Introduction
Lung cancer is the most commonly occurring type of
cancer and the leading cause of cancer-associated mortality in
adults worldwide (1). Malignant lung
tumors can be subdivided into small-cell lung carcinoma (SCLC) and
non-small cell lung carcinoma (NSCLC). NSCLC can be further
subdivided into adenocarcinoma, squamous cell carcinoma (SCC) and
large cell carcinoma using conventional histopathology. Progress in
the effectiveness of conventional treatment approaches to NSCLC,
including chemotherapy, radiotherapy and surgery, has plateaued.
The development of genetic testing technology and genotyping has
provided great insight into the molecular pathology of NSCLC. Known
driver mutations are identifiable in ~60% lung adenocarcinoma and
~80% of SCC (2,3). Such mutations can trigger activating
cascades in signaling pathways, including the
RAS-RAF-MEK-extracellular signal-regulated kinase/mitogen activated
protein kinase, phosphoinositide 3-kinase (PI3K)-AKT
serine/threonine kinase-mechanistic target of rapamycin, or janus
kinase-signal transducer and activator of transcription 1 pathways
(4–6),
leading to growth, proliferation, survival, and metastasis of
cancer cells. Successful targeted therapies involve the
identification and inhibition of these upregulated pathways by
small molecule inhibitors or receptor monoclonal antibodies (mAb).
Therapeutic targeting of the interaction between epidermal growth
factor receptor (EGFR) and members of its downstream pathway has
been most extensively studied. The two most common EGFR-activating
mutations are an exon 19 deletion and exon 21 L858R mutation, which
result in the constitutive activation of the receptor (7). It has been established that EGFR
activating mutations are more common in patients with specific
clinicopathological characteristics: Females, non-smokers, Asiatic
origin and adenocarcinoma histological subtype. Mutant EGFR can be
inhibited by small molecule tyrosine kinase inhibitors (TKIs),
including gefitinib, erlotinib and afatinib or mAbs, including
cetuximab and panitumumab. Multiple studies have demonstrated that
these drugs can significantly increase therapy response rate and
improve the progression free survival (PFS), and overall survival
(OS) times in patients with NSCLC (8–13). The
Food and Drug Administration (FDA) have approved gefitinib,
erlotinib and afatinib as the first-line drugs for patients
exhibiting EGFR, and the toxicity profiles of these drugs are
superior to those of standard chemotherapy drugs. However, all
patients with EGFR-mutation-derived NSCLC ultimately develop
resistance to TKI treatment. Almost half of the reported cases of
EGFR-TKI-resistance in NSCLC are due to the EGFR T790M mutation
(14–16). Other mechanisms of EGFR-TKI-resistance
include amplification of MET signaling (17,18),
amplification of Erb-B2 receptor tyrosine kinase 2 signaling
(19), mutation of PI3K catalytic
subunit a (20), mutation of B-Raf
proto-oncogene serine/threonine kinase (BRAF) (21) and NSCLC transformation to small-cell
lung cancer (which accounts for 3–15% of all NSCLC cases and is a
rare resistance mechanism which is poorly understood (22), and epithelial-mesenchymal transition
(23). TKI, AZD9291, has received FDA
approval and National Comprehensive Cancer Network guideline
recommendations for EGFR T790M-positive patients with non-small
cell lung cancer (24). However,
neoplastic tissue samples are currently required to screen patients
with the EGFR T790M mutation and to identify other molecular
features of patients with drug resistance. Furthermore, re-biopsies
following cancer progression are problematic due to factors,
including the high risk of surgery, the vulnerable health of the
patients and tumor heterogeneity.
Dead cells shed DNA into the bloodstream and when
this DNA is selectively isolated from the plasma, it is termed
cell-free DNA (cfDNA). In patients with cancerous tumors, a
fraction of the cfDNA is derived from tumor cells and is referred
as circulating tumor DNA (ctDNA). The presence of circulating
nucleic acids in peripheral circulation was discovered >60 years
ago by Mandel and Metais (25). Leon
et al (26) later described
the importance of circulating tumor DNA in cancer development. The
collection of ctDNA is more feasible than the collection of whole
tissue via biopsy, and it is sufficient for a general screening
test to characterize the genetic profile of patients with cancer.
Therefore, ctDNA has the potential to promote personalized cancer
therapy. In addition, particularly through real-time tumor
monitoring (27), unlike localized
tumor tissues, ctDNA reflects all molecular alterations of primary
tumors and metastases, potentially, inclusive of all heterogeneous
mutation profiles (28). ctDNA can
therefore be used for the early diagnosis of cancer (29,30),
real-time assessment of risk of tumor progression (31), dynamic monitoring of patient's
responses to treatment (32) and drug
resistance testing (33).
In the present study, a targeted sequencing approach
based on the Illumina platform was employed to detect and compare
NSCLC driver gene alterations in plasma samples from 32 Chinese
patients with advanced NSCLC. The purpose of this project was to
elucidate the association between TKI resistance and gene mutation
status, and to build a dynamic mutation detection system. A
particular aim was to detect the EGFR T790M mutation, which
predicts EGFR-TKI drug resistance through dynamic monitoring of
ctDNA. Using continuous detection methods such as that described in
the present study may be useful in the clinic to anticipate
required changes in treatment strategy by detecting the emergence
of drug resistant gene mutations. This has the potential to improve
the prognosis, quality of life and survival time of patients with
NSCLC, as well as to be cost-effective in terms of healthcare.
Materials and methods
Patients and ethical statements
The present study was approved by Shenzhen People's
Hospital Ethics Committee (Shenzhen, China) and the methods were
performed in accordance with their guidelines. Written informed
consent was obtained from all patients for the use of blood and
lung tumor tissue under the approval of the Ethics Committee. All
patient samples and medical data used in this study have been
irreversibly anonymized.
Patients with advanced or recurrent NSCLC derived
from EGFR mutations with a treatment plan to begin TKI treatment
were enrolled consecutively. The exclusion criteria were as
follows: i) Pregnant or lactating women; ii) patients who have
received prior treatment with an MEK inhibitor; and iii) patients
with a history of clinically significant interstitial lung disease
or pneumonitis.
A cohort of 32 patients with advanced NSCLC treated
with TKI drugs between November 2015 and December 2016 in Shenzhen
People's Hospital were enrolled into the present study. The
clinical characteristics of the patients are presented in Table I. Participants in the cohort,
including 18 females and 14 males, had been diagnosed with stage
IIIa to IV NSCLC. Patient blood samples were taken during TKI
treatment. EGFR TKI was treated until disease progression, and
blood samples were taken every 3 months. Treatment effectiveness
was assessed every 2 months according to the RECIST 1.1 criteria
(34). Testing for EGFR mutations in
blood plasma and imaging studies (including chest computational
tomography, brain magnetic resonance imaging, bone scans and
abdominal ultrasound) were performed by two independent research
teams who were blinded from each other's results until survival
outcomes were analyzed. Survival status was confirmed by telephone
follow-up every 3 months. The blood samples of patient H01 were
taken on the 24th December 2015 and the 7th of April 2016. The
blood samples of patient H02 were taken on the 31st of December
2015 and the 11th of April 2016.
 | Table I.Clinical characteristics of 32
patients with non-small cell lung cancer. |
Table I.
Clinical characteristics of 32
patients with non-small cell lung cancer.
| Characteristic | No. of patients
(%) |
|---|
| Age (years) |
|
| Mean
(standard deviation) | 59 (11.60) |
| Median
(range) | 62 (36–85) |
| Sex |
|
|
Male | 18 (56.20) |
|
Female | 14 (43.80) |
| Pathological
diagnosis |
|
|
Non-small cell lung
cancer | 32 (100.0) |
|
Adenocarcinoma | 14 (43.80) |
|
Unknown | 18 (56.20) |
| Tumor stage |
|
|
IIIA | 5 (15.60) |
|
IIIB | 3 (9.40) |
| IV | 24 (75.0) |
| Smoking
history |
|
|
Smoker | 10 (31.20) |
|
Non-smoker | 22 (68.80) |
| Treatment
history |
|
| No
previous treatment | 2 (6.25) |
|
Previous treatment | 30 (93.75) |
| Chemotherapy
history |
|
|
Chemotherapy undertaken | 25 (78.1) |
| No
chemotherapy undertaken | 7 (21.9) |
Plasma isolation and DNA
extraction
Patient blood samples were collected in tubes
containing EDTA and centrifuged at 1,600 × g for 10 min at 4°C
within 2 h of collection. The peripheral blood lymphocyte (PBL)
debris was stored at −20°C until further use. The supernatants were
further centrifuged at 10,000 × g for 10 min at 4°C, and plasma was
harvested and stored at −80°C until further use. DNA from PBLs was
extracted using the RelaxGene Blood DNA system (Tiangen Biotech
Co., Ltd., Beijing, China) and ctDNA was extracted from at least 2
ml plasma using the QIAamp Circulating Nucleic Acid kit (Qiagen,
Inc., Valencia, CA, USA) according to the manufacturers'
instructions. DNA was quantified with the Qubit 2.0 Fluorometer and
the Qubit dsDNA HS assay kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to manufacturer's instructions.
Next-generation sequencing (NGS)
library construction
DNA collected from PBLs was sheared using enzyme
dsDNA Fragmentase (New England BioLabs, Inc., Ipswich, MA, USA)
according to the manufacturer's instructions. Size selection for
150–250 bp fragments was performed with AMPure XP beads (Beckman
Coulter, Inc., Brea, CA, USA), which has a high recovery efficiency
(the amount of DNA harvested from the size selection procedure is
relatively large). DNA fragments and ctDNA were used to construct a
library using the KAPA Library Preparation kit (Kapa Biosystems,
Inc., Wilmington, MA, USA) according to the manufacturer's
instructions. Agencourt AMPure XP beads were used for the
purification of the DNA/ctDNA. Generally, following fragmentation,
the End Repair and 3′-end dA-tailing ensued. T-tailed adapters were
used and a 3′dA overhang was added enzymatically onto the
fragmented DNA sample, using the KAPA Library Preparation kit (Kapa
Biosystems, Inc.). Ligation was performed for 15 min at 20°C.
Single-step size selection was performed by adding 50 µl (1X)
PEG/NaCl SPRI solution buffer (Beckman Coulter, Inc.) to enrich for
ligated DNA fragments. The ligated fragments were then amplified
using 1X KAPA HiFi Hot Start Ready mix (Kapa Biosystems, Inc.) and
Pre-LM-PCR Oligos (Kapa Biosystems, Inc.) in 50µl reactions and 7–8
PCR cycles, depending on the input DNA mass. The thermocycling
conditions were as follows: Initial Denaturation, 98°C for 30 sec;
7–8 cycles at 98°C for 10 sec; 60°C for 10 sec; 68°C for 30 sec,
and the final extension at 68°C for 60 sec. Library purity and
concentration was assessed by Qubit 2.0 Fluorometer using the Qubit
dsDNA HS assay kit (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Fragment length was determined on
a 4200 Bioanalyzer using the DNA 1000 kit (both from Agilent
Technologies, Inc., Santa Clara, CA, USA).
Library design for hybrid
selection
Hybrid selection was performed with custom
biotinylated DNA probes for 104 genes associated with lung cancer
(Roche Diagnostics, Basel, Switzerland). The library of amplified
samples was hybridized with the SeqCap EZ Library (Roche
Diagnostics) according to the manufacturer's instructions for 16–20
h at 47°C. Following hybrid selection, the captured DNA fragments
were amplified with 12–14 cycles of PCR using 1X KAPA HiFi Hot
Start Ready mix and Post-LM-PCR Oligos in two separate 50 µl
reactions. The thermocycling conditions were as follows: Initial
denaturation, 98°C for 30 sec; 7–8 cycles at 98°C for 10 sec; 60°C
for 10 sec; 68°C for 30 sec, and the final extension at 68°C for 60
sec. The reactions were then pooled and purified using Agencourt
AMPure XP beads.
Denaturing and diluting libraries for
sequencing
Multiplexed libraries were denatured by 0.2M NaOH
and diluted by Tris-HCl according to the manufacturer's
instructions (Illumina, Inc., San Diego, CA, USA). The libraries
were sequenced using 150-bp paired-end runs using a NextSeq 500
system (Illumina, Inc.). All genes captured by NGS are listed in
Table II.
 | Table II.The gene list for sequencing in our
NGS method. |
Table II.
The gene list for sequencing in our
NGS method.
| Gene name | GenBank accession
no. |
|---|
| ABCB1 | NM_000927 |
| AKT1 | NM_005163 |
| ALK | NM_004304 |
| APC | NM_000038 |
| ATIC | NM_004044 |
| ATM | NM_000051 |
| ATRX | NM_000489 |
| BAG4 | NM_004874 |
| BCL2L11 | NM_006538 |
| BRAF | NM_004333 |
| BRCA1 | NM_007294 |
| BRCA2 | NM_000059 |
|
C10orf10 | NM_007021 |
| C8orf34 | NM_052958 |
| CBR3 | NM_001236 |
| CCND1 | NM_053056 |
| CD74 | NM_004355 |
| CDA | NM_001785 |
| CDK4 | NM_000075 |
| CDK6 | NM_001259 |
| CDKN2A | NM_000077 |
| CREBBP | NM_004380 |
| CTNNB1 | NM_001904 |
| CYP19A1 | NM_000103 |
| CYP1B1 | NM_000104 |
| CYP2C19 | NM_000769 |
| CYP2D6 | NM_000106 |
| CYP3A4 | NM_017460 |
| DDR2 | NM_006182 |
| DPYD | NM_000110 |
| EGFR | NM_005228 |
| EML4 | NM_019063 |
| ERBB2 | NM_004448 |
| ERBB3 | NM_001982 |
| ERBB4 | NM_005235 |
| ERCC1 | NM_001983 |
| EZR | NM_003379 |
| FGF19 | NM_005117 |
| FGF3 | NM_005247 |
| FGF4 | NM_002007 |
| FGFR1 | NM_015850 |
| FGFR2 | NM_000141 |
| FGFR3 | NM_000142 |
| FLT3 | NM_004119 |
| GSTP1 | NM_000852 |
| HRAS | NM_005343 |
| JAK1 | NM_002227 |
| JAK2 | NM_004972 |
| KDR | NM_002253 |
| KEAP1 | NM_012289 |
| KIT | NM_000222 |
| KMT2C | NM_170606 |
| KMT2D | NM_003482 |
| KRAS | NM_004985 |
| LRIG3 | NM_153377 |
| MALAT1 | NR_002819 |
| MAP2K1 | NM_002755 |
| MAP2K7 | NM_145185 |
| MET | NM_000245 |
| MLH1 | NM_000249 |
| MSH2 | NM_000251 |
| MTHFR | NM_005957 |
| MTOR | NM_004958 |
| MTRR | NM_002454 |
| NF1 | NM_000267 |
| NFE2L2 | NM_006164 |
| NOTCH1 | NM_017617 |
| NRAS | NM_002524 |
| NTRK1 | NM_002529 |
| NTRK3 | NM_002530 |
| PDE4DIP | NM_014644 |
| PDGFRA | NM_006206 |
| PIK3CA | NM_006218 |
| PTEN | NM_000314 |
| RAF1 | NM_002880 |
| RB1 | NM_000321 |
| RET | NM_020630 |
| ROS1 | NM_002944 |
| RRM1 | NM_001033 |
| SDC4 | NM_002999 |
| SETBP1 | NM_015559 |
| SLC34A2 | NM_006424 |
| SLIT1 | NM_003061 |
| SMARCA4 | NM_003072 |
| SMO | NM_005631 |
| SOD2 | NM_000636 |
| STAT3 | NM_003150 |
| STK11 | NM_000455 |
| STMN1 | NM_005563 |
| TACC3 | NM_006342 |
| TFG | NM_006070 |
| TOP2A | NM_001067 |
| TP53 | NM_000546 |
| TPM3 | NM_152263 |
| TRRAP | NM_003496 |
| TSC1 | NM_000368 |
| TSC2 | NM_000548 |
| TUBB3 | NM_006086 |
| TYMP | NM_001953 |
| TYMS | NM_001071 |
| UGT1A1 | NM_000463 |
| UMPS | NM_000373 |
| XPC | NM_004628 |
| XRCC1 | NM_006297 |
Variant calling and analysis
Sequencing data were demultiplexed and aligned to
the hg19 genome (GRch37) using Burrows-Wheeler Aligner (35) version 0.7.15-r1140. Pileup files for
properly paired reads with mapping quality >=60 were generated
using Samtools (http://www.htslib.org/) (36). Somatic variants were created using
VarScan2 (http://varscan.sourceforge.net/) (37) (-min-coverage=100; -min-var-freq=0.05;
-somatic-P-value=0.01; -strand-filter 1; otherwise, default
parameters were used). Allele Frequency (AFs) were calculated for
all Q30 bases. Using a custom Python script, previously identified
tumor DNA mutation were intersected with a SAMtools mpileup file
generated for each plasma DNA sample, and the number and frequency
of supporting reads were calculated for each mutation. For
screening plasma DNA without knowledge of tumor DNA, a mutation was
identified if >=4 mutant reads were revealed in plasma with
>=1 read on each strand, and no mutant reads were observed in
germline DNA or in a prior plasma sample with >=10-fold
coverage.
Results
Patient characteristics
A total of 14/32 patients (43.8%) presented with
adenocarcinoma, the remaining 18 patients (56.2%) were of unknown
histopathology. The majority of patients were non-smokers (22/32,
68.8%), and the majority of patients were treated with EGFR-TKI
(30/32, 93.75%).
The peripheral blood samples of these patients were
collected during the outpatient evaluation follow-up. Secondary
peripheral blood samples were collected from 4 patients, resulting
in a total of 36 plasma samples from 32 patients for EGFR mutation
testing.
Mutation spectrums of the 32 patients
with non-small cell lung cancer
To elucidate the association between the
effectiveness of EGFR-TKI treatment and gene status, NGS technology
was used to detect variations in ctDNA in the peripheral blood of a
cohort of 32 patients. Mutation spectrums were drawn for 36 samples
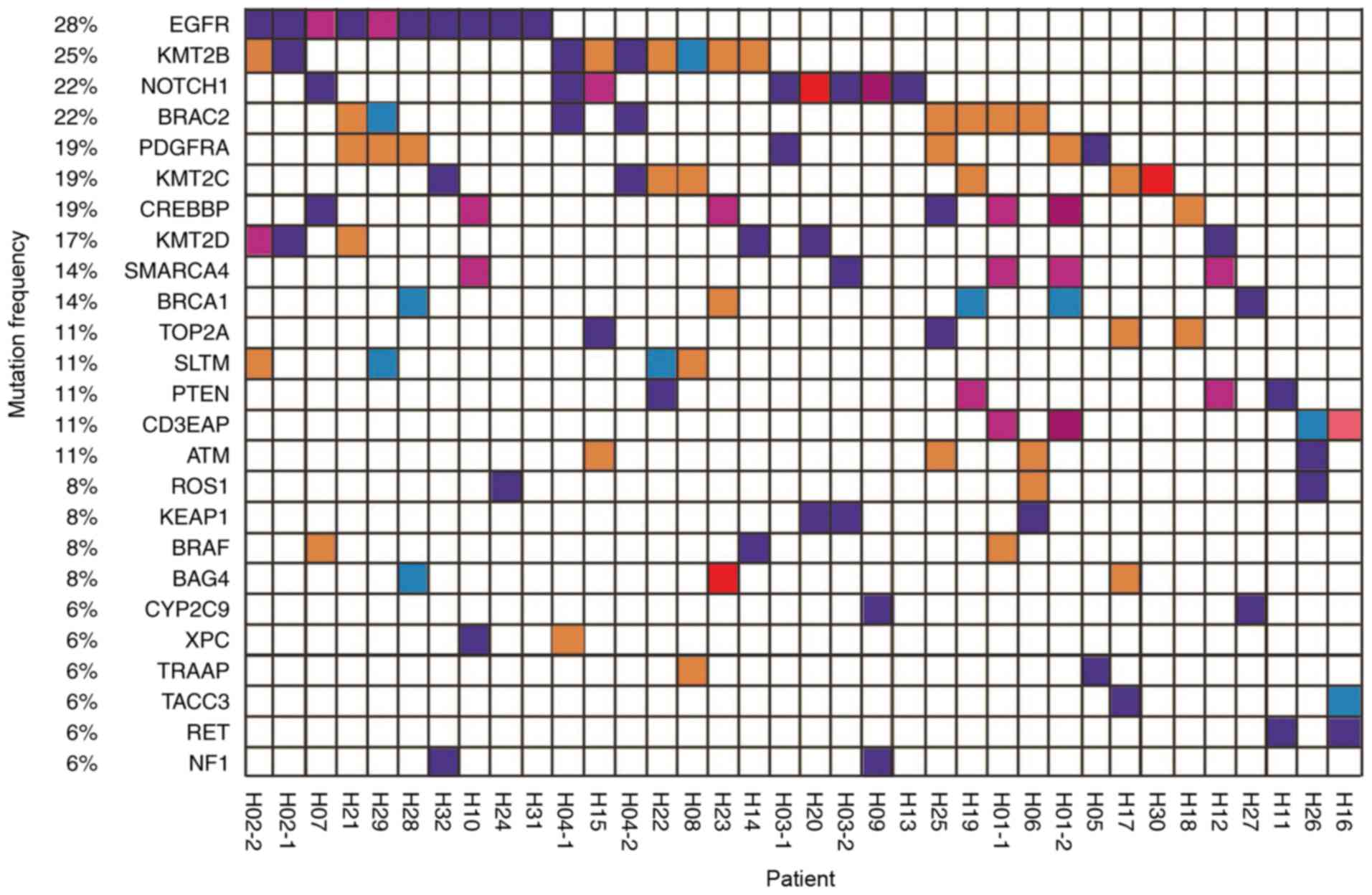
from these patients (Fig. 1).
All patients exhibited ‘driver gene’ mutations in
the ctDNA, and a number of patients exhibited actionable mutations
in genes including EGFR, ROS proto-oncogene receptor tyrosine
kinase and BRAF. Thus, it was demonstrated that the ctDNA tool is
useful for the molecular genotyping of patients with cancer.
Dynamic detection of EGFR gene
mutations in blood plasma
Patients were monitored for 24 months via
consultation, with blood collected every 3 months, in order to
create a dynamic resistance gene detection system. The somatic
mutation profile of all 32 patients is presented in Fig. 1. Their blood samples were collected
during TKI treatment and 4 underwent a second blood draw. The
results demonstrate that EGFR mutation rate was associated with
disease progression.
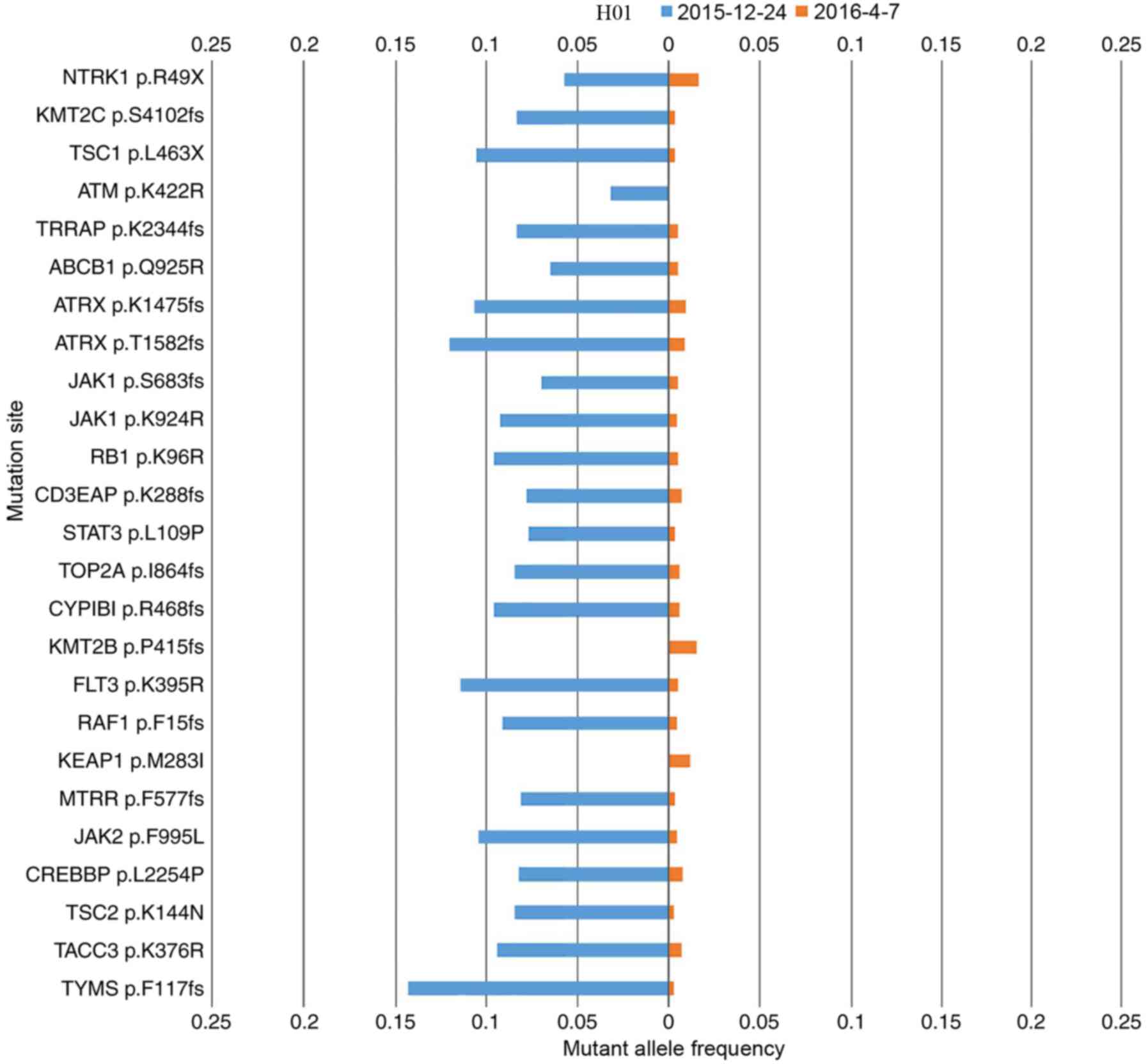
Patient H01 presented with adenocarcinoma and an
EGFR exon 19 deletion was also detected by ARMS-PCR prior to the
study. After 7 months of gefitinib treatment, the ctDNA of this
patient was measured, followed by a second measurement 4 months
later. The ctDNA mutation landscape for each measurement is
compared in Fig. 2. Upon the second
measurement, the EGFR exon 19 deletion was not identified, but the
mutation rate had declined, which was consistent with the partial
response (PR) clinical status of the patient following gefitinib
treatment. The mutation variation between first and second
measurements for patients H03, and H04 was similar to that of
H01.
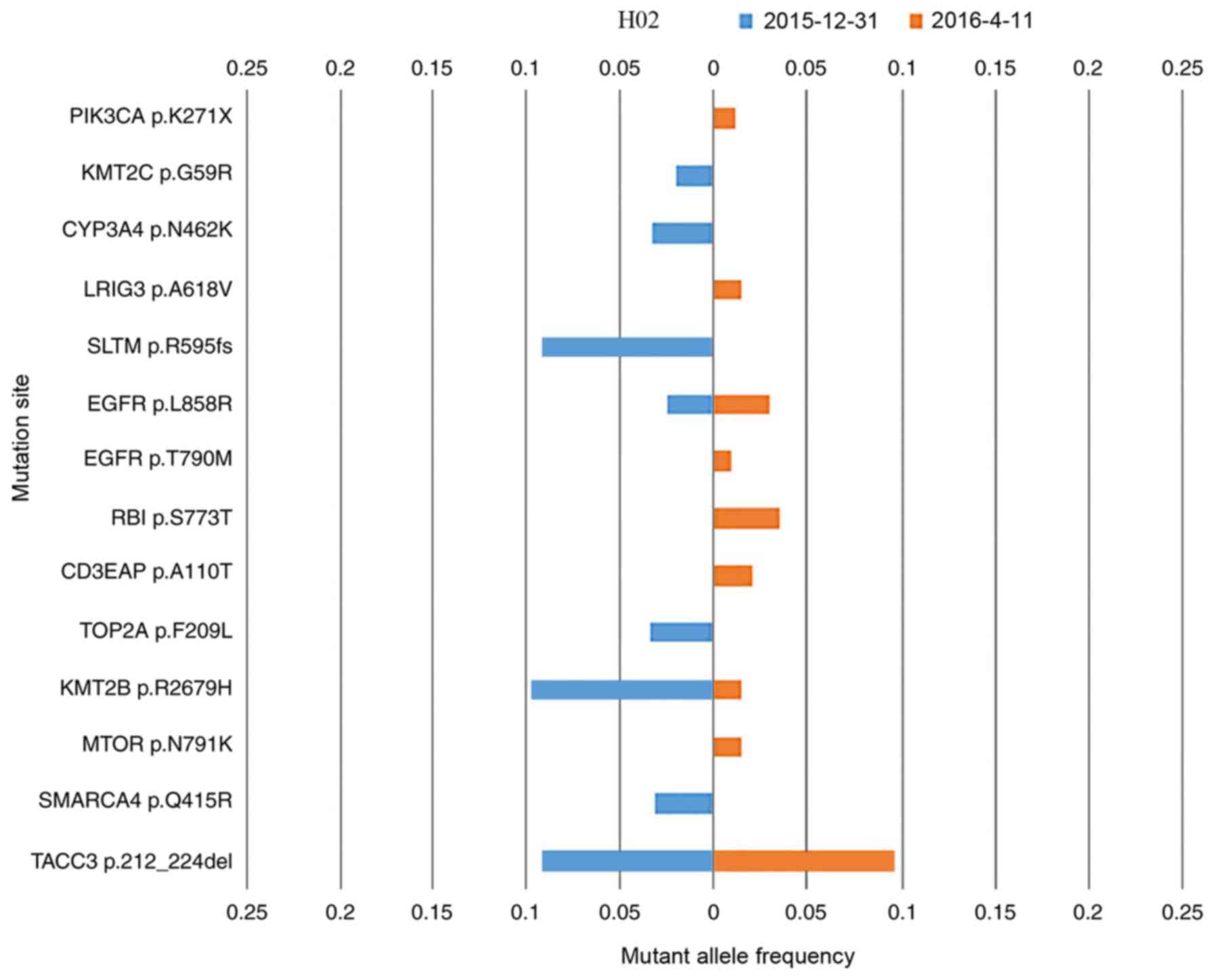
Patient H02 presented with adenocarcinoma; however,
no EGFR mutation was detected by ARMS-PCR. This patient was treated
with erlotinib blindly, which was initially effective. After 7
months of erlotinib treatment, the ctDNA of this patient was
measured, followed by a second measurement 3 months later. The
mutation landscapes for each measurement are compared in Fig. 3. The EGFR L858R
(NM_005228.4(EGFR):c.2573T>G (p.Leu858Arg)) mutation was
detected upon both measurements, and may explain the PR clinical
status following initial treatment with erlotinib. Furthermore, the
EGFR T790M [NM_005228.4(EGFR):c.2369C>T (p.Thr790Met)] mutation
was detected in the second measurement. EGFR T790M is detected as a
‘second-site mutation’ in >50% EGFR-mutation-derived types of
lung cancer that have developed resistance to erlotinib or
gefitinib (38). Patient H02
demonstrated a case of EGFR p.T790M-induced drug resistance. Other
novel driver gene mutations were detected in the second
measurement, which may explain the progressive disease clinical
status assigned at this stage. ARMS-PCR for two loci is sufficient
to detect the presence or absence of specific mutations; however,
NGS is able to identify high numbers of simultaneous mutations
(including common actionable mutations, EGFR p.L858R and EGFR
p.T790M (Fig. 3) which ARMS-PCR
cannot achieve (39).
Discussion
In the present study, a targeted sequencing approach
based on the Illumina platform was used to test variations in NSCLC
driver genes, including point mutations, insertions, deletions and
gene rearrangements simultaneously in plasma samples from 32
Chinese patients with advanced NSCLC. To elucidate the association
between drug effect and mutation status, mutation profiles were
constructed for 32 patients with NSCLC. Furthermore, the EGFR
status was dynamically monitored in order to construct a dynamic
resistance gene detection system.
As the results demonstrated, for patients with
NSCLC, a large number of gene mutations may occur. Besides these
common actionable mutations, including
NM_005228.4(EGFR):c.2573T>G (p.Leu858Arg), EGFR Exon 19
deletions and NM_005228.4(EGFR):c.2369C>T (p.Thr790Met), others
may also be used as biomarkers for prognosis. Single or small
numbers of target mutations can be detected using conventional
single locus testing methods such as ARMS-PCR; however, such
techniques do not indicate other, potentially unexpected, mutations
(40). On the other hand, NGS is able
to detect numerous gene mutations simultaneously (40,41). The
present study investigated >25 genes, constructed overall
mutation landscapes for individual patients and performed blood
collection rather than tissue biopsy. Another advantage of the
present study is the use of continuous samples from the same
individuals instead of isolated samples.
In recent years, ctDNA has been demonstrated to be
an effective material for tumor DNA monitoring. In the present
study, it was demonstrated that ctDNA may be used to measure tumor
burden (number of cancer cells, the size of a tumor or the amount
of cancer in the body). The concordance between ctDNA and tissue
immunohistochemistry testing of mutation variation is ~50%;
considering that the occurrence of novel mutations and the
disappearance of existing mutations is constant due to evolving
tumors, this is a relatively high similarity rate (42). This may be due to release of genomic
DNA from necrotic white blood cells into blood, and diluted ctDNA
in the plasma (43). The amount of
ctDNA in patients is also likely to be associated with metastasis,
vascularity, cellular turnover and response to therapy (44,45). In
addition, ctDNA may be influenced by clearance, degradation and
other physiological filtering events involving blood, and lymphatic
circulation (43).
While the sample size of 36 is not large, all
samples represented advanced NSCLC and were associated with
complete clinical information. Therefore, the samples used in the
present study were of high quality and consistency. The clinical
consequence of EGFR-mutation-derived NSCLC demonstrated
inter-patient variations following the initially rapid response to
EGFR-TKIs. For example, certain patients experience a rapid disease
flare just subsequent to the cessation of EGFR-TKI treatment, while
others do not (46). Repeated
treatment with an EGFR-TKI is effective in certain patients, while
ineffective in others (47). These
results indicate that the contribution of driver mutations vary
between patients and change during the clinical courses. Therefore,
the monitoring of EGFR gene mutation status is critical. Currently,
re-biopsy is recommended to achieve such monitoring by tissue
sample sequencing. However, tumor biopsy is usually invasive and
difficult to repeat in solid tumors. Since genetic changes appear
to accumulate over time in patients with NSCLC and different
portions of a tumor carry different genetic profiles, single biopsy
of a tumor is spatially and temporally limited, and may fail to
monitor dynamics of cancer progression (48,49). The
advantages of using ctDNA for dynamic genetic monitoring studies
are demonstrated in this study, which suggests that this method
should be applied more widely in clinical practice.
Acknowledgements
The authors would like to thank Liwei Deng and Cheng
Jin for their input to diagram presentation. The present study was
supported by the National Science Foundation of China (grant no.
61472411) for high performance servers and publication cost. The
Technology Development and Creative Design Program of Nanshan
Shenzhen (grant no. KC2015JSJS0028A) funded data collection, and
the Special Funds for Future Industries of Shenzhen (grant no.
JSGG20160229123927512) funded the sequencer and reagents.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alamgeer M, Ganju V and Watkins DN: Novel
therapeutic targets in non-small cell lung cancer. Curr Opin
Pharmacol. 13:394–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Savas P, Hughes B and Solomon B: Targeted
therapy in lung cancer: IPASS and beyond, keeping abreast of the
explosion of targeted therapies for lung cancer. J Thorac Dis. 5
Suppl 5:S579–S592. 2013.PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dearden S, Stevens J, Wu YL and Blowers D:
Mutation incidence and coincidence in non small-cell lung cancer:
Meta-analyses by ethnicity and histology (mutMap). Ann Oncol.
24:2371–2376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arteaga CL: The epidermal growth factor
receptor: From mutant oncogene in nonhuman cancers to therapeutic
target in human neoplasia. J Clin Oncol. 19 Suppl 1:32S–40S.
2001.PubMed/NCBI
|
|
7
|
Shigematsu H, Lin L, Takahashi T, Nomura
M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et
al: Clinical and biological features associated with epidermal
growth factor receptor gene mutations in lung cancers. J Natl
Cancer Inst. 97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang JC, Hirsh V, Schuler M, Yamamoto N,
O'Byrne KJ, Mok TS, Zazulina V, Shahidi M, Lungershausen J, Massey
D, et al: Symptom control and quality of life in LUX-Lung 3: A
phase III study of afatinib or cisplatin/pemetrexed in patients
with advanced lung adenocarcinoma with EGFR mutations. J Clin
Oncol. 31:3342–3350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang
Y, Li W, Hou M, Shi JH, Lee KY, et al: Afatinib versus cisplatin
plus gemcitabine for first-line treatment of Asian patients with
advanced non-small-cell lung cancer harbouring EGFR mutations
(LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet
Oncol. 15:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobayashi S, Boggon TJ, Dayaram T, Jänne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris MG and Varmus H: Acquired resistance of
lung adenocarcinomas to gefitinib or erlotinib is associated with a
second mutation in the EGFR kinase domain. PLoS Med. 2:e732005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balak MN, Gong Y, Riely GJ, Somwar R, Li
AR, Zakowski MF, Chiang A, Yang G, Ouerfelli O, Kris MG, et al:
Novel D761Y and common secondary T790M mutations in epidermal
growth factor receptor-mutant lung adenocarcinomas with acquired
resistance to kinase inhibitors. Clin Cancer Res. 12:6494–6501.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bean J, Brennan C, Shih JY, Riely G, Viale
A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, et al: MET
amplification occurs with or without T790M mutations in EGFR mutant
lung tumors with acquired resistance to gefitinib or erlotinib.
Proc Natl Acad Sci USA. 104:pp. 20932–20937. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Camidge DR, Pao W and Sequist LV: Acquired
resistance to TKIs in solid tumours: Learning from lung cancer. Nat
Rev Clin Oncol. 11:473–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohashi K, Sequist LV, Arcila ME, Moran T,
Chmielecki J, Lin YL, Pan Y, Wang L, de Stanchina E, Shien K, et
al: Lung cancers with acquired resistance to EGFR inhibitors
occasionally harbor BRAF gene mutations but lack mutations in KRAS,
NRAS, or MEK1. Proc Natl Acad Sci USA. 109:pp. E2127–E2133. 2012;
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sacher AG, Jänne PA and Oxnard GR:
Management of acquired resistance to epidermal growth factor
receptor kinase inhibitors in patients with advanced non-small cell
lung cancer. Cancer. 120:2289–2298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zakowski MF, Ladanyi M and Kris MG;
Memorial Sloan-Kettering Cancer Center Lung Cancer OncoGenome
Group, : EGFR mutations in small-cell lung cancers in patients who
have never smoked. N Engl J Med. 355:213–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cross DA, Ashton SE, Ghiorghiu S, Eberlein
C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ,
et al: AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated
resistance to EGFR inhibitors in lung cancer. Cancer Discov.
4:1046–1061. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mandel P and Metais P: Les acides
nucléiques du plasma sanguine chez l'homme. CR Seances Soc Biol
Fil. 142:241–243. 1948.(In Undetermined Language).
|
|
26
|
Leon SA, Shapiro B, Sklaroff DM and Yaros
MJ: Free DNA in the serum of cancer patients and the effect of
therapy. Cancer Res. 37:646–650. 1977.PubMed/NCBI
|
|
27
|
Qiu M, Wang J, Xu Y, Ding X, Li M, Jiang
F, Xu L and Yin R: Circulating tumor DNA Is effective for the
detection of EGFR mutation in non-small cell lung cancer: A
meta-analysis. Cancer Epidemiol Biomarkers Prev. 24:206–212. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schwarzenbach H, Hoon DS and Pantel K:
Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev
Cancer. 11:426–437. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen KZ, Lou F, Yang F, Zhang JB, Ye H,
Chen W, Guan T, Zhao MY, Su XX, Shi R, et al: Circulating tumor DNA
detection in early-stage non-small cell lung cancer patients by
targeted sequencing. Sci Rep. 6:319852016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beaver JA, Jelovac D, Balukrishna S,
Cochran R, Croessmann S, Zabransky DJ, Wong HY, Toro PV, Cidado J,
Blair BG, et al: Detection of cancer DNA in plasma of patients with
early-stage breast cancer. Clin Cancer Res. 20:2643–2650. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng D, Ye X, Zhang MZ, Sun Y, Wang JY,
Ni J, Zhang HP, Zhang L, Luo J, Zhang J, et al: Plasma EGFR T790M
ctDNA status is associated with clinical outcome in advanced NSCLC
patients with acquired EGFR-TKI resistance. Sci Rep. 6:209132016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sorensen BS, Wu L, Wei W, Tsai J, Weber B,
Nexo E and Meldgaard P: Monitoring of epidermal growth factor
receptor tyrosine kinase inhibitor-sensitizing and resistance
mutations in the plasma DNA of patients with advanced non-small
cell lung cancer during treatment with erlotinib. Cancer.
120:3896–3901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thress KS, Paweletz CP, Felip E, Cho BC,
Stetson D, Dougherty B, Lai Z, Markovets A, Vivancos A, Kuang Y, et
al: Acquired EGFR C797S mutation mediates resistance to AZD9291 in
non-small cell lung cancer harboring EGFR T790M. Nat Med.
21:560–562. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li H and Durbin R: Fast and accurate
long-read alignment with Burrows-Wheeler transform. Bioinformatics.
26:589–595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li H, Handsaker B, Wysoker A, Fennell T,
Ruan J, Homer N, Marth G, Abecasis G and Durbin R; 1000 Genome
Project Data Processing Subgroup, : The Sequence Alignment/Map
format and SAMtools. Bioinformatics. 25:2078–2079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koboldt DC, Larson DE and Wilson RK: Using
VarScan 2 for germline variant calling and somatic mutation
detection. Curr Protoc Bioinformatics. 44:15.4.1–17. 2013.
View Article : Google Scholar
|
|
38
|
Thress KS, Brant R, Carr TH, Dearden S,
Jenkins S, Brown H, Hammett T, Cantarini M and Barrett JC: EGFR
mutation detection in ctDNA from NSCLC patient plasma: A
cross-platform comparison of technologies to support the clinical
development of AZD9291. J Clin Oncol. 90:509–515. 2015.
|
|
39
|
Xu T, Kang X, You X, Dai L, Tian D, Yan W,
Yang Y, Xiong H, Liang Z, Zhao GQ, et al: Cross-platform comparison
of four leading technologies for detecting EGFR mutations in
circulating tumor dna from non-small cell lung carcinoma patient
plasma. Theranostics. 7:1437–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sacher AG, Paweletz C, Dahlberg SE, Alden
RS, O'Connell A, Feeney N, Feeney N, Mach SL, Jänne PA and Oxnard
GR: Prospective validation of rapid plasma genotyping for the
detection of EGFR and KRAS mutations in advanced lung cancer. JAMA
Oncol. 2:1014–1022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jänne PA, Yang JC, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fenizia F, De Luca A, Pasquale R, Sacco A,
Forgione L, Lambiase M, Iannaccone A, Chicchinelli N, Franco R,
Rossi A, et al: EGFR mutations in lung cancer: From tissue testing
to liquid biopsy. Future Oncol. 11:1611–1623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schwarzenbach H, Hoon DS and Pantel K:
Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev
Cancer. 11:426–437. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Diehl F, Schmidt K, Choti MA, Romans K,
Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al:
Circulating mutant DNA to assess tumor dynamics. Nat Med.
14:985–990. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kohler C, Barekati Z, Radpour R and Zhong
XY: Cell-free DNA in the circulation as a potential cancer
biomarker. Anticancer Res. 31:2623–2628. 2011.PubMed/NCBI
|
|
46
|
Chaft JE, Oxnard GR, Sima CS, Kris MG,
Miller VA and Riely GJ: Disease flare after tyrosine kinase
inhibitor discontinuation in patients with EGFR-mutant lung cancer
and acquired resistance to erlotinib or gefitinib: Implications for
clinical trial design. Clin Cancer Res. 17:6298–6303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nishino K, Imamura F, Morita S, Mori M,
Komuta K, Kijima T, Namba Y, Kumagai T, Yamamoto S, Tachibana I, et
al: A retrospective analysis of 335 Japanese lung cancer patients
who responded to initial gefitinib treatment. Lung Cancer.
82:299–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gerlinger M, Rowan AJ, Horswell S, Math M,
Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N,
Stewart A, et al: Intratumor heterogeneity and branched evolution
revealed by multiregion sequencing. N Engl J Med. 366:883–892.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang J, Fujimoto J, Zhang J, Wedge DC,
Song X, Zhang J, Seth S, Chow CW, Cao Y, Gumbs C, et al: Intratumor
heterogeneity in localized lung adenocarcinomas delineated by
multiregion sequencing. Science. 346:256–259. 2014. View Article : Google Scholar : PubMed/NCBI
|