Introduction
The ubiquitin proteasome system (UPS) regulates
diverse cellular processes, including cell proliferation, cell
cycle progression and apoptosis (1–3). The UPS
exerts its functions through the sequential action of three
enzymes, namely, the ubiquitin-activating E1 enzyme, the
ubiquitin-conjugating E2 enzyme and the ubiquitin-protein E3 ligase
(4,5).
Notably, E3 ligases determine substrate specificity for
ubiquitination and subsequent degradation (4,5).
In the human genome >600 putative E3 ubiquitin
ligases have been identified (6).
Within this group, the cullin-RING E3 ligase (CRL) complex family
is the largest. The CRL family contains eight members, including
CRL1, CRL2, CRL3, CRL4A, CRL4B, CRL5, CRL7 and CRL9 (6,7). CRL1,
also known as the S-phase kinase associated protein 1 (Skp1)-Cullin
1-F-box protein (SCF) E3 ligase complex, is the most
well-characterized CRL family member (6,8,9).
F-box proteins (FBPs) contain one subunit of an SCF
type of the E3 ubiquitin ligase complex, which serves as a
substrate recognition module (10).
F-box only protein (FBXO) 31 is a member of the FBP family, which
may serve an important role in tumorigenesis (11,12). To
the best of our knowledge, the present review is the first to
summarize the role of F-box only protein 31 (FBXO31) in different
types of cancer and explain its underlying mechanism of action,
thereby providing the rationale to design FBXO31-targeted
anticancer therapies.
F-box proteins
The first FBP to be identified was cyclin F, also
known as FBXO1, and was first described by Bai et al
(8) in 1996. Each FBP consists of at
least two major functional domains, a variable carboxy-terminal
domain that binds to specific substrates and the F-box motif, which
is responsible for the incorporation of the FBP into the SCF
complex (10). So far, a total of 69
human FBPs have been identified (10), which may be divided into three
subclasses according to the presence of specific substrate
recognition domains. These subclasses consist of the F-Box and WD
repeat domain containing (FBXW) subclass, which contains WD40
repeat domains and is comprised of 10 proteins, including FBXW7,
β-TRCP1 and β-TRCP2; the F-box and leucine rich repeat protein
(FBXL) subclass, which contains leucine-rich repeat domains and is
comprised of 22 proteins, including S-phase kinase-associated
protein 2 (SKP2), FBXL11 and FBXL19; and the FBXO subclass, which
contains various other domains and is comprised of 37 proteins,
including FBXO1, FBXO11 and FBXO31 (11,13–15).
FBPs serve a crucial role in various cellular
processes, including cell proliferation, the cell cycle, apoptosis,
migration, invasion and metastasis, suggesting that they may be
associated with tumorigenesis (11,16–18). Due
to the diversity of substrates targeted by FBPs for ubiquitination
and subsequent degradation, FBPs may be either oncogenic or tumor
suppressive. In some instances, depending on the tissue context,
FBPs may be used as prognostic markers and therapeutic targets in
certain types of cancer (10–12,19).
FBXO31
FBXO31, a member of the FBXO subclass of FBPs, is a
senescence-related gene located at chromosome 16q24.3 (20). The gene consists of 539 amino acids
and has a molecular weight of 60,533 Da (20). FBXO31 is highly expressed in adipose
and brain tissues, and expressed in low amounts in the bone marrow
at similar levels to other human tissues, including the stomach,
pancreas and breast (20).
FBXO31 contains a 40-amino acid F-box domain and is
a component of the SCF ubiquitin E3 ligases that mediate the
ubiquitination of targeted proteins for proteasomal degradation
(21) (Fig.
1). In SCF-FBXO31 E3 ligases, cullin 1 functions as a molecular
scaffold, which interacts with the adaptor subunit Skp1 at the
amino terminus and with the RING-finger protein ring-box 1 (Rbx1)
at the carboxyl terminus (10). Rbx1
recruits specific E2 ubiquitin-conjugating enzymes (UBCs),
including Ubc3, Ubc4 and Ubc5 (22).
Conversely, Skp1 binds to the FBXO31, which specifically recognizes
and binds to a number of substrates via a protein-protein
interaction domain (23). By
degrading various substrates, FBXO31 serves important roles in
multiple pathways, including neuronal development (24,25), DNA
damage response (26–29) and tumorigenesis (20,26,27,30–37).
FBXO31 was initially considered to be a candidate tumor suppressor,
as the decreased expression of FBXO31 was identified in breast
cancer (20). However, further
studies have determined that FBXO31 may be either oncogenic or
tumor suppressive.
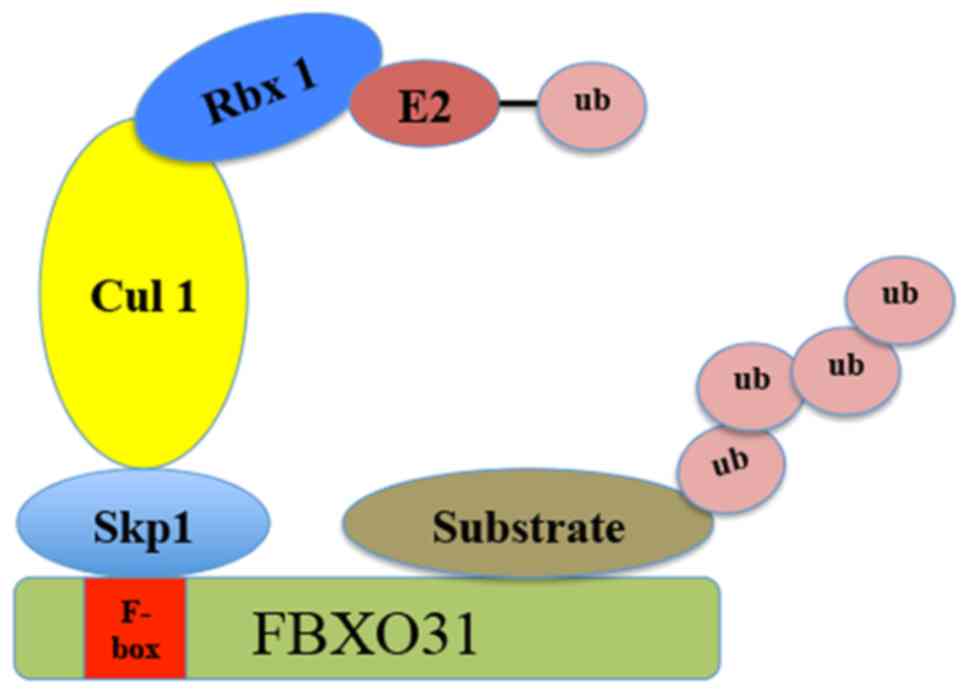 | Figure 1.Schematic illustration of Skp1-Cullin
1-F-box E3 ligase. The Skp1-Cullin1-F box complex consists of four
components: Skp1, Cul 1, Rbx1, along with the variable F-box
protein family that functions as the substrate recognition
component. FBXO31 is a member of the F-box protein family, which
recognizes the targeted substrates. FBXO31, F-box only protein 31;
Skp1, S-phase kinase associated protein 1; Rbx1, ring-box 1; Cul 1,
Cullin 1; ub, ubiquitin; E2, the ubiquitin-conjugating E2
enzyme. |
FBXO31 as a tumor suppressor
Breast cancer
Kumar et al (20) determined that FBXO31 may serve as a
tumor suppressor in breast cancer. The ectopic expression of FBXO31
induced cellular senescence in the breast cancer cell line MCF-7 in
an F-box domain-dependent manner. The overexpression of FBXO31
inhibited the ability of breast cancer cells to initiate colonies
on plastic culture dishes and inhibited the proliferation of breast
cancer cells (20). Additionally,
levels of FBXO31 mRNA were increased in finite life-span human
mammary epithelial cells and nonmalignant immortalized cells
compared with breast cancer cell line MCF-7 (20). Furthermore, although no tumor specific
mutations were identified, there was a trend associated with lower
FBXO31 expression in primary tumors compared with normal breast
tissue (20). Song et al
(38) also indicated that FBXO31
expression was reduced in the tumor tissues of 10 patients with
breast cancer, indicating that FBXO31 may serve a tumor suppressive
role.
The underlying mechanism for the tumor suppressive
function of FBX031 may be associated with the ubiquitination and
degradation of cell cycle-associated substrates. Johansson et
al (28) reported that FBXO31
targets chromatin licensing and DNA replication factor 1 (Cdt1), a
DNA replication licensing factor, for ubiquitination and
degradation in the G2 phase of the cell cycle. This process is
independent of SCF-Skp2 but dependent on the CRL4-damage-specific
DNA binding protein 1-associated ubiquitination of Cdt1 during the
S- and G2 phases of the cell cycle. Cdt1 stabilization in
FBXO31-depleted cells results in DNA re-replication, suggesting
that FBXO31 may function as a tumor suppressor (21). Jeffery et al (37) demonstrated that FBXO31 is required for
normal mitotic progression and genome stability as it caps forkhead
box protein M1 (FXOM1) levels during the G2/M transition.
Furthermore, it was suggested that FBXO31 targets mouse double
minute 2 homolog (MDM2) during ubiquitination and degradation,
leading to elevated p53 levels, cell cycle arrest and growth
inhibition, supporting the tumor suppressive role of FBXO31 in
breast cancer (27). In addition,
Manne et al (39) demonstrated
that FBXO31 targets the Snail family transcriptional repressor
protein 2 (Slug), which is involved in the epithelial-mesenchymal
transition, cell invasion, ubiquitination and degradation, and
therefore represses the growth of breast cancer cells. Studies have
determined that micro (mi)RNAs are dysregulated at all stages of
breast cancer and serve important roles in tumorigenesis, invasion
and metastasis (40,41). Liu et al (36) indicated that FBXO31 was the downstream
target gene of miRNA (miR)-210 in breast cancer, miR-210 and FBXO31
are inversely expressed in breast cancer cell lines T47D and MCF-7.
Subsequently, the tumor suppressive effect of miR-210
downregulation may be reversed by further depletion of FBXO31. A
study by Manne et al (39)
indicated that the oncogenic miRNAs miR93 and miR106a repressed
FBXO31 expression, thus impairing the degradation of Slug via
FBXO31.
Melanoma
A genome-wide RNA-interference screen initially
identified FBXO31 as a candidate gene required for the oncogenic
B-Raf serine/threonine protein (BRAF) to induce senescence in
primary melanocytes (42). Subsequent
results from the same group revealed that the ectopic expression of
FBXO31 inhibited the growth of SK-MEL-28 melanoma cells in
vitro and in mouse xenografts in vivo (43). It has been proposed that FBXO31
induces G1 arrest in melanoma cells by binding to cyclin D1, a key
regulator of G1/S phase transition (44) and promotes its ubiquitination and
subsequent degradation. Oncogene-induced senescence involves the
activation of DNA damage response pathways (45,46).
Additionally, FBXO31, a protein required for BRAF-induced
senescence (42), is involved in
mediating G1 arrest following DNA damage. It has been indicated
that FBXO31 expression is induced following exposure to various DNA
damaging agents, including ionizing radiation and is stabilized by
ataxia telangiectasia mutated-mediated phosphorylation (43). Induced FBXO31 subsequently binds to
and targets cyclin D1 for degradation, causing G1 arrest (43). However, FBXO31 knockdown abrogates G1
arrest following DNA damage and sensitizes melanoma cells to
radiation (43). These results
suggest that FBXO31 may serve as a radiosensitizing target.
Therefore, a small molecule that either inhibits cyclin D1
phosphorylation at Thr286 or disrupts FBXO31-cyclin D1 binding may
act as a radiosensitizer (47).
Hepatocellular carcinoma (HCC)
Loss of heterozygosity (LOH) at four microsatellite
loci on chromosome 16q24.3, the region harboring FBXO31, was
identified in 8.9–21.8% of HCC specimens (48). Huang et al (30) were the first to report the role of
FBXO31 in HCC. The results demonstrated that FBXO31 expression was
increased in the fetal liver and the fetal liver-derived cell line
L02; however, low expression was observed in the majority of HCC
cell lines including BEL-7402, BEL-7404, Hep3B and SMMC-7721.
Additionally, FBXO31 mRNA expression was downregulated in 93.75%
(30/32) of HCC specimens compared with adjacent healthy liver
tissues (30). Further experiments
revealed that the ectopic expression of FBXO31 inhibited cell
proliferation and colony formation in the HCC cell lines HepG2 and
Hep3B. These results suggest that the downregulation of cyclin D1
following FBXO31 overexpression is the proposed mechanism by which
FBXO31 inhibits the development of HCC.
Gastric cancer (GC)
Mori et al (49) demonstrated that LOH occurs frequently
in GC on chromosome band 16q24, including 16q24.3 where FBXO31 is
encoded. Zhang et al (32)
identified that levels of FBXO31 mRNA and protein were markedly
decreased in GC tissue compared with adjacent non-cancerous tissue.
In addition, immunohistochemical analyses revealed an association
between FBXO31 levels and tumor size, tumor infiltration, clinical
grade and patient prognosis. Furthermore, the results indicated
that the overall survival rates of patients with negative FBXO31
expression was significantly poorer than that of patients who were
FBXO31-positive. The results of previous studies using the GC cell
lines BGC-823 and HGC-27 suggest that FBXO31 overexpression
significantly decreases colony formation, induces G1 cell cycle
arrest and inhibits the expression of cyclin D1 protein. These
studies also indicate that these processes are dependent on the
F-box domain of FBXO31. Furthermore, it was demonstrated that the
ectopic expression of FBXO31 markedly inhibits xenograft tumor
growth in nude mice exhibiting tumors with low levels of cyclin D1
expression and high levels of FBXO31 expression. This suggests that
FBXO31 may function as a tumor suppressor by inhibiting cyclin D1
expression. FBXO31 knockdown induced the opposite effects (27).
Zhang et al (32) explored the role of miRNA on FBXO31
expression in GC and identified that miR-20a and miR-17 directly
bind to the 3′-untranslated region of FBXO31 to regulate FBXO31
expression. Furthermore, a strong negative correlation between
miR-20a or miR-17 and FBXO31 was observed in GC samples. These
results indicate that FBXO31 may suppress GC progression via the
miR-20a-miR-17-FBXO31-cyclin D1 signaling pathway.
Ovarian cancer
Launonen et al (34) assessed the frequency of LOH in 78
tumor specimens of epithelial ovarian cancer and identified that
LOH at 16q24.3, a region where the FBXO31 gene is located, was
associated with advanced tumor grade, serous histology, a more
advanced tumor stage and metastasis. These results suggest that
FBXO31 may be a potential tumor suppressor in ovarian cancer,
however, further studies focusing on the role of FBXO31 in ovarian
cancer are required to explore this.
Prostate cancer
Härkönen et al (35) detected the frequency of LOH in 74
tumor specimens collected from 33 patients with prostate cancer and
indicated that the frequency of LOH at 16q was 68% (21/31) for
primary prostate tumors, 90% (28/31) for locally recurrent tumors
and 75% (9/12) for tumors of metastatic origin. Furthermore, the
region 16q24.3, where FBXO31 is located, exhibited a significant
development of LOH during disease recurrence, suggesting that this
region harbors gene(s) associated with prostate cancer progression.
However, multiple tumor suppressor genes are located at this region
with FBXO31 (50–54) and no studies thus far have detected
the expression and function of FBXO31 in prostate cancer;
therefore, FBXO31 may only be regarded as a potential tumor
suppressor.
Colon cancer
FBXO31 binds to MDM2 and targets it for
ubiquitination and degradation following DNA damage (27), causing an increase in p53 expression.
MDM2 and p53 expression following DNA damage did not significantly
change in HCT116 colon cancer cells following FBXO31 knockdown;
however, the mitotic index of these cells was markedly higher than
that of control HCT116 cells at 18 and 24 h following γ-irradiation
(27). In addition, the difference in
mitotic index was correlated with the expression of p53 and the p53
target gene p21, which serves a critical role in p53-mediated
growth arrest (55). These results
suggest that FBXO31 may be a potential tumor suppressor in colon
cancer.
FBXO31 as an oncogene
Esophageal squamous cell carcinoma
(ESCC)
Kogo et al (31) indicated that the high expression of
FBXO31 mRNA was associated with increased tumor invasion and a more
advanced clinical stage. Furthermore, the study suggested that
FBXO31 expression was an independent prognostic factor for 5-year
overall in patients with ESCC following surgery, indicating that
FBXO31 may act as an oncogene in ESCC. Cyclin D1 is a
well-characterized oncogene in ESCC (56–58) and
FBXO31 may mediate the ubiquitination and degradation of cyclin D1
(43); therefore the association
between cyclin D1 and FBXO31 expression in ESCC was evaluated. The
results of immunohistochemistry indicated that cyclin D1 and FBXO31
expression were increased in ESCC tissue. Additionally, a
comparative genomic hybridization array indicated that cases with
high cyclin D1 amplification exhibited elevated FBXO31 expression,
suggesting that FBXO31 may degrade cyclin D1 as it moves from the
nucleus to the cytoplasm (31).
However, Liu et al (26)
demonstrated that FBXO31 knockdown does not affect cyclin D1
protein expression, nor does it rescue DNA damage-induced cyclin D1
degradation in ESCC cell lines. Furthermore, the role of FBPs,
including FBXO4, FBXO31, Skp2 and FBXW8, in regulating cyclin D1
stability was re-evaluated (59). The
results indicated that FBXO31 knockdown did not impair cyclin D1
proteolysis in mouse embryonic fibroblasts, suggesting that FBXO31
may only regulate cyclin D1 stability in a cell type-specific
manner. Therefore, the molecular mechanism involved in
overexpression of FBXO31 in ESCC remains unclear. Liu et al
(26) demonstrated that FBXO31
reduces stress-induced cell apoptosis by inducing lys-48-linked
polyubiquitination and the degradation of mitogen-activated protein
kinase kinase 6 (MKK6) in ESCC, suggesting that FBXO31 may serve an
oncogenic role in ESCC by modulating cell apoptosis.
Lung cancer
Huang et al (33) analyzed the expression of FBXO31 in 50
patients with lung cancer (36 patients had adenocarcinoma and 14
patients had squamous cell cancer). The results indicated that
levels of FBXO31 mRNA were increased in lung cancer tissues
compared with non-cancerous lung tissues. Furthermore, the
increased expression of FBXO31 was significantly associated with
tumor size, tumor infiltration, clinical stage and lymph node
metastasis. Additionally, the ectopic expression of FBXO31 promoted
the growth, metastasis and invasion of A549 cells, whereas FBXO31
knockdown inhibited these processes. The results of a
tumorigenicity assay conducted in nude mice demonstrated that
FBXO31 promotes tumor growth in vivo. Therefore, these
results suggest that FBXO31 may function as an oncogene in lung
cancer.
Conclusions and perspectives
FBXO31 was initially identified in 2005 and is a
poorly understood member of the FBP family (20). Furthermore, only a limited number of
studies have assessed its role in various diseases, including
cancer (20,24–37). With
the F-box motif, FBXO31 has the ability to bind to several
substrates for ubiquitination and degradation and may therefore be
associated with various functions, including cell cycle
progression, DNA damage and cell proliferation (25–28,37,39,43).
A total of 7 FBXO31-associated substrates have been identified,
including Cdt1 (28), MDM2 (27), cyclin D1 (43), MKK6 (26), FXOM1 (37), Par6c (25) and Slug (39) (Fig.
2).
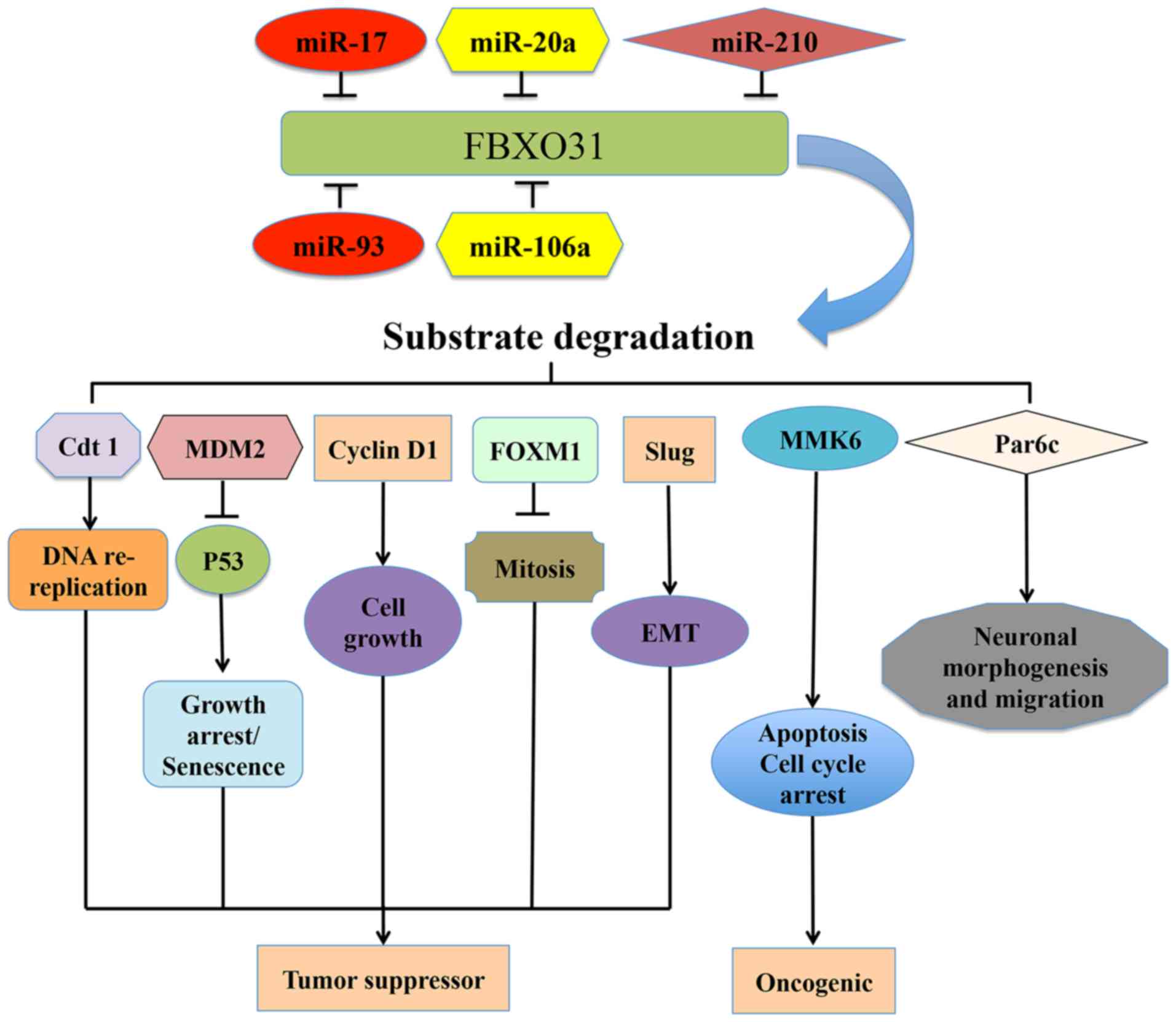 | Figure 2.Upstream regulators of FBXO31 and its
major downstream targets that contribute to human diseases,
including cancer. FBXO31 coordinates the ubiquitin-dependent
proteolysis of several proteins, including Cdt1, MDM2, cyclin D1,
MKK6, FOXM1, Par6c, Slug and determines their functions in various
types of cancer. Various miRNAs, including miR-17, miR-20a,
miR-210, miR93 and miR106a regulate the expression of FBXO31.
FBXO31, F-box only protein 31; MDM2, mouse double minute 2 homolog;
MKK6, mitogen-activated protein kinase kinase 6; FXOM1, forkhead
box protein M1; cdt1, chromatin licensing and DNA replication
factor 1; miR, microRNA; Slug, Snail family transcriptional
repressor protein 2. |
In conclusion, the present review suggests that
FBXO31 may act a tumor suppressor or oncogene depending on the cell
type or tissue context. Furthermore, compared with other
well-characterized FBPs, including Skp2, FBXW7 and β-TrCP, current
understanding of FBXO31 remains limited. Therefore, further studies
are warranted to fully elucidate the role of FBXO31 in various
human diseases, including its involvement in the regulation of
tumorigenesis and to identify novel substrates and cellular
pathways regulated by FBXO31.
Acknowledgements
The present study was supported by National Science
Foundation of China grants (grant no. 81502630) and partly
supported by the Chinese National Key Disciplines. The authors
would also like to thank Dr. Brian J. North from Beth Israel
Deaconess Medical Center, Harvard Medical School (Boston, MA, USA),
for assisting in the editing of the manuscript.
Glossary
Abbreviations
Abbreviations:
|
FBXO31
|
F-box only protein 31
|
|
SCF
|
Skp1-Cullin1-F box
|
|
UPS
|
ubiquitin proteasome system
|
|
CRL
|
Cullin-RING E3 ligase
|
|
FBPs
|
F-box proteins
|
|
MDM2
|
mouse double minute 2 homolog
|
|
FXOM1
|
Forkhead box protein M1
|
|
HCC
|
hepatocellular carcinoma
|
|
LOH
|
loss of heterozygosity
|
|
GC
|
gastric cancer
|
|
ESCC
|
esophageal squamous cell carcinoma
|
|
MKK6
|
mitogen-activated protein kinase
kinase 6
|
References
|
1
|
Hershko A and Ciechanover A: The ubiquitin
system. Annu Rev Biochem. 67:425–479. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Varshavsky A: The ubiquitin system, an
immense realm. Annu Rev Biochem. 81:167–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bett JS: Proteostasis regulation by the
ubiquitin system. Essays Biochem. 60:143–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ciechanover A, Orian A and Schwartz AL:
Ubiquitin-mediated proteolysis: Biological regulation via
destruction. Bioessays. 22:442–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smalle J and Vierstra RD: The ubiquitin
26S proteasome proteolytic pathway. Annu Rev Plant Biol.
55:555–590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Bengtson MH, Ulbrich A, Matsuda A,
Reddy VA, Orth A, Chanda SK, Batalov S and Joazeiro CA: Genome-wide
and functional annotation of human E3 ubiquitin ligases identifies
MULAN, a mitochondrial E3 that regulates the organelle's dynamics
and signaling. PLoS One. 3:e14872008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deshaies RJ and Joazeiro CA: RING domain
E3 ubiquitin ligases. Annu Rev Biochem. 78:399–434. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bai C, Sen P, Hofmann K, Ma L, Goebl M,
Harper JW and Elledge SJ: SKP1 connects cell cycle regulators to
the ubiquitin proteolysis machinery through a novel motif, the
F-box. Cell. 86:263–274. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frescas D and Pagano M: Deregulated
proteolysis by the F-box proteins SKP2 and beta-TrCP: Tipping the
scales of cancer. Nat Rev Cancer. 8:438–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Liu P, Inuzuka H and Wei W: Roles
of F-box proteins in cancer. Nat Rev Cancer. 14:233–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong J, Lv L and Huo J: Roles of F-box
proteins in human digestive system tumors (Review). Int J Oncol.
45:2199–2207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng N, Zhou Q, Wang Z and Wei W: Recent
advances in SCF ubiquitin ligase complex: Clinical implications.
Biochim Biophys Acta. 1866:12–22. 2016.PubMed/NCBI
|
|
13
|
Cenciarelli C, Chiaur DS, Guardavaccaro D,
Parks W, Vidal M and Pagano M: Identification of a family of human
F-box proteins. Curr Biol. 9:1177–1179. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin J, Cardozo T, Lovering RC, Elledge SJ,
Pagano M and Harper JW: Systematic analysis and nomenclature of
mammalian F-box proteins. Genes Dev. 18:2573–2580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Winston JT, Koepp DM, Zhu C, Elledge SJ
and Harper JW: A family of mammalian F-box proteins. Curr Biol.
9:1180–1182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diaz VM and de Herreros AG: F-box
proteins: Keeping the epithelial-to-mesenchymal transition (EMT) in
check. Semin Cancer Biol. 36:71–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heo J, Eki R and Abbas T: Deregulation of
F-box proteins and its consequence on cancer development,
progression and metastasis. Semin Cancer Biol. 36:33–51. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Randle SJ and Laman H: F-box protein
interactions with the hallmark pathways in cancer. Semin Cancer
Biol. 36:3–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng N, Wang Z and Wei W:
Ubiquitination-mediated degradation of cell cycle-related proteins
by F-box proteins. Int J Biochem Cell Biol. 73:99–110. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar R, Neilsen PM, Crawford J, McKirdy
R, Lee J, Powell JA, Saif Z, Martin JM, Lombaerts M, Cornelisse CJ,
et al: FBXO31 is the chromosome 16q24.3 senescence gene, a
candidate breast tumor suppressor, and a component of an SCF
complex. Cancer Res. 65:11304–11313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Skowyra D, Craig KL, Tyers M, Elledge SJ
and Harper JW: F-box proteins are receptors that recruit
phosphorylated substrates to the SCF ubiquitin-ligase complex.
Cell. 91:209–219. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye Y and Rape M: Building ubiquitin
chains: E2 enzymes at work. Nat Rev Mol Cell Biol. 10:755–764.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cardozo T and Pagano M: The SCF ubiquitin
ligase: Insights into a molecular machine. Nat Rev Mol Cell Biol.
5:739–751. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mir A, Sritharan K, Mittal K, Vasli N,
Araujo C, Jamil T, Rafiq MA, Anwar Z, Mikhailov A, Rauf S, et al:
Truncation of the E3 ubiquitin ligase component FBXO31 causes
non-syndromic autosomal recessive intellectual disability in a
Pakistani family. Hum Genet. 133:975–984. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vadhvani M, Schwedhelm-Domeyer N,
Mukherjee C and Stegmüller J: The centrosomal E3 ubiquitin ligase
FBXO31-SCF regulates neuronal morphogenesis and migration. PLoS
One. 8:e575302013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu J, Han L, Li B, Yang J, Huen MS, Pan
X, Tsao SW and Cheung AL: F-box only protein 31 (FBXO31) negatively
regulates p38 mitogen-activated protein kinase (MAPK) signaling by
mediating lysine 48-linked ubiquitination and degradation of
mitogen-activated protein kinase kinase 6 (MKK6). J Biol Chem.
289:21508–21518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malonia SK, Dutta P, Santra MK and Green
MR: F-box protein FBXO31 directs degradation of MDM2 to facilitate
p53-mediated growth arrest following genotoxic stress. Proc Natl
Acad Sci USA. 112:pp. 8632–8637. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johansson P, Jeffery J, Al-Ejeh F, Schulz
RB, Callen DF, Kumar R and Khanna KK: SCF-FBXO31 E3 ligase targets
DNA replication factor Cdt1 for proteolysis in the G2 phase of cell
cycle to prevent re-replication. J Biol Chem. 289:18514–18525.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shiloh Y: FBXO31: A new player in the
ever-expanding DNA damage response orchestra. Sci Signal.
2:pe732009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang HL, Zheng WL, Zhao R, Zhang B and Ma
WL: FBXO31 is down-regulated and may function as a tumor suppressor
in hepatocellular carcinoma. Oncol Rep. 24:715–720. 2010.PubMed/NCBI
|
|
31
|
Kogo R, Mimori K, Tanaka F, Komune S and
Mori M: FBXO31 determines poor prognosis in esophageal squamous
cell carcinoma. Int J Oncol. 39:155–159. 2011.PubMed/NCBI
|
|
32
|
Zhang X, Kong Y, Xu X, Xing H, Zhang Y,
Han F, Li W, Yang Q, Zeng J, Jia J and Liu Z: F-box protein FBXO31
is down-regulated in gastric cancer and negatively regulated by
miR-17 and miR-20a. Oncotarget. 5:6178–6190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang HL, Jiang Y, Wang YH, Chen T, He HJ,
Liu T, Yang T, Yang LW, Chen J, Song ZQ, et al: FBXO31 promotes
cell proliferation, metastasis and invasion in lung cancer. Am J
Cancer Res. 5:1814–1822. 2015.PubMed/NCBI
|
|
34
|
Launonen V, Mannermaa A, Stenbäck F, Kosma
VM, Puistola U, Huusko P, Anttila M, Bloigu R, Saarikoski S,
Kauppila A and Winqvist R: Loss of heterozygosity at chromosomes 3,
6, 8, 11, 16, and 17 in ovarian cancer: Correlation to
clinicopathological variables. Cancer Genet Cytogenet. 122:49–54.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Härkönen P, Kyllönen AP, Nordling S and
Vihko P: Loss of heterozygosity in chromosomal region 16q24.3
associated with progression of prostate cancer. Prostate.
62:267–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu D, Xia H, Wang F, Chen C and Long J:
MicroRNA-210 interacts with FBXO31 to regulate cancer proliferation
cell cycle and migration in human breast cancer. Onco Targets Ther.
9:5245–5255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeffery JM, Kalimutho M, Johansson P,
Cardenas DG, Kumar R and Khanna KK: FBXO31 protects against genomic
instability by capping FOXM1 levels at the G2/M transition.
Oncogene. 36:1012–1022. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song Q, Jing H, Wu H, Zhou G, Kajiyama T
and Kambara H: Gene expression analysis on a photodiode array-based
bioluminescence analyzer by using sensitivity-improved SRPP.
Analyst. 135:1315–1319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Manne RK, Agrawal Y, Bargale A, Patel A,
Paul D, Gupta NA, Rapole S, Seshadri V, Subramanyam D, Shetty P and
Santra MK: A microRNA/Ubiquitin ligase feedback loop regulates
slug-mediated invasion in breast cancer. Neoplasia. 19:483–495.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bahrami A, Aledavood A, Anvari K,
Hassanian SM, Maftouh M, Yaghobzade A, Salarzaee O, ShahidSales S
and Avan A: The prognostic and therapeutic application of microRNAs
in breast cancer: Tissue and circulating microRNAs. J Cell Physiol.
Jan 21–2017.(Epub ahead of print).
|
|
41
|
Nassar FJ, Nasr R and Talhouk R: MicroRNAs
as biomarkers for early breast cancer diagnosis, prognosis and
therapy prediction. Pharmacol Ther. 172:34–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wajapeyee N, Serra RW, Zhu X, Mahalingam M
and Green MR: Oncogenic BRAF induces senescence and apoptosis
through pathways mediated by the secreted protein IGFBP7. Cell.
132:363–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Santra MK, Wajapeyee N and Green MR: F-box
protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest
after DNA damage. Nature. 459:722–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alao JP: The regulation of cyclin D1
degradation: Roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bartkova J, Rezaei N, Liontos M,
Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E,
Niforou K, Zoumpourlis VC, et al: Oncogene-induced senescence is
part of the tumorigenesis barrier imposed by DNA damage
checkpoints. Nature. 444:633–637. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Di Micco R, Fumagalli M, Cicalese A,
Piccinin S, Gasparini P, Luise C, Schurra C, Garre' M, Nuciforo PG,
Bensimon A, et al: Oncogene-induced senescence is a DNA damage
response triggered by DNA hyper-replication. Nature. 444:638–642.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jia L and Sun Y: F-box proteins FBXO31 and
FBX4 in regulation of cyclin D1 degradation upon DNA damage.
Pigment Cell Melanoma Res. 22:518–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin YW, Lee IN, Chen CH, Huang GT, Lee HS,
Lee PH, Lu FJ and Sheu JC: Deletion mapping of chromosome 16q24 in
hepatocellular carcinoma in Taiwan and mutational analysis of the
17-beta-HSD gene localized to the region. Int J Cancer. 93:74–79.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mori Y, Matsunaga M, Abe T, Fukushige S,
Miura K, Sunamura M, Shiiba K, Sato M, Nukiwa T and Horii A:
Chromosome band 16q24 is frequently deleted in human gastric
cancer. Br J Cancer. 80:556–562. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kremmidiotis G, Baker E, Crawford J, Eyre
HJ, Nahmias J and Callen DF: Localization of human cadherin genes
to chromosome regions exhibiting cancer-related loss of
heterozygosity. Genomics. 49:467–471. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Whitmore SA, Settasatian C, Crawford J,
Lower KM, McCallum B, Seshadri R, Cornelisse CJ, Moerland EW,
Cleton-Jansen AM, Tipping AJ, et al: Characterization and screening
for mutations of the growth arrest-specific 11 (GAS11) and C16orf3
genes at 16q24.3 in breast cancer. Genomics. 52:325–331. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pullman WE and Bodmer WF: Cloning and
characterization of a gene that regulates cell adhesion. Nature.
356:529–532. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Graña X, Claudio PP, De Luca A, Sang N and
Giordano A: PISSLRE, a human novel CDC2-related protein kinase.
Oncogene. 9:2097–2103. 1994.PubMed/NCBI
|
|
54
|
Powell JA, Gardner AE, Bais AJ, Hinze SJ,
Baker E, Whitmore S, Crawford J, Kochetkova M, Spendlove HE,
Doggett NA, et al: Sequencing, transcript identification, and
quantitative gene expression profiling in the breast cancer loss of
heterozygosity region 16q24.3 reveal three potential
tumor-suppressor genes. Genomics. 80:303–310. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Waldman T, Kinzler KW and Vogelstein B:
p21 is necessary for the p53-mediated G1 arrest in human cancer
cells. Cancer Res. 55:5187–5190. 1995.PubMed/NCBI
|
|
56
|
Nakagawa H, Zukerberg L, Togawa K, Meltzer
SJ, Nishihara T and Rustgi AK: Human cyclin D1 oncogene and
esophageal squamous cell carcinoma. Cancer. 76:541–549. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shinozaki H, Ozawa S, Ando N, Tsuruta H,
Terada M, Ueda M and Kitajima M: Cyclin D1 amplification as a new
predictive classification for squamous cell carcinoma of the
esophagus, adding gene information. Clin Cancer Res. 2:1155–1161.
1996.PubMed/NCBI
|
|
58
|
Hou X, Liang RB, Wei JC, Xu Y, Fu JH, Luo
RZ, He JH, Zhang LJ, Lin P and Yang HX: Cyclin D1 expression
predicts postoperative distant metastasis and survival in
resectable esophageal squamous cell carcinoma. Oncotarget.
7:31088–31096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kanie T, Onoyama I, Matsumoto A, Yamada M,
Nakatsumi H, Tateishi Y, Yamamura S, Tsunematsu R, Matsumoto M and
Nakayama KI: Genetic reevaluation of the role of F-box proteins in
cyclin D1 degradation. Mol Cell Biol. 32:590–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
















