Introduction
Non-small cell lung cancer (NSCLC) accounts for 85%
of pulmonary malignancies (1) and is
one of the leading causes of cancer-associated mortality worldwide.
Recurrent and metastatic disease is the main cause of mortality,
and the 5-year survival rate for lung cancer is 15% (2). Therefore, in order to improve the
prognosis for patients with NSCLC, it is important to understand
the underlying molecular mechanisms that contribute to tumor
recurrence and metastases, and to identify the relevant markers and
targets for treatments.
The epithelial-mesenchymal transition (EMT) is a
complex but reversible process whereby epithelial cells acquire a
mesenchymal phenotype (3). EMT leads
to the loss of the epithelial cell marker E-cadherin, which is a
cell-cell junction protein. Loss of E-cadherin is associated with
NSCLC progression and metastasis, and with a poor prognosis for
patients with NSCLC (4). Therefore,
determining an effective method to inhibit EMT in NSCLC may
significantly improve the treatment of NSCLC.
Eukaryotic translation initiation factor 5A (EIF5A)
is a protein involved in numerous intracellular processes. EIF5A
has been demonstrated to serve a role in translation initiation,
translation elongation, transcription, mRNA turnover and
nucleocytoplasmic transport (5).
EIF5A arises in two forms (6,7); EIF5A1 serves an essential role in
non-malignant cells, although it has also been implicated in cancer
(8). EIF5A2 is a purported oncogene
that is less widely expressed in normal tissue (5,9,10); however, EIF5A2 expression has been
associated with a number of cancer types, including lung cancer.
Downregulation of EIF5A2 prevents EMT in NSCLC (11), while overexpression of EIF5A2 is an
adverse prognostic marker of survival for patients with stage I
NSCLC (12). In addition, it has been
demonstrated that inhibition of EIF5A2 enhances NSCLC sensitivity
to chemotherapeutics, prevents or reverses EMT, and reduces the
migration and invasion capabilities of NSCLC cells (13).
The role of EIF5A2 as an oncogene has been well
established in a number of types of cancer, including NSCLC.
However, its role in NSCLC cells is not clear. In the present
study, the role of EIF5A2 in NSCLC was investigated.
Materials and methods
Patients and specimens
A total of 47 paired tumor tissue samples and
adjacent normal tissues were obtained from primary lung cancer
patients undergoing surgical treatment between May 2008 and
December 2010 at the Affiliated Hospital of Zunyi Medical College
(Zunyi, China). Among the 47 patients with NSCLC, there were 25
males and 22 females aged between 47 and 71 years old, with a mean
age of 60 years old. None of the patients had received adjuvant
therapy prior to surgery. Following removal, the tissue specimens
were immediately fixed in 4% formaldehyde and embedded in paraffin
for immunohistochemical staining. All the tissues were processed
within 15 min of removal. Each sample was frozen and stored at
−80°C. The tumor tissue samples were confirmed by pathological
diagnosis, while paired non-cancerous adjacent tisssues were
dissected at least 2 cm away from the tumor border and were
confirmed to lack tumor cells by microscopy. The patient
clinicopathological features are presented in Table I. The present study was approved by
the Ethics Committee of the Affiliated Hospital of Zunyi Medical
College, and written informed consent was obtained from each
patient enrolled in the study.
 | Table I.Clinicopathological characteristics of
patients with non-small cell lung cancer. |
Table I.
Clinicopathological characteristics of
patients with non-small cell lung cancer.
|
| EIF5A2 expression
level |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristic | Low (n=21) | High (n=26) | P-valuea |
|---|
| Sex |
|
| 1.0000 |
|
Female | 9 | 11 |
|
|
Male | 12 | 15 |
|
| Age, years |
|
| 0.5506 |
|
≤60 | 7 | 12 |
|
|
>60 | 14 | 14 |
|
| Location of
tumor |
|
| 0.3806 |
|
Left | 8 | 14 |
|
|
Right | 13 | 12 |
|
| Histology |
|
| 0.3881 |
|
Adenocarcinoma | 10 | 16 |
|
|
Squamous cell carcinoma | 11 | 10 |
|
| Tumor size, cm |
|
| 0.0175a |
| ≤3 | 16 | 10 |
|
|
>3 | 5 | 16 |
|
| Lymph node
metastasis |
|
| 0.0200a |
|
Yes | 7 | 18 |
|
| No | 14 | 8 |
|
| Clinical stage |
|
| 0.7674 |
|
I–II | 13 | 14 |
|
|
III | 8 | 12 |
|
Immunohistochemical staining of EIF5A2 expression.
Immunohistochemical staining of EIF5A2 was performed to assess
relative EIF5A2 expression in NSCLC tissue compared with adjacent
normal tissue, according to a standard immunoperoxidase staining
protocol. EIF5A2 immunohistochemistry was performed on 4-µm thick,
formalin-fixed, paraffin-embedded tissue sections. A rabbit
polyclonal antibody anti-EIF5A2 (1:100; cat. no. GTX110510; GeneTex
Inc., Irvine, CA, USA) was used for staining (14) and incubated at 37°C for 2 h, followed
by incubation 1 h with horseradish peroxidase-conjugated goat
anti-rabbit IgG (1:500; cat. no. ab7090; Abcam, Cambridge, MA,
USA), then 3,3′-diaminobenzidine (DAB) staining solution (1:25,
cat. no. 070004-D; Beijing CellChip Biotechnology Co., Ltd.,
Beijing, China) for 10 min at room temperature. The
immunohistochemical staining was evaluated using a
semi-quantitative score method as follows: 0, 0%; 1, <5%; 2,
~5-50%; and 3, >50% stained cells. In addition, the staining
intensity was scored as 0 (−), 1 (+), 2 (++) or 3 (+++). The final
score was defined as the sum of the two parameters, and the samples
were grouped as negative (0), weak (1–2), moderate
(3) and strong (4–6) staining.
Negative and weak staining were defined as low expression of
EIF5A2, and moderate and strong staining were defined as high
expression of EIF5A2. Only the final immunoreaction scores of the
moderate and strong groups were considered as positive for
expression. The EIF5A2 expression results are presented in Table I.
Cell culture and transfection
The human bronchial epithelial HBE cell line and
five NSCLC cell lines (A549, H23, Calu-3, H1299 and H460) were
purchased from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). Cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), supplemented with 100 U/ml penicillin, 100 mg/ml
streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
10% fetal calf serum (Gibco, Thermo Fisher Scientific, Inc.). Cells
were maintained at 37°C with 5% CO2 in a humidified
incubator.
Knockdown of EIF5A2 gene expression in NSCLC cells
was accomplished using small interfering (si)RNAs targeted against
EIF5A2, which were designed and synthesized by Shanghai GenePharma
Co., Ltd. (Shanghai, China). The sequence of EIF5A2 siRNA was as
follows: 5′-GGAUCUUAAACUGCCAGAATT-3′, 5′-UUCUGGCAGUUUAAGAUCCTT-3′.
EIF5A2 siRNA (siRNA1, 5 µmol/ml; siRNA2, 10 µmol/ml; siRNA3, 15
µmol/ml) or negative control siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′;
5′-ACGUGACACGUUCGGAGAATT-3′) was transfected into the H1299 and
H460 cells using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The transfection medium was replaced with culture medium 6 h after
transfection. All subsequent experiments were performed 24 h after
transfection and repeated three times.
Cell proliferation assay
The cell proliferation assay was performed using
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), according to the manufacturer's protocol. The
H1299 and H460 cells treated with EIF5A2 siRNA or control siRNA
were seeded on a 96-well microplate at a density of
5×103 cells/well. Cell proliferation was assessed at 24,
48 and 72 h. CCK-8 solution (10 µl) was added to each well, and the
cells were incubated at 37°C for an additional 3 h. Optical density
(OD) was determined at a wavelength of 450 nm using an MRX TC II
microplate reader (Dynex Technologies, Chantilly, VA, USA).
Cell migration and invasion assays. For the
migration assay, H1299 and H460 cells (2×105) were
resuspended in 100 µl serum-free RPMI medium and loaded into the
upper chamber of a modified Boyden chamber (Neuro Probe, Inc.,
Gaithersburg, MD, USA). The lower chamber was filled with RPMI
medium and vascular endothelial growth factor (50 ng/ml). The
chamber was incubated at 37°C for 24 h. The lower side of the
filter was washed with PBS and fixed with 4% paraformaldehyde. The
cells were stained with hematoxylin and counted in three random
high-power fields in each well under an Olympus inverted microscope
(IX70).
For the invasion assay, the cells (2×105)
were resuspended in 100 µl of serum-free RPMI medium and placed
into the upper chamber, which contained an 8-µm microporous
membrane insert (Costar; Corning Inc., Corning, NY, USA) coated
with Matrigel® (BD Biosciences, Franklin Lakes, NJ,
USA). The lower chamber contained 10% FBS plus RPMI-1640 medium.
The chamber was incubated at 37°C for 24 h. Following this, the
lower side of the filter was washed with PBS and fixed with 4%
paraformaldehyde at 37°C for 30 min. The cells were stained with
hematoxylin at 37°C for 15 min and eosin at 37°C for 5 min. The
invading cells were imaged and counted using an inverted phase
contrast microscope.
Flow cytometry
The apoptosis of cells was assessed using flow
cytometry. H1299 and H460 cells were respectively plated in 6-well
plates at a density of 5×105 cells/well. Cells were
harvested and fixed with cold 75% ethanol at 4°C overnight. The
fixed cells were collected and labeled with 10 µg/ml anti-Annexin
V-fluorescein isothiocyanate (FITC; eBioscience, Inc., San Diego,
CA, USA) and propidium iodide (PI; eBioscience, Inc.) at 4°C for 1
h. The degree of apoptosis was expressed as the percentage of cells
stained with Annexin V-FITC/PI and analyzed by fluorescence
activated cell sorting using a FACscan flow cytometer (BD
Biosciences FACScan) and WinList software (version 7.0; Verity
Software House, Inc., Topsham, ME, USA).
Western blot analysis
The non-cancerous human bronchial epithelial cell
line, in addition to the NSCLC A549, H23, Calu-3, H1299 and H460
cell lines, were resuspended in 300 µl cell lysis buffer (Cell
Signaling Technology, Inc., Danvers, MA, USA) with 1X protease
inhibitors and 1 mM phenylmethanesulfonyl fluoride (Sigma-Aldrich;
Merck KGaA). The protein concentration was quantified using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.). A total of 40 µg of protein was loaded in each well and run
on a 10% SDS-PAGE gel, and the proteins were transferred to
polyvinylidene fluoride membranes (Merck KGaA). The membranes were
blocked with Tris-buffered saline (TBS) and with TBS containing
0.1% Tween-20 and 5% bovine serum albumin at 4°C overnight. Western
blotting was performed using primary antibodies against EIF5A2
(dilution, 1:500; cat. no. 17069-1-AP; ProteinTech Group, Inc.,
Chicago, IL, USA), ww (dilution, 1:5,000 in 5% milk; cat. no. I-19;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Vimentin (cat.
no. 13614), E-cadherin (cat. no. 5296), Ras-related C3 botulinum
toxin substrate 1 (Rac1; cat. no. 2465), apoptosis regulator Bcl-2
(Bcl-2; cat. no. 2872) and myc proto-oncogene protein (c-Myc; cat.
no. 9402; all dilutions, 1:1,000; Cell Signaling Technology, USA)
and incubated overnight at 4°C. Subsequently, the secondary goat
anti-rabbit antibodies (1:4,000, cat. no. NEF812001EA; PerkinElmer,
Inc., Waltham, MA, USA) were added and incubated for 1 h at room
temperature. The blots were then re-probed with horseradish
peroxidase-conjugated secondary antibody and visualized using the
SuperSignal West Pico chemiluminescent substrate (Pierce; Thermo
Fisher Scientific, Inc.). Autoradiographs were scanned using
ImageQuant LAS 4010 Imaging System (GE Healthcare, Chicago, IL,
USA) and analyzed semi-quantitatively. All images are
representative of at least three separate experiments.
Statistical analysis
Quantitative values are presented as the mean ±
standard deviation. Student's t-test was performed to determine
statistically significant differences between groups. Statistical
analysis was performed using SPSS software (version 16.0; SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
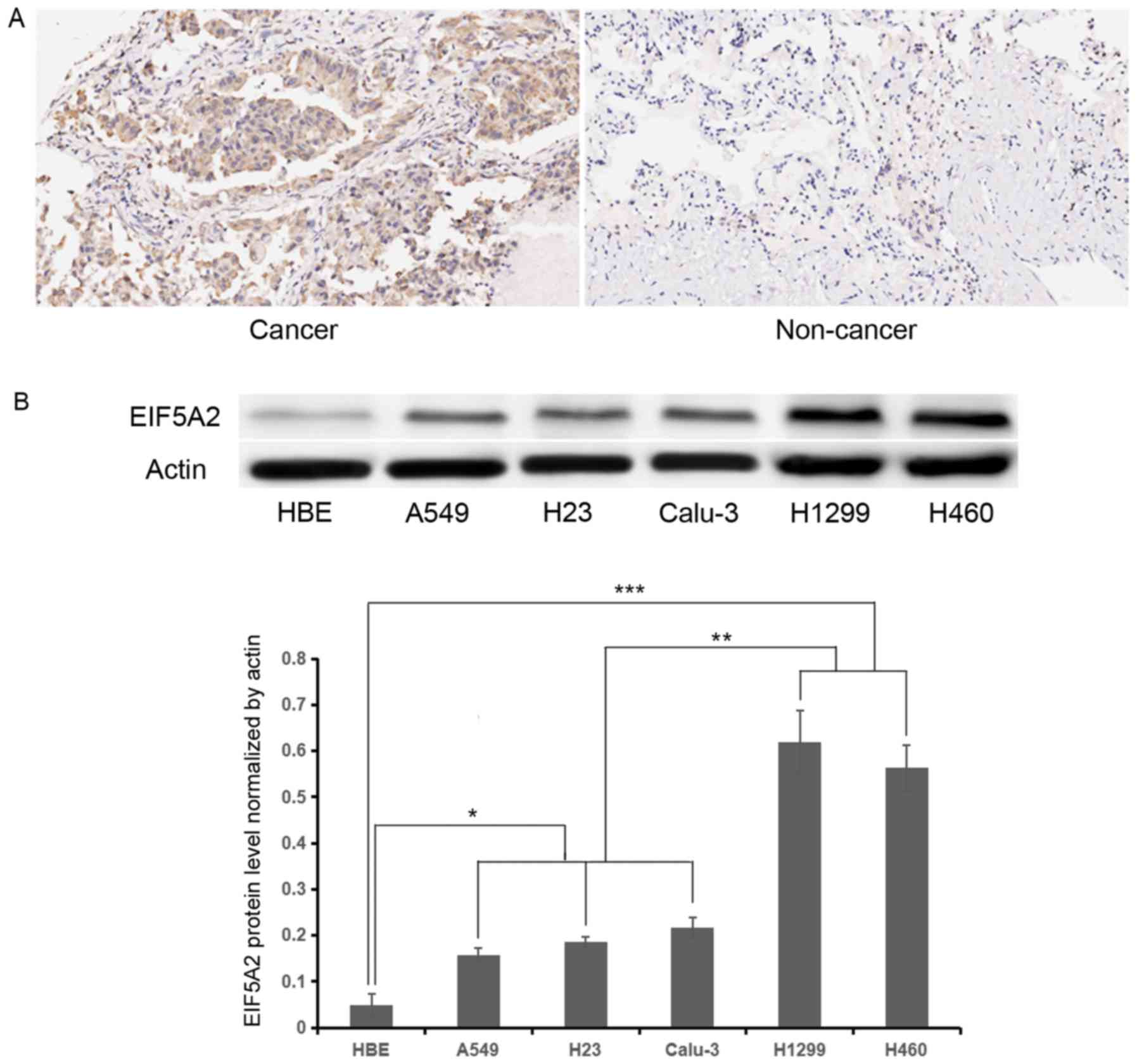
EIF5A2 expression is upregulated in
NSCLC cells
To assess whether or not EIF5A2 is upregulated in
NSCLC samples and cells, immunohistochemical staining and western
blot analysis were performed. Immunohistochemical staining
demonstrated stronger EIF5A2 expression (score: Strong, 5–6) in
NSCLC tissue compared with adjacent benign lung tissue (score:
Negative or weak, 0–1) (Fig. 1A). A
total of 26 patients with NSCLC (>50%) exhibited high expression
of EIF5A2. Western blot analysis demonstrated that the relative
EIF5A2 expression normalized by actin were 0.05±0.03, 0.16±0.02,
0.19±0.01, 0.22±0.02, 0.62±0.07, 0.56±0.05 in the HBE, A549, H23,
Calu-3, H1299 and H460 cell lines, respectively. Compared with the
benign human bronchial epithelial HBE cell line, increased EIF5A2
expression was observed in all NSCLC lines (P<0.05), and H1299
and H460 demonstrated higher EIF5A2 expression compared with in
other NSCLC lines (P<0.01; Fig.
1B).
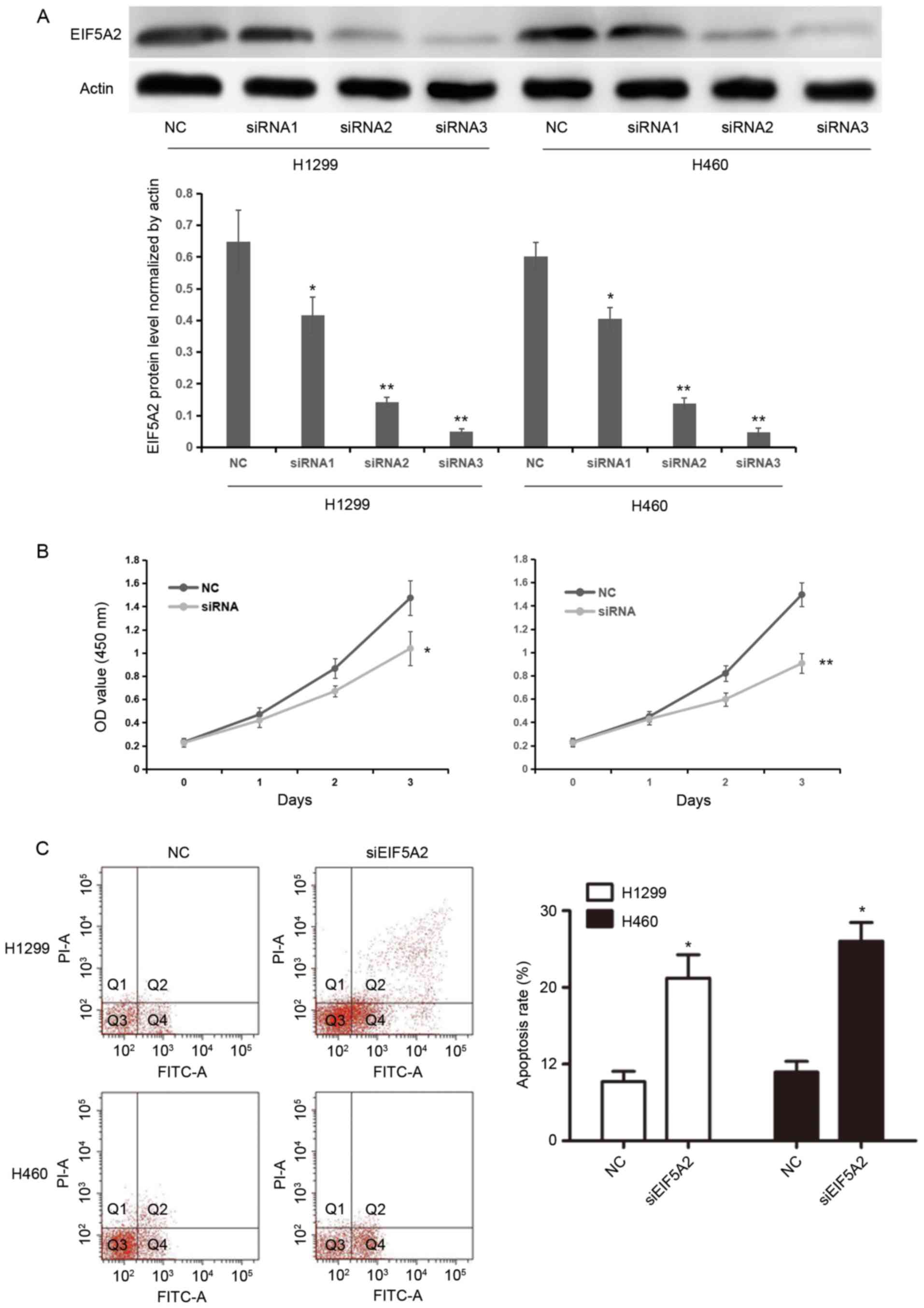
EIF5A2 silencing inhibits cell growth
and induces apoptosis of NSCLC cells in vitro
EIF5A2 siRNAs (siRNA1, 5 µmol/ml; siRNA2, 10
µmol/ml; siRNA3, 15 µmol/ml) were separately transfected into H1299
or H460 cells in order to silence EIF5A2 expression. The silencing
efficacy of three concentrations of EIF5A2 siRNA was confirmed by
western blotting analysis. EIF5A2 protein levels normalized by
actin were 0.64±0.10, 0.42±0.06, 0.14±0.02, 0.05±0.01 in NC,
siRNA1, siRNA2 and siRNA3 groups of the H1299 cell line,
respectively; whereas, EIF5A2 protein levels were 0.60±0.04,
0.40±0.04, 0.14±0.02 and 0.05±0.01 in NC, siRNA1, siRNA2 and siRNA3
groups of the H460 cell line, respectively. EIF5A2 expression was
significantly inhibited in the H1299 and H460 cell lines treated
with EIF5A2 siRNA compared with those treated with control siRNA
(P<0.05), thus confirming the knockdown (Fig. 2A). Following comparison of the
knockdown efficiency of three concentration of EIF5A2 siRNA, 10
µmol/ml EIF5A2 siRNA was chosen for further study.
Cell proliferation was then assessed in H1299 and
H460 cells treated with EIF5A2 siRNA and compared with cells
treated with control siRNA. The OD values for H1299 cells treated
with EIF5A2 siRNA were 0.23±0.03, 0.47±0.06, 0.87±0.08 and
1.47±0.15 at 0, 1, 2 and 3 days, respectively, while the OD values
for H1299 cells treated with control siRNA were 0.23±0.04,
0.42±0.06, 0.67±0.05 and 1.04±0.15. For H460 cells treated with
EIF5A2 siRNA, the OD values were 0.23±0.04, 0.45±0.04, 0.82±0.07
and 1.50±0.10 at 0, 1, 2 and 3 days, respectively, while for
control siRNA transfected cells, the OD values were 0.22±0.03,
0.43±0.05, 0.60±0.06 and 0.91±0.08, respectively. Cells treated
with EIF5A2 siRNA exhibited significantly reduced cellular
proliferation 3 days after transfection compared with the cells
treated with control siRNA (H1299, P<0.05; H460, P<0.01;
Fig. 2B).
The apoptosis rate of cells following transfection
was determined by flow cytometry. At 72 h post-siRNA transfection,
the apoptosis rates of the H1299 and H460 cells transfected with
EIF5A2 siRNA were significantly higher compared with those of H1299
and H460 cells treated with control siRNA (21.22±3.05 and
26.03±2.43 vs. 7.71±1.34 and 8.96±1.41, respectively; P<0.05;
Fig. 2C).
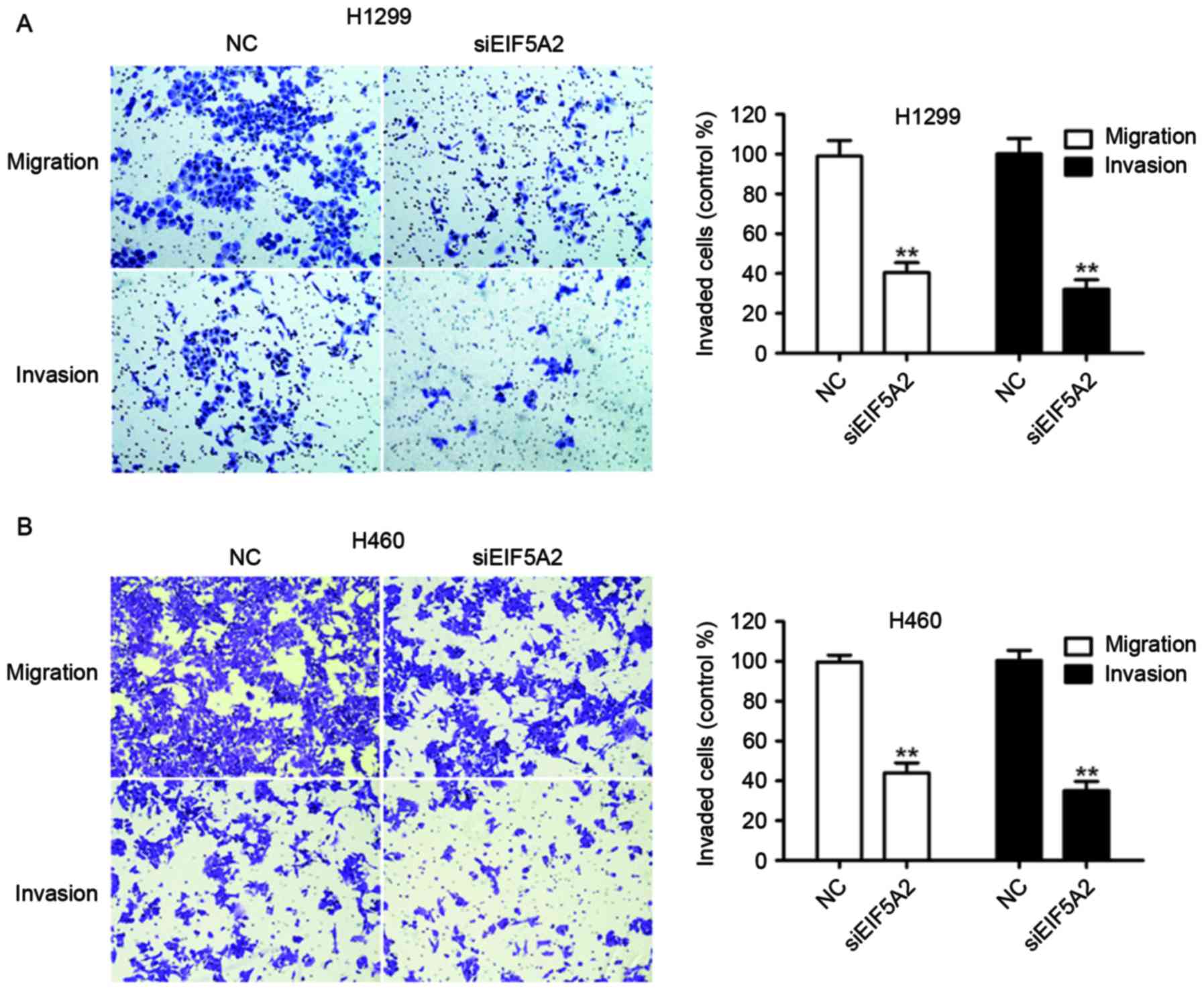
Knockdown of EIF5A2 inhibits NSCLC
cell migration and invasion in vitro
Migration and invasion assays were performed to
assess the effect of EIF5A2 silencing on the metastatic capacity of
NSCLC cells. H1299 cells treated with EIF5A2 siRNA exhibited a
significantly decreased ability to migrate and invade compared with
H1299 cells treated with control siRNA (40.43±4.98 vs. 97.00±4.37%;
P<0.01) and (32.19±4.75 vs. 98.12±5.06%, P<0.01),
respectively (Fig. 3A). Similarly,
H460 cells treated with EIF5A2 siRNA demonstrated a significantly
decreased ability to migrate and invade compared with H460 cells
treated with control siRNA (40.58±4.38 vs. 99.70±3.46%, P<0.01;
Fig. 3B) and (34.04±3.26 vs.
100.43±5.11%, P<0.01).
EIF5A2 regulates the expression of
c-Myc, Bcl-2, Rac1 and E-cadherin in NSCLC cells
To investigate the underlying molecular mechanism of
the effect of EIF5A2 on cell proliferation, apoptosis rate and
metastatic capacity, expression levels of various proteins were
measured. Western blot analysis revealed that the protein
expression levels of Vimentin, E-cadherin, Rac1, Bcl-2 and c-Myc
normalized to actin were 0.92±0.02, 0.03±0.02, 0.62±0.22, 0.42±0.22
and 0.49±0.20, respectively, in the control group of H1299 cell
line; whereas, in the siEIF5A2 group they were 0.2±0.08, 0.17±0.05,
0.22±0.03, 0.22±0.02 and 0.20±0.04, respectively. In the H460 cell
line, the protein expression levels of Vimentin, E-cadherin, Rac1,
Bcl-2 and c-Myc normalized to actin were 0.96±0.02, 0.10±0.02,
0.47±0.06, 0.24±0.01 and 0.86±0.04, respectively, in control group;
whereas, in the siEIF5A2 group they were 0.34±0.10, 0.23±0.03,
0.16±0.04, 0.14±0.01 and 0.20±0.07, respectively. These results
demonstrated that H1299 and H460 cells treated with EIF5A2 siRNA
expressed lower levels of Vimentin, Rac1, Bcl-2 and c-Myc, and
higher levels of E-cadherin compared with the H1299 and H460 cells
treated with control siRNA (P<0.05; Fig. 4).
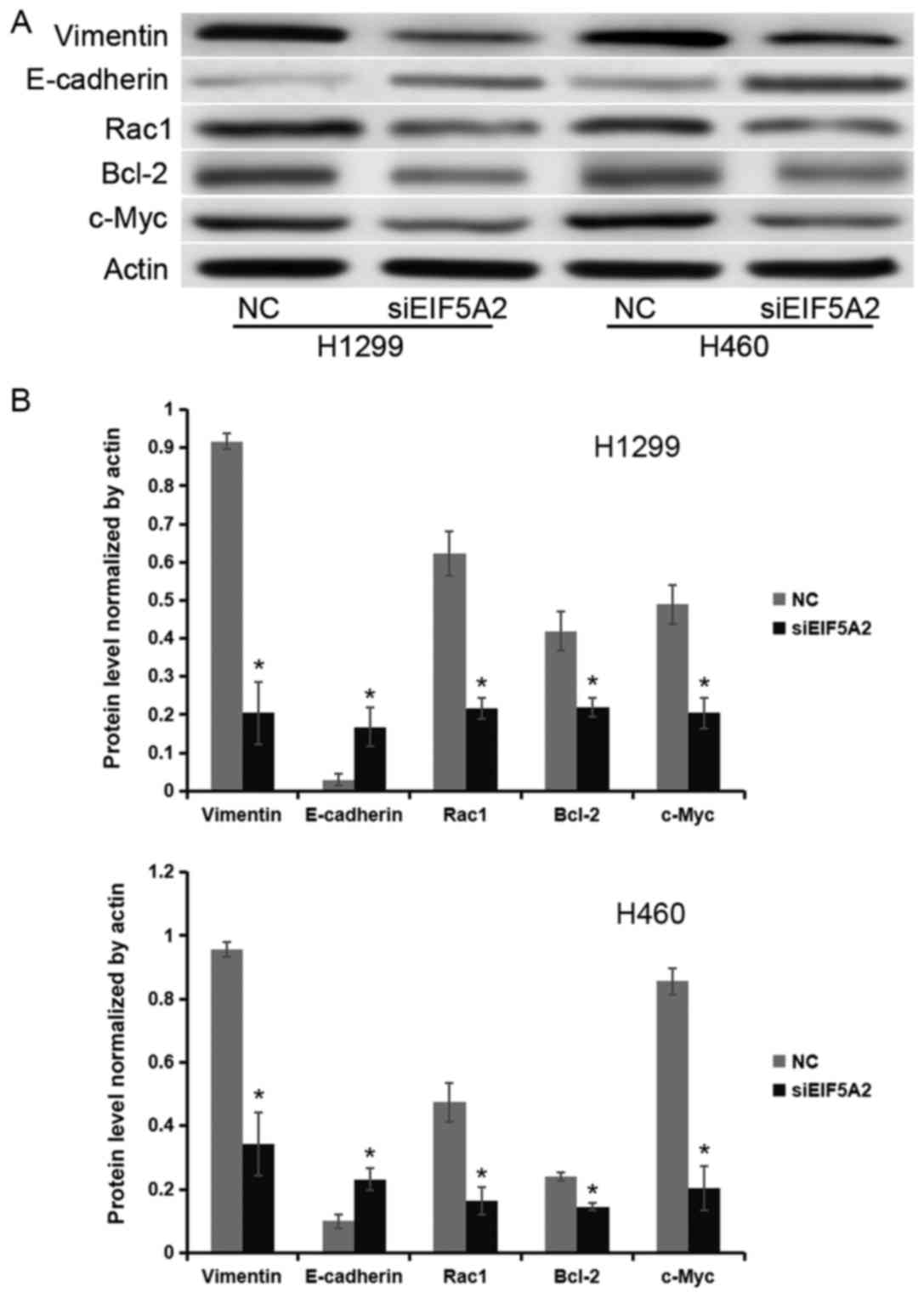 | Figure 4.EIF5A2 regulates the expression of
c-Myc, Bcl-2, Rac1 and E-cadherin in non-small cell lung cancer
cells. (A) Western blot analysis and (B) quantification of the
western blots demonstrated reduced expression of Rac1, Bcl-2, and
c-Myc, and increased expression of E-cadherin in H1299 and H460
cells treated with the EIF5A2 siRNA (siEIF5A2) compared with cells
treated with control siRNA. Rac1, Ras-related C3 botulinum toxin
substrate 1; Bcl-2, apoptosis regulator Bcl-2; c-Myc, myc
proto-oncogene protein; EIF5A2, eukaryotic translation initiation
factor 5A; NC, control; siRNA, small interfering RNA.
*P<0.05. |
Discussion
EIF5A2 has been implicated in a number of cancers,
including bladder cancer, colorectal cancer, pancreatic
adenocarcinoma, esophageal squamous cell carcinoma, gastric
adenocarcinoma, breast cancer, prostate adenocarcinoma,
hepatocellular carcinoma, ovarian cancer and melanoma (9,14–22). Patients with esophageal squamous cell
carcinoma, bladder cancer and pancreatic adenocarcinoma in which
EIF5A2 is overexpressed have been demonstrated to exhibit
statistically significantly shorter survival times (14,17,20).
Furthermore, patients with hepatocellular carcinoma in which EIF5A2
is overexpressed have a greater number of metastases and are more
likely to present with venous invasion (23,24). These
effects on survival times suggest that EIF5A2 is not only important
in malignant transformation, but also may serve as a driver of
tumor progression.
Previous studies have demonstrated that EIF5A2
exhibits its tumorigenic effects via activation of the
phosphoinositide-3-kinase/Rac-α serine/threonine-protein kinase
signaling pathway (15,25) and its induction of matrix
metalloproteinase 2 protein expression (26). In addition, EIF5A2 reduces expression
of E-cadherin and increases expression of Vimentin (26,27), and
may activate transforming protein RhoA precursor/Rac1 signaling
pathways (24). Additionally, a
previous study identified zinc finger protein Gli1 as a putative
transcription factor for EIF5A2 (28).
Data from the present study demonstrated that EIF5A2
was overexpressed in NSCLC cells compared with cells from adjacent
non-cancerous lung tissues, which is in agreement with the results
of previous studies (11,13). NSCLC cells treated with EIF5A2 siRNA
exhibited significantly reduced proliferation, significantly
increased rates of apoptosis, and significantly reduced abilities
to migrate and invade. In addition, the underlying molecular
mechanisms by which EIF5A2 exhibits its effects were investigated.
In NSCLC cells treated with EIF5A2 siRNA, the expression of the
tumorigenic proteins Rac1, Bcl-2, and c-Myc was decreased compared
with that of NSCLC cells treated with control siRNA. Rac1 has been
widely implicated in cancer cell invasion and metastasis, Bcl-2 has
been demonstrated to be an anti-apoptotic protein that can promote
tumorigenesis and Myc overexpression stimulates gene amplification,
potentially by stimulating DNA replication (29–31).
Furthermore, in NSCLC cells treated with EIF5A2 siRNA in the
present study, the anti-malignant protein E-cadherin was
overexpressed, and the EMT marker Vimentin was downregulated
compared with the expression levels found in the NSCLC cells
treated with control siRNA.
The results from the present study suggest that
EIF5A2 may serve as a therapeutic target for the treatment of
NSCLC, for which an EIF5A2 siRNA may be effective. SiRNA is not
widely used due to challenges with successfully delivering the
siRNA to the tumor; however, a previous study demonstrated that not
only was delivery of EIF5A2 interference RNA to a tumor in
vivo possible, but that it also resulted in tumor suppression
(14). The aforementioned study
examined the effects of EIF5A2 siRNA on bladder cancer, therefore
further research is required to confirm that EIF5A2 siRNA could be
delivered to NSCLC cells in vivo. Using EIF5A2 siRNA as a
treatment is also limited by potential side effects. EIF5A2
silencing in non-target benign tissue could cause unanticipated
side effects and such side effects would require thorough
investigation prior to the wide use of siRNA as a therapy for
NSCLC.
Other types of treatment that inhibit EIF5A2 may
prove more technically feasible in terms of their ability to reach
a tumor in vivo. MicroRNA-30b (miR-30b) and miR-125b have
been demonstrated to suppress gastric adenocarcinoma cells and
hepatocellular carcinoma cells respectively via their direct
inhibitory effects on EIF5A2 translation (20,22).
Therefore, any compound that increases miR-30b and/or miR-125b
transcription has the potential to serve as a successful therapy
for NSCLC. In addition, the compound N1-guanyl-1,7-diaminoheptane
(GC7) is a direct EIF5A2 inhibitor and therefore has the potential
to be an effective therapy for NSCLC (17–19,21).
While EIF5A2 inhibitors have the potential to serve
as individual targeted chemotherapeutics, a successful therapy
regimen may require the inclusion of a mix of targeted and more
traditional drugs. Prior studies have demonstrated that EIF5A2
inhibition lowers the chemoresistance of colorectal cancer cells to
doxorubicin, breast cancer cells to doxorubicin (18), hepatocellular carcinoma cells to
5-fluorouracil (32), hepatocellular
carcinoma cells to doxorubicin (33),
bladder cancer cells to doxorubicin (34) and esophageal squamous cell carcinoma
cells to 5-fluorouracil, docetaxel and taxol (35). While this effect on chemoresistance
would require confirmation in NSCLC cells, these prior results
suggest that the role of EIF5A2 inhibitors in NSCLC treatment may
be adjunctive as opposed to primary in nature.
Additionally, EIF5A2 could serve as a marker of
disease severity; EIF5A2 has been suggested to be a potential
biomarker for esophageal squamous cell carcinoma (24) and melanoma (23). Data from the present study revealed
that NSCLC cells that overexpressed EIF5A2 exhibited a greater
malignant tendency compared with NSCLC cells that expressed lower
levels of EIF5A2. Therefore, the level of EIF5A2 expression in a
patient's tumor may predict the aggressiveness of that tumor. In a
patient, EIF5A2 levels would have to be determined via biopsy,
although if the protein is secreted in the blood, serum tests may
also prove useful. Patients with aggressive tumors expressing high
levels of EIF5A2 could be treated with more aggressive therapeutic
strategies, including pneumonectomy or lobectomy with mediastinal
lymph node dissection, or prolonged chemotherapeutic regimens.
Patients with less aggressive tumors expressing lower levels of
EIF5A2 could be treated more conservatively, with less aggressive
chemotherapeutics or less extensive resections.
In the present study, five NSCLC cell lines (A549,
H23, Calu-3, H1299 and H460) were examined. All the cell lines
overexpressed EIF5A2 compared with the non-cancerous human
bronchial epithelium HBE cell line; however, H1299 and H460 cells
expressed the greatest amount of EIF5A2 compared with the other
NSCLC cell lines. Subsequent experiments in the present study were
then performed on the H1299 and H460 cell lines only. Therefore,
while the present study has demonstrated that silencing of EIF5A2
has an inhibitory effect on the malignant potential of cells from
the H1299 and H460 cell lines, this may not be the case for other
NSCLC cell lines. Different tumor cells can exhibit different
phenotypic characteristics, leading to heterogeneity in tumors,
therefore, while EIF5A2 may serve as a potential therapeutic target
or marker of disease severity for certain patients with NSCLC's, it
may not do so for other patients.
The data from the present study suggest that EIF5A2
serves as a tumorigenic protein in NSCLC primarily by increasing
cell proliferation, inhibiting apoptosis and promoting metastasis.
Knockdown of EIF5A2 reverses these tumorigenic effects, suggesting
that EIF5A2 inhibitors may serve as effective therapies for
NSCLC.
References
|
1
|
D'Addario G, Früh M, Reck M, Baumann P,
Klepetko W and Felip E; ESMO Guidelines Working Group, : Metastatic
non-small-cell lung cancer: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 21 Suppl
5:v116–v119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosell R, Felip E, Taron M, Majo J, Mendez
P, Sanchez-Ronco M, Queralt C, Sanchez JJ and Maestre J: Gene
expression as a predictive marker of outcome in stage IIB-IIIA-IIIB
non-small cell lung cancer after induction gemcitabine-based
chemotherapy followed by resectional surgery. Clin Cancer Res.
10:4215s–4219s. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mathews MB and Hershey JW: The translation
factor eIF5A and human cancer. Biochim Biophys Acta. 1849:836–844.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishfaq M, Maeta K, Maeda S, Natsume T, Ito
A and Yoshida M: The role of acetylation in the subcellular
localization of an oncogenic isoform of translation factor eIF5A.
Biosci Biotechnol Biochem. 76:2165–2167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tong Y, Park I, Hong BS, Nedyalkova L,
Tempel W and Park HW: Crystal structure of human eIF5A1: Insight
into functional similarity of human eIF5A1 and eIF5A2. Proteins.
75:1040–1045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujimura K, Wright T, Strnadel J, Kaushal
S, Metildi C, Lowy AM, Bouvet M, Kelber JA and Klemke RL: A
hypusine-eIF5A-PEAK1 switch regulates the pathogenesis of
pancreatic cancer. Cancer Res. 74:6671–6681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clement PM, Henderson CA, Jenkins ZA,
Smit-McBride Z, Wolff EC, Hershey JW, Park MH and Johansson HE:
Identification and characterization of eukaryotic initiation factor
5A-2. Eur J Biochem. 270:4254–4263. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jenkins ZA, Hååg PG and Johansson HE:
Human eIF5A2 on chromosome 3q25-q27 is a phylogenetically conserved
vertebrate variant of eukaryotic translation initiation factor 5A
with tissue-specific expression. Genomics. 71:101–109. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu GD, Shi XB, Sun LB, Zhou QY, Zheng DW,
Shi HS, Che YL, Wang ZS and Shao GF: Down-regulation of eIF5A-2
prevents epithelial-mesenchymal transition in non-small-cell lung
cancer cells. J Zhejiang Univ Sci B. 14:460–467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He LR, Zhao HY, Li BK, Liu YH, Liu MZ,
Guan XY, Bian XW, Zeng YX and Xie D: Overexpression of eIF5A-2 is
an adverse prognostic marker of survival in stage I non-small cell
lung cancer patients. Int J Cancer. 129:143–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu G, Yu H, Shi X, Zhou Q, Zheng D, Shi H,
Li N, Zhang X and Shao G: Cisplatin sensitivity is enhanced in
non-small cell lung cancer cells by regulating
epithelial-mesenchymal transition through inhibition of eukaryotic
translation initiation factor 5A2. BMC Pulm Med. 14:1742014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei YX, Chen G, You L and Zhao YP:
Expression of eukaryotic translation initiation factor 5A2 in
pancreatic adenocarcinoma and its correlation with the prognosis.
Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 35:634–638. 2013.(In Chinese).
PubMed/NCBI
|
|
15
|
Khosravi S, Wong RP, Ardekani GS, Zhang G,
Martinka M, Ong CJ and Li G: Role of EIF5A2, a downstream target of
Akt, in promoting melanoma cell invasion. Br J Cancer. 110:399–408.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Ma Y, Xie C, Wu Z, Kang Z, Fang Z,
Su B and Guan M: EphA6 promotes angiogenesis and prostate cancer
metastasis and is associated with human prostate cancer
progression. Oncotarget. 6:22587–22597. 2015.PubMed/NCBI
|
|
17
|
Li Y, Fu L, Li JB, Qin Y, Zeng TT, Zhou J,
Zeng ZL, Chen J, Cao TT, Ban X, et al: Increased expression of
EIF5A2, via hypoxia or gene amplification, contributes to
metastasis and angiogenesis of esophageal squamous cell carcinoma.
Gastroenterology. 146:1701–1713.e9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Liu R, Fu P, Du F, Hong Y, Yao M,
Zhang X and Zheng S: N1-Guanyl-1,7-diaminoheptane sensitizes
estrogen receptor negative breast cancer cells to doxorubicin by
preventing epithelial-mesenchymal transition through inhibition of
eukaryotic translation initiation factor 5A2 activation. Cell
Physiol Biochem. 36:2494–2503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meng QB, Kang WM, Yu JC, Liu YQ, Ma ZQ,
Zhou L, Cui QC and Zhou WX: Overexpression of eukaryotic
translation initiation factor 5A2 (EIF5A2) correlates with cell
aggressiveness and poor survival in gastric cancer. PLoS One.
10:e01192292015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei JH, Cao JZ, Zhang D, Liao B, Zhong WM,
Lu J, Zhao HW, Zhang JX, Tong ZT, Fan S, et al: EIF5A2 predicts
outcome in localised invasive bladder cancer and promotes bladder
cancer cell aggressiveness in vitro and in vivo. Br J Cancer.
110:1767–1777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu X, Liu H, Zhang H, Dai W, Guo C, Xie C,
Wei S, He S and Xu X: Sonic hedgehog-GLI family zinc finger 1
signaling pathway promotes the growth and migration of pancreatic
cancer cells by regulating the transcription of eukaryotic
translation initiation factor 5A2. Pancreas. 44:1252–1258. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu W, Cai MY, Tong ZT, Dong SS, Mai SJ,
Liao YJ, Bian XW, Lin MC, Kung HF, Zeng YX, et al: Overexpression
of EIF5A2 promotes colorectal carcinoma cell aggressiveness by
upregulating MTA1 through C-myc to induce
epithelial-mesenchymaltransition. Gut. 61:562–575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shek FH, Fatima S and Lee NP: Implications
of the use of eukaryotic translation initiation factor 5A (eIF5A)
for prognosis and treatment of hepatocellular carcinoma. Int J
Hepatol. 2012:7609282012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang DJ, Dong SS, Ma NF, Xie D, Chen L, Fu
L, Lau SH, Li Y, Li Y and Guan XY: Overexpression of eukaryotic
initiation factor 5A2 enhances cell motility and promotes tumor
metastasis in hepatocellular carcinoma. Hepatology. 51:1255–1263.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Z, Yu T, Zhou B, Wei J, Fang Y, Lu J,
Guo L, Chen W, Liu ZP and Luo J: Mg(II)-Catechin nanoparticles
delivering siRNA targeting EIF5A2 inhibit bladder cancer cell
growth in vitro and in vivo. Biomaterials. 81:125–134. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bao Y, Lu Y, Wang X, Feng W, Sun X, Guo H,
Tang C, Zhang X, Shi Q and Yu H: Eukaryotic translation initiation
factor 5A2 (eIF5A2) regulates chemoresistance in colorectal cancer
through epithelial mesenchymal transition. Cancer Cell Int.
15:1092015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tian SB, Yu JC, Liu YQ, Kang WM, Ma ZQ, Ye
X and Yan C: MiR-30b suppresses tumor migration and invasion by
targeting EIF5A2 in gastric cancer. World J Gastroenterol.
21:9337–9347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsang FH, Au V, Lu WJ, Shek FH, Liu AM,
Luk JM, Fan ST, Poon RT and Lee NP: Prognostic marker microRNA-125b
inhibits tumorigenic properties of hepatocellular carcinoma cells
via suppressing tumorigenic molecule eIF5A2. Dig Dis Sci.
59:2477–2487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen QY, Zheng Y, Jiao DM, Chen FY, Hu HZ,
Wu YQ, Song J, Yan J, Wu LJ and Lv GY: Curcumin inhibits lung
cancer cell migration and invasion through Rac1-dependent signaling
pathway. J Nutr Biochem. 25:177–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coultas L and Strasser A: The role of the
Bcl-2 protein family in cancer. Semin Cancer Biol. 13:115–123.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khaleghian M, Shakoori A, Razavi AE and
Azimi C: Relationship of amplification and expression of the C-MYC
gene with survival among gastric cancer patients. Asian Pac J
Cancer Prev. 16:7061–7069. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang FW, Cai MY, Mai SJ, Chen JW, Bai HY,
Li Y, Liao YJ, Li CP, Tian XP, Kung HF, et al: Ablation of EIF5A2
induces tumor vasculature remodeling and improves tumor response to
chemotherapy via regulation of matrix metalloproteinase 2
expression. Oncotarget. 5:6716–6733. 2014.PubMed/NCBI
|
|
33
|
Lou B, Fan J, Wang K, Chen W, Zhou X,
Zhang J, Lin S, Lv F and Chen Y: N1-guanyl-1,7-diaminoheptane (GC7)
enhances the therapeutic efficacy of doxorubicin by inhibiting
activation of eukaryotic translation initiation factor 5A2 (eIF5A2)
and preventing the epithelial-mesenchymal transition in
hepatocellular carcinoma cells. Exp Cell Res. 319:2708–2717. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang J, Yu H, Shen M, Wei W, Xia L and
Zhao P: N1-guanyl-1,7-diaminoheptane sensitizes bladder cancer
cells to doxorubicin by preventing epithelial-mesenchymal
transition through inhibition of eukaryotic translation initiation
factor 5A2 activation. Cancer Sci. 105:219–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang H, Li XD, Zhou Y, Ban X, Zeng TT, Li
L, Zhang BZ, Yun J, Xie D, Guan XY and Li Y: Stemness and
chemotherapeutic drug resistance induced by EIF5A2 overexpression
in esophageal squamous cell carcinoma. Oncotarget. 6:26079–26089.
2015.PubMed/NCBI
|