Introduction
Colon cancer refers to the malignant lesions of
colonic epithelium due to various environmental or genetic
carcinogenic factors, ranking third most common among
gastrointestinal tumors and also one of the most common malignant
tumors (1). The majority of colon
cancers is sporadic, and caused by risk factors, including aging,
sex, environmental factors, obesity, diabetes, high fat diet,
sitting for prolonged durations, insufficient intake of cellulose,
smoking and drinking (2). Patients
with intestine inflammatory diseases also possess a high risk of
developing colon cancer, accounting for over two thirds of colon
cancer cases. Furthermore, the risk of colon cancer is
significantly associated with illness duration (2% within 10 years
vs. 18% within 30 years) and the severity of inflammatory diseases
(3). Similar with other cancer types,
the cause of colon cancer remains unknown. Studies have reported
that the incidence and development of colon cancer involve multiple
stages, and is caused by numerous factors (2,4). In the
last 20 years, it has been demonstrated that colon cancer is
associated with environmental factors, diet, lifestyle and genetic
factors (2). Carcinogens combined
with genetic factors may lead to cell mutations and thereby
gradually cause cancer.
Autophagy refers to the process of transporting
substrates to lysosomes, which are then degraded and digested by
lysosomal enzymes (5). It is an
important way to degrade unnecessary proteins in eukaryotic cells,
and is essential to the maintenance of homeostasis, growth,
differentiation, development and environmental adaptability of
cells (6). If the process of
autophagy is disturbed, and large protein molecules with abnormal
structures and damaged organelles cannot be removed, they
accumulate in cells, affecting the normal development of cells,
thus leading to various acute and chronic diseases, including
cancer, neurodegenerative disease and myopathy as well as aging
(7).
Microtubule-associated protein 1A/1B-light chain 3
(LC3), a homology of the yeast ATG8 (Aut7/Apg8) gene in mammalian
cells, serves functions in the late stage of autophagosome
formation, namely the extension of the pre-autophagosome structure
(8). There are three subtypes of LC3
in mammalian cells, LC3A, LC3B and LC3C, and each subtype may be
subdivided into type I and type II, among which only LC3B is
associated with autophagy (8).
Pro-LC3 is not processed in cells and is cleaved by Atg4 to form
cytoplasmic LC3I. Following cleavage and ubiquitination, LC3I is
evenly distributed in the cytoplasm and binds to phosphatidyl
ethanolamine on the vesicle membrane of autophagosomes to form
LC3-II, which has membrane binding capacity (9). LC3-II is located in the membrane of
autophagosomes and pro-autophagosomes, when autophagy is activated,
LC3-II increases with the increase in autophagosomes in cells
(9). As LC3-II is located on the
somatic membrane of autophagosomes, the concentration of LC3-II is
considered to be directly proportional to the number of
autophagosomes (10). Western
blotting is used to detect the ratio of LC3 II/LC3I gray level,
which quantitatively assesses the number of autophagosomes, and
thus may be used as an effective index to detect the activity of
autophagy (10).
Phosphatidylinositol 3 kinase (PI3K), a type of
esterase that is activated when cells are stimulated by growth
factors and other stimulatory factors, and its substrate
3,4-diphosphate phosphatidyl inositol is phosphorylated into
3,4,5-triphosphate phosphatidyl inositol (11). PI3K passes a mitotic signal to P70S6K1
via RAC-α serine/threonine-protein kinase (Akt) and mechanistic
target of rapamycin (mTOR), to upregulate the translation of major
proteins, including cyclin, and promote the progression of G1, so
as to prolong cell cycle (11). In a
previous study on colon and prostate cancer, it was demonstrated
that inhibiting the activity of PI3K may significantly reduce the
expression of CyclinD1 and cell cycle dependent protein kinase 4
(CDK4), as well as the phosphorylation of Rb (11). In addition, PI3K inhibition induced
CDKs to suppress the expression of p21CIP1, blocking the G1 stage
and thereby stopping cell cycle progression (11).
Cellular autophagy is a relatively conservative
subcellular metabolism pathway in eukaryotes, and its underlying
molecular mechanism is complex and highly conservative (12). The mTOR-dependent pathway, namely the
PI3K/Akt/mTOR signaling pathway and its associated factors regulate
cell autophagy (13). The
transduction pathway stimulates upstream stimulatory factors,
including various nutrients and growth factors to integrate. It
then activates a series of downstream effectors to regulate a large
number of activities, serving a key role in regulating cell
proliferation, growth and differentiation. PI3K and its downstream
molecule Akt constitute a signaling pathway, which reduces
apoptosis, promotes the survival of cells and activates downstream
mTOR to regulate autophagy (13). At
present, numerous studies on the association between the
PI3K/Akt/mTOR signaling pathway and autophagy have occurred,
confirming that is serves important roles in regulating autophagy
(14).
Scutellaria is a famous traditional Chinese
medicine in China, with wogonoside and neobaicalein as its specific
constituents (15). The former is a
yellow crystal without an obvious melting point (15). It turns to red brown color when heated
to 230°C and decomposes at 302°C. In recent years, there has been
increasing international studies on wogonoside leading to a more
profound understanding of its effects, Scutellaria exhibits
anti-oxidation, free radical removal and anti-tumor effects
(16). It effectively inhibits the
activity of hepatocellular carcinoma cells, colorectal cancer
cells, cervical cancer cells, breast cancer cells and other tumor
cells (17). In recent years, the
study of the molecular mechanism of traditional Chinese medicine
and its effective constituents in inhibiting tumors have made a lot
of progress, resulting in various findings, including the
inhibition of tumor growth by inducing cell apoptosis, cell
toxicity, regulating cell signal transduction, inducing cell
differentiation, reversing multidrug resistance and suppressing the
activity of telomerase (16–18). In the present study, the potential
anticancer effect of wogonoside on human colon cancer cells was
demonstrated.
Materials and methods
Cell culture
Human colon cancer cells LOVO cells were cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (both from Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C with 5% CO2. Wogonoside was purchased
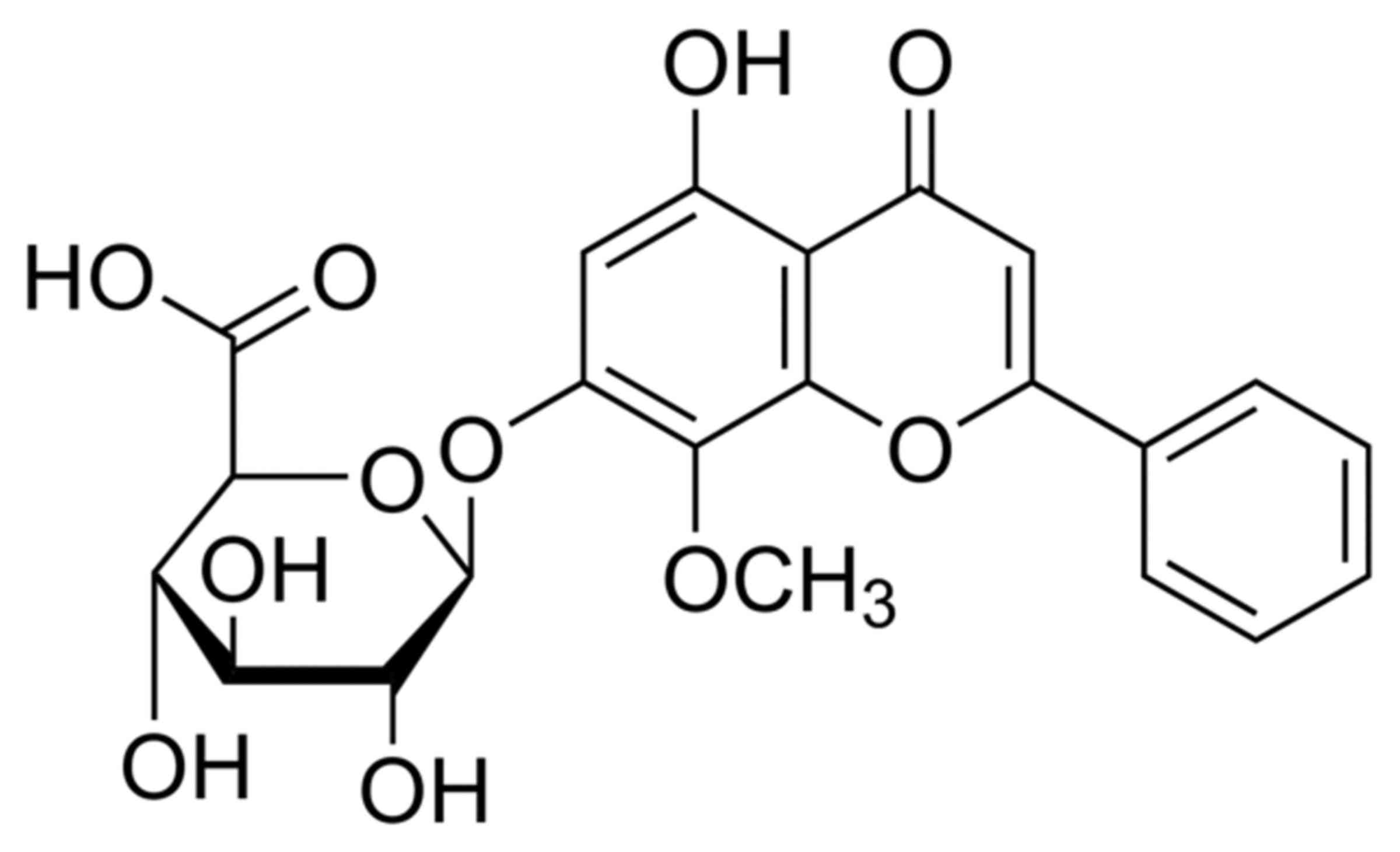
from Shanghai Tauto Biotech Co., Ltd. (Shanghai, China) and its
structural formula is presented in Fig.
1.
Cell viability assays
LOVO cells (2×104 cells/well) were seeded
into 96-well culture plates and cultured for 12 h. Wogonoside
(0.00, 1.95, 3.91, 7.81, 15.63, 31.25, 62.50, 125.00, 250.00,
500.00 and 1,000.00 µM; control group is 0.00 µM of wogonoside) was
added to the cells and the cells were cultured for a further 24, 48
or 72 h. Cell viability was assessed using a cell counting kit-8
(CCK-8) assay (Beyotime Institute of Biotechnology, Shanghai,
China). Following treatment with wogonoside, 10 µl of CCK-8 was
added to cells and incubated for 30 min. Absorbance value was read
using a microplate reader (Multiskan MK3; Bio-Rad Laboratories,
Inc., Hercules, CA, USA) at 450 nm.
Flow cytometry analysis
LOVO cells (1×106 cells/well) were seeded
into 96-well culture plates and cultured for 12 h. Wogonoside was
added to the cells and the cells were cultured for a further 48 h,
collected by trypsinization and washed twice with PBS. Cells were
collected via centrifugation at 1,000 × g for 5 min at room
temperature, and stained with Annexin V and propidium iodide (BD
Biosciences, San Jose, CA, USA) for 30 min at room temperature in
the dark. Cell apoptosis was measured using a FACScan laser flow
cytometer (FACSCalibur; BD Biosciences) and analyzed using Image
Lab 3.0 (Bio-Rad Laboratories, Inc.).
Immunofluorescence
Cells (1×106 cells/well) were seeded into
96-well culture plates and cultured for 12 days. Wogonoside was
added to the cells and the cells were cultured for an additional 48
h at 37°C with 5% CO2 and washed with PBS 3 times for 5
min. Cells were fixed with 4% paraformaldehyde for 15 min at room
temperature and blocked with 5% BSA-TBST for 1 h at room
temperature. Cells were incubated with LC3 (cat. no. 12741; 1:100;
Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight
and washed with PBST for 15 min. Cells were incubated with
488-conjugated anti-rabbit secondary antibodies for 1 h at 37°C and
observed using a fluorescence microscope at magnification, ×10
equipped with a CoolSNAP-Pro color digital camera (Media
Cybernetics, Rockville, MD, USA).
Western blotting
LOVO cells (1×106 cells/well) were seeded
into 96-well culture plates and cultured for 12 days. Wogonoside
was added to the cells and the cells were cultured for a further 48
h, collected by trypsinization and lysed in lysis buffer (Beyotime
Institute of Biotechnology) at 4°C for 30 min. Miscible liquids
were elucidated by centrifugation at 10,000 × g for 15 min at 4°C.
The protein concentration was measured using a bicinchoninic acid
assay (Beyotime Institute of Biotechnology). A total of 50 µg
protein/lane was separated by 10 or 12% SDS-PAGE and transferred
onto polyvinylidene fluoride membranes (EMD Millipore, Billerica,
MA, USA). Membranes were blocked with 5% not-fat milk for 1 h at
37°C and incubated with the following primary antibodies: Caspase-3
(cat. no. 9665; 1:1,000 dilution), caspase-9 (cat. no. 9508;
1:1,000 dilution), apoptosis regulator Bax (Bax; cat. no. 14796;
1:1,000 dilution), apoptosis regulator Bcl-2 (Bcl-2; cat. no. 3498;
1:1,000 dilution), LC3 (cat. no. 13118; 1:2,000 dilution), PI3K
(cat. no. 4249; 1:1,000 dilution), AKT (cat. no. 4685; 1:1,000
dilution), p-AKT (cat. no. 4060; 1:2,000 dilution), p-mTOR (cat.
no. 5536; 1:1,000 dilution), p-p70 S6 kinase (p70S6K; cat. no.
9204; 1:2,000 dilution), p62 (cat. no. 23214; 1:2,000 dilution; all
from Cell Signaling Technology) and GAPDH (cat. no. AF1186; 1:5,000
dilution; Beyotime Institute of Biotechnology) overnight at 4°C
followed anti-rabbit horseradish peroxidase-conjugated secondary
antibodies (cat. no. 7074; 1:5,000; Cell Signaling Technology,
Inc.) for 1 h at 37°C. Bands were detected using an enhanced
chemiluminescence western blot detection kit (GE Healthcare,
Chicago, IL, USA) and analyzed using Image Pro Plus software
(version 3.0; Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation using SPSS 20.0 (IBM Corp, Armonk, NY, USA). The
statistical significance of the differences between treatments was
assessed using one-way analysis of variance followed by
Student-Newman-Keuls test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Wogonoside inhibits the growth of
colon cancer cells
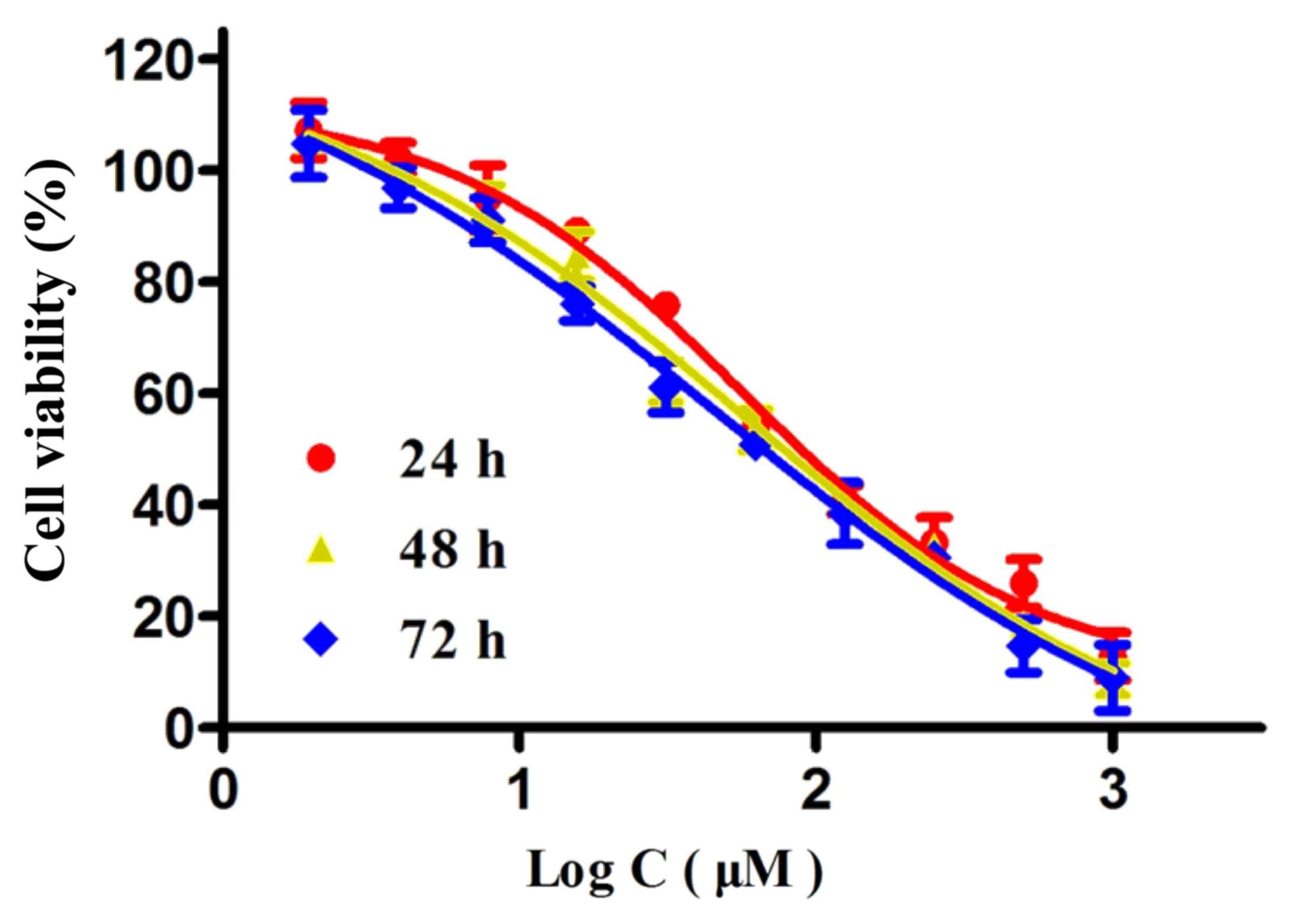
To investigate the anticancer effect of wogonoside
on the viability of colon cancer cells, LOVO cells were treated
with different concentrations of wogonoside for 24, 48 and 72 h. As
presented in Fig. 2, wogonoside
suppressed the viability of LOVO cells in a dose- and
time-dependent manner, compared with the control group.
Wogonoside induces apoptosis in colon
cancer cells
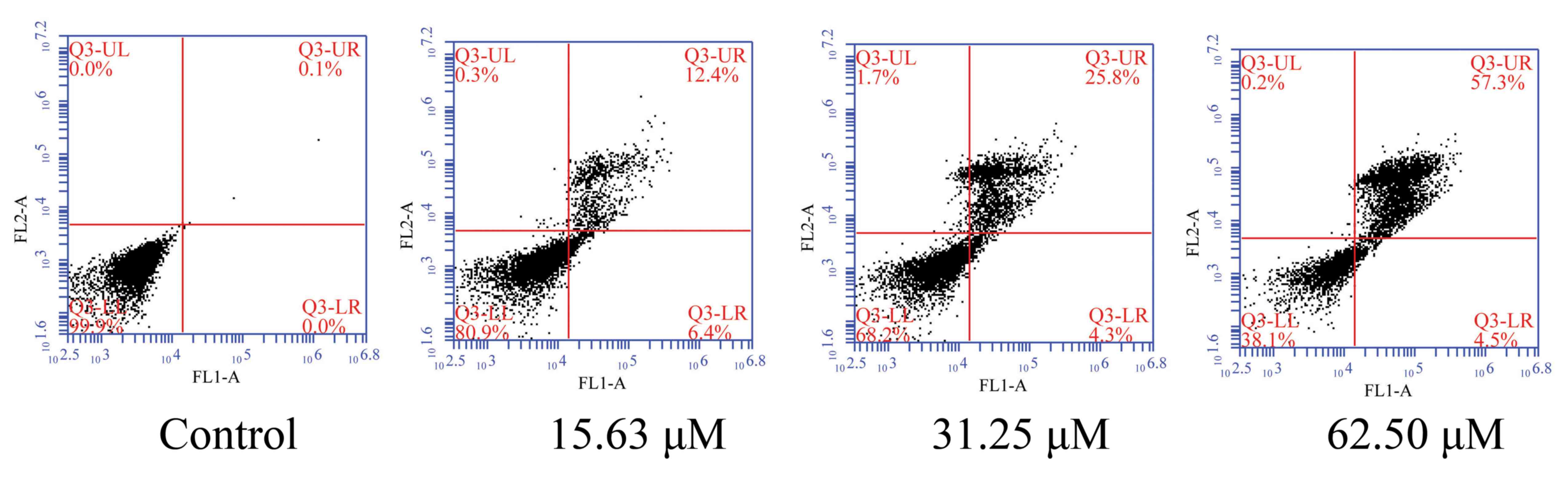
To evaluate the effect of wogonoside on the death of
colon cancer cells, LOVO cells were treated with different
concentrations of wogonoside for 48 h. It was demonstrated that
wogonoside significantly induced cell apoptosis in LOVO cells in a
dose-dependent manner, compared with the control group (Fig. 3).
Wogonoside induces caspase-3 and
caspase-9 protein expression in colon cancer cells
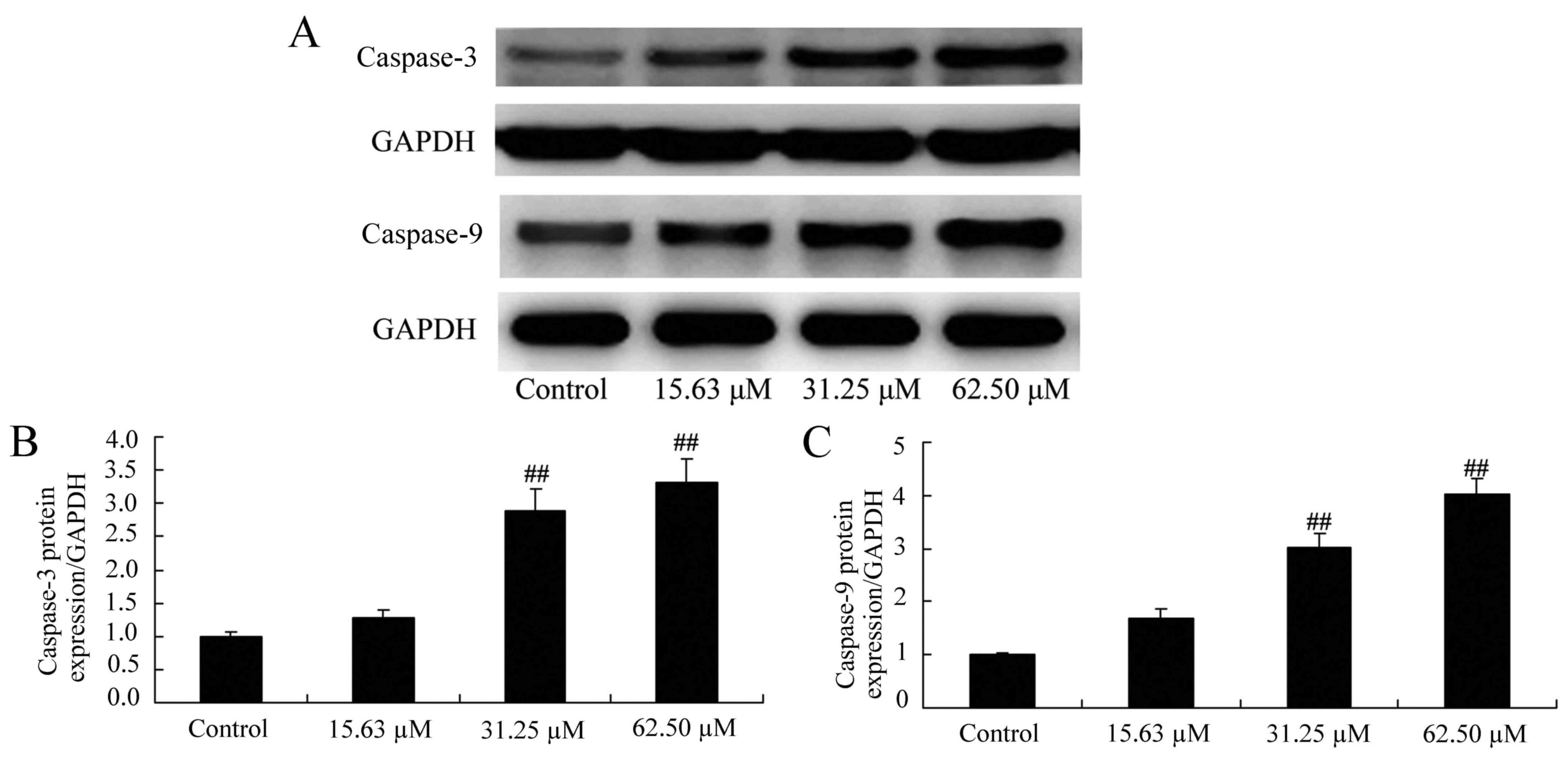
To evaluate the effect of wogonoside on the
apoptotic mechanisms in colon cancer cells, caspase-3 and caspase-9
protein expression were detected in LOVO cells following wogonoside
treatment. As presented in Fig. 4,
wogonoside (31.25 or 62.50 µM) significantly increased caspase-3
and caspase-9 protein expression in LOVO cells, compared with the
control group.
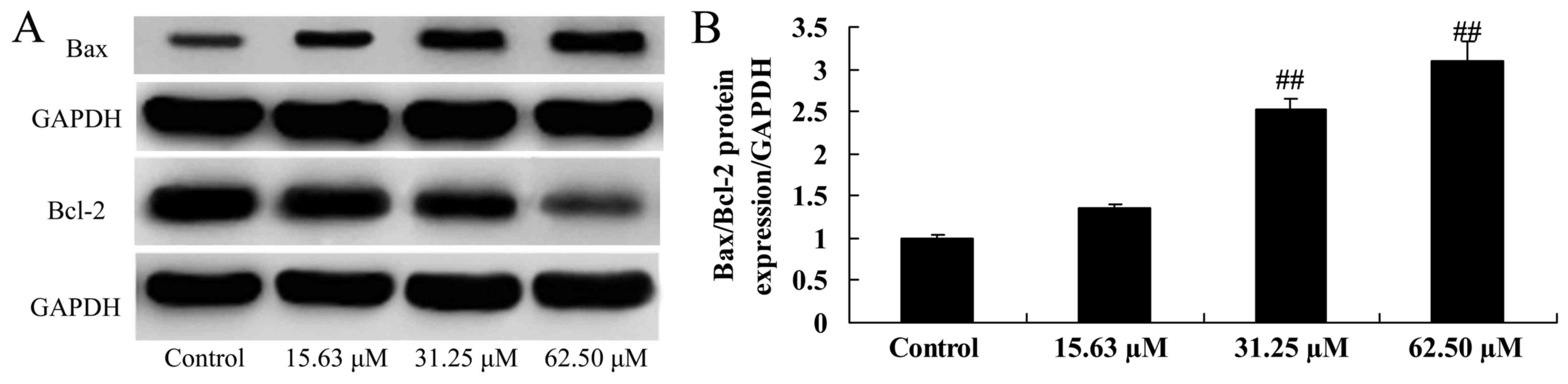
Wogonoside induces Bax/Bcl-2 protein
expression in colon cancer cells
Whether Bax/Bcl-2 participates in the anticancer
effect of wogonoside was further investigated. Wogonoside (31.25 or
62.50 µM) significantly increased the Bax/Bcl-2 protein expression
ratio in LOVO cells, compared with the control group (Fig. 5).
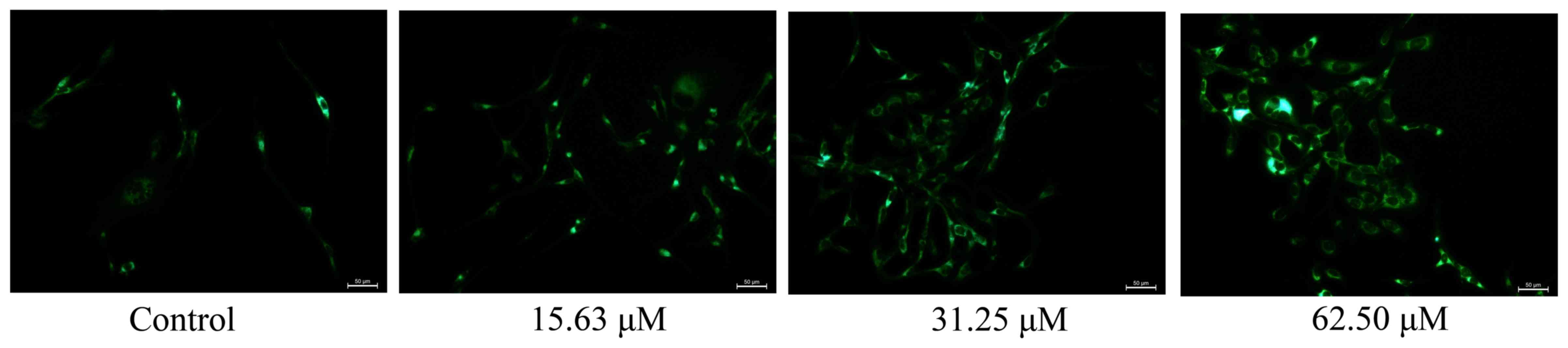
Wogonoside induces mitochondrial
autophagy in colon cancer cells
To examine the effect of wogonoside on mitochondrial
autophagy in colon cancer cells, LOVO cells were treated with
different concentrations of wogonoside for 48 h. Fig. 6 demonstrates that wogonoside markedly
induced mitochondrial autophagy (LC3 protein expression) of LOVO
cells in a dose-dependent manner, compared with the control
group.
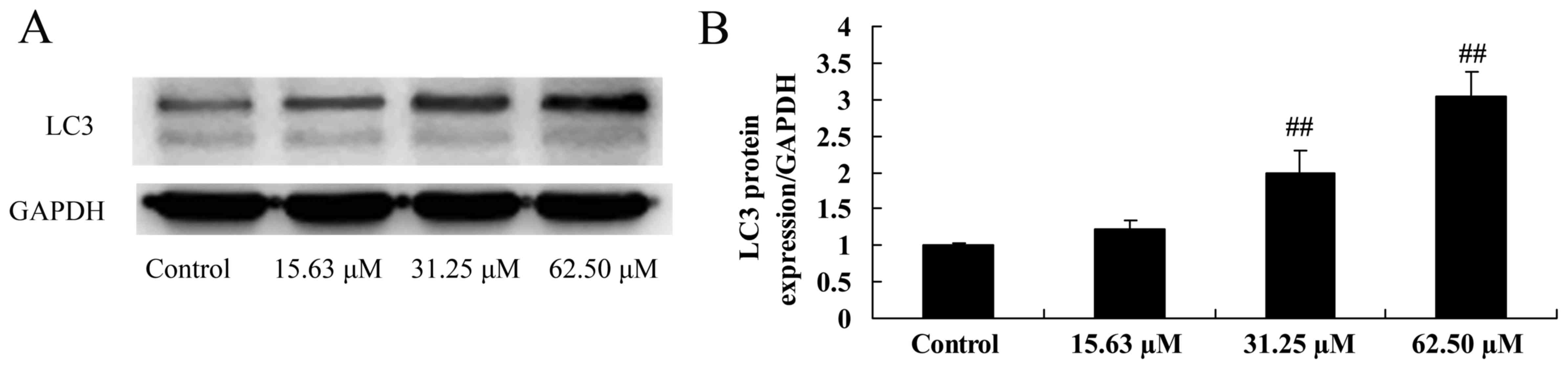
Wogonoside induces LC3 protein
expression in colon cancer cells
Next, the effect the mechanism of wogonoside on
autophagy in colon cancer cell was examined, LC3 protein expression
was measured following treatment of LOVO cells with different
concentrations of wogonoside at 48 h. Fig. 7 indicates that wogonoside (31.25 or
62.50 µM) significantly promoted LC3 protein expression in LOVO
cells in a dose-dependent manner, compared with the control
group.
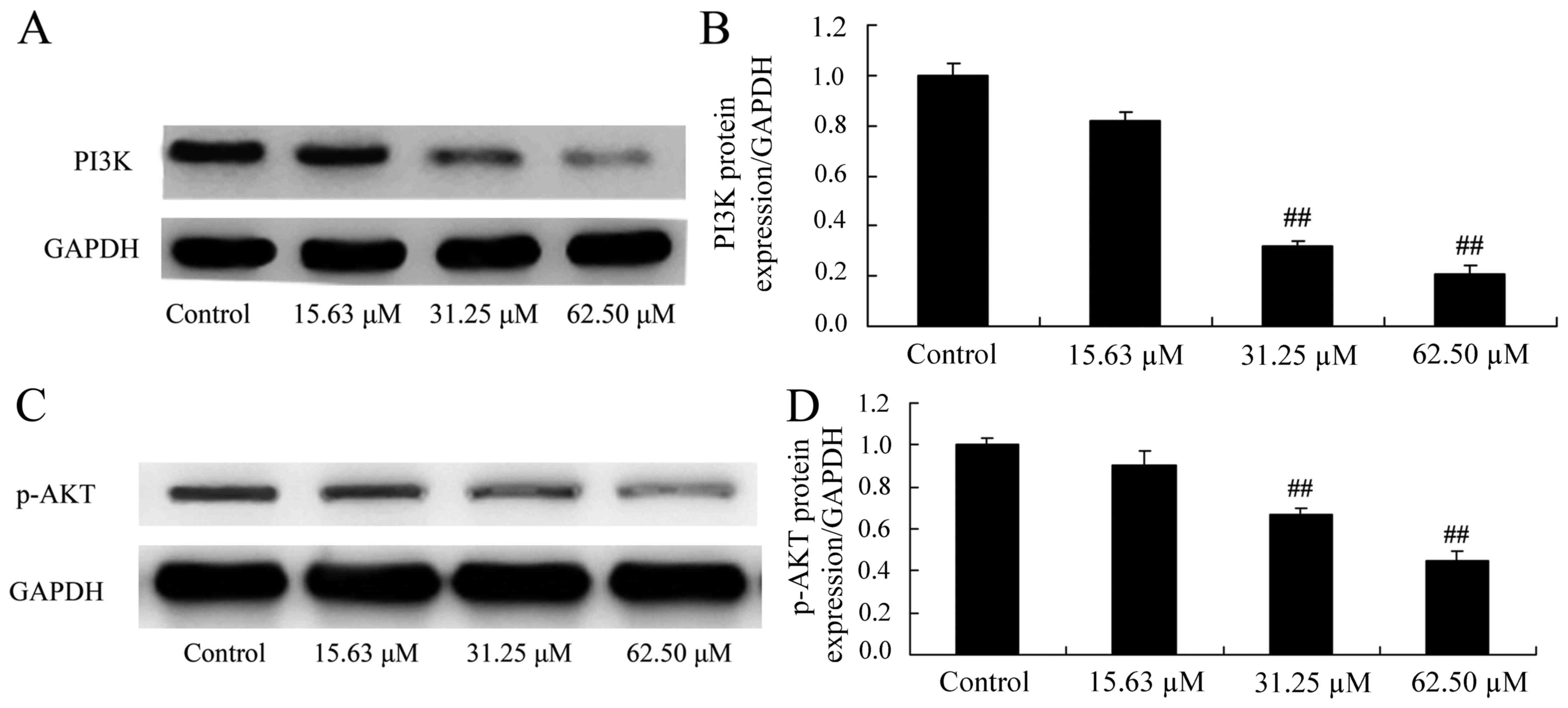
Wogonoside induces PI3K/AKT protein
expression in colon cancer cells
The mechanisms of wogonoside underlying the
apoptosis of colon cancer cells via the PI3K/AKT signaling pathway
were investigated in LOVO cells. PI3K and p-AKT protein expression
was significantly suppressed in LOVO cells treated with 31.25 or
62.50 µM of wogonoside in a dose-dependent manner, compared with
the control group (Fig. 8).
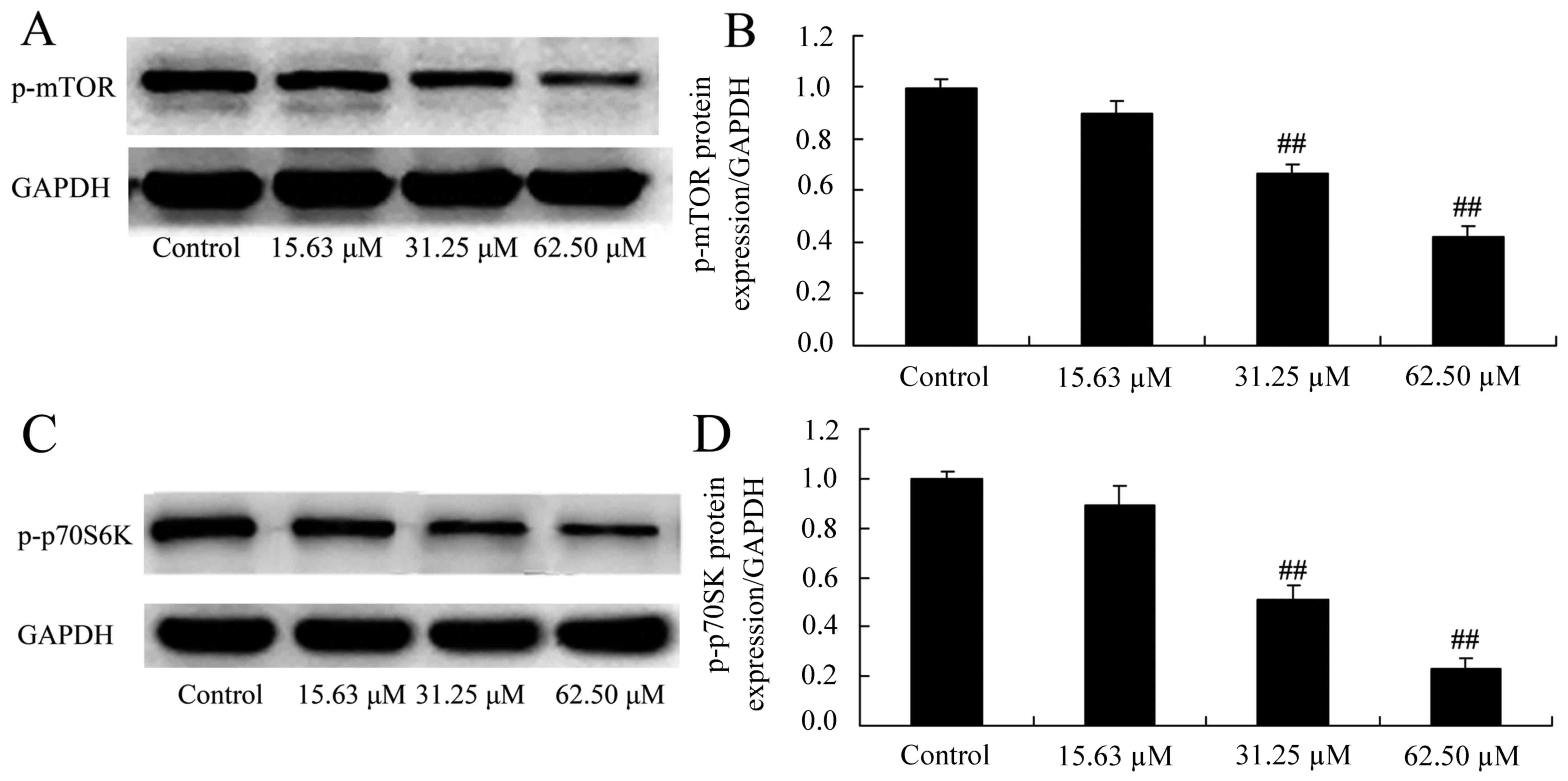
Wogonoside induces mTOR/p70S6K protein
expression in colon cancer cells
The mechanisms of wogonoside underlying the
apoptosis of colon cancer cells via the mTOR/p70S6K signaling
pathway were investigated in LOVO cells. Consistently, p-mTOR and
p-p70S6K protein expression was significantly suppressed in LOVO
cells treated with 31.25 or 62.50 µM of wogonoside in a
dose-dependent manner, compared with the control group (Fig. 9).
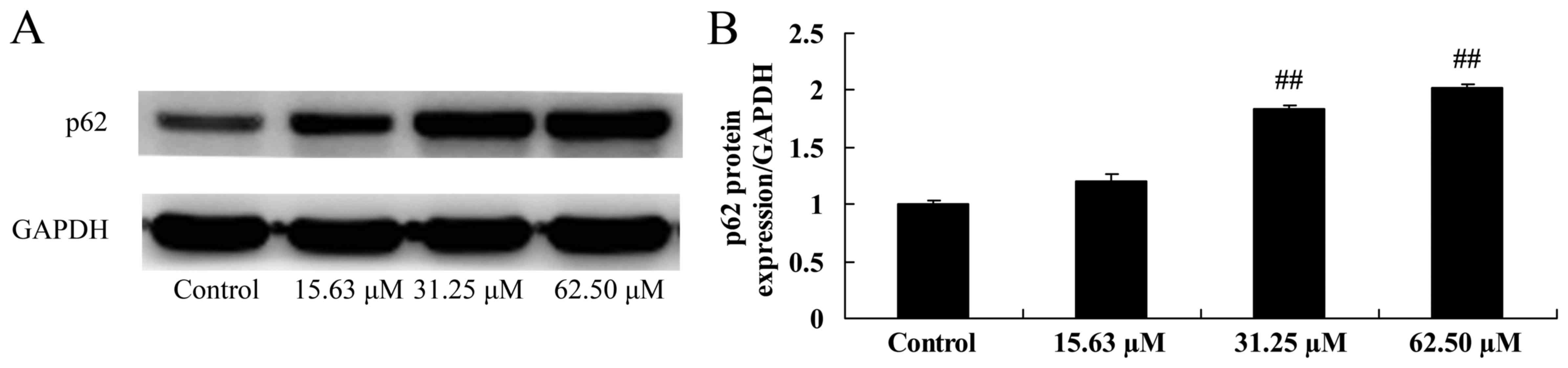
Wogonoside induces p62 protein
expression of colon cancer cell
The mechanisms of wogonoside underlying the
apoptosis of colon cancer cells via the p62 pathway were
investigated in LOVO cells. The results demonstrated that 31.25 or
62.50 µM of wogonoside significantly induced p-p62 protein
expression in LOVO cells, compared with the control group (Fig. 10).
Discussion
Autophagy serves an important role in the incidence
and development of colon cancer. Autophagy may induce apoptosis and
tolerance to nutritional deficiency of colon cancer cells, and
elevated autophagy activity may lead to colon cancer (19). Studies have identified that the
expression of Atg key proteins, including LC3 and Beclin-1 increase
during the early, and advanced stages of colon cancer (7). This may enhance the tolerance of cells
located at the tumor core to hypoxia and nutritional deficiency,
thus increasing their resistant to cell death (7). In certain cases, the upregulation of
autophagy activity may protect colon cancer cells from harm due to
chemotherapeutic drugs or selective cell death (20). Therefore, studying the changes in
autophagy and autophagy-related genes at the molecular level is of
significance to clarify the pathogenesis of colon cancer and
identify therapeutic strategies. The present data demonstrated that
wogonoside significantly suppressed cell viability, induced cell
apoptosis, and increased caspase-3, caspase-9, LC3 protein
expression as well as the Bax/Bcl-2 ratio in LOVO cells in a
dose-dependent manner. Huang et al (17) demonstrated that wogonoside inhibits
angiogenesis via suppressing Wnt/β-catenin pathway in breast
cancer. Sun et al (21)
suggested that wogonoside induces autophagy through regulating the
MAPK-mTOR signaling pathway in MDA-MB-231 cells.
The PI3K/Akt/mTOR signaling pathway serves an
important role in the incidence and development of malignant
tumors, and drug resistance by inducing the survival,
differentiation and angiogenesis of tumor cells, thus, it has
become a novel target for the intervention therapy of tumor
(22). In numerous malignant tumor
types, including breast cancer and non-small cell lung cancer, the
PI3K/Akt signaling pathway may prevent the death of cancer cells
from chemotherapy or radiotherapy-induced apoptosis (12). Selectively inhibiting PI3K or Akt
activity to decrease the phosphorylation of Akt may increase the
sensitivity of cells towards apoptosis induced by chemotherapy and
radiotherapy (23). For lymphoma with
high expression levels of Akt, mTOR inhibitors significantly
increase the chemotherapy-induced apoptosis rate of cancer cells
(24). In the present study,
wogonoside was observed to significantly suppress PI3K and p-AKT
protein expression in LOVO cells. Sun et al (25) suggested that wogonoside prevents
colitis-associated colorectal carcinogenesis through inhibiting
nuclear transcription facto-κB (NF-κB) activation via the PI3K/Akt
signaling pathway.
Akt is able to regulate multiple genes associated
with apoptosis and thereby inhibit cell apoptosis (24). Transcription factor forkhead box O1, a
member of the forkhead family, performs numerous activities
including the following: Inactivating Akt by translocating it from
the nucleus to the cytoplasm; downregulating Fas ligand to induce
apoptosis; phosphorylating proapoptotic protein BAD and caspase-9
to inhibit their activity; activating NF-κB to promote the
transcription of apoptosis-inhibiting genes; suppressing the
release of cytochrome C; and maintaining the integrity of
mitochondria, thereby inhibiting cell death (26). Akt is also able to send survival
signals by phosphorylating mTOR, and its downstream molecules,
including P70S6K and eukaryotic translation initiation factor
4E-binding protein 1, thus inhibiting the apoptosis of cells that
do not depend on p53 (22). Activated
PI3K/Akt may further activate its downstream molecule mTOR through
the tuberous sclerosis 1/2 complex. mTOR signaling pathway is
essential to cell growth, as activated mTOR signaling pathway
inhibits apoptosis induced by various stimuli, and promote cell
cycle progression and cell survival and proliferation (27). The mTOR signaling pathway not only
serves an important role in the growth and proliferation of normal
cells, but is also associated with the process whereby normal cells
become cancerous, as well as the growth and proliferation of cancer
cells (28). In addition, it is
involved in angiogenesis, and serves an important role in tumor
formation, invasion and metastasis (28). In the present, the results
demonstrated that wogonoside significantly suppressed p-mTOR
protein expression in LOVO cells. Sun et al (21) suggested that wogonoside induces
autophagy through regulating the mitogen-activated protein
kinase-mTOR signaling pathway in MDA-MB-231 cells.
P70S6K, the kinase of 40S ribosomal small subunit
protein S6 and one of the downstream targets in the PI3K/Akt/mTOR
signaling pathway, directly phosphorylates 40S ribosomal protein S6
to promote the translation of mRNA essential in the Gl-S stage of
the cell cycle, as well as the expression of proteins associated
with cell growth and differentiation, thus serving an important
role in the regulation of cell growth and proliferation (29,30). The
mTOR/P70S6K signaling pathway serves an essential role in cell
growth, differentiation, proliferation, migration and survival
(29). Zhang et al (31) reported wogonoside induces
autophagy-related apoptosis in human glioblastoma cells through the
PI3K/AKT/mTOR/p70S6K signaling pathway.
In conclusion, the present study demonstrated that
wogonoside significantly inhibits cell growth, induces apoptosis
and mitochondrial-mediated autophagy of colon cancer cells, which
is regulated by the PI3K/AKT/mTOR/p70S6K pathway. Therefore, these
findings provide a basis for future investigations aimed at
elucidating the anticancer effect of wogonoside on apoptosis and
autophagy in colon cancer therapy.
Acknowledgements
The present study was supported by the Natural
Science Foundation of the Institutions of Higher Education from the
Education Department of Anhui Province (grant no. KJ2017A261) and
Natural Science Foundation of Wannan Medical College (grant no.
WK2016F08).
References
|
1
|
Liu C, Huang Z, Jiang H and Shi F: The
sirtuin 3 expression profile is associated with pathological and
clinical outcomes in colon cancer patients. Biomed Res Int.
2014:8712632014.PubMed/NCBI
|
|
2
|
Chen WK, Ren L, Wei Y, Zhu DX, Miao CH and
Xu JM: General anesthesia combined with epidural anesthesia
ameliorates the effect of fast-track surgery by mitigating
immunosuppression and facilitating intestinal functional recovery
in colon cancer patients. Int J Colorectal Dis. 30:475–481. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Declercq J, Jacobs B, Biesmans B, Roth A,
Klingbiel D, Tejpar S and Creemers JW: Single nucleotide
polymorphism (rs4932178) in the P1 promoter of FURIN is not
prognostic to colon cancer. Biomed Res Int. 2015:3212762015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hestetun KE, Brydøy M, Myklebust MP and
Dahl O: Nuclear maspin expression as a predictive marker for
fluorouracil treatment response in colon cancer. Acta Oncol.
54:470–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar A, Singh B, Sharma PR, Bharate SB,
Saxena AK and Mondhe DM: A novel microtubule depolymerizing
colchicine analogue triggers apoptosis and autophagy in HCT-116
colon cancer cells. Cell Biochem Funct. 34:69–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buchser WJ, Laskow TC, Pavlik PJ, Lin HM
and Lotze MT: Cell-mediated autophagy promotes cancer cell
survival. Cancer Res. 72:2970–2979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu S, Wang X, Chen J and Chen Y: Autophagy
of cancer stem cells is involved with chemoresistance of colon
cancer cells. Biochem Biophys Res Commun. 434:898–903. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coker-Gürkan A, Arisan ED, Obakan P,
Akalin K, Özbey U and Palavan-Unsal N: Purvalanol induces
endoplasmic reticulum stress-mediated apoptosis and autophagy in a
time-dependent manner in HCT116 colon cancer cells. Oncol Rep.
33:2761–2770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JM, Huang S, Wu TT, Foster NR and
Sinicrope FA: Prognostic impact of Beclin 1, p62/sequestosome 1 and
LC3 protein expression in colon carcinomas from patients receiving
5-fluorouracil as adjuvant chemotherapy. Cancer Biol Ther.
14:100–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiong HY, Guo XL, Bu XX, Zhang SS, Ma NN,
Song JR, Hu F, Tao SF, Sun K, Li R, et al: Autophagic cell death
induced by 5-FU in Bax or PUMA deficient human colon cancer cell.
Cancer Lett. 288:68–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zou H, Li L, Garcia Carcedo I, Xu ZP,
Monteiro M and Gu W: Synergistic inhibition of colon cancer cell
growth with nanoemulsion-loaded paclitaxel and PI3K/mTOR dual
inhibitor BEZ235 through apoptosis. Int J Nanomedicine.
11:1947–1958. 2016.PubMed/NCBI
|
|
12
|
Zhu L, Derijard B, Chakrabandhu K, Wang
BS, Chen HZ and Hueber AO: Synergism of PI3K/Akt inhibition and Fas
activation on colon cancer cell death. Cancer Lett. 354:355–364.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang ZG, Wang Y, Huang Y, Lu Q, Zheng L,
Hu D, Feng WK, Liu YL, Ji KT, Zhang HY, et al: bFGF regulates
autophagy and ubiquitinated protein accumulation induced by
myocardial ischemia/reperfusion via the activation of the
PI3K/Akt/mTOR pathway. Sci Rep. 5:92872015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao ZX, Yang YT, Yu S, Li YZ, Wang WW,
Huang J, Xie XF, Xiong L, Lei S and Peng C: Pogostone induces
autophagy and apoptosis involving PI3K/Akt/mTOR axis in human
colorectal carcinoma HCT116 cells. J Ethnopharmacol. 202:20–27.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai Y, Li S, Li T, Zhou R, Wai AT and Yan
R: Oral pharmacokinetics of baicalin, wogonoside, oroxylin A
7-O-beta-d-glucuronide and their aglycones from an aqueous extract
of Scutellariae Radix in the rat. J Chromatogr B Analyt Technol
Biomed Life Sci. 1026:124–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang YZ, Tang YZ and Liu YH: Wogonoside
displays anti-inflammatory effects through modulating inflammatory
mediator expression using RAW264.7 cells. J Ethnopharmacol.
148:271–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang Y, Zhao K, Hu Y, Zhou Y, Luo X, Li
X, Wei L, Li Z, You Q, Guo Q and Lu N: Wogonoside inhibits
angiogenesis in breast cancer via suppressing Wnt/β-catenin
pathway. Mol Carcinog. 55:1598–1612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu C, Zhang Z, Zhang H, Zhen Z, Calway T,
Wang Y, Yuan CS and Wang CZ: Pretreatment of baicalin and
wogonoside with glycoside hydrolase: A promising approach to
enhance anticancer potential. Oncol Rep. 30:2411–2418. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pigna E, Berardi E, Aulino P, Rizzuto E,
Zampieri S, Carraro U, Kern H, Merigliano S, Gruppo M, Mericskay M,
et al: Aerobic exercise and pharmacological treatments counteract
cachexia by modulating autophagy in colon cancer. Sci Rep.
6:269912016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwatra D, Subramaniam D, Ramamoorthy P,
Standing D, Moran E, Velayutham R, Mitra A, Umar S and Anant S:
Methanolic extracts of bitter melon inhibit colon cancer stem cells
by affecting energy homeostasis and autophagy. Evid Based
Complement Alternat Med. 2013:7028692013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Y, Zou M, Hu C, Qin Y, Song X, Lu N
and Guo Q: Wogonoside induces autophagy in MDA-MB-231 cells by
regulating MAPK-mTOR pathway. Food Chem Toxicol. 51:53–60. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang LL, Mu GG, Ding QS, Li YX, Shi YB,
Dai JF and Yu HG: Phosphatase and Tensin Homolog (PTEN) represses
colon cancer progression through inhibiting paxillin transcription
via PI3K/AKT/NF-kappaB pathway. J Biol Chem. 290:15018–15029. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tahir AA, Sani NF, Murad NA, Makpol S,
Ngah WZ and Yusof YA: Combined ginger extract & Gelam honey
modulate Ras/ERK and PI3K/AKT pathway genes in colon cancer HT29
cells. Nutr J. 14:312015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng Y, Lin ZM, Ge N, Zhang DL, Huang J
and Kong F: Ursolic acid induces apoptosis of prostate cancer cells
via the PI3K/Akt/mTOR pathway. Am J Chin Med. 43:1471–1486. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Y, Zhao Y, Wang X, Zhao L, Li W, Ding
Y, Kong L, Guo Q and Lu N: Wogonoside prevents colitis-associated
colorectal carcinogenesis and colon cancer progression in
inflammation-related microenvironment via inhibiting NF-κB
activation through PI3K/Akt pathway. Oncotarget. 7:34300–34315.
2016.PubMed/NCBI
|
|
26
|
Pal I and Mandal M: PI3K and Akt as
molecular targets for cancer therapy: Current clinical outcomes.
Acta Pharmacol Sin. 33:1441–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gulhati P, Bowen KA, Liu J, Stevens PD,
Rychahou PG, Chen M, Lee EY, Weiss HL, O'Connor KL, Gao T and Evers
BM: mTORC1 and mTORC2 regulate EMT, motility, and metastasis of
colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res.
71:3246–3256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qazi AK, Hussain A, Khan S, Aga MA, Behl
A, Ali S, Singh SK, Taneja SC, Shah BA, Saxena AK, et al:
Quinazoline based small molecule exerts potent tumour suppressive
properties by inhibiting PI3K/Akt/FoxO3a signalling in experimental
colon cancer. Cancer Lett. 359:47–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu S, Kounenidakis M, Schmidt EM,
Deshpande D, Alkahtani S, Alarifi S, Föller M, Alevizopoulos K,
Lang F and Stournaras C: Rapid activation of
FAK/mTOR/p70S6K/PAK1-signaling controls the early
testosterone-induced actin reorganization in colon cancer cells.
Cell Signal. 25:66–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu Q, Liu LZ, Qian X, Chen Q, Jiang Y, Li
D, Lai L and Jiang BH: MiR-145 directly targets p70S6K1 in cancer
cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res.
40:761–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Wang H, Cong Z, Xu J, Zhu J, Ji X
and Ding K: Wogonoside induces autophagy-related apoptosis in human
glioblastoma cells. Oncol Rep. 32:1179–1187. 2014. View Article : Google Scholar : PubMed/NCBI
|