Introduction
Gastric cancer is a common disease and a leading
cause of cancer-associated mortality. According to a statistical
study, ~70% of patients with gastric cancer in 2006 had lymph node
metastasis at the time of diagnosis, leading to a median overall
survival time of 16.7 months (1).
Despite the standardization of surgical techniques and multimodal
therapy, the postoperative survival of patients with advanced
gastric cancer remains low in China due to the rates of invasion
and metastasis (2).
Epithelial-mesenchymal transition (EMT) is a process
by which cells lose epithelial characteristics and acquire
mesenchymal properties, including the loss of all cell-cell
contact, increased motility and invasion (3). Previous studies have indicated that the
EMT phenotype is associated with advanced gastric cancer stages,
that EMT is a key gastric cancer progression driver and that it
serves a fundamental role during the early stages of gastric cancer
invasion and metastasis (4).
Investigating the potential mechanisms that modulate gastric cancer
cell EMT and characterizing novel EMT regulators will increase the
understanding of gastric cancer biology; it may also aid
identification of novel biomarkers for the early detection of
gastric cancer and of potentially efficient targets for
preventative and curative anti-gastric cancer intervention
approaches, preventing local and distant invasions (5).
Interleukin-6 (IL-6) is a cytokine that participates
in acute inflammation (6). A previous
study has indicated that IL-6 led to EMT-associated changes via
zinc finger protein SNAI (Snail) signaling pathway activation, and
is involved in tumor progression, which is an important factor in
tumor development (7). Elevated
levels of IL-6 are associated with poor prognosis for a number of
types of cancer (8).
Previous data have indicated that protein inhibitor
of activated signal transducer and activator of transcription 1
(PIAS1), a downstream target protein of the signal transducer and
activator of transcription (STAT) signaling pathway inhibitor, is
associated with the anti-inflammatory response through the negative
regulation of the STAT1 signaling pathway, which mediates
inflammatory cell adhesion and inhibits inflammatory injury
(9). A previous study demonstrated
that the expression of PIAS1 in certain cancer cells was
significantly downregulated or lost altogether (10). Therefore, PIAS1 functions as an
inflammatory inhibitor and serves a role in the inhibition of
cancer cell growth. A previous study indicated that the PIAS1
expression was primarily observed in the tissues of patients with
gastric cancer, indicating that PIAS1 may be involved in the
pathogenesis of cancer and verifying that PIAS1 may act as a marker
for the preclinical detection and clinical assessment of patients
with gastric cancer (11).
Concurrently, the overexpression of PIAS1 protein was also
demonstrated to inhibit the migration and invasion of gastric
cancer cells (11). However, the
mechanisms of its effect of gastric cancer were unclear.
On the basis of the aforementioned previous results,
we hypothesized that the effects of PIAS1 may be due to the
suppression of EMT by the phosphatidylinositol 3-kinase
(PI3K)/serine/threonine kinase (Akt)/Snail signaling pathway and
downregulated matrix metalloproteinase 9 (MMP-9) expression, which
causes suppression of cell growth in vitro. To examine this
hypothesis, the gastric cancer SGC7901 cell line was used, and the
migration response of cells with upregulated PIAS1 expression was
studied, indicating its potential effective mechanism for gastric
cancer treatment.
Materials and methods
Reagents
A total of 10 rabbit or mouse monoclonal antibodies
against epithelial (E)-cadherin (cat. no. SC71009), vimentin (cat.
no. SC6260) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA), Snail (cat. no. 3879), Twist-related protein 1
(Twist 1; cat. no. 46702), PIAS1 (cat. no. 3550), PI3K (cat. no.
4292), phosphorylated (P)-PI3K (cat. no. 4228), Akt (cat. no.
4685), P-Akt (cat. no. 4060) and MMP-9 (cat. no. 2270) were
purchased from Cell Signaling Technology (CST; Beverly, MA, USA).
Mouse monoclonal antibody against GAPDH (cat. no. AF1186) and
horseradish peroxidase-conjugated goat anti-rabbit or mouse IgG
were purchased from Shanghai Biyuntian Bio-Technology Co., Ltd.
(Shanghai, China). Recombinant human IL-6 was produced by R&D
Systems, Inc. (Minneapolis, MN, USA).
The human gastric cancer SGC7901 cell line used in
the present study was provided by Ruijin Hospital, Shanghai
Jiaotong University School of Medicine (Shanghai, China) and
maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.), with addition of 100 U/ml
penicillin and 100 µg/ml streptomycin (Sigma-Aldrich; Merck, KGaA,
Darmstadt, Germany) at 37°C and 5% CO2.
Construction of expression
plasmid
A replication-defective adenovirus serotype 5/F35
(Ad5/F35) was used as the vector (AGTC Gene Technology Co., Ltd.,
Beijing, China). Ad5/F35-PIAS1 was constructed by Shanghai Hongming
Biotechnology Co. Ltd. (Shanghai, China) as follows: PIAS1 cDNA
containing the full-length translated regions was obtained using
the polymerase chain reaction with the following primers: Forward
(EcoR V site is underlined);
5′-GCCGATATCATGGCGGACAGTGCGGAACTAAAGCAAATG-3′ and
5′-ATTAAGCTTTCAGTCCAACGAGATAATGTCTGGTATAGT-3′ (reverse, HindIII
site is underlined). The sequence of PIAS1 was deposited in the
GenBank database (cat no. AF167160.1) prior to being sub-cloned
into 5 ul PDC316-MCMV-EGFP transfer plasmid (Microbix Biosystems
Inc., Ontario, Canada) with green fluorescence by insertion of a
fragment of green fluorescence protein (GFP) as previously
described (12). This plasmid was
co-transfected [multiplicity of infection (MOI), 10] into 293 cells
(Ruijin Hospital, Shanghai Jiaotong University School of Medicine,
Shanghai, China) using lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), that were grown in Dulbecco modified
Eagle medium plus 10% fetal calf serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), penicillin, and streptomycin at 37°C in a 5%
CO2 incubator. Additionally, an Ad5/F35 plasmid
containing an empty expression cassette was constructed for use as
a control. All of the viral constructs were similar, with the
exception of the PIAS1 gene, and the production and purification
procedures were identical to our previous study (13).
Cell culture and gene
transduction
When the SGC7901 cells reached 70% confluence, the
process of transfection of Ad5/F35-PIAS1 (MOI, 10) was performed in
Ad5/F35-PIAS1-treated cells. Concurrently, an additional group of
SGC7901 cells were transfected with MOI 5 of the empty
Ad5/F35-vector as Ad5/F35-null-treated cells. A third group of
cells that underwent PBS treatment served as control cells. The
samples were harvested for additional experiments at 24 h following
transduction.
Fluorescence microscopy
The Ad5/F35-PIAS1-treated cells,
Ad5/F35-null-treated cells and control cells were placed on glass
slides and covered with a cover glass and the fluorescence of GFP
was observed using an Olympus IX51 fluorescence microscope (Olympus
Corporation, Tokyo, Japan).
Establishment of cell inflammatory
microenvironment and groups
In the present study, the cells were divided into
three groups: i) The IL-6-treated group: IL-6 (R&D Systems,
Inc.). was added to FBS-free medium at a concentration of 50 ng/ml
for 72 h in SGC7901 cells; ii) the Ad5/F35-PIAS1+IL-6-treated
group: Ad5/F35-PIAS1 (MOI10) was added to SGC7901 cells following
48 h of IL-6 treatment; iii) the Ad5/F35-null+IL-6-treated group:
Ad5/F35-null (MOI 5) was added to SGC7901 cells after 48 h of IL-6
treatment. The samples were harvested for additional experiments at
24 h following transduction.
Assessment of cell viability by MTT
assay
A total of 2×103 SGC7901 cells from each
group were seeded onto a 96-well plate. After 24 h, 20 µl MTT (5
mg/ml) was added to each well. After 4 h, 100 µl dimethyl sulfoxide
was added to each well subsequent to removal of the medium.
Finally, the absorbance was detected with an enzyme calibrator at
570 nm and the cell viability (%) was calculated: (A570
of study group/A570 of control group) ×100.
PIAS1 expression in cells by reverse
transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from cells of each group
using a TRIzol® reagent kit (Life Technologies; Thermo
Fisher Scientific, Inc.). Samples were analyzed with the Tiangen
PCR purification kit (Tiangen Biotech Co., Ltd., Beijing, China),
and the cDNA obtained from this reaction was mixed with a 50-µl
reaction volume containing: 1X PCR buffer with 1.5 mM
MgCl2, 0.2 mM deoxynucleotide triphosphates (dNTPs),
0.05 U/µl Taq polymerase and 2 µM human PIAS1 gene-specific primers
(PIAS1 forward primer: 5′-CCACGCCTTCCTGCTGTAGA-′3; PIAS1 reverse
primer: 5′-TATCACACAGGCAGTCTTAGAT-′3) and amplified in an automated
thermal cycler. The conditions of RT-PCR were as follows: 1 cycle
for 5 min at 95°C; then 35 cycles for 45 sec at 94°C, 45 sec at
55°C, and 1 min at 72°C; then 1 cycle for 10 min at 72°C. The PCR
products were separated by electrophoresis using 1.2% agarose gels
and stained with ethidium bromide. The densities of cDNA bands were
analyzed by scanning densitometry using the Image Analysis Gel Doc
2000 system and Quantity One software (version 4.4.0; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The band densities were
normalized to the GAPDH (the primer sequence of GAPDH, the forward
primer: 5′GGCTGAGAACGGGAAGCTTGTC′3; the reverse primer:
5′CAGCCTTCTCCATGGTGGTGAAGA′3) band densities and the results were
expressed as a ratio.
Scratch wound-healing assay
To measure cell motility, 4×105 SGC7901
cells from each group were seeded in 6-well plates. A central
linear wound was created by scraping the cell monolayer with a 200
µl sterile pipette tip. The media were carefully changed to remove
any floating cells and cells were cultured at 5% CO2 and
37°C for 24 h prior to fixation with 4% paraformaldehyde for 1 h at
room temperature. The migration of cells into the empty areas in
the scraped region was observed at 24 h and images of each plate
were captured with a light inverted microscope (Olympus IX53;
magnification, ×200; Olympus Corporation, Tokyo, Japan) fitted with
a digital camera (Olympus cellSens Standard; Olympus Corporation).
The wound at 0 h was considered to be 100% of the average gap, and
the percentage of the cleaned area at 24 h compared with time 0 was
measured using Image Pro plus version 6.2 software (Media
Cybernetics, Rockville, MD, USA).
Cell invasion assay
For the determination of cell invasion capabilities,
Transwell chambers (Corning Incorporated, Corning, NY, USA) were
used. The Transwell chamber membranes were covered with 75 µl
Matrigel (2 mg/ml; BD Biosciences, Franklin Lakes, NJ, USA) and
incubated for 2 h at 37°C. The SGC7901 cells from each group were
grown in RPMI-1640 (10% FBS) medium were trypsinized and suspended
at a density of 1×106/ml in serum-free RPMI-1640 medium.
A total of 200 µl cell suspension was placed into the upper
compartment of the Transwell chambers. The lower compartment of the
chambers was filled with 600 µl RPMI-1640 containing 10% FBS. The
Transwell chamber systems were incubated in the humidified
incubator at 37°C and 5% CO2 for 24 h. Following
incubation, non-invaded cells and Matrigel were removed from the
top of the chamber with a cotton bud. At the bottom of the cell
membrane, invaded cells were washed three times with PBS, fixed
with 4% paraformaldehyde for 1 h at room temperature, prior to the
paraformaldehyde being discarded. Subsequently, the membranes were
air-dried and stained with 1% Giemsa solution for 15 min at room
temperature. A total of 5 random fields were observed under an
inverted microscope (magnification, ×200) and the invasion rate was
calculated to find the number of the invaded cells. The data
representing the average cells of 5 fields were compared between
the experimental and control groups. Each experiment was repeated
three times.
EMT alterations of cells
The cell morphology in each group was observed in
each of 5 randomly selected high-power fields using an inverted
light microscope (Olympus IX53; magnification, ×200; Olympus
Corporation) fitted with a digital camera (Olympus cellSens
Standard; Olympus Corporation).
Western blotting analysis
The SGC7901 cells from each group were washed twice
with PBS and then homogenized in radio immunoprecipitation assay
buffer. Following centrifugation at 12,000 × g at 4°C for 10 min,
the supernatant was collected and stored at 80°C. The protein
concentration of each sample was determined by BCA protein assay.
Each sample was adjusted up to a desired protein content of 40 µg
per lane, denatured in loading buffer and separated by
electrophoresis on 9% SDS-PAGE at 100 V for 120 min. The separated
proteins were transferred to polyvinylidene difluoride membrane
using transfer buffer at 200 mA for 90 min. The membranes were
blocked with 5% non-fat dry milk powder in TBS-0.1% Tween-20 for 1
h at room temperature, washed three times for 10 min each in
TBS-0.1% Tween-20, and incubated with primary antibodies against
E-cadherin, Snail, Twist 1, vimentin, PI3K, P-PI3K, Akt, P-Akt and
MMP-9 with 1:1,000 dilution in TBS-0.1% Tween-20 overnight at 4°C.
Membranes were washed three times for 10 min in TBS-0.1% Tween, the
membranes were incubated with a second antibody, horseradish
peroxidase-conjugated goat anti-rabbit IgG (cat. no. A0208;
dilution, 1:500) or goat anti-mouse IgG (cat. no. A0216; dilution,
1:500) for 1 h at room temperature. Subsequent to washing three
times with PBS, the membranes were detected using an Amersham
enhanced chemiluminescence Western Blotting Detection kit
(Amersham; GE Healthcare, Chicago, IL, USA), and then underwent
densitometry using the Image Analysis Gel Doc 2000 system and
Quantity One software version 4.4.0. GAPDH was determined in the
similar manner with anti-GAPDH antibody (dilution, 1:500) as an
endogenous control for other proteins.
Statistical analysis
All data were expressed as the mean ± standard
deviation. Statistics were performed by SPSS version 13.0 (SPSS,
Inc., Chicago, IL, USA). One-way analysis of variance with
Dunnett's multiple comparison tests was used to perform comparisons
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
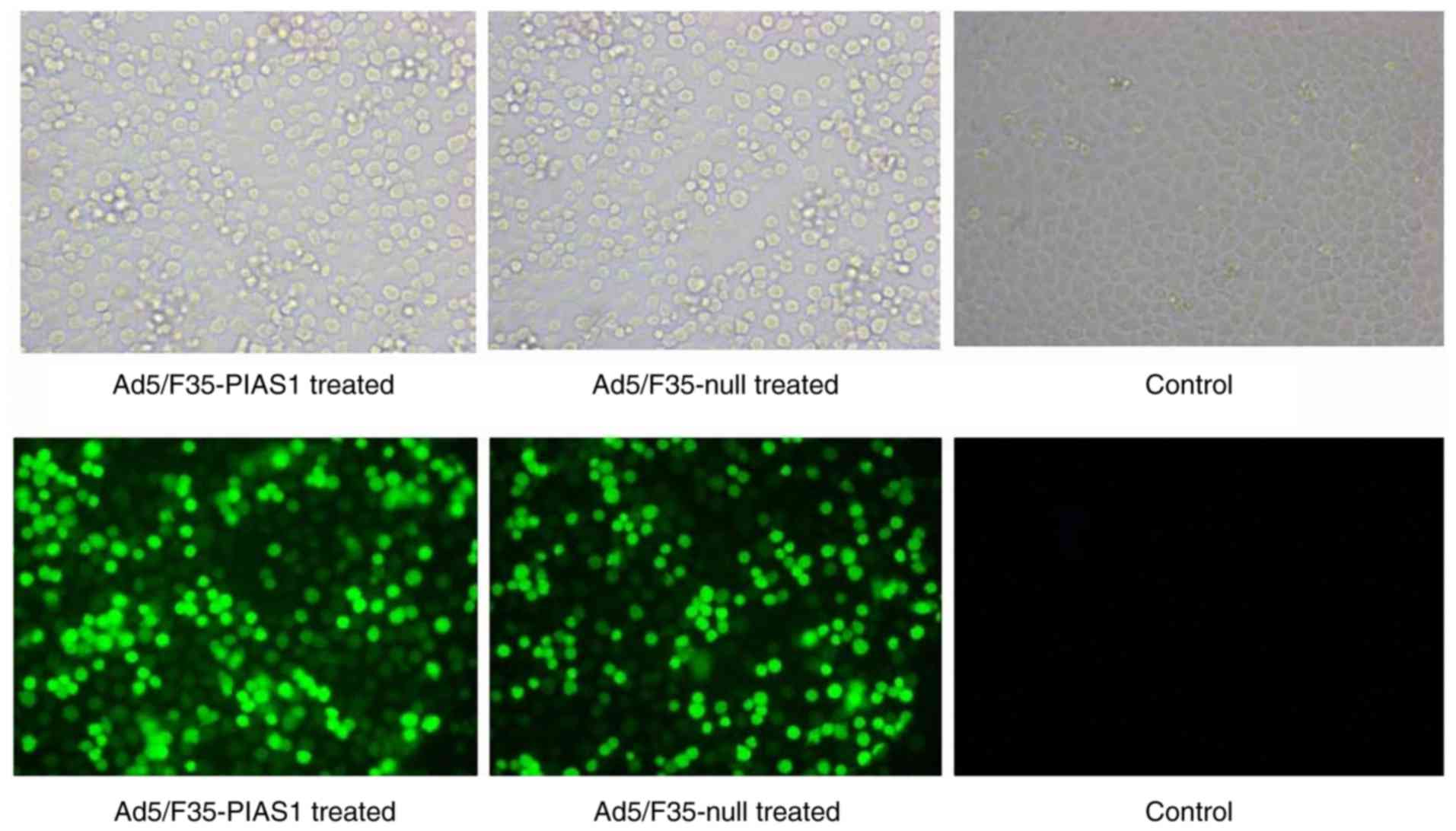
Transfection capability of
Ad5/F35-PIAS1
According to the results of immunofluorescence
microscopy, the cells of Ad5/F35-PIAS1 and Ad5/F35-null
transfection exhibited high fluorescent intensity, but the control
cells demonstrated a fluorescent intensity (Fig. 1).
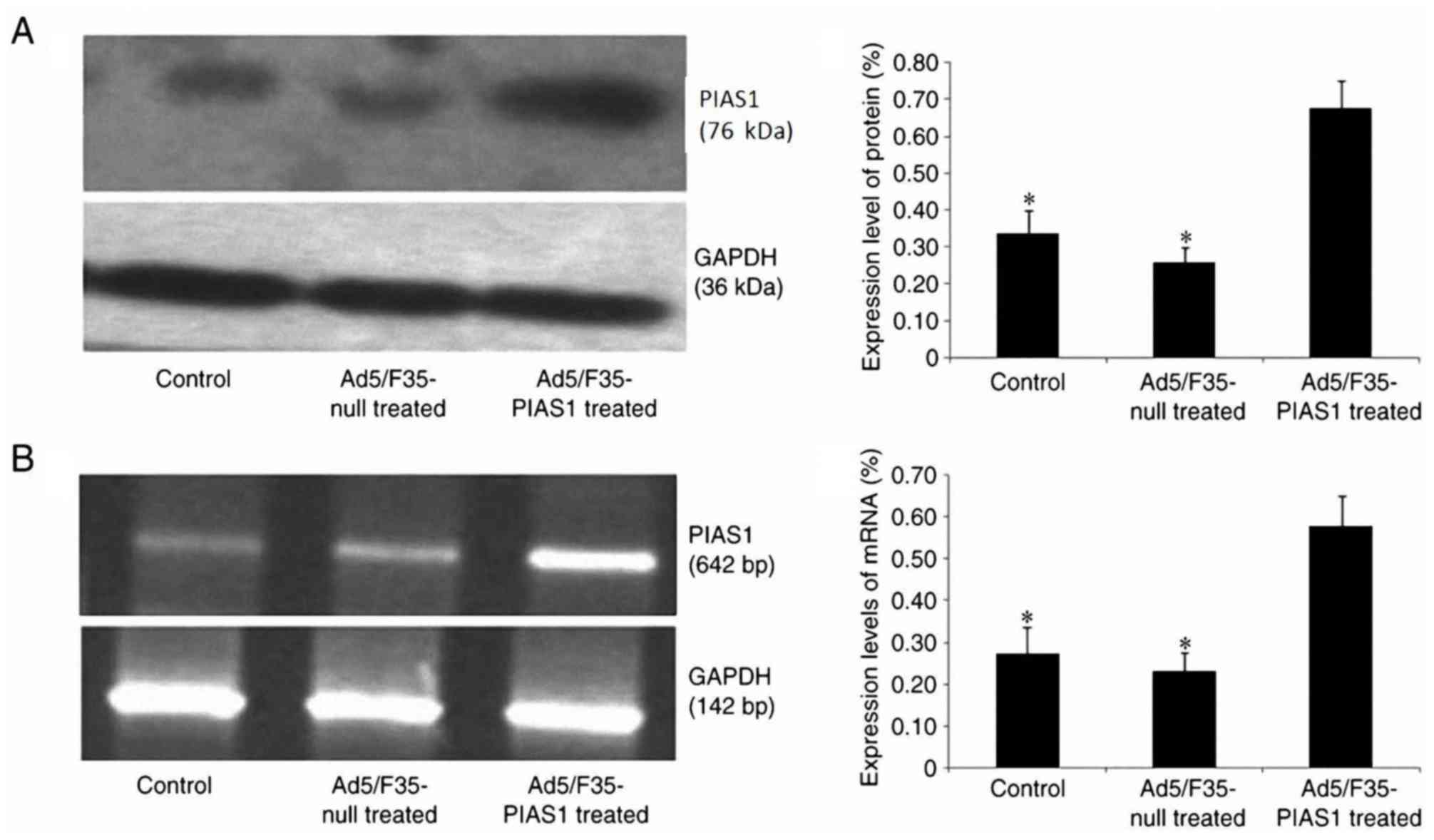
PIAS1 gene mRNA and protein expression
in gastric cancer cell
According to western blotting and RT-PCR analyses,
following 24 h of Ad5/F35-PIAS1 transfection, the PIAS1 protein
(Fig. 2A) and mRNA (Fig. 2B) expression levels in SGC7901 cells
were significantly increased when compared with control and
Ad5/F35-null-transfected cells (P<0.01).
Assessment of cell viability
The cell viability rate of Ad5/F35-PIAS1+IL-6 cells
(42.2±12.3%) was significantly decreased compared with that of IL-6
(72.4±11.2%) or Ad5/F35-null+IL-6 (69.7±9.8%)-treated cells
(P<0.01) (Data not shown).
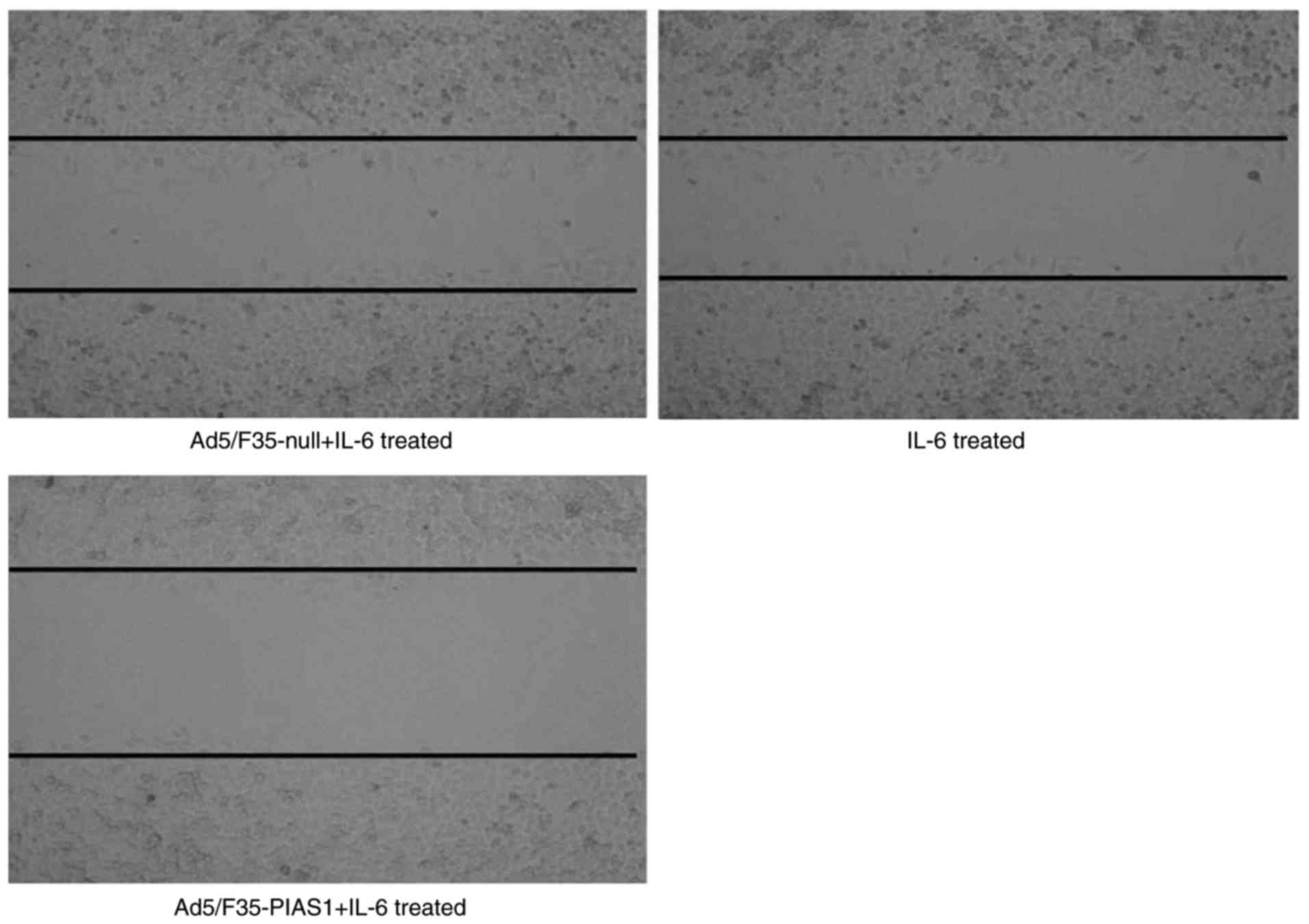
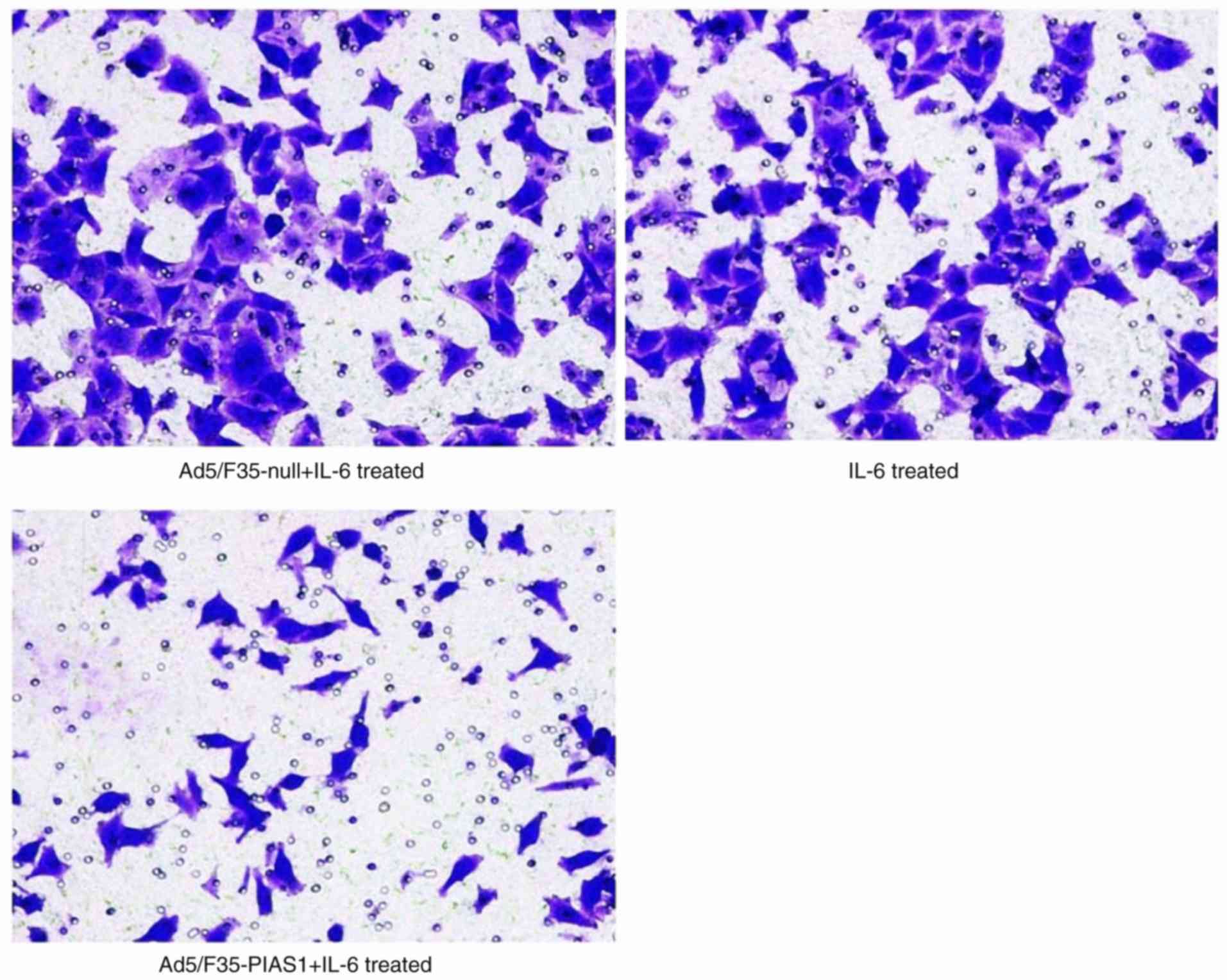
Cell invasion and migration assay
IL-6 treatment increased the migratory activity of
the SGC7901 cells, and treatment with Ad5/F35-PIAS1 inhibited
IL-6-induced migration. The scratch wound healing assay indicated
that Ad5/F35-PIAS1+IL-6-treated cells (9.80±2.22%) exhibited a
slower migration rate compared with IL-6 (19.03±2.70%) and
Ad5/F35-null+IL-6-treated cells (18.42±4.42%; Fig. 3). Similarly, the Transwell migration
assay demonstrated that the Ad5/F35-PIAS1+IL-6-treated cells
(28.44±3.57) were associated with significantly lower migration
than IL-6- (54.44±7.74) and Ad5/F35-null+IL-6-treated cells
(53.22±11.50; P<0.01; Fig. 4).
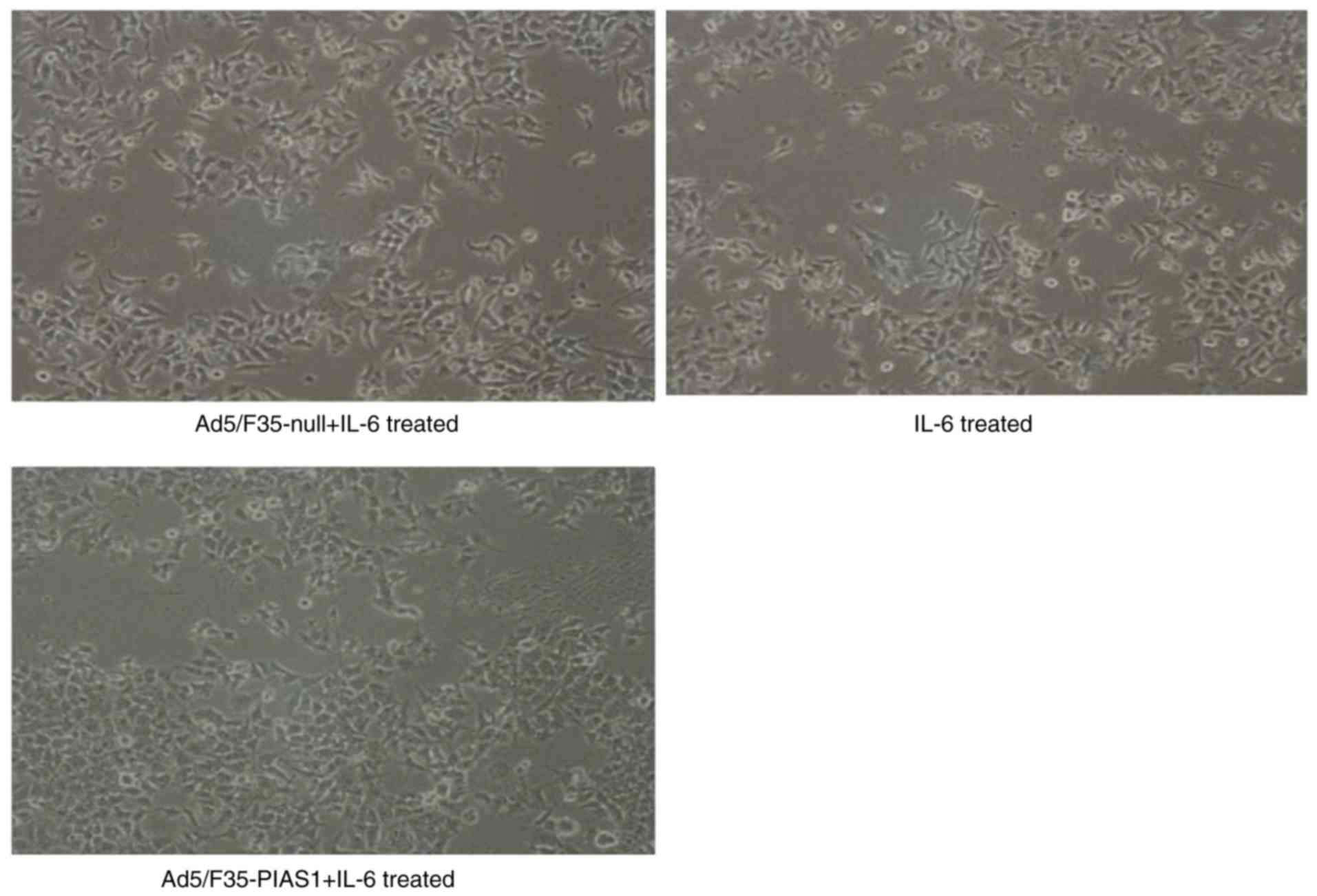
EMT alterations in gastric cancer
cells
The spacing of IL-6- and Ad5/F35-null+IL-6-treated
SGC7901 cells was more spread out and had lost their cell-cell
contacts, whereas the Ad5/F35-PIAS1+IL-6-treated cells remained
tightly attached with typical epithelial cell characteristics
(Fig. 5).
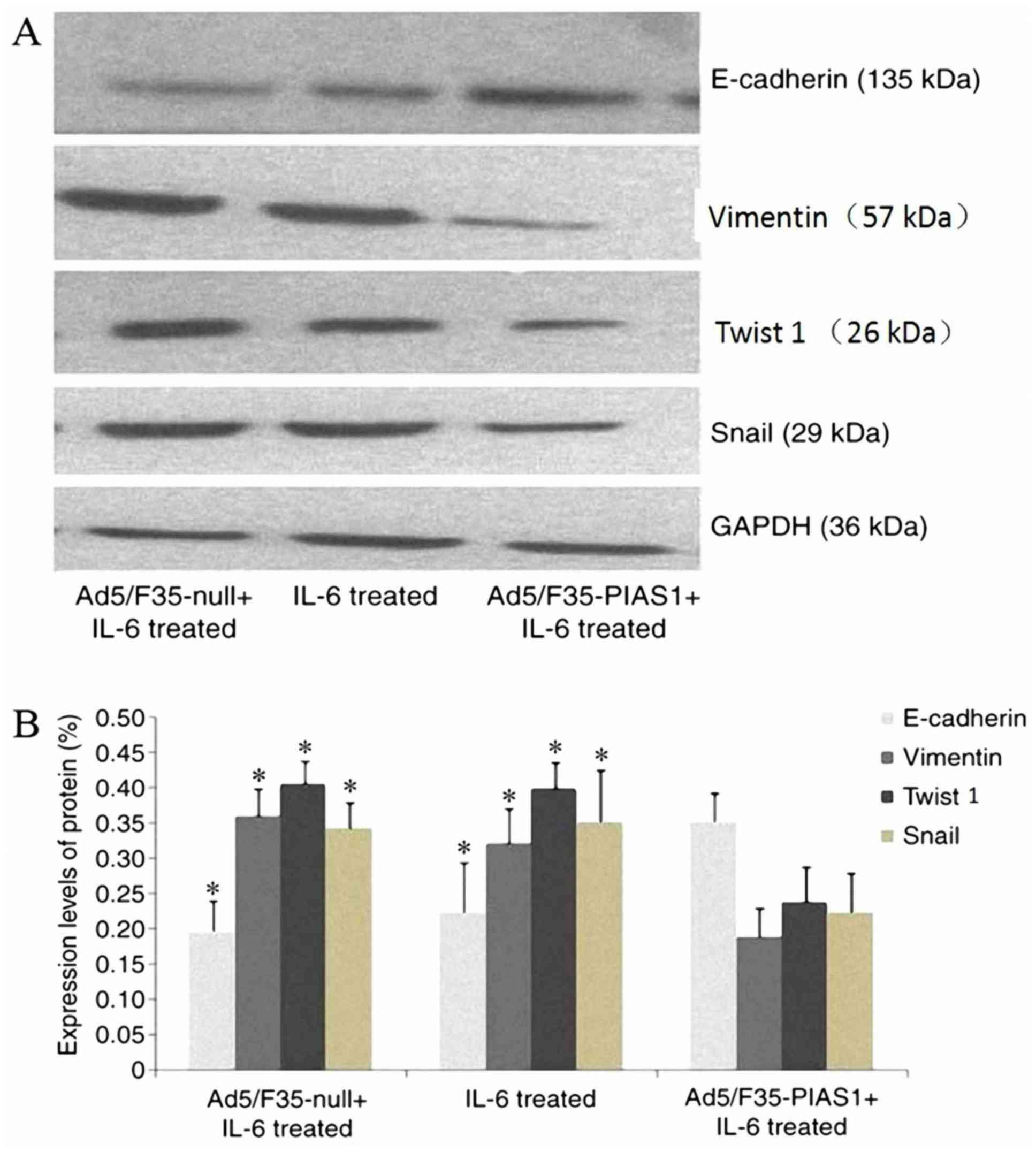
Effect of PIAS1 on EMT in gastric
cancer
To confirm the effect of PIAS1 on the change in EMT
of gastric cancer cells, western blotting was performed for EMT
markers; the results indicated that the protein expression of
Snail, Twist 1 and vimentin were decreased in
Ad5/F35-PIAS1+IL-6-treated cells; however, the E-cadherin protein
expression level was increased when compared with those of IL-6 and
Ad5/F35-null+IL-6-treated cells (P<0.01, respectively; Fig. 6).
PIAS1 suppresses EMT by inhibiting the
PI3K/Akt-MMP-9 signaling pathway
As demonstrated by western blotting, IL-6 promoted
the activation of PI3K-Akt and increased MMP-9 protein expression.
However, a decrease in the expression levels of total PI3K and Akt
protein was observed in the Ad5/F35-PIAS1+IL-6-treated cells when
compared with those of IL-6 and Ad5/F35-null+IL-6-treated cells
(P<0.01, respectively). Densitometry results also suggested that
the expression levels of MMP-9 protein in the
Ad5/F35-PIAS1+IL-6-treated cells were significantly decreased
compared with that of the IL-6 and Ad5/F35-null+IL-6-treated cells
(P<0.01, respectively; Fig.
7).
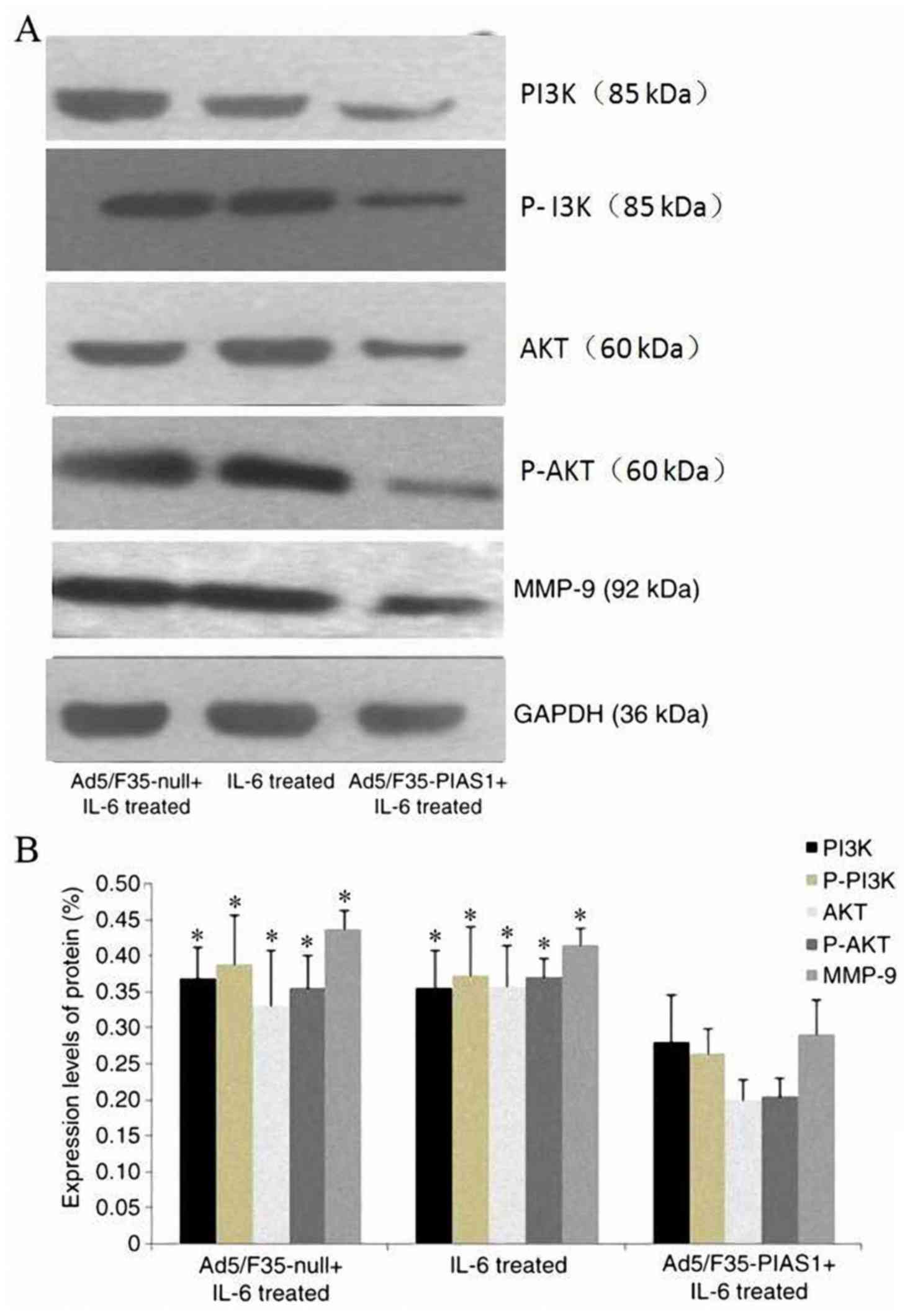 | Figure 7.Expression levels of proteins involved
in P-PI3K, PI3K, P-Akt, Akt and MMP-9 in SGC7901 cells incubated
with Ad5/F35-null+IL-6, IL-6, Ad5/F35-PIAS1+IL-6 were investigated
using (A) western blotting and (B) densitometric analysis of the
western blotting data. *P<0.01 vs. Ad5/F35-PIAS1+IL-6 treated
group. PI3K, phosphatidylinositol 3-kinase; P-PI3K, phosphorylated
PI3K; Akt, serine/threonine kinase; P-Akt, phosphorylated Akt;
MMP-9, matrix metallopeptidase 9; PIAS1, protein inhibitor of
activated signal transducer and activator of transcription 1; IL-6,
interleukin-6. |
Discussion
Gastric cancer was the fifth most common cancer
world wide, comprising 6.8% of the total number of new cases
diagnosed in 2012 (14). Despite
previous advances in surgical techniques and the development of
chemotherapy and radiotherapy, the mortality of gastric cancer
remains high, with a 5-year survival rate of <30% (15). To provide data that will enable the
development of novel therapeutic strategies, it is crucial to
elucidate the molecular mechanisms that promote the migration and
invasion properties of gastric cancer. Metastasis is defined as the
process of dissemination of cancer cells from their origin to a
distant organ, a complex process involving several stages, which
are as follows: i) The activation of EMT; ii) local invasion; iii)
intravasation; iv) the ability to survive in the bloodstream; v)
extravasation, whereby tumor cells exit the bloodstream; and vi)
establishment of tumor cells in the tissues of a distant organ
(16).
EMT is a multistage reprogramming process that is
also important in the physiological process of embryogenesis.
During EMT, epithelial cells are closely arranged and undergo a
phenotypic alteration to acquire a mesenchymal phenotype, which
involves a loss of polarity and intercellular adhesion,
cytoskeletal disorganization that promotes motility and remodeling
of the surrounding microenvironment, which is also commonly
identified in various physiological processes, including wound
healing, inflammation and fibrosis (17). A previous study has also demonstrated
that EMT is associated with the acquisition of malignant
characteristics in gastric cancer cells, suggesting that EMT is a
vital step in tumor progression and metastasis (18). An additional previous study has
indicated that the mechanism of EMT expression is regulated by
various factors and signaling pathways, including E-cadherin,
Snail, Twist 1 and vimentin (19).
The levels of Twist 1, Snail and vimentin expression are all
upregulated in patients with gastric cancer, whereas the level of
E-cadherin is decreased in these patients (20). A study has indicated that the
reduction of E-cadherin is considered to be an important EMT
feature, which serves critical roles in EMT by changing the
components of intercellular adhesion and regulating diverse
signaling pathways activated by the PI3K/Akt signaling pathway
(21).
The cascade reaction of PI3K/Akt is one of the vital
intracellular signal transduction systems, participating in
numerous physiological progressions, such as cell growth,
proliferation, differentiation and apoptosis. The constitutive
activation of the PI3K/Akt signaling pathway has been noted during
the malignant transformation of various cell lines and implicated
in carcinogenesis and metastatic potential of human cancer
(22). A previous study identified
that PI3K/Akt modulated cancer metastasis via the regulation of EMT
(23). IL-6 is a potent inflammatory
cytokine that is released by inflammatory cells and cancer cells.
IL-6 mediates several important physiological functions, including
the control of the acute-phase inflammatory response and the
support of cell growth and survival (24). Several studies have suggested that
IL-6 is a key mediator of the development of metastatic potential
in cancer cells (25,26). Previous studies have indicated that
IL-6 promotes the initial steps of cancer metastasis, potentially
by upregulating MMP-9 through the PI3K/Akt signal pathway (27,28), and
it has been demonstrated that IL-6 induces EMT through the
expression of molecular markers (29,30).
Therefore, the present study evaluated the expression of the
primary biomarkers of EMT using western blotting and RT-PCR in
SGC7901 cells treated with IL-6. Expression of the epithelial
marker E-cadherin was downregulated, whereas the associated
transcription factors involved in EMT, namely vimentin and Twist 1,
were upregulated. Upon stimulation with IL-6, SGC7901 cells
secreted MMP-9 and induced invasiveness. These data indicate that
during cancer pathogenesis, elevated levels of IL-6 may promote
metastasis by acting on the inflammatory microenvironment. This
finding is consistent with previous data, which indicate that IL-6
promotes tumor growth and malignant progression in gastric cancer,
and that the induction of EMT is associated with tumor progression
and the poor prognosis of patients with gastric cancer (31). This indicates that activating the IL-6
pathway also serves an important role in tumor
microenvironment.
PIAS1 was originally identified as an inhibitor of
STAT1 (32). It is well-known that
activated STAT factors may regulate gene expression and thereby
affect cell differentiation, proliferation, angiogenesis and
apoptosis. PIAS1 already has been identified as a negative
regulator of tumor suppressors, including p53 and p73 (33). To address whether PIAS1 targeting
could be used to improve gastric cancer therapy, PIAS1 expression
in primary tumors of all stages in metastatic lesions from patient
tissues was analyzed. The data from the present study were
complemented by functional experiments following PIAS1 upregulation
expression in vitro. A previous study demonstrated that a
loss of PIAS1 led to the enhanced proliferation of tumor cells,
while another study found an association between the reduced
expression of PIAS1 and gastric cancer development (11,34).
Similar to these data, the elevated PIAS1 expression in gastric
cancer was observed in the present study, and a pro-proliferative
role for the protein in this malignancy was proposed. On the basis
of the data of the present study, we hypothesized that the elevated
expression of PIAS1 in gastric cells impairs the transcriptional
activity of the PI3K/Akt signaling pathway. The present study also
confirms the effect of elevated PIAS1 expression on EMT in gastric
cancer.
The results of present study indicated that
IL-6-treated cells exhibited significantly increased migratory and
invasive capabilities, as assessed by scratch wound healing and
invasion assays. Transfection with the Ad5/F35-PIAS1 plasmid
inhibited IL-6-induced migration, and the scratch wound healing
assay indicated that Ad5/F35-PIAS1+IL-6-treated cells exhibited a
notably slower viability recovery rate compared with the IL-6 and
Ad5/F35-null+IL-6-treated cells. Similarly, the results of the
Transwell invasion assay indicated that the
Ad5/F35-PIAS1+IL-6-treated cells were significantly associated with
decreased rates of migration compared with the other groups. These
results indicated that PIAS1 was important for gastric cancer cell
invasion and cell migration. Consequently, PIAS1 led to a reduction
in the activation of the PI3K/Akt signaling pathway and MMP-9
protein expression in SGC7901 cells. Therefore, these cells may
inhibit the initiation of EMT, leading to the increased expression
of E-cadherin, and consequently a downregulation of Snail, Twist 1
and vimentin protein expression.
Taken together, these data confirm that PIAS1
upregulation causes the inhibition of EMT in an inflammatory
microenvironment, which consequently results in decreased cell
migration. On the basis of the present data, it was concluded that
PIAS1 may be a promising novel target for treatment of gastric
cancer.
References
|
1
|
Röcken C: Ways to personalized medicine
for gastric cancer. Pathologe. 34:403–412. 2013.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim HS, Kim JH, Kim JW and Kim BC:
Chemotherapy in elderly patients with gastric cancer. J Cancer.
7:88–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang SS, Jiang J, Liang XH and Tang YL:
Links between cancer stem cells and epithelial-mesenchymal
transition. Onco Targets Ther. 8:2973–2980. 2015.PubMed/NCBI
|
|
4
|
Huang L, Wu RL and Xu AM:
Epithelial-mesenchymal transition in gastric cancer. Am J Transl
Res. 7:2141–2158. 2015.PubMed/NCBI
|
|
5
|
Peng Z, Wang CX, Fang EH, Wang GB and Tong
Q: Role of epithelial-mesenchymal transition in gastric cancer
initiation and progression. World J Gastroenterol. 20:5403–5410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka T and Kishimoto T: The biology and
medical implications of interleukin-6. Cancer Immunol Res.
2:288–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang C, Yang G, Jiang T, Zhu G, Li H and
Qiu Z: The effects and mechanisms of blockage of STAT3 signaling
pathway on IL-6 inducing EMT in human pancreatic cancer cells in
vitro. Neoplasma. 58:396–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodriguez JA, Huerta-Yepez S, Law IK,
Baay-Guzman GJ, Tirado-Rodriguez B, Hoffman JM, Iliopoulos D,
Hommes DW, Verspaget HW, Chang L, et al: Diminished expression of
CRHR2 in human colon cancer promotes tumor growth and EMT via
persistent IL-6/Stat3 signaling. Cell Mol Gastroenterol Hepatol.
1:610–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dadakhujaev S, Salazar-Arcila C, Netherton
SJ, Chandhoke AS, Singla AK, Jirik FR and Bonni S: A novel role for
the SUMO E3 ligase PIAS1 in cancer metastasis. Oncoscience.
1:229–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heo KS, Chang E, Takei Y, Le NT, Woo CH,
Sullivan MA, Morrell C, Fujiwara K and Abe J: Phosphorylation of
protein inhibitor of activated STAT1 (PIAS1) by MAPK-activated
protein kinase-2 inhibits endothelial inflammation via increasing
both PIAS1 transrepression and SUMO E3 ligase activity.
Arterioscler Thromb Vasc Biol. 33:321–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen P, Zhao D, Sun Y, Huang L, Zhang S
and Yuan Y: Protein inhibitor of activated STAT-1 is downregulated
in gastric cancer tissue and involved in cell metastasis. Oncol
Rep. 28:2149–2155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen P, Huang LY and Yuan YZ: Construction
and identification of recombinant adenovirus with rat protein
inhibitor of activated STAT1 gene. Chin J Bilogicals. 24:431–434.
2011.
|
|
13
|
Chen P, Huang L, Sun Y and Yuan Y:
Upregulation of PIAS1 protects against sodium taurocholate-induced
severe acute pancreatitis associated with acute lung injury.
Cytokine. 54:305–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng H, Huang X, Fan J, Wang L, Xia Q,
Yang X, Wang Z and Liu L: A variant of estrogen receptor-alpha,
ER-alpha36 is expressed in human gastric cancer and is highly
correlated with lymph node metastasis. Oncol Rep. 24:171–176.
2010.PubMed/NCBI
|
|
16
|
Mihmanli M, Ilhan E, Idiz UO, Alemdar A
and Demir U: Recent developments and innovations in gastric cancer.
World J Gastroenterol. 22:4307–4320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao J, Yang C, Guo S and Wu Y: GM130
regulates epithelial-to-mesenchymal transition and invasion of
gastric cancer cells via snail. Int J Clin Exp Pathol.
8:10784–10791. 2015.PubMed/NCBI
|
|
18
|
Choi YJ, Kim N, Chang H, Lee HS, Park SM,
Park JH, Shin CM, Kim JM, Kim JS, Lee DH and Jung HC: Helicobacter
pylori-induced epithelial-mesenchymal transition, a potential role
of gastric cancer initiation and an emergence of stem cells.
Carcinogenesis. 36:553–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lander R, Nordin K and LaBonne C: The
F-box protein Ppa is a common regulator of core EMT factors Twist,
Snail, Slug, and Sip1. J Cell Biol. 194:17–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tania M, Khan MA and Fu J: Epithelial to
mesenchymal transition inducing transcription factors and
metastatic cancer. Tumour Biol. 35:7335–7342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu W, Yang Z and Lu N: A new role for the
PI3K/Akt signaling pathway in the epithelial-mesenchymal
transition. Cell Adh Migr. 9:317–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin K, Wang L, Zhang X, He Z, Xia Y, Xu J,
Wei S, Li B, Li Z, Sun G, et al: Netrin-1 promotes gastric cancer
cell proliferation and invasion via the receptor neogenin through
PI3K/AKT signaling pathway. Oncotarget. 8:51177–51189. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Willems L, Tamburini J, Chapuis N, Lacombe
C, Mayeux P and Bouscary D: PI3K and mTOR signaling pathways in
cancer: New data on targeted therapies. Curr Oncol Rep. 14:129–138.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ho LJ, Luo SF and Lai JH: Biological
effects of interleukin-6: Clinical applications in autoimmune
diseases and cancers. Biochem Pharmacol. 97:16–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fisher DT, Appenheimer MM and Evans SS:
The two faces of IL-6 in the tumor microenvironment. Semin Immunol.
26:38–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nguyen DP, Li J and Tewari AK:
Inflammation and prostate cancer: The role of interleukin 6 (IL-6).
BJU Int. 113:986–992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SO, Yang X, Duan S, Tsai Y, Strojny
LR, Keng P and Chen Y: IL-6 promotes growth and
epithelial-mesenchymal transition of CD133+ cells of non-small cell
lung cancer. Oncotarget. 7:6626–6638. 2016.PubMed/NCBI
|
|
28
|
Hsu CP, Chen YL, Huang CC, Chou CC, Liu
CL, Hung CH, Kao TY and Chung YC: Anti-interleukin-6 receptor
antibody inhibits the progression in human colon carcinoma cells.
Eur J Clin Invest. 41:277–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nowak DG, Cho H, Herzka T, Watrud K,
DeMarco DV, Wang VM, Senturk S, Fellmann C, Ding D, Beinortas T, et
al: MYC Drives Pten/Trp53-deficient proliferation and metastasis
due to IL6 Secretion and AKT suppression via PHLPP2. Cancer Discov.
5:636–651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li D, Sun X, Yan D, Huang J, Luo Q, Tang H
and Peng Z: Piwil2 modulates the proliferation and metastasis of
colon cancer via regulation of matrix metallopeptidase 9
transcriptional activity. Exp Biol Med (Maywood). 237:1231–1240.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim MA, Lee HS, Lee HE, Kim JH, Yang HK
and Kim WH: Prognostic importance of epithelial-mesenchymal
transition-related protein expression in gastric carcinoma.
Histopathology. 54:442–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu WL, Ma YL, Hsieh DY, Liu YC and Lee
EH: STAT1 negatively regulates spatial memory formation and
mediates the memory-impairing effect of Aβ.
Neuropsychopharmacology. 39:746–758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kahyo T, Nishida T and Yasuda H:
Involvement of PIAS1 in the sumoylation of tumor suppressor p53.
Mol Cell. 8:713–718. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei J, Costa C, Ding Y, Zou Z, Yu L,
Sanchez JJ, Qian X, Chen H, Gimenez-Capitan A, Meng F, et al: mRNA
expression of BRCA1, PIAS1, and PIAS4 and survival after
second-line docetaxel in advanced gastric cancer. J Natl Cancer
Inst. 103:1552–1556. 2011. View Article : Google Scholar : PubMed/NCBI
|