Introduction
Nasopharyngeal carcinoma (NPC) is a head and neck
malignant tumor that is common to multiple provinces in
southeastern China and Asia, and northern Africa (1). Radiotherapy targeting the nasopharyngeal
tumor and draining lymph node echelons remains a common treatment
for NPC (2,3). Although early stage NPC may be
effectively treated by radiotherapy alone, treatments of regional
advanced NPC (Union for International Cancer Control Stage III/IV)
require improvements; the incidence rate of a local residual tumor
following radical radiotherapy is 7.0–13.0% and the local recurrent
rate ranges from 16.8 to 23.0% (4–8). To reduce
the rate of local recurrence and distant metastasis, combination
treatments, including chemoradiotherapy and molecular targeted
therapy are particularly important for cases of locally advanced
head and neck squamous-cell carcinoma (HNSCC) (9). Potential therapeutic targets for HNSCC,
including epidermal growth factor receptor (EGFR), have been
researched over the past decade. High EGFR expression is associated
with poor prognosis in head and neck squamous-cell carcinoma and
with resistance to radiotherapy (10,11). A
previous study demonstrated that nimotuzumab combined with
radiotherapy or chemotherapy was associated with improved
locoregional tumor control and survival compared with standard
chemoradiotherapy in patients with locally advanced HNSCC (12). Cetuximab has been used with
radiotherapy or in combination with platinum 5-fluorouracil in
advanced or recurrent patients with HNSCC (13–16).
However, the use of molecular targeted drugs has been hindered: The
molecular targeted therapy causes skin toxicity, and the rates of
treatment sensitivity require improvement. In addition, the effect
of the drugs when combined with chemotherapy or radiotherapy
remains incompletely understood. Pfister et al (12) revealed that combining cetuximab with
radiotherapy or chemoradiotherapy resulted in drug-associated
toxicities in HNSCC; the early trial was subsequently suspended.
Numerous other anti-EGFR agents are currently being assessed in
phases II and III clinical trials in different HNSCC therapeutic
settings (17). None of these
molecule-targeting drugs have yet been approved by the Food and
Drug Administration for use in patients with HNSCC due to the
limited improvement on survival with which they are associated.
The present study assessed which factors influenced
the survival of 135 patients with locally advanced NPC following
radiotherapy. The present study revealed that targeted treatment
functions were an independent negative prognostic factor in
patients with locally advanced NPC.
Materials and methods
Patient characteristics
The population of the present study comprised 135
patients (99 males, 36 females; 76≥45 years, 59<45 years) with
locally advanced NPC who received radical radiotherapy in the
Department of Radiation Oncology, Xiangya Hospital, Central South
University (Changsha, China) between August 2008 and January 2012.
The inclusion criteria were as follows: i) Patients exhibited
pathologically proved poorly differentiated squamous-cell
carcinoma; ii) patients were staged in III–IVA according to the 7th
American Joint Committee on Cancer (AJCC) staging system (18) with no evidence of distant metastasis
at the time of diagnosis; and iii) patients did not receive any
other anticancer treatments prior to primary radiotherapy or
undergo an operation following radiotherapy. The duration of
overall survival was calculated from the date of radiotherapy
completion to the date of mortality in the patient or the date of
the last follow-up. The duration of local relapse-free survival was
calculated from the date of radiotherapy completion to the date of
tumor local recurrence. The duration of disease metastasis-free
survival was calculated from the date of radiotherapy completion to
the date of tumor distant metastasis (19,20).
Intensity modulated radiation
therapy
In accordance with the International Commission on
Radiation Units and Measurements (ICRU) reports nos. 50 and 62
(21,22), the primary tumor was named GTVnx, the
positive lymph nodes were named GTVnd, and the retropharyngeal
lymph nodes were included under the name GTVnx. Clinical target
volume 1 (CTV1) included a 5–10 mm extension around the GTVnx and
other high-risk regions, including the parapharyngeal space, the
inferior part of sphenoid sinus, the posterior 1/3 of the nasal
cavity, the posterior 1/3 of the maxillary sinus, the skull base,
the clivus, the oval foramen, the lacerated foramen, and high-risk
lymphatic drainage areas, including the retropharyngeal lymph
nodes, and levels II, III, and Va of the upper cervical lymph
nodes. CTV2 included the low-risk lymphatic drainage levels IV and
Vb. The corresponding planning target volume (PTV) included a 3 mm
margin around the CTV. A total dose of 62.72–80.64 Gy in 33
fractions to the PGTVnx (GTVnx + 3 mm margin), 69.96–72.6 Gy in 33
fractions to the PGTVnd (GTVnd + 3 mm margin), 59.4–64.0 Gy in 33
fractions to the PTV1 and 50.0–54.0 Gy in 28 fractions to the PTV2
were prescribed. All patients were treated with 1 fraction daily, 5
days/week, for 5–6 weeks (23,24). Dose
limits for the critical tissue structures and the plan evaluation
were designed according to the Radiation Therapy Oncology Group
0225 (25). The patients were
re-examined by magnetic resonance imaging (MRI) following either
the completion of radiotherapy or the radiation dose reaching ~70
Gy (26). All patients were exposed
to 6MV beams and 9 fixed-gantry (0, 40, 80, 120, 160, 200, 240, 280
and 320°) angles were designated. Plans were all normalized so that
95% of the target received ≥100% prescription dose.
Chemotherapy
To inhibit NPC progression during treatment
planning, 131 of the patients received a platinum-based
chemotherapy regimen. The chemotherapy regimens included Taxol (120
mg/m2 on day 1 + 80 mg/m2
Cislatin/Nedaplatinon day 2; for 3 weeks/cycle), Gemcitaine (1
g/m2 on day 1 and 8+80 mg/m2
Cislatin/Nedaplatinon day 1, for 3 weeks/cycle) or 5-Fu [4
g/m2 continuous infusion (CI) >96 h + 80
mg/m2 Cislatin/Nedaplatinon day 1, for 3 weeks/cycle],
for 2–6 cycles. The remaining 4 patients did not undergo
chemotherapy since they were unwilling or unable. Neoadjuvant
chemotherapy was administered when the waiting time for
radiotherapy >1 week or to decrease the size of large tumors.
Following the completion of radiotherapy, adjuvant chemotherapy was
administered to the patients at stage N2/N3 and those with existing
residual disease, as detected using MRI or physical
examination.
Sensitization treatment
Intravenously, 76 patients received 750 mg sodium
glycididazole/m2/fraction, 3–5 fractions/week for 5–6
weeks, within a 1 or 2 h window, to ensure that the drug remained
active during administration.
Target treatment
A monoclonal antibody treatment of EGFR was
administered to 20 of the patients who were able to purchase the
drug. Of these, 16 received 100–200 mg nimotuzumab/week for 6–8
weeks, while 4 received 400 mg cetuximab/m2 as an
initial dose and 250 mg cetuximab/m2/week for 6–8 weeks,
the duration of treatment was determined by the patient response to
the drug.
Immunohistochemical analysis
Biopsy specimens from the 20 patients with NPC who
had been receiving targeted drugs were 10% formalin-fixed and
paraffin-embedded. Samples were fixed in 10% formalin at room
temperature for 48 h. For the immunohistochemical detection of
EGFR, 4 µm tissue sections were deparaffinized in xylene and
subsequently exposed to microwaves in a microwave oven (750 W) at
60–70°C for 15 min in EDTA buffer (pH 9.0, G1202). Following
cooling for 30 min and washing in PBS, endogenous peroxidase was
blocked with 3% hydrogen peroxide for 25 min. Specimens were
subsequently incubated at room temperature in a humidified box with
PBS containing 10% normal goat serum for 30 min. Specimens were
incubated overnight at 4°C with the anti-EGFR antibody (cat. no.
bs-0165R, 1:200; BIOSS, Beijing, China). A horseradish
peroxidase-labeled goat anti-mouse IgG secondary antibody (dilution
1:1,000, cat. no. K5007; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA) was then incubated with the tissue for 30 min at
37°C. Immunostaining was detected using the ChemMate kit (cat. no.
K5007, ready-to-use; Dako; Agilent Technologies, Inc.) with
3,3′-diaminobenzidine as the chromogen. For the negative control,
the primary antibody was replaced with non-immune isotypic
antibodies. The protocol was repeated in triplicate.
Evaluation of staining
The stained sections were viewed separately by two
pathologists (Department of Pathology, Xiangya Hospital, Central
South University, Changsha, China) who were blinded to the clinical
or clinicopathological status of the cases. EGFR expression was
evaluated by scanning the entire tissue specimen under low power
magnification (×40) and subsequently confirmed under high power
magnification (×400). The result (positive or negative) was
diagnosed through stereological cell counts. The absence of
positive cells resulted in a negative diagnosis. When <25% of
the cells were positive the diagnosis was weakly positive. A
moderately positive diagnosis was made when the proportion of
positive cells was 25–50%. A strongly positive diagnosis was made
when >50% of the cells were positive. According to this method
of assessment, staining scores of negative and weakly positive were
regarded as representing tumors with decreased EGFR expression,
while staining scores of moderately and strongly positive were
regarded as representing tumors with increased EGFR expression.
Follow-up
The follow-up methods included direct telephone
calls to the patients or their families, or hospital visits for the
patients. Follow-up was measured from the first day of treatment to
the last follow-up date or the date of patient mortality. Following
radiotherapy, follow-up examinations were conducted once every 3
months in the first 2 years, once every 6 months in years 2 to 5,
and once annually following this. MRI of the nasopharynx and neck
region was performed once annually for patients with no residual
tumors and once every 3–6 months for patients with residual tumors,
as described previously (27).
Recurrence was defined as regrowth of the tumor following
disappearance for ≥1 month. The follow-up began at August 2008 and
ended in September 2015. The median follow-up for survivors was 48
months (range, 2–75 months). In addition, only 129 of the 135
patients were eligible for survival analysis due the loss of
follow-up for 6 patients who were treated as mortalities. The
follow-up rate was 95.6%.
Statistical analysis
All statistical analysis was performed using
Statistical Analysis System 9.3 software. Survival curves were
calculated using Kaplan-Meier estimates and differences were
compared using the log-rank test. Univariate and multivariate
survival analysis was performed according to the Cox proportional
hazards model. For all statistical tests, P≤0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics of
patients
The clinical characteristics of 135 patients with
locally advanced NPC were presented in Table I. Of these patients, 59 were <45
years and 76 were ≥45 years; 19 exhibited AJCC (7th) stage III NPC
and 116 exhibited AJCC (7th) stage IVA NPC. Histologically, all
patients were classified as exhibiting poorly differentiated
squamous-cell carcinoma according to the World Health Organization
classification (28). Of the 135
patients enrolled in the present study, 20 received EGFR monoclonal
antibody treatment and the remainder did not.
 | Table I.Characteristics and Kaplan-Meier
analysis of 135 patients with locally advanced nasopharyngeal
carcinoma. |
Table I.
Characteristics and Kaplan-Meier
analysis of 135 patients with locally advanced nasopharyngeal
carcinoma.
| Parameters | n | χ2 | P-value |
|---|
| Sex |
| 0.0704 | 0.7907 |
|
Male | 99 |
|
|
|
Female | 36 |
|
|
| Age (years) |
| 5.3076 | 0.0212 |
|
<45 | 59 |
|
|
|
≥45 | 76 |
|
|
| AJCC (7th)
stage |
| 0.3251 | 0.5685 |
|
III | 19 |
|
|
|
IVA | 116 |
|
|
| Chemotherapy
(platinum-based) |
| 0.2491 | 0.6177 |
|
Yes | 131 |
|
|
| No | 4 |
|
|
| Prescribed dose,
Gy |
| 0.8668 | 0.3518 |
|
≤73.92 | 114 |
|
|
|
>73.92 | 21 |
|
|
| Targeted
treatment |
| 4.9193 | 0.0266 |
|
Yes | 20 |
|
|
| No | 115 |
|
|
| Sensitization
treatment |
| 0.5548 | 0.4564 |
|
Yes | 76 |
|
|
| No | 59 |
|
|
Only 129 of the 135 patients underwent survival
analysis. In total, 4/129 patients developed local recurrence
(3.1%), 20/129 patients developed distant metastasis (15.5%), and
1/129 patients developed recurrence and distant metastasis (0.8%).
Mortality was recorded in 22/129 patients (17.1%); 17 of these
mortality cases were due to tumor recurrence and metastasis, 3 were
due to tumor-associated complications (nasopharyngeal hemorrhage, 2
cases; septic shock, 1 case), 1 was due to malnutrition systemic
failure and 1 was due to unknown causes.
Univariate and multivariate
Coxregression analyses
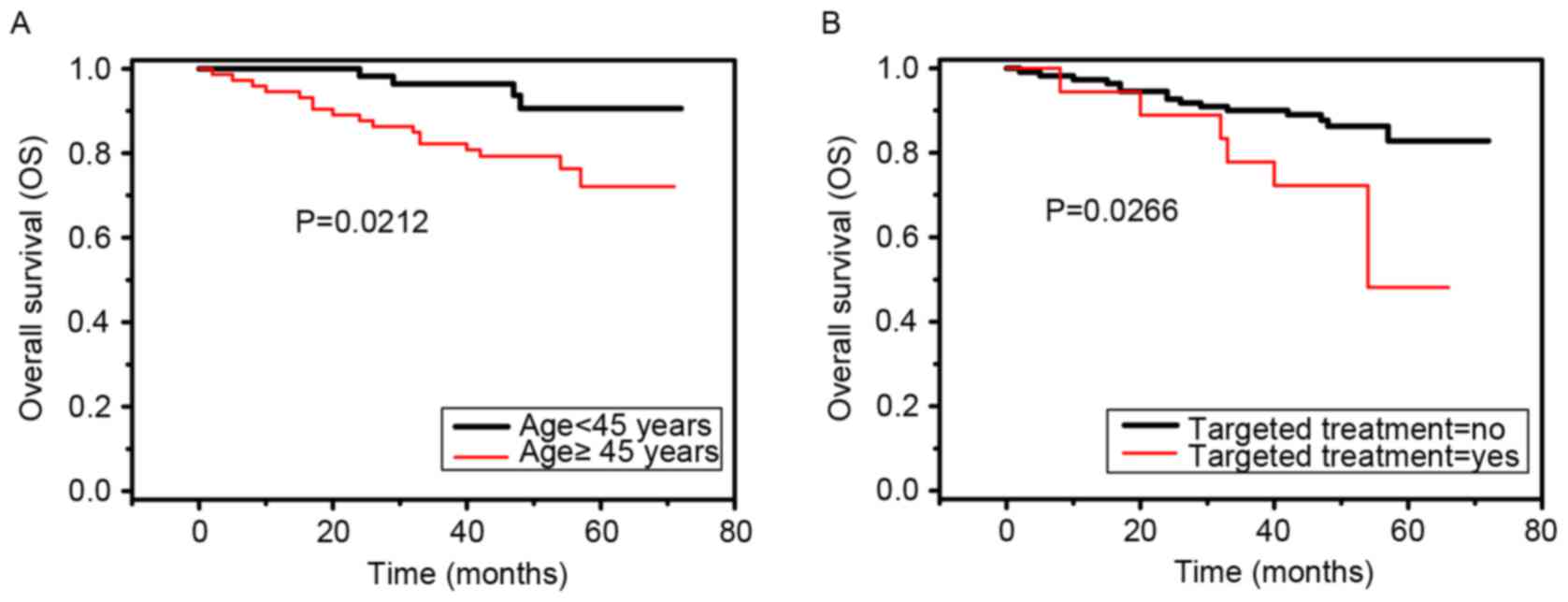
Univariate analyses revealed that patient age
(χ2=5.3076, P=0.0212) and targeted treatment
(χ2=4.9193, P=0.0266) were negative prognostic
predictors of overall survival in patients with locally advanced
NPC (Fig. 1A and B). Multivariate Cox
regression analysis indicated that targeted treatment [hazard ratio
(95% confidence interval), 2.642 (1.001, 6.972); P=0.0497] was a
negative prognostic predictor of overall survival in patients with
locally advanced NPC (Table II).
 | Table II.Multivariate Cox regression analysis
of 135 patients with locally advanced nasopharyngeal carcinoma. |
Table II.
Multivariate Cox regression analysis
of 135 patients with locally advanced nasopharyngeal carcinoma.
| Variables | Subset | Hazard ratio (95%
CI) | P-value |
|---|
| Age, years | <45 vs. ≥45 | 2.836 (0.932,
8.631) | 0.0664 |
| Targeted
treatment | Yes vs. No | 2.642 (1.001,
6.972) | 0.0497 |
| Prescribed dose,
Gy | ≤73.92 vs.
>73.92 | 0.528 (0.117,
2.377) | 0.4050 |
Immunostaining analysis
To further evaluate the clinical significance of
EGFR, the protein expression levels of EGFR in 20 paraffin-embedded
NPC biopsy specimens from 20 patients with locally advanced NPC
receiving targeted treatment were determined using
immunohistochemical analysis. The present study defined patients
for which tumor size had decreased by <40% following radiation
treatment as radiation-resistant, and those for which tumor size
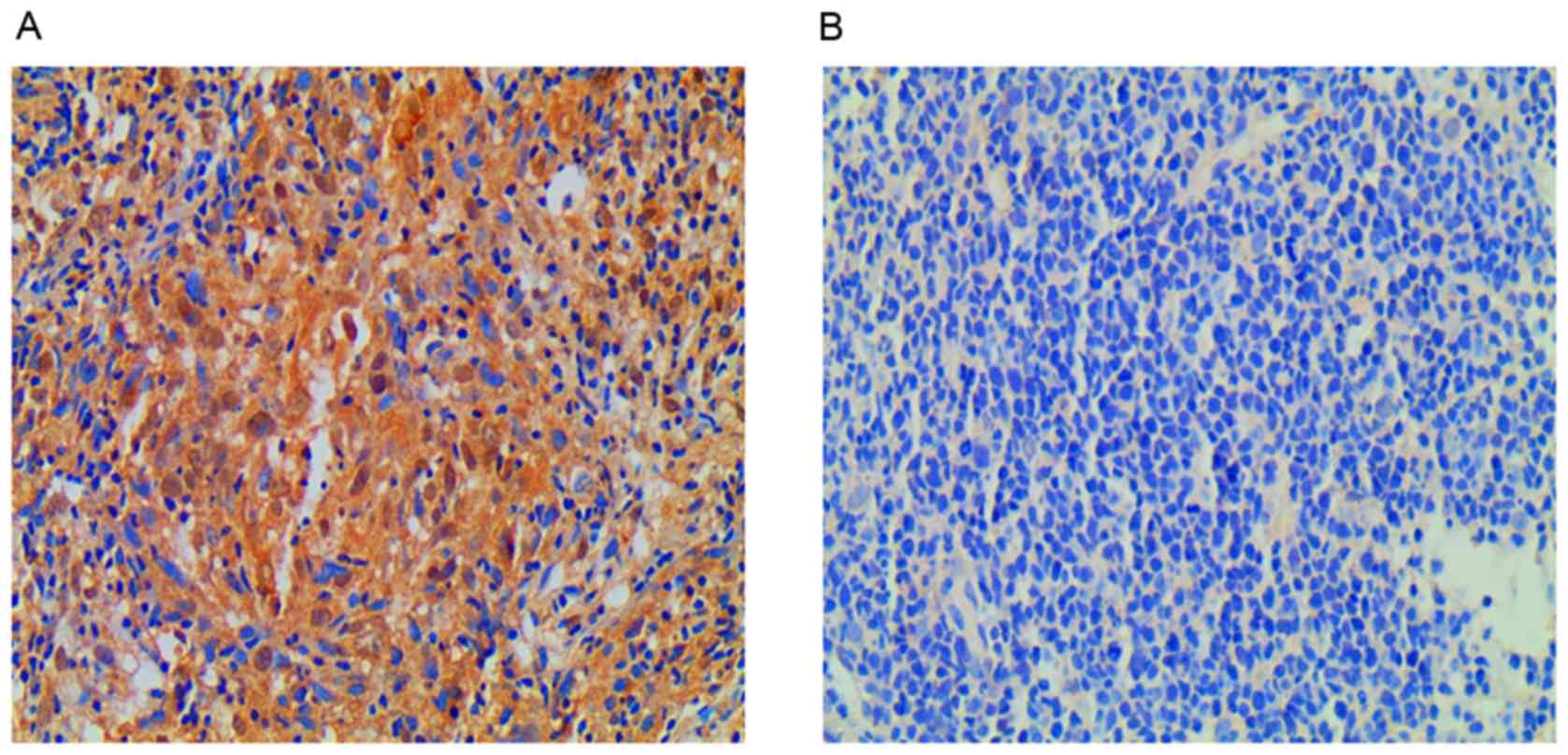
had decreased by >60% as radiation-sensitive (29). Of the 20 patients, 2 belonged to the
radiation-resistant group and revealed increased expression of EGFR
compared with the radiation-sensitive group; tumor size decreased
in these patients by 37.1 and 34.6% compared with initial size. The
remaining 18 patients belonged to the radiation-sensitive group,
with 17 demonstrating decreased expression of EGFR and 1 revealing
increased expression of EGFR: Tumor size decreased by 73–100% in
the radiation-sensitive group, compared with initial size. EGFR was
located in the nasopharyngeal carcinoma cell cytoplasm. There was
decreased EGFR expression in 17 of the 20 patients (85%) and
increased EGFR expression in the remaining 3 patients (15%). The
average staining intensity was increased in the radiation-resistant
group compared with the radiation-sensitive group (Fig. 2A and B).
Discussion
EGFR-specific monoclonal antibodies, including
cetuximab and nimotuzumab, have been researched in multiple types
of cancer, including sarcoma (30),
gliomas (31), and non small cell
lung cancer (NSCLC) (32). These
antibodies may inhibit the downstream growth-signaling pathway and
may serve as a marker of tumorigenesis in NSCLC and glioma
(33). However, few studies have
assessed the use of targeted drugs in patients with NPC when
combined with chemotherapy or radiation.
The present study enrolled 135 patients with locally
advanced NPC, of which 20 received targeted treatment. The present
study demonstrated that targeted treatment functions as an
independent negative prognostic factor in patients with locally
advanced NPC. Immunostaining analysis identified that the staining
intensity of the radiation-resistant group was increased compared
with that of the radiation-sensitive group. Although 1 patient in
the radiation-sensitive group revealed increased expression of
EGFR, the staining intensity of the tissue derived from the patient
was relatively decreased compared with that from the patients of
the radiation-resistant group. This result supported studies by Pan
et al (34) and Ma et
al (35), which demonstrated that
EGFR overexpression was associated with radiation resistance in
head and neck cancer, and prognostically associated with a
decreased local control rate and survival. Multiple studies have
revealed that ~80% of primary NPC biopsies demonstrate increased
expression of EGFR (36,37). In the present study, there were 2/20
patients who received targeted treatment that belonged to
radiation-resistant group, these patients exhibited EGFR over
expression. A total of 18/20 who received targeted treatment
belonged to the radiation-sensitive group, only 1 patient in this
group exhibited EGFR over expression. In the present study, only
15% of the patients demonstrated increased expression of EGFR.
No consensus has yet been reached regarding the
effectiveness of an EGFR-targeting treatment in patients with NPC.
Pfister et al (12) suggested
that combining cetuximab and radiotherapy or chemotherapy may
improve loco-regional tumor control and the survival rate compared
with conventional chemoradiotherapy in locally advanced HNSCC.
Cohen et al (17) revealed
that cetuximab, which may act as a radiosensitizer, when combined
with conventional chemoradiotherapy with cisplatin remained an
ineffective treatment for patients with HNSCC. This result may
differ from the present study due to EGFR mutation status. Although
EGFR mutations are reported to have a decreased prevalence (0–1%)
in patients with NPC compared with other NPC-associated genes
(38,39), they may serve an important function in
tumor development where they do occur; for example, the
mitogen-activated protein kinase/extracellular signal-regulated
kinase pathway is downstream of EGFR and may lead to cetuximab
resistance in patients with NPC (40,41).
Numerous drugs are in the early stages of
development for HNSCC treatment, including novel anti-EGFR
small-molecule tyrosine kinase inhibitors, EGFR antisense
molecules, and multiple add-on therapies to radiation and
chemotherapy intended to decrease resistance to anti-EGFR agents
(42–45). In addition, numerous other anti-EGFR
agents are currently being assessed in phases II and III clinical
trials in different HNSCC therapeutic settings (46,47). As
the targeted treatment group only had 3/20 patients with high
expression of EGFR, the majority of the patients exhibited low
expression of EGFR; consequently, those patients were not sensitive
to cetuximab and nimotuzumab. Multiple studies have revealed that
almost 80% of primary NPC biopsies indicate high expression of EGFR
(36,37). EGFR of >25% was associated with a
significantly poorer treatment outcome. The 5-year disease-specific
survival, relapse-free survival, loco-regional relapse-free, and
distant metastasis-free rates in patients with an EGFR of >25%
were 48, 36, 60, and 55%, respectively. The corresponding rates in
patients with an EGFR of <25% were 86, 80, 93, and 86%,
respectively. The differences were all statistically significant,
with the exception of distant metastasis. In multivariate analysis,
EGFR extent was the only independent factor that predicted disease
relapse, loco-regional failure, and mortality due to cancer
(48). The present study hypothesized
that treatment insensitivity was the primary reason for this
unsatisfied curative effect. Collectively, the results obtained
from these previously studies were similar to those presented in
the present study. Results of the present study demonstrated that
<25% EGFR in patients with NPC is associated with reduced
overall survival. The prognosis was not only concerned with the
positive expression rate of EGFR; however, it also had an
association with the amount of EGFR. The possible efficacy of
molecular targeted therapy against EGFR is a promising treatment
strategy, future studies examining the expression of EFGR prior to
treatment in patients with advance-stage NPC are required. Of
course, the number of subjects should not be discounted; only 2/20
patients belonged to the radiation resistance group, and therefore
the sample size was too small to perform meaningful statistical
analysis independently. Validating the results of the present study
requires further biopsy collections to assess the expression of
EGFR in patients with NPC and whether EGFR expression is associated
with radiation resistance. The limitations of the present study
were as follows: On one hand, the volume of nasopharyngeal tumor
tissue was small (<0.5 cm3), meaning that they were
difficult to isolate under the nasopharyngeal microscope.
Additionally, targeted treatment was not included as a part of the
standard therapy: Only patients with local advanced NPC who could
afford the treatment were able to select the targeted drugs. The
present study suggested that treatment with cetuximab and
nimotuzumab may be associated with the expression of EGFR. However,
it may not be effective in patients with low expression of EGFR,
despite this EGFR remains an attractive target for treating certain
types of cancer.
Acknowledgements
The present study was supported by National Natural
Science and Technology Major Foundation of China (grant no.
SQ2017ZX090361); National Natural Science Foundation of China
(grant nos. 81372792 and 81602683); Hunan Department of Scienceand
Technology Foundation (grant nos. 2016SK2007 and 2015JJ4055).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang JT, See LC, Liao CT, Ng SH, Wang CH,
Chen IH, Tsang NM, Tseng CK, Tang SG and Hong JH: Locally recurrent
nasopharyngeal carcinoma. Radiother Onco1. 54:135–142. 2000.
View Article : Google Scholar
|
|
4
|
Stoker SD, van Diessen JNA, de Boer JP,
Karakullukcu B, Leemans CR and Tan IB: Current treatment options
for local residual nasopharyngeal carcinoma. Curr Treat Options
Oncol. 14:475–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qiu S, Lin S, Tham IW, Pan J, Lu J and Lu
JJ: Intensity-modulated radiation therapy in the salvage of locally
recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys.
83:676–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee AW, Sze WM, Au JS, Leung SF, Leung TW,
Chua DT, Zee BC, Law SC, Teo PM, Tung SY, et al: Treatment results
for nasopharyngeal carcinoma in the modern era: The Hong Kong
experience. Int J Radiat Oncol Biol Phys. 61:1107–1116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeh SA, Tang Y, Lui CC, Huang YJ and Huang
EY: Treatment outcomes and late complications of 849 patients with
nasopharyngeal carcinoma treated with radiotherapy alone. Int J
Radiat Oncol Biol Phys. 62:672–679. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baujat B, Audry H, Bourhis J, Chan AT,
Onat H, Chua DT, Kwong DL, Al-Sarraf M, Chi KH, Hareyama M, et al:
Chemotherapy in locally advanced nasopharyngeal carcinoma: An
individual patient data meta-analysis of eight randomized trials
and 1753 patients. Int J Radiat Oncol Biol Phys. 64:47–56. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vermorken JB and Specenier P: Optimal
treatment for recurrent/metastatic head and neck cancer. Ann Oncol.
21 Suppl 7:vii252–vii261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bianco C, Tortora G, Bianco R, Caputo R,
Veneziani BM, Caputo R, Damiano V, Troiani T, Fontanini G, Raben D,
et al: Enhancement of antitumor activity of ionizing radiation by
combined treatment with the selective epidermal growth factor
receptor-tyrosine kinase inhibitor ZD1839 (Iressa). Clin Cancer
Res. 8:3250–3258. 2002.PubMed/NCBI
|
|
11
|
Ai Z, Wang J, Wang Y, Lu L, Tong J and
Teng Y: Overexpressed epidermal growth factor receptor
(EGFR)-induced progestin insensitivity in human endometrial
carcinoma cells by the EGFR/mitogen-activated protein kinase
signaling pathway. Cancer. 116:3603–3613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pfister DG, Su YB, Kraus DH, Wolden SL,
Lis E, Aliff TB, Zahalsky AJ, Lake S, Needle MN and Shaha AR:
Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy
for locoregionally advanced squamous cell head and neck cancer: A
pilot phase II study of a new combined-modality paradigm. J Clin
Oncol. 24:1072–1078. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du Y, Peyser ND and Grandis JR:
Integration of molecular targeted therapy with radiation in head
and neck cancer. Pharmacol Ther. 142:88–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonner JA, Harari PM, Giralt J, Cohen RB,
Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, et al:
Radiotherapy plus cetuximab for locoregionally advanced head and
neck cancer: 5-year survival data from a phase 3 randomised trial,
and relation between cetuximab-induced rash andsurvival. Lancet
Oncol. 11:21–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cohen RB: Current challenges and clinical
investigations of epidermal growth factor receptor (EGFR) and ErbB
family-targeted agents in the treatmentof head and neck squamous
cell carcinoma (HNSCC). Cancer Treat Rev. 40:567–577. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang XQ, Chen X, Xie XX, Zhou Q, Li K, Li
S, Shen LF and Su J: Co-expression of CD147 and GLUT-1 indicates
radiation resistance and poor prognosis in cervical squamous cell
carcinoma. Int J Clin Exp Pathol. 7:1651–1666. 2014.PubMed/NCBI
|
|
20
|
Zhao Y, Shen L, Chen X, Qian Y, Zhou Q,
Wang Y, Li K, Liu M, Zhang S and Huang X: High expression of PKM2
as a poor prognosis indicatoris associated with radiation
resistance in cervical cancer. Histol Histopathol. 30:1313–1320.
2015.PubMed/NCBI
|
|
21
|
International Commission on Radiation
Units and Measurements (ICRU), . ICRU Report: Prescribing,
recording, and reporting photon beam therapy. 50. ICRU; Bethesda,
MD: 1993
|
|
22
|
International Commission on Radiation
Units and Measurements (ICRU), . ICRU Report: Prescribing,
recording, and reporting photon beam therapy. 62. ICRU; Bethesda,
MD: 1999, (supplement to ICRU report 50).
|
|
23
|
Zhao Y and Shen L, Huang X, Jing D, Huang
D, Fu J, Li Z, Zhang G and Shen L: High expression of Ki-67 acts a
poor prognosis indicator in locally advanced nasopharyngeal
carcinoma. Biochem Biophys Res Commun. 494:390–396. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Q, He Y, Zhao Y, Wang Y, Kuang W and
Shen L: A study of 358 cases of locally advanced nasopharyngeal
carcinoma receiving intensity-modulated radiation therapy:
Improving the seventh edition of the American joint committee on
cancer T-staging system. Biomed Res Int.
2017:14196762017.PubMed/NCBI
|
|
25
|
Radiation Therapy Oncology Group, . A
Phase II Study of Intensity Modulated Radiation Therapy
(IMRT)+/− Chemotherapy for Nasopharyngeal Cancer. RTOG
protocol 0225. 2014, https://www.rtog.org
|
|
26
|
He Y, Zhou Q, Shen L, Zhao Y, Lei M, Wei
R, Shen L and Cao S: A retrospective study of the prognostic value
of MRI-derived residual tumors at the end ofintensity-modulated
radiotherapy in 358 patients with locally-advanced nasopharyngeal
carcinoma. Radiat Oncol. 10:892015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Liu LZ, Mao YP, Chen L, Tang LL,
Zhou GQ, Sun Y, Yue D, Lin AH, Li L and Ma J: Prognostic value
ofmagnetic resonance imaging-detected cranial nerve invasion in
nasopharyngeal carcinoma. Br J Cancer. 110:1465–1471. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsu HC, Chen CL, Hsu MM, Lynn TC, Tu SM
and Huang SC: Pathology of nasopharyngeal carcinoma. Proposal of a
new histologic classification correlated with prognosis. Cancer.
59:945–951. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang S, Chen J, Guo Y, Lin H, Zhang Z,
Feng G, Hao Y, Cheng J, Liang P, Chen K, et al: Identification of
prognostic biomarkers for response to radiotherapy by DNA
microarray in nasopharyngeal carcinoma patients. Int J Oncol.
40:1590–1600. 2012.PubMed/NCBI
|
|
30
|
Little SE, Bax DA, Rodriguez-Pinilla M,
Natrajan R, Messahel B, Pritchard-Jones K, Vujanic GM, Reis-Filho
JS and Jones C: Multifaceted dysregulation of the epidermal growth
factor receptor pathway in clear cell sarcoma of the kidney. Clin
Cancer Res. 13:4360–4364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ekstrand AJ, James CD, Cavenee WK, Seliger
B, Pettersson RF and Collins VP: Genes for epidermal growth factor
receptor, transforming growth factor alpha, and epidermal growth
factor and their expression in human gliomas in vivo. Cancer Res.
51:2164–2172. 1991.PubMed/NCBI
|
|
32
|
Qi DL, Cui Y, Wang QS, Huang CB, Xu J,
Yang YZ, Xin L, Tian Y and Qi XA: A clinical trail on docetaxel and
carboplatin therapy with or without nimotuzumab for the treatment
of advanced nonsmall cell lung cancer. J Cancer Res Ther. 11 Suppl
1:C32–C37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shin DM, Ro JY, Hong WK and Hittelman WN:
Dysregulation of epidermal growth factor receptor expression in
premalignant lesions during head and neck tumorigenesis. Cancer
Res. 54:3153–3159. 1994.PubMed/NCBI
|
|
34
|
Pan J, Tang T, Xu L, Lu JJ, Lin S, Qiu S,
Chen G and K Tham IW: Prognostic significance of expression of
cyclooxygenase-2, vascular endothelial growth factor, and epidermal
growth factor receptor in nasopharyngeal carcinoma. Head Neck.
35:1238–1247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma BB, Poon TC, To KF, Zee B, Mo FK, Chan
CM, Ho S, Teo PM, Johnson PJ and Chan AC: Prognostic significance
of tumor angiogenesis, Ki67, p53 oncoprotein, epidermal growth
factor receptor and HER2 receptor protein expression in
undifferentiated nasopharyngeal carcinoma-a prospective study. Head
Neck. 25:864–872. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soo R, Putti T, Tao Q, Goh BC, Lee KH,
Kwok-Seng L, Tan L and Hsieh WS: Overexpression of cyclooxygenase-2
in nasopharyngeal carcinoma and association with epidermal growth
factor receptor expression. Arch Otolaryngol Head Neck Surg.
131:147–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan J, Kong L, Lin S, Chen G, Chen Q and
Lu JJ: The clinical significance of coexpression of
cyclooxygenases-2, vascular endothelial growth factors, and
epidermal growth factor receptor in nasopharyngeal carcinoma.
Laryngoscope. 118:1970–1975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee SC, Lim SG, Soo R, Hsieh WS, Guo JY,
Putti T, Tao Q, Soong R and Goh BC: Lack of somatic mutations in
EGFR tyrosine kinase domain in hepatocellular and nasopharyngeal
carcinoma. Pharmacogenet Genomics. 16:73–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang ZC, Fu S, Wang F, Wang HY, Zeng YX
and Shao JY: Oncogene mutational profile in nasopharyngeal
carcinoma. Onco Targets Ther. 7:457–467. 2014.PubMed/NCBI
|
|
40
|
Zuo Q, Shi M, Chen J and Liao W: The Ras
signaling pathway mediates cetuximab resistance in nasopharyngeal
carcinoma. Biomed Pharmacother. 65:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fernández-Medarde A and Santos E: Ras in
cancer and developmental diseases. Genes Cancer. 2:344–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bozec A, Etienne-Grimaldi MC, Fischel JL,
Sudaka A, Toussan N, Formento P and Milano G: The mTOR-targeting
drug temsirolimus enhances the growth-inhibiting effects of the
cetuximabbevacizumab-irradiation combination on head and neck
cancer xenografts. Oral Oncol. 47:340–344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chung CH, Wang H, Tsottles N, Gourin CG,
Agrawal N, Molinolo A, Gutkind S, Forastiere AA and Marur S: A
phase I study of everolimus in combination with cetuximab and
cisplatin as first-line therapy in recurrent and metastatic (R/M)
head and neck squamous cell carcinoma (HNSCC). J Clin Oncol Oncol
Clin Oncol. 30 Suppl:2012.
|
|
44
|
Argiris A, Ohr J, Kubicek GJ, Duvvuri U,
Heron DE, Kotsakis AP, Spencer C, Kim S, Grandis JR, Johnson JT, et
al: Phase II randomized trial of radiotherapy (RT), cetuximab (E),
and pemetrexed (Pem) with or without bevacizumab (B) in locally
advanced squamous cell carcinoma of the head and neck (SCCHN). J
Clin Oncol. 31 Suppl:S6043. 2013.
|
|
45
|
Xu M, Mizoguchi I, Morishima N, Chiba Y,
Mizuguchi J and Yoshimoto T: Regulation of antitumor immune
responses by the IL-12 family cytokines, IL-12, IL-23, and IL-27.
Clin Dev Immunol. 2010:8324542010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yoo DS, Kirkpatrick JP, Craciunescu O,
Broadwater G, Peterson BL, Carroll MD, Clough R, MacFall JR, Hoang
J, Scher RL, et al: Prospective trial of synchronous bevacizumab,
erlotinib, and concurrent chemoradiation in locally advanced head
and neck cancer. Clin Cancer Res. 18:1404–1414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Argiris A, Kotsakis AP, Hoang T, Worden
FP, Savvides P, Gibson MK, Gyanchandani R, Blumenschein GR Jr, Chen
HX, Grandis JR, et al: Cetuximab and bevacizumab: Preclinical data
and phase II trial in recurrent or metastatic squamous cell
carcinoma of the head and neck. Ann Oncol. 24:220–225. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu MT, Chen MK, Huang CC and Huang CY:
Prognostic value of molecular markers and implication for molecular
targeted therapies in nasopharyngeal carcinoma: An update in an era
of new targeted molecules development. World J Oncol. 6:243–261.
2015. View Article : Google Scholar : PubMed/NCBI
|