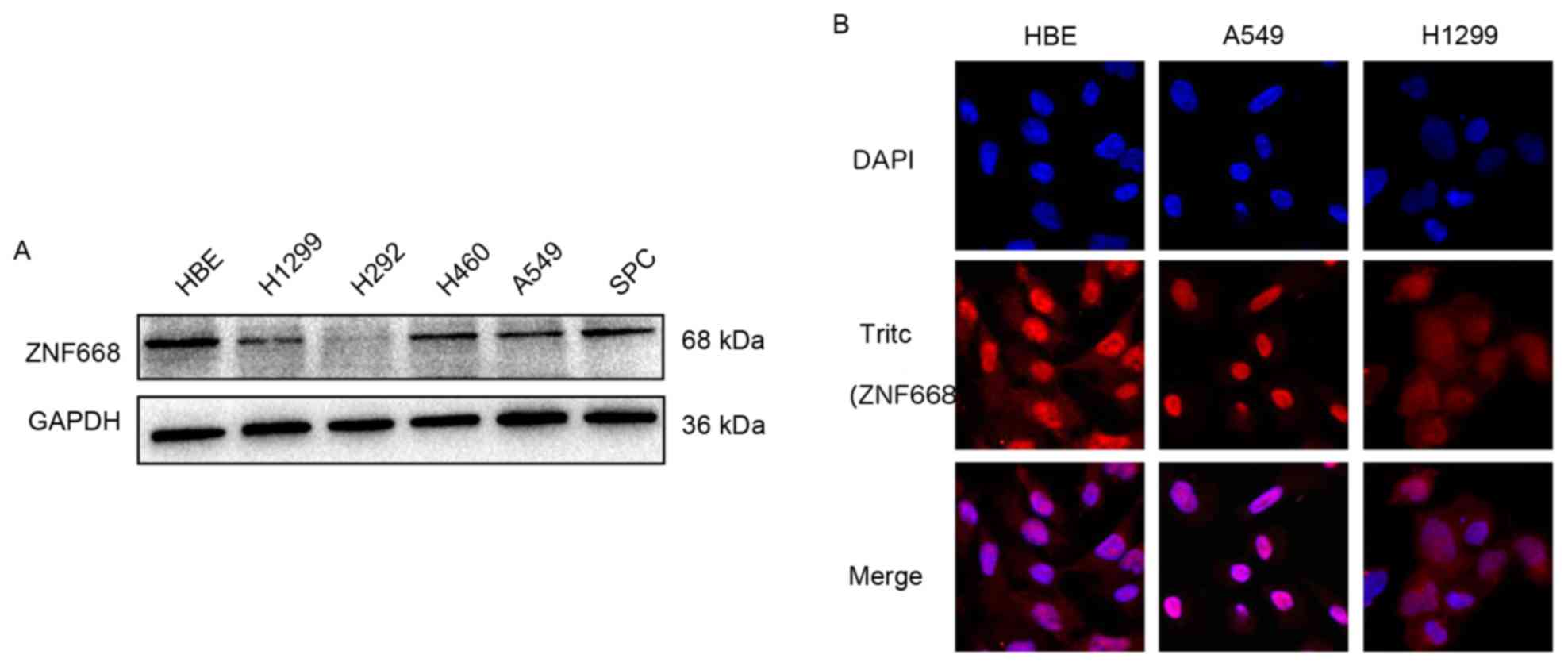
ZNF668 protein expression was decreased in NSCLC
cell lines (5/5) compared with normal HBE cells (Fig. 2A). ZNF668 was primarily localized in
the nuclei, whereas weak cytoplasmic expression was observed in
A549, H1299 and HBE cells (Fig.
2B).
ZNF668 suppresses NSCLC cell invasion
and migration
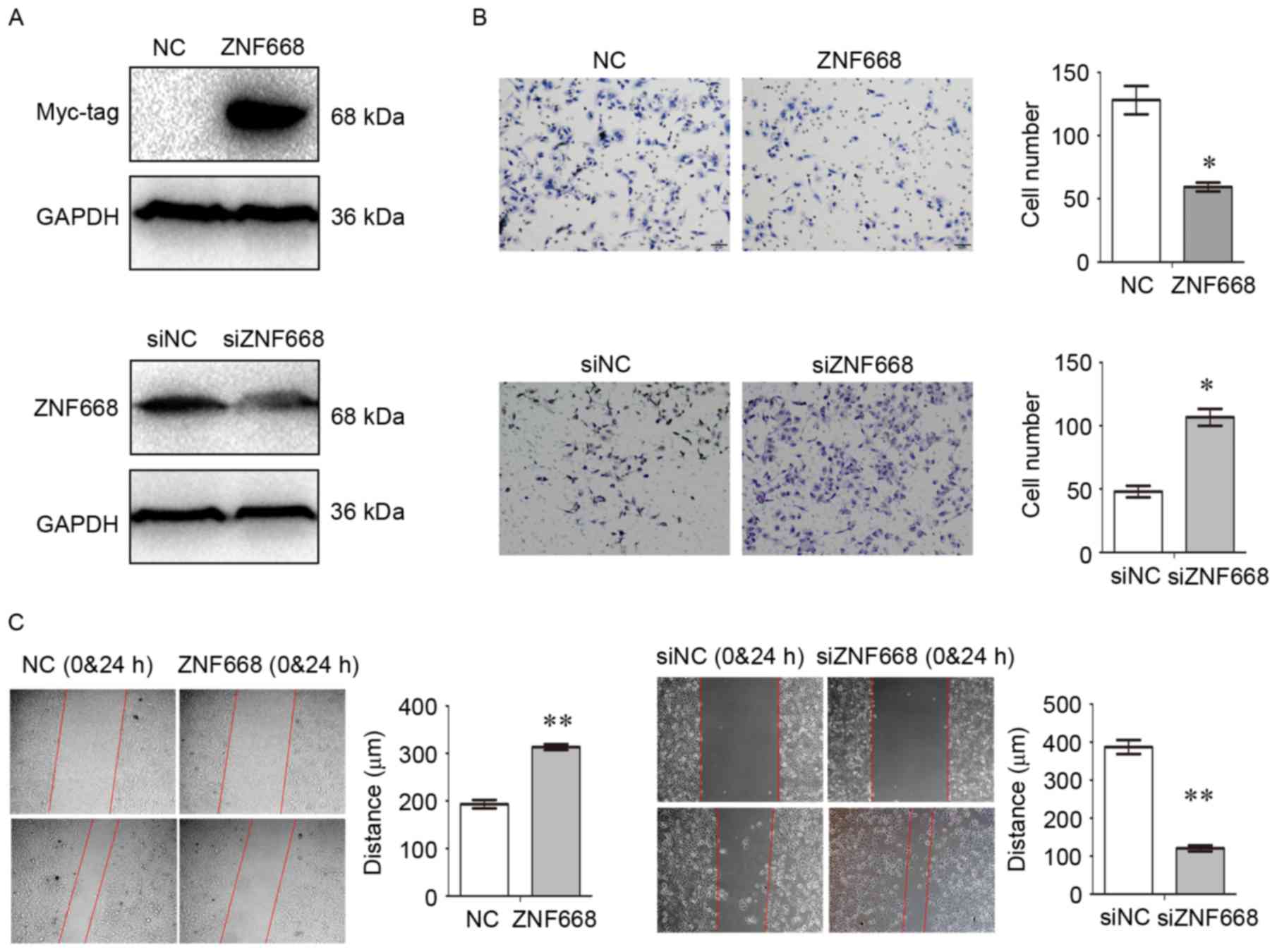
ZNF668 expression was manipulated by transfecting a
ZNF668-expressing plasmid in A549 cells and by siRNA-mediated gene
knockdown in HBE cells (Fig. 3A).
Transwell assays demonstrated a significant decrease in cell
invasion following ZNF668 overexpression, whereas increased
invasion was observed following ZNF668 depletion (Fig. 3B). Wound healing assays demonstrated
that migration was suppressed in A549 cells overexpressing ZNF668,
whereas migration of HBE cells was enhanced when ZNF668 expression
was inhibited (Fig. 3C).
ZNF668 downregulates Snail and
upregulates E-cadherin and ZO-1 expression
ZNF668 was demonstrated to inhibit the invasion and
migration of NSCLC cells. Therefore, the expression of
epithelial-mesenchymal transition (EMT)-associated proteins was
investigated following ZNF668 overexpression in A549 cells or
silencing in HBE cells. ZNF668 overexpression downregulated Snail
and increased the expression of E-cadherin and ZO-1. By contrast,
ZNF668 depletion resulted in increased Snail expression and
decreased E-cadherin and ZO-1 expression levels (Fig. 4). The expression of additional
EMT-associated proteins, including occludin, α-catenin, β-catenin,
Slug, Vimentin, N-cadherin and Fibronectin, was not altered by
ZNF668 manipulation.
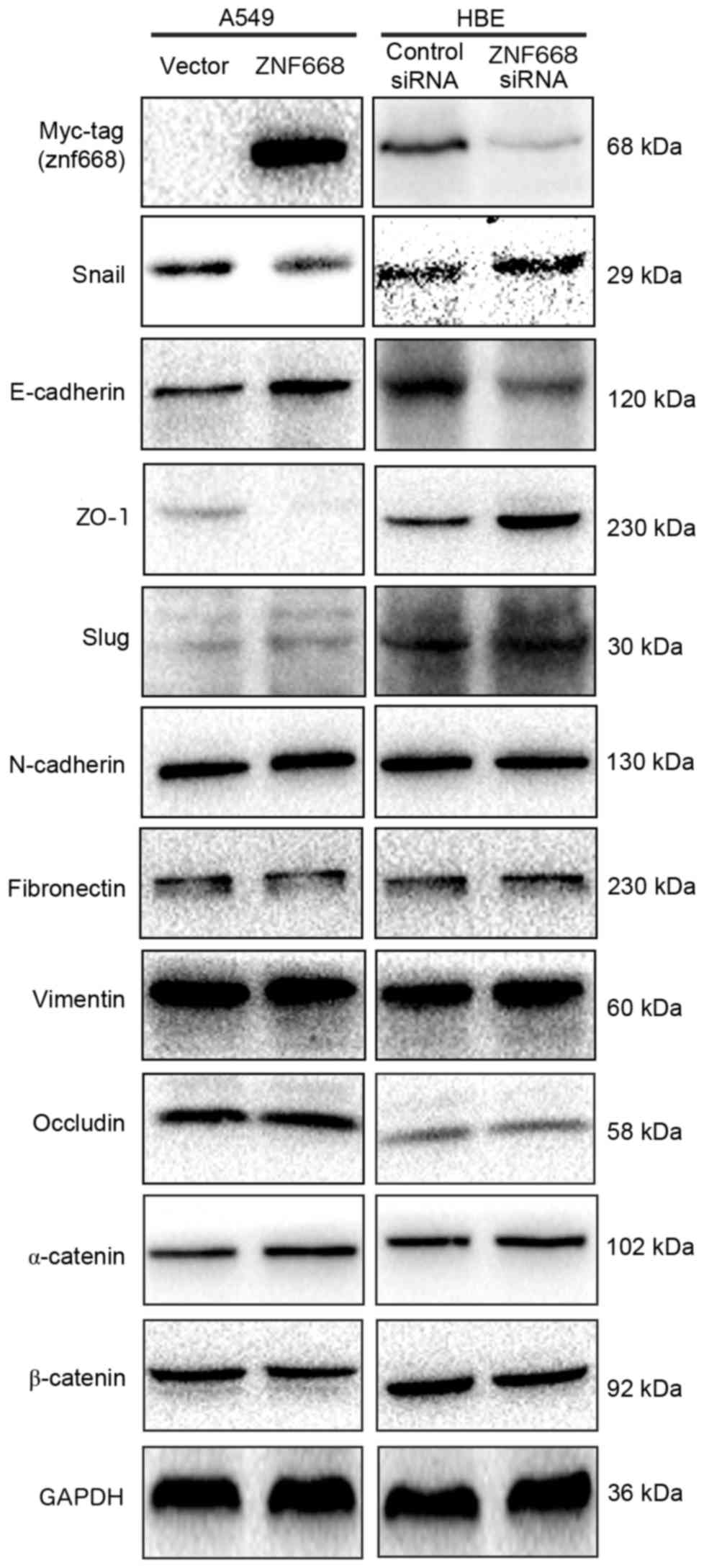 | Figure 4.Snail, E-cadherin and ZO-1 expression
was dependent on ZNF668 expression in lung cancer cells. Snail
expression was decreased, whereas E-cadherin and ZO-1 expression
levels increased following ZNF668 overexpression. The opposite
effect was observed following ZNF668 silencing. Data represent at
least three independent experiments generating similar results.
ZNF668, zinc finger protein 668; E-cadherin, epithelial-cadherin;
ZO-1, zonula occludens-1; si, small interfering; NC, negative
control; N-cadherin, neural cadherin; Slug, snail family
transcriptional repressor 2; Snail, snail family transcriptional
repressor 1. |
Discussion
The results of the present study demonstrated that
ZNF668 is downregulated in human NSCLC, and that ZNF668
overexpression suppresses the migration and invasion of lung cancer
cells through the downregulation of Snail and upregulation of
E-cadherin and ZO-1.
Due to the fact that ZNF668 is a newly discovered
protein, its expression profile and subcellular localization is not
well characterized. Using immunohistochemistry, it was demonstrated
that ZNF668 is strongly expressed in the nuclei of normal lung
tissues, whereas decreased expression was observed in the majority
of the tested NSCLC samples. Western blot analysis and
immunofluorescent staining in cell lines were consistent with the
data obtained from the clinical tissue samples. The results of the
present study are in agreement with previously reported data,
indicating that ZNF668 primarily has a nuclear localization in
breast cancer cells (9). Additional
statistical analysis revealed that loss of ZNF668 expression was
associated with positive lymph node metastasis and advanced TNM
stage in patients with NSCLC; indicating that ZNF668 may serve a
critical suppressive function during the development of NSCLC.
However, in certain cases, ZNF668 immunoreactivity in the cytoplasm
and nuclei was observed. The function of cytosolic ZNF668
expression renders further investigation.
ZNF668 has previously been demonstrated to inhibit
p53 degradation by binding MDM2, resulting in suppression of breast
cancer cell proliferation (9).
However, to the best of our knowledge, its effect on invasion and
migration of human tumors has not been thoroughly investigated. The
results of the present study demonstrated that overexpression of
ZNF668 significantly impaired the invasive and migratory ability of
lung cancer cells and resulted in Snail downregulation and
E-cadherin and ZO-1 upregulation. Snail directly binds to
E-cadherin and suppresses its expression. Previous studies have
demonstrated that transforming growth factor-β, mitogen activated
protein kinase, Wnt and phosphoinositide 3-kinase-protein kinase B
are the key signaling pathways involved in Snail activation
(13–19). Furthermore, Lim et al (20) demonstrated that p53 inhibits the
invasion of hepatocellular carcinoma cells by decreasing Snail
expression. Further research is required to investigate whether the
induced downregulation of Snail following ZNF668 overexpression is
associated with the activation of these signaling pathways.
The present study indicated that ZNF668 expression
is decreased in NSCLC samples and loss of ZNF668 expression is
associated with increased TNM stage and positive lymph node
metastasis. Furthermore, ZNF668 overexpression decreases the
expression of Snail, while it increases E-cadherin and ZO-1
expression, thus suppressing the invasion and migration of NSCLC
cells.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81472805, 81402369
and 81301837).
References
|
1
|
Capaldo CT, Farkas AE and Nusrat A:
Epithelial adhesive junctions. F1000Prime Rep. 6:12014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ivanov AI and Naydenov NG: Dynamics and
regulation of epithelial adherens junctions: Recent discoveries and
controversies. Int Rev Cell Mol Biol. 303:27–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brooke MA, Nitoiu D and Kelsell DP:
Cell-cell connectivity: Desmosomes and disease. J Pathol.
226:158–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Xu HT, Dai SD, Wei Q, Yuan XM and
Wang EH: Reduction of p120(ctn) isoforms 1 and 3 is significantly
associated with metastatic progression of human lung cancer. APMIS.
115:848–562007. View Article : Google Scholar
|
|
5
|
Liu Y, Li QC, Miao Y, Xu HT, Dai SD, Wei
Q, Dong QZ, Dong XJ, Zhao Y, Zhao C and Wang EH: Ablation of
p120-catenin enhances invasion and metastasis of human lung cancer
cells. Cancer Sci. 100:441–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Wang Y, Zhang Y, Miao Y, Zhao Y,
Zhang PX, Jiang GY, Zhang JY, Han Y, Lin XY, et al: Abnormal
expression of p120-catenin, E-cadherin, and small GTPases is
significantly associated with malignant phenotype of human lung
cancer. Lung Cancer. 63:375–382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Runkle EA and Mu D: Tight junction
proteins: From barrier to tumorigenesis. Cancer Lett. 337:41–48.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balda MS and Matter K: Tight junctions and
the regulation of gene expression. Biochim Biophys Acta.
1788:761–767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu R, Peng G, Dai H, Breuer EK,
Stemke-Hale K, Li K, Gonzalez-Angulo AM, Mills GB and Lin SY:
ZNF668 functions as a tumor suppressor by regulating p53 stability
and function in breast cancer. Cancer Res. 71:6524–6534. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu R, Wang E, Peng G, Dai H and Lin SY:
Zinc finger protein 668 interacts with Tip60 to promote H2AX
acetylation after DNA damage. Cell Cycle. 12:2033–41. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: WHO Classification of Tumours of the Lung,
Pleura, Thymus and Heart. Lyon: International Agency for Research
on Cancer; 2015
|
|
12
|
Goldstraw P: Updated staging system for
lung cancer. Surg Oncol Clin N Am. 20:655–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De Craene B, van Roy F and Berx G:
Unraveling signalling cascades for the Snail family of
transcription factors. Cell Signal. 17:535–547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Herreros AG, Peiró S, Nassour M and
Savagner P: Snail family regulation and epithelial mesenchymal
transitions in breast cancer progression. J Mammary Gland Biol
Neoplasia. 15:135–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katoh M and Katoh M: Cross-talk of WNT and
FGF signaling pathways at GSK3beta to regulate beta-catenin and
SNAIL signaling cascades. Cancer Biol Ther. 5:1059–1064. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Julien S, Puig I, Caretti E, Bonaventure
J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A
and Larue L: Activation of NF-kappaB by Akt upregulates Snail
expression and induces epithelium mesenchyme transition. Oncogene.
26:7445–7456. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SO and Kim MR: [6]-Gingerol prevents
disassembly of cell junctions and activities of MMPs in invasive
human pancreas cancer cells through ERK/NF-κB/Snail signal
transduction pathway. Evid Based Complement Alternat Med.
2013:7618522013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5(pii): E172016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lim SO, Kim H and Jung G: p53 inhibits
tumor cell invasion via the degradation of snail protein in
hepatocellular carcinoma. FEBS Lett. 584:2231–2236. 2010.
View Article : Google Scholar : PubMed/NCBI
|