Introduction
Gastric cancer remains the second most common type
of malignant cancer in China, despite the incidence decreasing
worldwide (1,2). In addition, the majority of patients
with gastric cancer in China are diagnosed with late-stage gastric
cancer (3).
Among the patterns of metastasis, peritoneal seeding
is the most common and most life-threatening type of gastric
cancer, and is considered to be the terminal stage of gastric
cancer (4). Despite often short and
poor survival rates among patients with gastric cancer with
peritoneal seeding, there exists a marked heterogeneity in the
survival duration. Therefore, there has been increasing interest in
investigating the prognostic factors and allowing more accurate
stratification for the patients, which are likely to improve
clinical practice and possibly contribute to more rational study
design and analysis.
The assessment of performance status as the Eastern
Cooperative Oncology Group Performance Status (ECOG PS) is a simple
tool to evaluate a patient's physical condition and is also a
common prognostic factor predicting treatment survival rates
(5). However, the ECOG PS assessment
is subjective and biased. Ando et al reported that
performance status assessments differed significantly among
oncologists, nurses and patients, with the assessment by
oncologists being most optimistic and that by patients the least
(6). Therefore, the selection of ECOG
PS as a prognostic factor remains problematic, and more objective
and reliable prognostic scores are required to reflect clinical
outcome in patients with advanced cancer.
There is increasing evidence that the systemic
inflammatory response, as evidenced by the elevation of C-reactive
protein (CRP), is critical in patients with advanced cancer
(7,8).
Furthermore, Forrest et al reported that the Glasgow
Prognostic Score (GPS), the combination of serum CRP and serum
albumin, was a reliable, objective scoring tool for predicting
survival rates in patients with inoperable non-small cell lung
cancer (9). Additionally, several
studies have demonstrated that GPS is associated with prognosis
independent of age, stage and performance status in various types
of malignancy (10–16).
Crumley et al reported that the GPS was
superior to performance status as a prognostic factor in patients
receiving palliative chemotherapy for gastroesophageal cancer
(10). However, whether GPS is a
superior prognostic factor to ECOG PS in predicting the survival
rates of patients with gastric cancer with peritoneal seeding
remains to be elucidated. Therefore, the present study aimed to
compare GPS with ECOG PS in predicting the outcome of gastric
cancer with peritoneal seeding.
Patients and methods
Patients
Between May 2006 and March 2014, the present study
recruited 384 consecutive patients, who were diagnosed with gastric
adenocarcinoma with peritoneal seeding, at Sun Yat-sen University
Cancer Center. The treatment, including gastrectomy, was performed
following the provision of written informed consent from patients.
The present study was approved by the independent Institute
Research Ethics Committee at the Sun Yat-sen University Cancer
Center (Guangdong, China) and was performed according to the
principles expressed in the Declaration of Helsinki.
The demographic information of the patients was
collected for analysis. Only patients with an entire set of
laboratory data were included in the present study. Patients who
had evidence of infection, and those who received preoperative
chemotherapy or radiotherapy were excluded.
The ECOG PS was evaluated by the definition of the
ECOG criteria. Peritoneal seeding was classified according to the
first English edition of the Japanese classification of gastric
carcinoma (17). Multisite distant
metastasis was defined as concurrent extra-regional lymph node
metastasis, hepatic metastasis, lung metastasis or other metastases
excluding peritoneal seeding. The first-line chemotherapy regimens
included various agents, including 5-fluorouracil, taxane,
irinotecan, oxaliplatin and capecitabine.
GPS estimation
The GPS was estimated according to a previous
description (9). The patients were
assigned a score of 2 if they presented with elevated CRP (>10
mg/l) and hypoalbuminemia (<35 mg/l), a score of 1 if presenting
with only one of these biochemical abnormalities, and a score of 0
if neither of these abnormalities were present.
Statistical analysis
The categorical variables are presented as numbers
and percentages, and were compared using χ2 tests.
Unadjusted Kaplan-Meier survival curves were generated to compare
differences in overall survival (OS) between different groups with
log-rank testing. Prognostic factors were first analyzed by
univariate analysis, with which P<0.05 was entered into
multivariate analysis using Cox proportional hazard models. The
forward selection method was used for multivariate Cox proportional
analysis. In the present study, receiver operating characteristic
(ROC) curves were also constructed to assess sensitivity,
specificity and areas under the curves (AUCs) with a 95% confidence
interval (CI). P<0.05 (two-sided) was considered to indicate a
statistically significant difference. The statistical analyses
described above were performed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA).
The patients were monitored every 3 months for 2
years, and at intervals of 6–12 months thereafter until lost to
follow up or mortality. The regular follow-up period ranged between
0.1 and 52.2 months (median, 9.77 months).
Results
Patient characteristics
The classified clinical and laboratory
characteristics of the 384 gastric cancer patients with peritoneal
seeding are shown in Table I. There
were no significant differences in OS in terms of gender
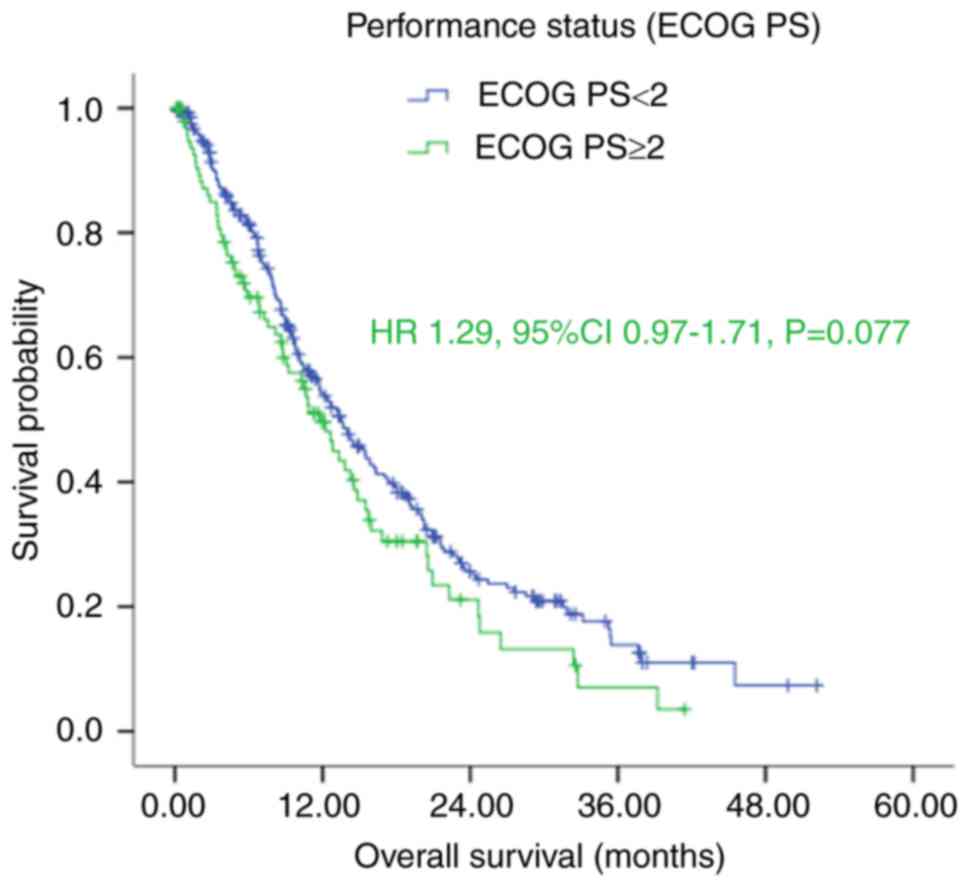
(male/female), ECOG PS (<2/≥2) (Fig.
1), tumor location (cardia/middle/antrum), signet ring cell
carcinoma (yes/no), or CA72-4 (<5.3/≥5.3 U/ml). By contrast,
significant differences in OS were observed in terms of age, tumor
size, ascites, carcinoembryonic antigen (CEA), CA19-9, albumin,
CRP, peritoneal seeding classification, multisite distant
metastasis, palliative gastrectomy, first-line chemotherapy and GPS
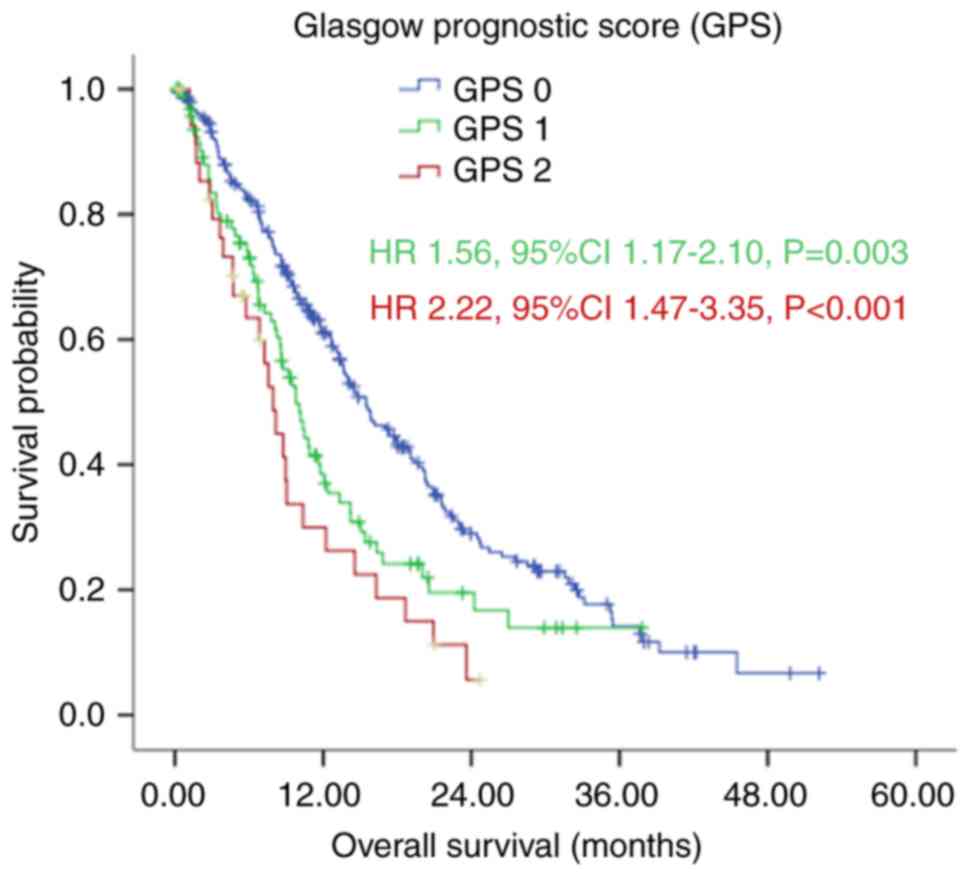
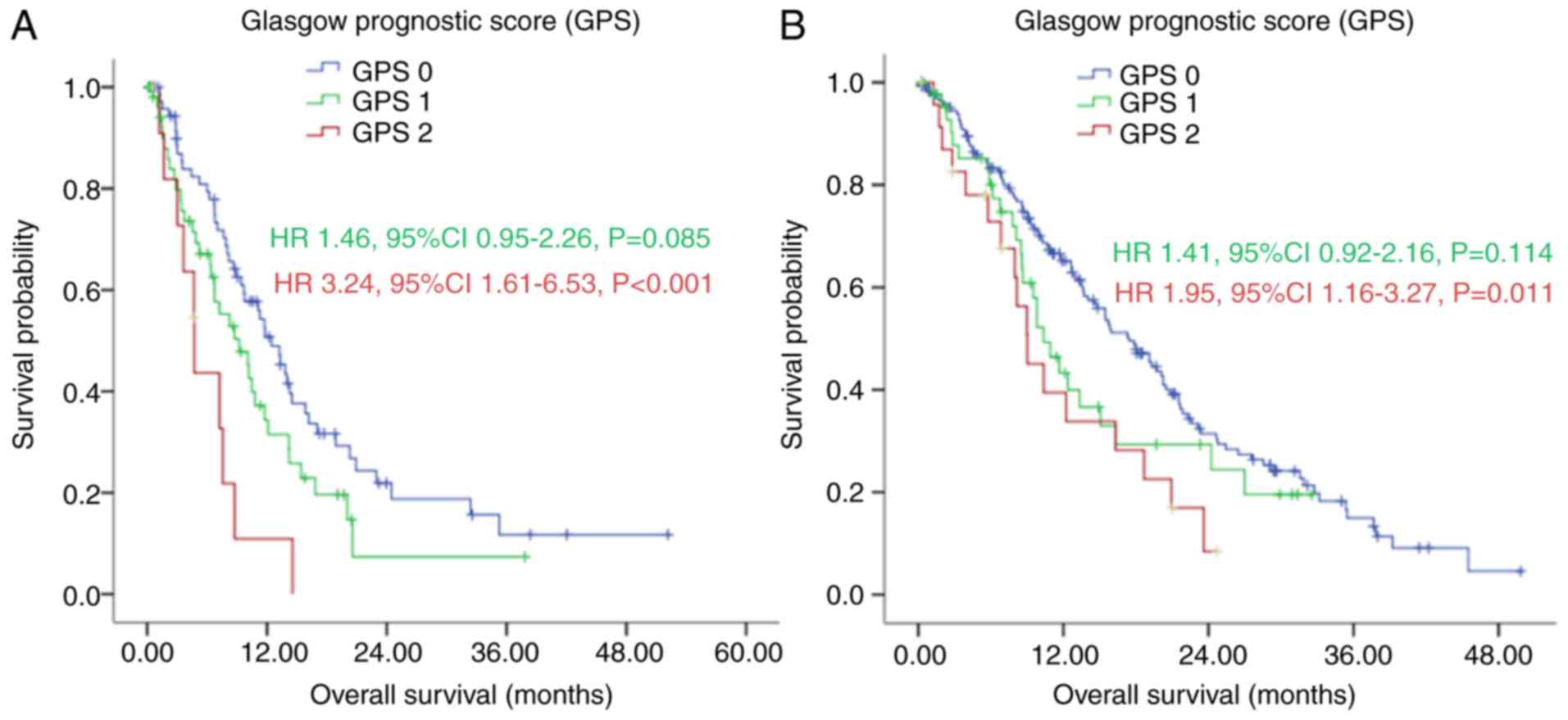
(Fig. 2).
 | Table I.Classified clinical and laboratory
characteristics associated with OS. |
Table I.
Classified clinical and laboratory
characteristics associated with OS.
| Characteristic | Patients (n) | OS (months), median
(95% CI) | P-value |
|---|
| Age (years) |
|
| 0.019 |
|
<65 | 319 | 14.03
(12.07–16.00) |
|
| ≥65 | 65 | 10.37
(6.78–13.96) |
|
| Gender (n) |
|
| 0.285 |
| Male | 210 | 12.23
(9.94–14.53) |
|
|
Female | 174 | 13.70
(11.99–15.41) |
|
| ECOG PS (n) |
|
| 0.076 |
|
<2 | 285 | 13.67
(11.39–15.94) |
|
| ≥2 | 99 | 11.80
(9.40–14.20) |
|
| Tumor location |
|
| 0.514 |
|
Cardia | 96 | 11.73
(8.78–14.69) |
|
|
Middle | 142 | 13.13
(10.07–16.20) |
|
|
Antrum | 138 | 14.23
(11.37–17.10) |
|
| Tumor size
(cm) |
|
| 0.038 |
|
<5 | 150 | 14.27
(10.83–17.71) |
|
| ≥5 | 202 | 11.27
(8.72–13.81) |
|
| SRCC |
|
| 0.052 |
|
Yes | 136 | 15.83
(12.19–19.47) |
|
| No | 244 | 11.77
(9.51–14.02) |
|
| Ascites |
|
| 0.001 |
|
Yes | 160 | 9.73
(8.21–11.25) |
|
| No | 224 | 15.73
(13.01–18.46) |
|
| CEA (ng/ml) |
|
| 0.005 |
|
<5 | 270 | 14.50
(12.90–16.10) |
|
| ≥5 | 101 | 9.80
(7.27–12.33) |
|
| CA19-9 (U/ml) |
|
| <0.001 |
|
<35 | 223 | 15.50
(12.76–18.25) |
|
|
≥35 | 141 | 10.90
(7.95–13.86) |
|
| CA72-4 (U/ml) |
|
| 0.125 |
|
<5.3 | 161 | 14.00
(11.55–16.46) |
|
|
≥5.3 | 154 | 12.10
(8.82–15.38) |
|
| Albumin (g/l) |
|
| 0.001 |
|
<35 | 63 | 8.97
(7.51–10.42) |
|
|
≥35 | 321 | 14.03
(12.34–15.73) |
|
| CRP (mg/l) |
|
| <0.001 |
|
<10 | 274 | 14.57
(12.80–16.34) |
|
|
≥10 | 110 | 8.73
(7.30–10.17) |
|
| Peritoneal
seeding |
|
| 0.001 |
|
P1/P2 | 200 | 15.47
(13.73–17.20) |
|
| P3 | 184 | 10.00
(8.64–11.36) |
|
| Multisite distant
metastasis |
|
| 0.002 |
|
Yes | 142 | 10.17
(7.75–12.59) |
|
| No | 241 | 15.47
(12.66–18.27) |
|
| Palliative
gastrectomy |
|
| <0.001 |
|
Yes | 164 | 19.10
(15.85–22.35) |
|
| No | 219 | 9.80
(8.31–11.29) |
|
| First-line
chemotherapy |
|
| <0.001 |
| Yes | 279 | 15.40
(13.70–17.10) |
|
| No | 105 | 6.40
(4.66–8.14) |
|
| GPS |
|
| <0.001 |
| 0 | 247 | 15.50
(13.09–17.91) |
|
| 1 | 101 | 10.07
(8.29–11.84) |
|
| 2 | 36 | 7.97
(6.47–9.46) |
|
The associations between clinicopathological
characteristics and GPS in patients with gastric cancer with
peritoneal seeding are shown in Table
II. Age, gender, ascites, CA72-4, albumin, CRP, classification
of peritoneal seeding, multisite distant metastasis and palliative
gastrectomy were closely associated with the GPS classification.
Compared with the GPS 0 and GPS 1 patients, the GPS 2 patients
appeared to have higher levels of tumor marker and CRP, and had a
higher frequency of ascites and multisite distant metastasis with
more severe peritoneal seeding.
 | Table II.Clinicopathlogical characteristics of
384 patients with gastric cancer with peritoneal metastasis
according to GPS. |
Table II.
Clinicopathlogical characteristics of
384 patients with gastric cancer with peritoneal metastasis
according to GPS.
| Characteristic | GPS 0 (%) | GPS 1 (%) | GPS 2 (%) | P-value |
|---|
| Patients (n) | 247 (64.3) | 101 (26.3) | 36 (9.4) |
|
| Age (years) |
|
|
| 0.020 |
|
<65 | 215 (87.0) | 77 (76.2) | 27 (75.0) |
|
|
≥65 | 32 (13.0) | 24 (23.8) | 9 (25.0) |
|
| Gender (n) |
|
|
| 0.010 |
|
Male | 121 (49.0) | 65 (64.4) | 24 (66.7) |
|
|
Female | 126 (51.0) | 36 (35.6) | 12 (33.3) |
|
| ECOG PS (n) |
|
|
| 0.131 |
|
<2 | 191 (77.3) | 71 (70.3) | 23 (63.9) |
|
| ≥2 | 56 (22.7) | 30 (29.7) | 13 (36.1) |
|
| Tumor location |
|
|
| 0.636 |
|
Cardia | 66 (27.4) | 23 (23.2) | 7 (19.4) |
|
|
Middle | 89 (36.9) | 36 (36.4) | 17 (47.2) |
|
|
Antrum | 86 (35.7) | 40 (40.4) | 12 (33.3) |
|
| Tumor size
(cm) |
|
|
| 0.102 |
|
<5 | 105 (46.3) | 36 (38.7) | 9 (28.1) |
|
| ≥5 | 122 (53.7) | 57 (61.3) | 23 (71.9) |
|
| SRCC |
|
|
| 0.234 |
|
Yes | 156 (63.4) | 61 (61.6) | 27 (77.1) |
|
| No | 90 (36.6) | 38 (38.4) | 8 (22.9) |
|
| Ascites |
|
|
| <0.001 |
|
Yes | 167 (67.6) | 47 (46.5) | 10 (27.8) |
|
| No | 80 (32.4) | 54 (53.5) | 26 (72.2) |
|
| CEA (ng/ml) |
|
|
| 0.114 |
|
<5 | 181 (75.7) | 70 (70.0) | 19 (59.4) |
|
| ≥5 | 58 (24.3) | 30 (30.0) | 13 (40.6) |
|
| CA19-9 (U/ml) |
|
|
| 0.715 |
|
<35 | 147 (62.8) | 58 (58.6) | 18 (58.1) |
|
|
≥35 | 87 (37.2) | 41 (41.4) | 13 (41.9) |
|
| CA72-4 (U/ml) |
|
|
| 0.006 |
|
<5.3 | 116 (56.9) | 37 (45.1) | 8 (27.6) |
|
|
≥5.3 | 88 (43.1) | 45 (54.9) | 21 (72.4) |
|
| Albumin (g/l) |
|
|
| <0.001 |
|
<35 | 0 (0.0) | 27 (26.7) | 36 (100.0) |
|
|
≥35 | 247 (100.0) | 74 (73.3) | 0 (0.0) |
|
| CRP (mg/l) |
|
|
| <0.001 |
|
<10 | 247 (100.0) | 74 (73.3) | 0 (0.0) |
|
|
≥10 | 0 (0.0) | 27 (26.7) | 36 (100.0) |
|
| Peritoneal
seeding |
|
|
| 0.001 |
|
P1/P2 | 145 (58.7) | 44 (43.6) | 11 (30.6) |
|
| P3 | 102 (41.3) | 57 (56.4) | 25 (69.4) |
|
| Multisite distant
metastasis |
|
|
| <0.001 |
|
Yes | 75 (30.5) | 55 (54.5) | 12 (33.3) |
|
| No | 171 (69.5) | 46 (45.5) | 24 (66.7) |
|
| Palliative
gastrectomy |
|
|
| 0.001 |
|
Yes | 122 (49.6) | 32 (31.7) | 10 (27.8) |
|
| No | 124 (50.4) | 69 (68.3) | 26 (72.2) |
|
| First-line
chemotherapy |
|
|
| 0.242 |
|
Yes | 186 (75.3) | 70 (69.3) | 23 (63.9) |
|
| No | 61 (24.7) | 31 (30.7) | 13 (36.1) |
|
Survival rates
The results of the Kaplan-Meier analysis
demonstrated that patients with good performance status (ECOG
<2) had longer median OS, compared with those with poor
performance status (ECOG ≥2), with an OS of 13.67 (95% CI:
11.39–15.94), vs. 11.80 (95% CI: 9.40–14.20) months, respectively.
However, this difference was not significant (P=0.076; Fig. 1 and Table
I).
However, the Kaplan-Meier analysis demonstrated that
patients in the GPS 0 group had a significantly longer median OS,
compared with those in the GPS 1 and GPS 2 group, with median OS
rates of 15.50 (95% CI: 13.09–17.91), 10.07 (95% CI: 8.29–11.84)
and 7.97 (95% CI: 6.47–9.46) months, respectively (P<0.001;
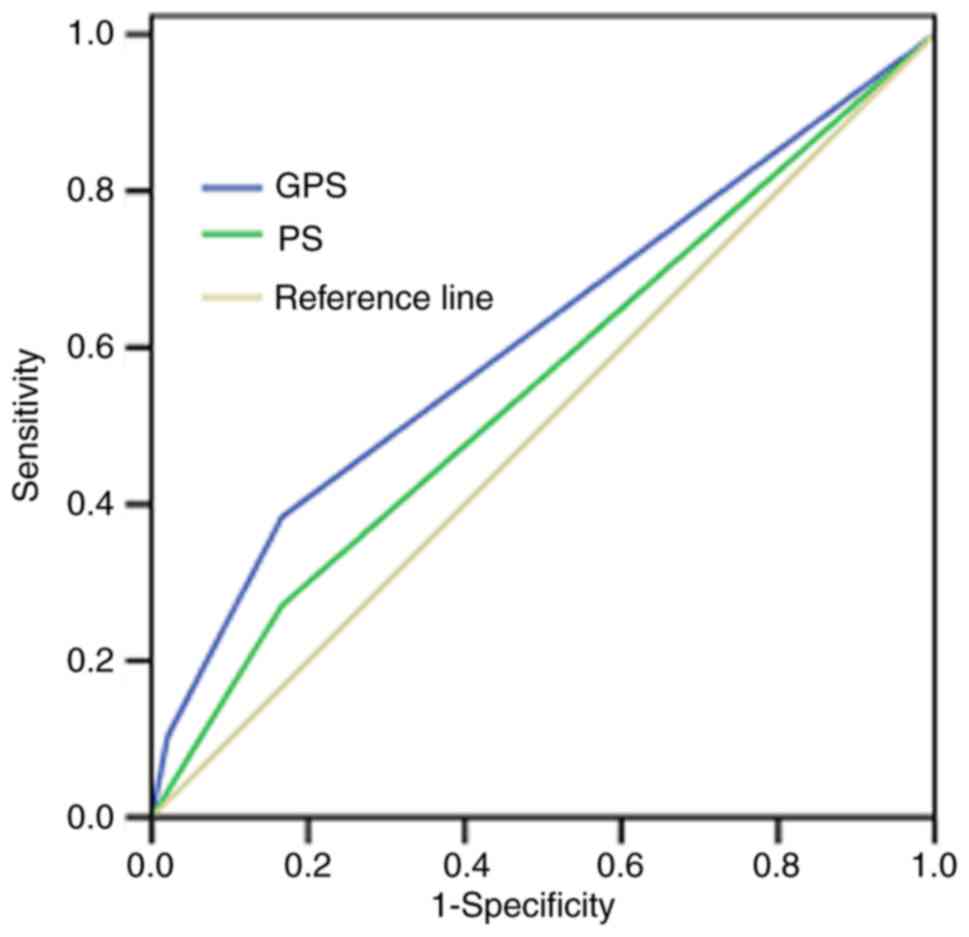
Fig. 2 and Table I). The ROC curves also showed that the
AUC of GPS was 0.613 (P=0.011), whereas the AUC of ECOG PS was
0.552 (P=0.243; Fig. 3).
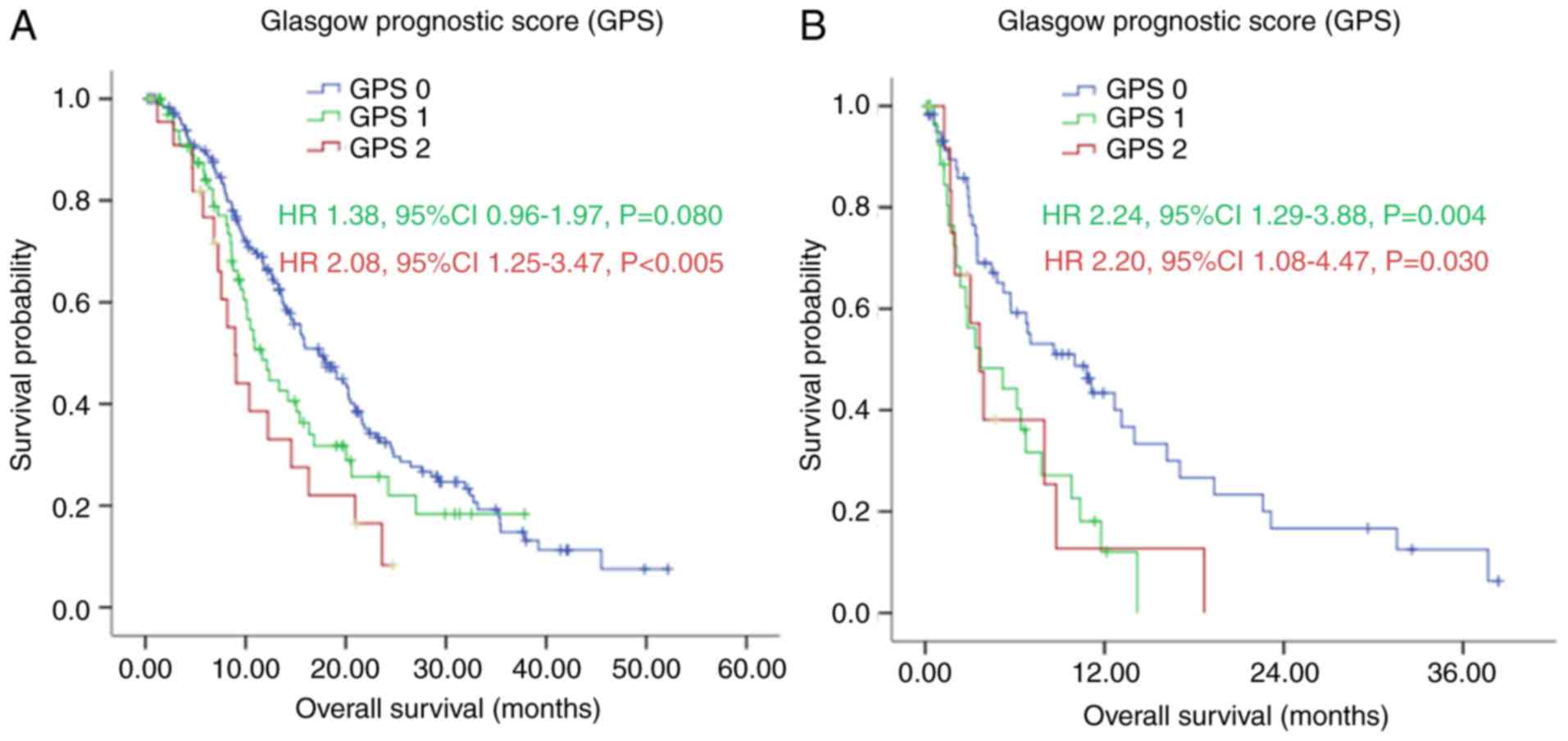
In the subgroup analysis, with first-line
chemotherapy, the median OS of patients in the GPS 0 group was
significantly longer, compared with the median OS of patients in
the GPS 1 and GPS 2 group [17.40 (95% CI: 14.47–20.33), vs. 11.67
(95% CI: 8.50–14.84), vs. 8.97 (95% CI: 7.20–10.73) months,
respectively], as shown in Fig. 4A
(P=0.008). Without first-line chemotherapy, patients in the GPS 0
group also had a significantly longer median OS, compared with
those in the GPS 1 and GPS 2 groups [10.00 (95% CI: 5.05–14.95),
vs. 3.73 (95% CI: 0.00–7.58), vs. 3.63 (95% CI: 2.30–4.97) months,
respectively], as shown in Fig. 4B
(P=0.005).
In patients with multisite distant metastasis, the
median OS of the GPS 0 group was longer, compared with the median
OS in the GPS 1 and GPS 2 group [12.43 (95% CI: 9.75–15.12), vs.
9.20 (95% CI: 5.75–12.65), vs. 4.73 (95% CI: 3.07–6.40) months
(Fig. 5A; P=0.002). Without multisite
distant metastasis, the GPS 0 group also had a longer median OS,
compared with the median OS in the GPS 1 and GPS 2 groups [17.40
(95% CI: 14.12–20.68), vs. 10.37 {95% CI: 7.97–12.76), vs. 9.03
(95% CI: 6.12–11.95) months, as shown in Fig. 5B (P=0.019).
Univariate and multivariate
analyses
In the univariate survival analysis, age (P=0.020),
tumor size (P=0.039), ascites (P<0.001), CEA (P=0.006), CA19-9
(P<0.001), albumin (P=0.001), CRP (P<0.001), classification
of peritoneal seeding (P=0.001), multisite distant metastasis
(P=0.002), palliative gastrectomy (P<0.001), first-line
chemotherapy (P<0.001) and GPS (P<0.001) were associated with
OS (Table III). The multivariate
survival analysis demonstrated that CA19-9 (P<0.001), palliative
gastrectomy (P<0.001), first-line chemotherapy (P<0.001) and
GPS (P<0.001) remained the prognostic factors in predicting the
OS (Table III).
 | Table III.Univariate and multivariate of
analyses of overall survival in patients with gastric cancer with
peritoneal metastasis. |
Table III.
Univariate and multivariate of
analyses of overall survival in patients with gastric cancer with
peritoneal metastasis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95%CI) | P-value | HR (95%CI) | P-value |
|---|
| Age (years) |
| 0.020 |
|
|
|
<65 | 1 |
|
|
|
|
≥65 | 1.44
(1.60–1.96) |
|
|
|
| Gender (n) |
| 0.285 |
|
|
|
Male | 1 |
|
|
|
|
Female | 0.87
(0.68–1.12) |
|
|
|
| ECOG PS (n) |
| 0.077 |
|
|
|
<2 | 1 |
|
|
|
| ≥2 | 1.29
(0.97–1.71) |
|
|
|
| Tumor location |
| 0.515 |
|
|
|
Cardia | 1 |
|
|
|
|
Middle | 1.07
(0.77–1.47) | 0.696 |
|
|
|
Antrum | 0.90
(0.65–1.25) | 0.536 |
|
|
| Tumor size
(cm) |
| 0.039 |
|
|
|
<5 | 1 |
|
|
|
| ≥5 | 1.32
(1.01–1.71) |
|
|
|
| SRCC |
| 0.053 |
|
|
| No | 1 |
|
|
|
|
Yes | 0.77
(0.59–1.00) |
|
|
|
| Ascites |
| <0.001 |
|
|
| No | 1 |
|
|
|
|
Yes | 1.53
(1.19–1.97) |
|
|
|
| CEA (ng/ml) |
| 0.006 |
|
|
|
<5 | 1 |
|
|
|
| ≥5 | 1.47
(1.12–1.93) |
|
|
|
| CA19-9 (U/ml) |
| <0.001 |
| <0.001 |
|
<35 | 1 |
| 1 |
|
|
≥35 | 1.62
(1.25–2.10) |
| 1.63
(1.25–2.12) |
|
| CA72-4 (U/ml) |
| 0.126 |
|
|
|
<5.3 | 1 |
|
|
|
|
≥5.3 | 1.24
(0.94–1.62) |
|
|
|
| Albumin (g/l) |
| 0.001 |
|
|
|
<35 | 1 |
|
|
|
|
≥35 | 0.59
(0.43–0.82) |
|
|
|
| CRP (mg/l) |
| <0.001 |
|
|
|
<10 | 1 |
|
|
|
|
≥10 | 1.72
(1.30–2.27) |
|
|
|
| Peritoneal
seeding |
| 0.001 |
|
|
|
P1/P2 | 1 |
|
|
|
| P3 | 1.50
(1.17–1.92) |
|
|
|
| Multisite distant
metastasis |
| 0.002 |
|
|
| No | 1 |
|
|
|
|
Yes | 1.49
(1.15–1.93) |
|
|
|
| Palliative
gastrectomy |
| <0.001 |
| <0.001 |
| No | 1 |
| 1 |
|
|
Yes | 0.51
(0.40–0.66) |
| 0.56
(0.43–0.73) |
|
| First-line
chemotherapy |
| <0.001 |
| <0.001 |
| No | 1 |
| 1 |
|
|
Yes | 0.43
(0.32–0.57) |
| 0.40
(0.30–0.54) |
|
| GPS |
| <0.001 |
| 0.006 |
| 0 | 1 |
| 1 |
|
| 1 | 1.56
(1.17–2.10) | 0.003 | 1.47
(1.08–1.98) | 0.013 |
| 2 | 2.22
(1.47–3.35) | <0.001 | 1.76
(1.13–2.73) | 0.012 |
Discussion
There is substantial evidence that tumor-related
factors and host-related factors, including poor performance
status, weight loss and systemic inflammatory response, can
determine the outcomes of patients with malignant cancer (15,18).
However, the assessments of weight loss and performance status are
subjective and biased. By contrast, in the present study,
univariate and multivariate analysis demonstrated that an
inflammatory prognostic score, as evidenced by the GPS, was
superior to performance status (ECOG PS) as a prognostic factor in
predicting the outcome of patients with gastric cancer with
peritoneal dissemination.
It is generally recognized that cancer-related
inflammation can assist in malignant cancer cell proliferation and
survival, accelerating angiogenesis and metastasis, destroying the
adaptive immune responses of the patients, and finally altering the
responses of patients to hormones and chemotherapy treatment
(19). CRP is an important acute
phase protein and a sensitive marker of the systemic inflammatory
response. Additionally, CRP can be expressed in malignant cancer
cells (7,20,21). CRP
synthesis is generally induced by several chemokines and cytokines,
including tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1) and
IL-6, from the liver or cancer tissues (22,23).
However, serum CRP measurement is more convenient and stable,
compared with cytokine and chemokine measurement. Several studies
have revealed that elevated CRP is associated with poor survival
rates in certain types of malignant cancer, including breast
cancer, hepatocellular carcinoma and gastric cancer (8,24,25). In accordance with other studies
(8,24), the present study showed that elevated
CRP correlated with poorer prognosis in patients with gastric
cancer and peritoneal seeding.
Lien et al reported that preoperative serum
albumin levels are associated with resectability and survival rates
in patients with gastric cancer (26). Serum albumin is not only an indicator
used to recognize the nutritional status of patients, but is also
useful for predicting the prognostic outcome of cancer patients
(5,27). Patients with gastric cancer with
peritoneal seeding often develop hypoalbuminemia due to oral intake
deficiency, overconsumption, bleeding and ascites. In the present
study, univariate analysis revealed that hypoalbuminemia was
significantly associated with poor prognosis. However, when CRP and
albumin were placed in the multivariate analysis, neither of them
was associated with OS, which indicated the insufficiency of serum
CRP and albumin alone as a prognostication. Therefore, GPS, the
combination of serum albumin and CRP, was superior in predicting
the outcome of gastric cancer with peritoneal seeding.
The mechanism by which GPS affect cancer survival
rates remains to be fully elucidated. However, in addition to
reflecting the presence of a systemic inflammatory response, GPS
may also reflect the declining nutrition status of patients with
advanced stage disease, which affects their tolerance and
compliance to therapeutic regimens (10). In the present study, it was also noted
that a higher GPS correlated significantly with higher levels of
tumor markers, increased frequency of ascites, multisite distant
metastasis and more severe peritoneal seeding, suggesting that a
higher GPS was correlated with a more aggressive disease phenotype.
However, certain therapeutic regimes can lead to elevated CRP or
weight loss and malnourishment, and thus reduced albumin levels.
Therefore, whether the poorer survival rate of patients was due to
these host-associated factors or cancer-associated factors also
remains to be elucidated. Of note, the present study found that
there were no significant differences among the GPS 0, GPS 1 and
GPS 2 groups regarding the period of first-line chemotherapy and
response to chemotherapy (Table IV),
which was a contradiction to the findings of other studies
(12,20). This suggested that patients with a
higher GPS should also receive active palliative chemotherapy.
Although anti-inflammatory treatment with low-dose aspirin lowers
the incidence of colorectal adenomas and the mortality rate of
several common types of cancer (28,29),
whether anti-inflammatory treatment can improve the outcome of
patients with advanced cancer remains to be elucidated.
Furthermore, whether a higher GPS is a cause or a consequence of
cancer progression also remains unclear.
 | Table IV.Response to chemotherapy of 278
patients with gastric cancer with peritoneal metastasis according
to GPS. |
Table IV.
Response to chemotherapy of 278
patients with gastric cancer with peritoneal metastasis according
to GPS.
| Characteristic | GPS 0 | GPS 1 | GPS 2 | P-value |
|---|
| Patients (n) | 185 | 70 | 23 |
|
| Period of
chemotherapy, mean (n, 95% CI) | 5.39
(4.82–5.97) | 5.06
(4.14–5.97) | 4.96
(3.58–6.33) | 0.762 |
| DCR (CR+PR+SD) n
(%) | 48 (25.9) | 19 (27.1) | 3 (13) | 0.368 |
Irrespective of the mechanisms involved, the results
of the present study showed that GPS was a simple, objective and
reliable survival predictor for gastric cancer with peritoneal
seeding, which was true for those receiving palliative chemotherapy
and for those who were not.
The present study was a substantially retrospective
study, which is a potential limitation. Although the data in the
present study were from a high-volume institution, the results
require cautious interpretation and a large-scale prospective study
is required to validate the results.
In conclusion, the present study indicated that the
GPS is superior to performance status (ECOG PS) as a prognostic
factor in predicting the outcome of patients with gastric cancer
with peritoneal seeding.
Acknowledgements
This study was supported in part by a grant from
National Natural Science Foundation of China (grant no. 81302144)
and the Guangdong Science and Technology Department (grant no.
2012B0617000879).
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen S, Li YF, Feng XY, Zhou ZW, Yuan XH
and Chen YB: Significance of palliative gastrectomy for late-stage
gastric cancer patients. J Surg Oncol. 106:862–871. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bando E, Yonemura Y, Takeshita Y,
Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T,
Nishimura G and Miwa K: Intraoperative lavage for cytological
examination in 1,297 patients with gastric carcinoma. Am J Surg.
178:256–262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sachlova M, Majek O and Tucek S:
Prognostic value of scores based on malnutrition or systemic
inflammatory response in patients with metastatic or recurrent
gastric cancer. Nutr Cancer. 66:1362–1370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ando M, Ando Y, Hasegawa Y, Shimokata K,
Minami H, Wakai K, Ohno Y and Sakai S: Prognostic value of
performance status assessed by patients themselves, nurses, and
oncologists in advanced non-small cell lung cancer. Br J Cancer.
85:1634–1639. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nozoe T, Korenaga D, Futatsugi M, Saeki H,
Maehara Y and Sugimachi K: Immunohistochemical expression of
C-reactive protein in squamous cell carcinoma of the
esophagus-significance as a tumor marker. Cancer Lett. 192:89–95.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hashimoto K, Ikeda Y, Korenaga D, Tanoue
K, Hamatake M, Kawasaki K, Yamaoka T, Iwatani Y, Akazawa K and
Takenaka K: The impact of preoperative serum C-reactive protein on
the prognosis of patients with hepatocellular carcinoma. Cancer.
103:1856–1864. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Forrest LM, McMillan DC, McArdle CS,
Angerson WJ and Dunlop DJ: Evaluation of cumulative prognostic
scores based on the systemic inflammatory response in patients with
inoperable non-small-cell lung cancer. Br J Cancer. 89:1028–1030.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crumley AB, McMillan DC, McKernan M,
McDonald AC and Stuart RC: Evaluation of an inflammation-based
prognostic score in patients with inoperable gastro-oesophageal
cancer. Br J Cancer. 94:637–641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishizuka M, Nagata H, Takagi K, Horie T
and Kubota K: Inflammation-based prognostic score is a novel
predictor of postoperative outcome in patients with colorectal
cancer. Ann Surg. 246:1047–1051. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crumley AB, Stuart RC, McKernan M,
McDonald AC and McMillan DC: Comparison of an inflammation-based
prognostic score (GPS) with performance status (ECOG-ps) in
patients receiving palliative chemotherapy for gastroesophageal
cancer. J Gastroenterol Hepatol. 23:e325–e329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roxburgh CS, Crozier JE, Maxwell F, Foulis
AK, Brown J, McKee RF, Anderson JH, Horgan PG and McMillan DC:
Comparison of tumour-based (Petersen Index) and inflammation-based
(Glasgow Prognostic Score) scoring systems in patients undergoing
curative resection for colon cancer. Br J Cancer. 100:701–706.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kinoshita A, Onoda H, Imai N, Iwaku A,
Oishi M, Fushiya N, Koike K, Nishino H and Tajiri H: Comparison of
the prognostic value of inflammation-based prognostic scores in
patients with hepatocellular carcinoma. Br J Cancer. 107:988–993.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roxburgh CS and McMillan DC: Role of
systemic inflammatory response in predicting survival in patients
with primary operable cancer. Future Oncol. 6:149–163. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Y and Huang D: The value of the
systematic inflammation-based Glasgow Prognostic Score in patients
with gastric cancer: A literature review. J Cancer Res Ther.
10:799–804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Japanese Research Society for Gastric
Cancer, . Japanese Classification of Gastric Carcinoma. 1st
English. Tokyo: Kanehara & Co, Ltd; 1995
|
|
18
|
Andreyev HJ, Norman AR, Oates J and
Cunningham D: Why do patients with weight loss have a worse outcome
when undergoing chemotherapy for gastrointestinal malignancies? Eur
J Cancer. 34:503–509. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwang JE, Kim HN, Kim DE, Choi HJ, Jung
SH, Shim HJ, Bae WK, Hwang EC, Cho SH and Chung IJ: Prognostic
significance of a systemic inflammatory response in patients
receiving first-line palliative chemotherapy for recurred or
metastatic gastric cancer. BMC Cancer. 11:4892011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du Clos TW: Function of C-reactive
protein. Ann Med. 32:274–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du Clos TW and Mold C: C-reactive protein:
An activator of innate immunity and a modulator of adaptive
immunity. Immunol Res. 30:261–277. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Slaviero KA, Clarke SJ and Rivory LP:
Inflammatory response: An unrecognised source of variability in the
pharmacokinetics and pharmacodynamics of cancer chemotherapy.
Lancet Oncol. 4:224–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McMillan DC, Elahi MM, Sattar N, Angerson
WJ, Johnstone J and McArdle CS: Measurement of the systemic
inflammatory response predicts cancer-specific and non-cancer
survival in patients with cancer. Nutr Cancer. 41:64–69. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Imaoka H, Mizuno N, Hara K, Hijioka S,
Tajika M, Tanaka T, Ishihara M, Yogi T, Tsutsumi H, Fujiyoshi T, et
al: Evaluation of modified glasgow prognostic score for pancreatic
cancer: A retrospective cohort study. Pancreas. 45:211–217. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lien YC, Hsieh CC, Wu YC, Hsu HS, Hsu WH,
Wang LS, Huang MH and Huang BS: Preoperative serum albumin level is
a prognostic indicator for adenocarcinoma of the gastric cardia. J
Gastrointest Surg. 8:1041–1048. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee J, Lim T, Uhm JE, Park KW, Park SH,
Lee SC, Park JO, Park YS, Lim HY, Sohn TS, et al: Prognostic model
to predict survival following first-line chemotherapy in patients
with metastatic gastric adenocarcinoma. Ann Oncol. 18:886–891.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baron JA, Cole BF, Sandler RS, Haile RW,
Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R,
Burke CA, et al: A randomized trial of aspirin to prevent
colorectal adenomas. N Engl J Med. 348:891–899. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rothwell PM, Fowkes FG, Belch JF, Ogawa H,
Warlow CP and Meade TW: Effect of daily aspirin on long-term risk
of death due to cancer: Analysis of individual patient data from
randomised trials. Lancet. 377:31–41. 2011. View Article : Google Scholar : PubMed/NCBI
|