Introduction
Mitochondria are eukaryotic organelles that play a
vital role in numerous cellular functions such as oxidative
adenosine triphosphate production, calcium homeostasis, and
programmed cell death (1). In
addition, a recent study showed that mitochondria are involved in
innate immune response to RNA viruses (2).
Retinoic acid-inducible gene-I (RIG-I)-like
receptors (RLRs) are pattern-recognition receptors (PRRs) that
recognize pathogen-associated molecular patterns. RLRs function as
cytosolic virus sensors and play an important role in antiviral
immunity (3). RLRs include RIG-I,
melanoma differentiation-associated gene 5 (MDA5), and laboratory
of genetics and physiology 2 (LGP2). RIG-I and MDA5 contain an
N-terminal domain consisting of tandem caspase activation and
recruitment domains (CARDs), a central DExD/H box RNA helicase
domain, and a C-terminal regulatory domain. In contrast, LGP2 lacks
CARDs. RIG-I and MDA5 show structural and functional similarities
but recognize different RNA viruses (4). RIG-I recognizes relatively short
double-stranded RNAs (dsRNAs), 5′-triphosphate single-stranded
RNAs, and hepatitis C viruses. In contrast, MDA5 recognizes long
dsRNAs (5) and picornaviruses. Once
RIG-I and MDA5 sense an RNA virus invasion, they interact with
mitochondrial antiviral-signaling protein (MAVS), an adaptor
protein on the mitochondrial membrane, to induce antiviral cytokine
type I interferons (IFNs). Therefore, RLRs function as
mitochondria-mediated antiviral immune systems.
Recent studies have shown that RLR activation in
cancer cells exerts antitumor effects (6–8). Besch
et al reported that RIG-I and MDA5 activation in human
melanoma cells initiates a proapoptotic signaling pathway in a type
I IFN-independent manner (7). They
also showed that treatment with RLR ligands exerts antitumor
effects in immunodeficient mice, suggesting that RLR activation
exerts antitumor effects in the absence of immune activation. Yuan
et al reported that treatment with 5′-triphosphate siRNA
against the gene encoding vascular endothelial growth factor
exerted multiple antitumor effects, including induction of
antitumor immunity through RIG-I-mediated innate immune response
and induction of apoptosis, in non-small cell lung cancer (NSCLC)
cells (8). Collectively, these
studies indicate that RLRs can be potentially used for treating
cancer by inducing antitumor immunity and cytotoxicity against
cancer cells.
Radiation is an effective treatment for cancer
therapy and is used for treating various cancers such as lung
cancers. Our recent study showed that ionizing radiation affected
the expression of Toll-like receptors, a type of PRRs, and response
to their agonists in human monocytic THP1 cell-derived macrophages
(9). However, ionizing radiation
exerts a limited effect on RLR expression and response to their
agonists in human monocytic THP1 cell-derived macrophages (10). These results suggest that RLR agonists
can be used as effective immunostimulants during radiation therapy.
However, it is unclear whether ionizing radiation affects the
cytotoxicity of RLR agonists.
Lung cancer is the leading cause of cancer-related
death over the world, and NSCLC accounts for 85% of all cases of
lung cancer. Since the overall 5-year survival rate of patients
with NSCLC remains lower than 15% (11), development of more effective
anticancer strategies is essential for the treatment of NSCLC.
Therefore, we investigated the effects of RLR agonists and ionizing
radiation cotreatment on cytotoxicity against human NSCLC
cells.
Materials and methods
Reagents
Propidium iodide (PI) and dimethyl sulfoxide were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Z-Val-Ala-Asp (OMe)-CH2F (Z-VAD-fmk) was purchased from
Peptide Institute, Inc. (Osaka, Japan). Poly(I:C)-LMW/LyoVec™ and
poly(I:C)-HMW/LyoVec™ (hereafter referred to as ‘poly[I:C]-LMW’ and
‘poly[I:C]-HMW’, respectively) were purchased from InvivoGen (San
Diego, CA, USA). Anti-RIG-I antibody (no. 4200), anti-MDA5 antibody
(no. 5321), anti-MAVS antibody (no. 3993), anti-caspase-3 antibody
(no. 9662), anti-β-actin antibody (no. 4967), and anti-rabbit
horseradish peroxidase (HRP)-linked IgG antibody were purchased
from Cell Signaling Technology Japan, K.K. (Tokyo, Japan). Ambion's
Silencer® Select Pre-designed siRNA against the gene
encoding RIG-I (ID: s24144), Silencer® Select
Pre-designed siRNA against the gene encoding MDA5 (ID: s34498), and
Silencer® Select Negative Control 1 siRNA were purchased
from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Cell culture and treatment
Human NSCLC cells A549 and H1299 were purchased from
Riken Bio-Resource Center (Tsukuba, Japan) and American Type
Culture Collection (ATCC; Manassas, VA, USA), respectively. A549
cells were maintained in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA) supplemented with 1%
penicillin/streptomycin (Gibco®; Gibco; Thermo Fisher
Scientific, Inc.) and 10% heat-inactivated fetal bovine serum (FBS;
Japan Bioserum Co., Ltd., Nagoya, Japan) at 37°C in a humidified
atmosphere of 5% CO2. H1299 cells were maintained in
RPMI-1640 medium (Gibco®; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 1% penicillin/streptomycin and
10% heat-inactivated FBS at 37°C in a humidified atmosphere of 5%
CO2.
Cells were seeded in 35-mm culture dishes
(6.0×104 cells) or 60-mm culture dishes
(1.2×105 cells; Iwaki, Chiba, Japan) and were cultured
overnight to promote their adherence to the dish. On the next day,
the cells were treated with RLR agonist poly(I:C)-LMW or
poly(I:C)-HMW (125–1,000 ng/ml) for indicated time periods. Next,
the cells were harvested using 0.1%
trypsin-ethylenediaminetetraacetic acid (Gibco®; Gibco;
Thermo Fisher Scientific, Inc.), and the number of viable cells was
counted using trypan blue dye exclusion assay.
In some experiments, the cells were preincubated
with 50 µM Z-VAD-fmk (a pan-caspase inhibitor) for 1 h, followed by
treatment with 250 ng/ml poly(I:C)-HMW.
Clonogenic survival assay
Cells were seeded in 60-mm culture dishes and were
cultured overnight. On the next day, 250 ng/ml poly(I:C)-HMW were
added to the culture medium before 1 h irradiation, and then
exposed to X-ray. After X-ray irradiation, the cells were incubated
for 14 h in the presence of poly(I:C)-HMW. Next, the cells were
washed twice with a fresh medium and their culture medium was
replaced with a fresh medium. After replacement, the cells were
incubated for 8–11 days. Next, the cells were fixed with methanol
and were stained with Giemsa solution (Wako Pure Chemical
Industries, Ltd., Osaka, Japan). Colonies containing >50 cells
were counted. The surviving fraction at each dose was calculated
with respect to the plating efficiency of the non-irradiated
control. The survival curves were fitted to a linear-quadratic
model: SF=exp (−αD-βD2), where SF is the
surviving fraction and D is the physical dose, by data
analysis software Origin Pro 9.0J (OriginLab Co., Northampton, MA,
USA).
siRNA transfection
A549 cells were transfected with target siRNAs or
control siRNA by using Lipofectamine RNAiMAX (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. The final concentration of the siRNAs was 10 nM.
After incubation for 24 h, the transfected cells were harvested and
were used for performing subsequent analyses.
In vitro irradiation
The cells were irradiated (150 kVp, 20 mA, 0.5-mm Al
filter, and 0.3-mm Cu filter) by using an X-ray generator
(MBR-1520R-3; Hitachi Medical Corporation, Tokyo, Japan) at a
distance of 45 cm from the focus and at a dose rate of 1.00–1.04
Gy/min.
Sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and western blotting
Harvested cells were lysed in 1X Laemmli sample
buffer (Bio-Rad Laboratories, Inc., Hercules, CA, USA) containing
2.5% 2-mercaptoethanol through sonication, and the obtained cell
lysates were boiled for 10 min. Protein concentration of the cell
lysates was determined using XL-Bradford assay kit (APRO Science,
Tokushima, Japan) and SmartSpec™ plus spectrophotometer (Bio-Rad
Laboratories, Inc.). Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and western blotting were performed, as reported
previously (12). The following
primary antibodies were used: anti-RIG-I antibody (dilution,
1:3,000), anti-MDA5 antibody (dilution, 1:3,000), anti-MAVS
antibody (dilution, 1:3,000), anti-caspase-3 antibody (dilution,
1:3,000), and anti-actin antibody (dilution, 1:4,000). The
following secondary antibody was used: HRP-linked anti-rabbit IgG
antibody (dilution, 1:10,000). Antigens were visualized using ECL
Prime Western Blotting Detection System (GE Healthcare UK Ltd.,
Buckinghamshire, UK). Blots were stripped using a Stripping
Solution (Wako Pure Chemical Industries, Ltd.).
Analysis of cell death
Cell death was analyzed using Annexin V-FITC
(BioLegend Inc., San Diego, CA, USA), PI, and Annexin V binding
buffer (BioLegend Inc.), as reported previously (13). Stained cells were analyzed by
performing flow cytometry (Cytomics FC500; Beckman Coulter, Inc.,
Brea, CA, USA). In the Annexin V/PI quadrant gating, Annexin
V−/PI−, Annexin V+/PI−,
and Annexin V+/PI+ were used to identify the
fraction of viable cells, early apoptotic cells, and late
apoptotic/necrotic cells, respectively.
Detection of γ-H2AX by performing flow
cytometry
Harvested cells were fixed overnight in ice-cold 70%
methanol at −20°C. The fixed cells were washed using a wash buffer
(WB; PBS containing 0.5% bovine serum albumin) and were treated
with a WB containing 0.25% Triton X-100 on ice for 5 min. After
washing with the WB, cell pellets were incubated for 1 h at room
temperature with anti-phosphorylated histone H2AX monoclonal
antibody (JBW301; Upstate Biotechnology, Lake Placid, NY, USA)
diluted to 300-folds by using the WB containing 0.25% Triton X-100.
The labeled cells were washed with the WB and were treated in the
dark for 1 h at room temperature with Alexa Fluor
488®-conjugated anti-mouse IgG secondary antibody
(Molecular Probes; Thermo Fisher Scientific, Inc.) diluted to
400-folds by using the WB containing 0.25% Triton X-100. The
stained cells were washed with the WB and were analyzed by
performing flow cytometry.
Statistical analysis
Data are presented as mean ± standard error (SE).
Comparisons between control and experimental groups were performed
using a two-sided Student's t-test or a two-sided Mann-Whitney's
U-test depending on the data distribution. Multiple data were
analyzed using one-factor analysis of variance followed by
Tukey-Kramer test. Differences were considered significant at
P<0.05. All statistical analyses were performed using Excel 2010
software (Microsoft Corporation, Redmond, WA, USA) with an add-in
software Statcel 3 (The Publisher OMS Ltd., Tokyo, Japan).
Results
RLR agonists exert cytotoxicity
against NSCLC cells
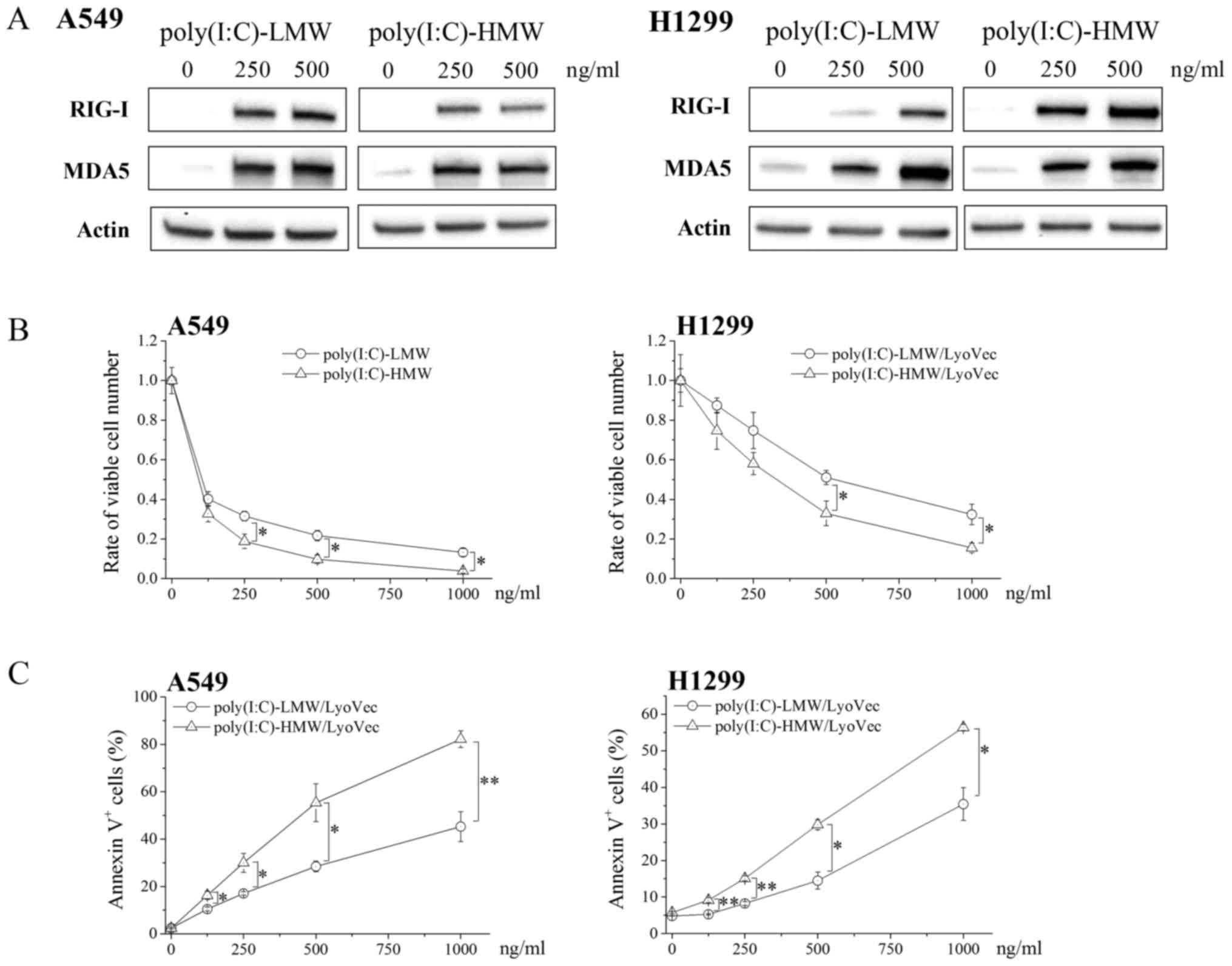
We first examined the cytotoxicity of RLR agonists
against A549 and H1299 cells. In the present study,
poly(I:C)-LyoVec™, a complex of a synthetic dsRNA analogue
poly(I:C) and transfection reagent LyoVec™, was used as an RLR
agonist. RIG-I and MDA5 expression was undetectable or negligible
in untreated A549 and H1299 cells and strongly increased in RLR
agonists poly(I:C)-LMW- and poly(I:C)-HMW-treated A549 and H1299
cells (Fig. 1A). Moreover,
poly(I:C)-LMW and poly(I:C)-HMW treatment significantly decreased
the number of viable cells and increased the percentages of Annexin
V+ cells in a dose-dependent manner (Fig. 1B and C, respectively). Because
poly(I:C)-HMW exerted a stronger cytotoxic effect than
poly(I:C)-LMW, we used poly(I:C)-HMW in all subsequent
experiments.
Effects of the RLR agonist and
ionizing radiation cotreatment on cytotoxicity against NSCLC
cells
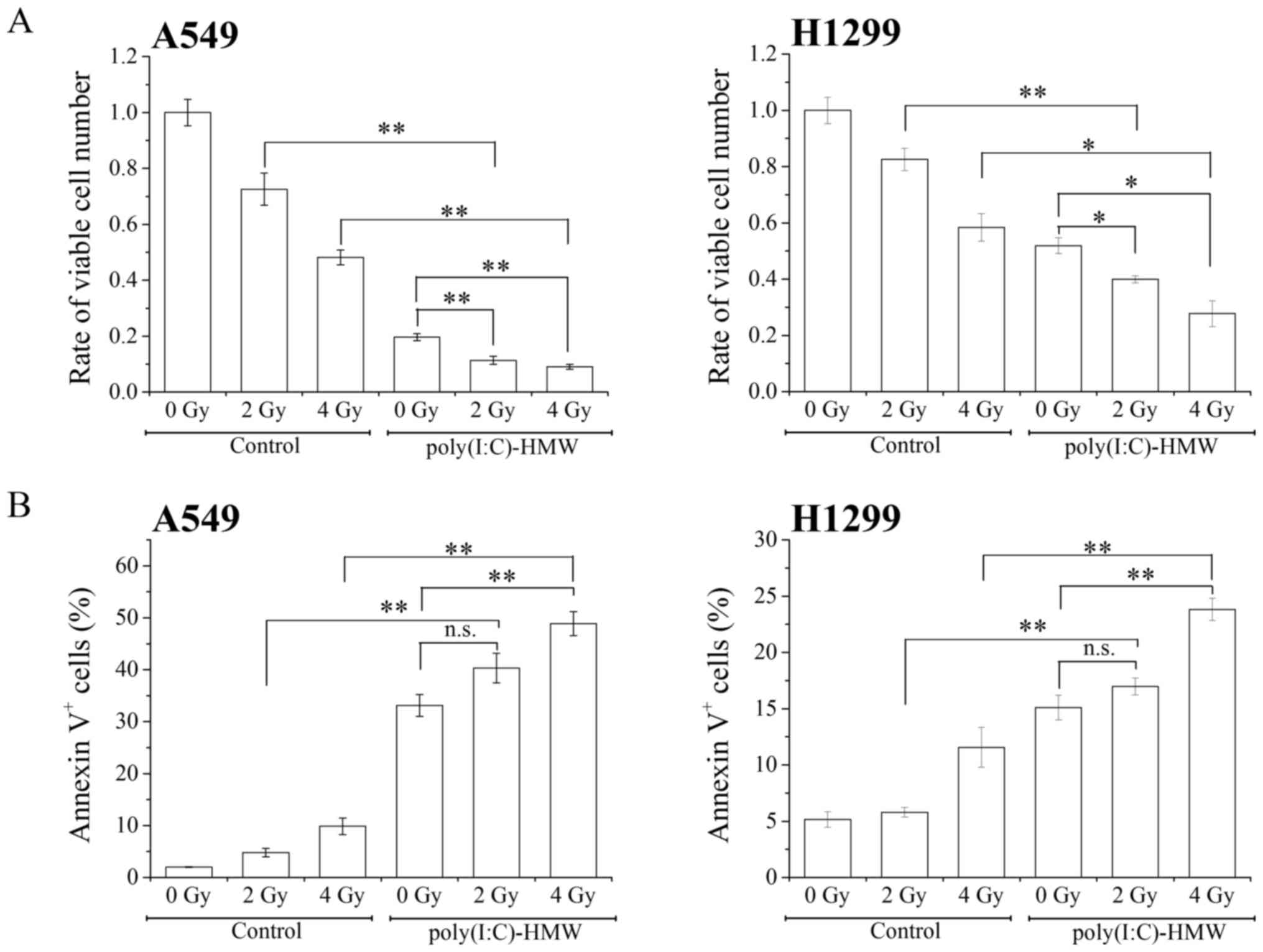
We next examined the effect of the RLR agonist and
ionizing radiation cotreatment on cytotoxicity against NSCLC cells.
Cotreatment with poly(I:C)-HMW and X-ray irradiation (2 and 4 Gy)
significantly decreased the number of viable cells compared with
treatment with poly(I:C)-HMW or X-ray irradiation alone (Fig. 2A). Furthermore, cotreatment with
poly(I:C)-HMW and 4 Gy X-ray irradiation increased the percentages
of Annexin V+ cells compared with treatment with
poly(I:C)-HMW or X-ray irradiation alone (Fig. 2B). These results indicate that the RLR
agonist and ionizing radiation cotreatment effectively induced the
cytotoxicity against NSCLC cells.
Radiosensitizing effects of the RLR
agonist on NSCLC cells
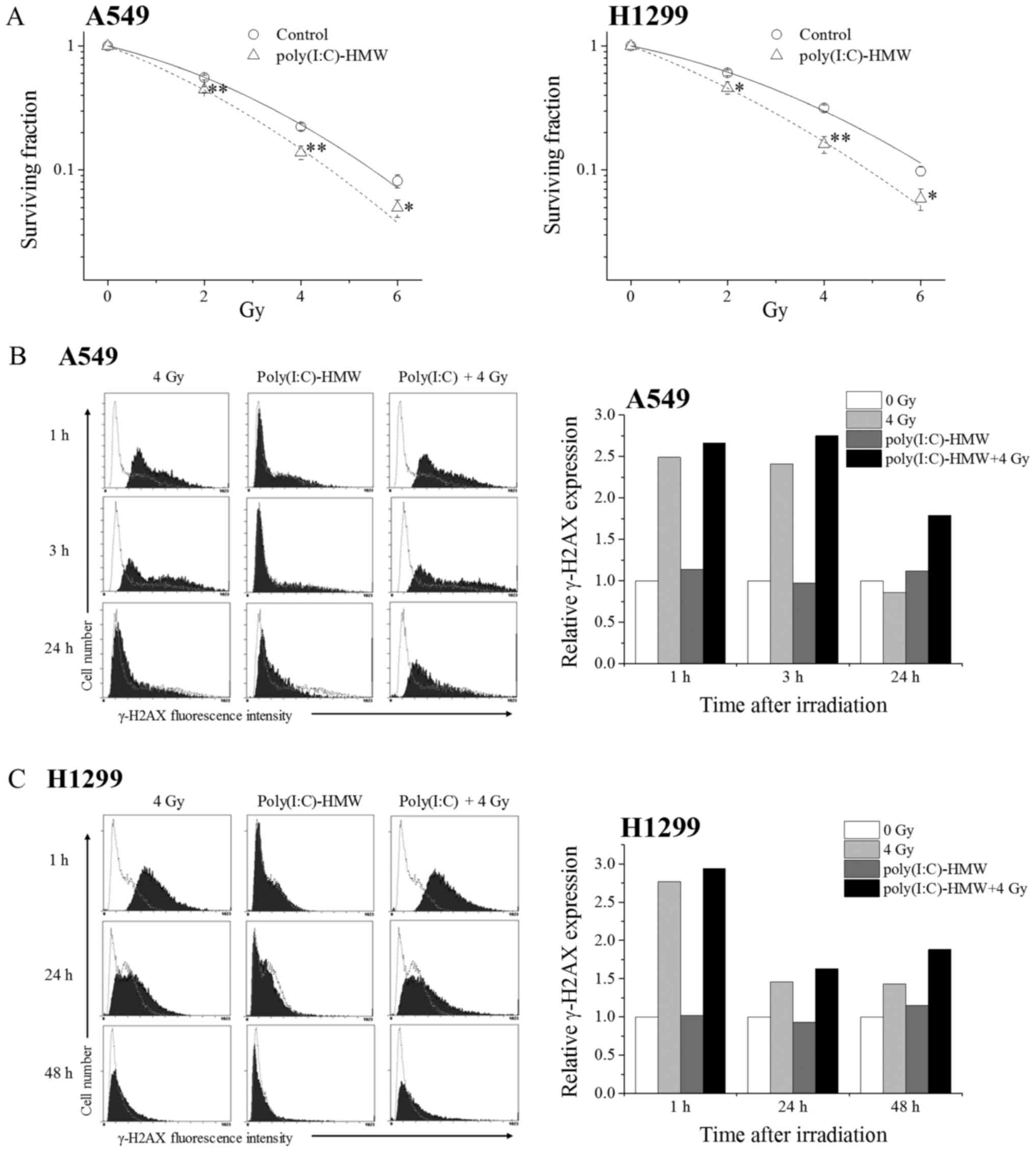
We next examined the radiosensitizing effects of
poly(I:C)-HMW on NSCLC cells. Clonogenic cell survival decreased in
irradiated A549 and H1299 cells pretreated with poly(I:C)-HMW
(Fig. 3A). As shown in Table I, the radiation dose that 37% of cells
will survival (D37) was reduced from 3.02 Gy in the
control to 2.35 Gy by the treatment with poly(I:C)-HMW in A549 cell
(P<0.01). Similarly, the D37 was reduced from 3.47 Gy
in the control to 2.46 Gy by the treatment with poly(I:C)-HMW in
H1299 cells (P<0.05). The sensitizer enhancement ratio (SER)
judged by the D37 in the A549 and H1299 cells were 1.28
and 1.41, respectively. These results suggest that the RLR agonist
exerted radiosensitizing effects on NSCLC cells.
 | Table I.Summary of survival curve
parameters. |
Table I.
Summary of survival curve
parameters.
| Cell | Treatment | α
(Gy−1) | β
(Gy−2) | D37
(Gy) | SER
(D37) |
|---|
| A549 | Control | 0.216 | 0.037 | 3.02 |
|
|
| Poly(I:C)-HMW | 0.341 | 0.034 | 2.35 | 1.28 |
| H1299 | Control | 0.182 | 0.030 | 3.47 |
|
|
| Poly(I:C)-HMW | 0.339 | 0.026 | 2.46 | 1.41 |
To investigate mechanisms underlying the
radiosensitizing effects of poly(I:C)-HMW, we analyzed the repair
of DNA double-stranded breaks (DSBs). Histone H2AX undergoes
phosphorylation at serine 139 (γ-H2AX) immediately after DSB
induction and undergoes dephosphorylation after DSB repair
(14). Therefore, we analyzed γ-H2AX
expression in NSCLC cells treated with 4 Gy X-ray irradiation
and/or poly(I:C)-HMW. Treatment with the ionizing irradiation
increased γ-H2AX expression in A549 cells irrespective of
poly(I:C)-HMW treatment (Fig. 3B). No
significant difference was observed in γ-H2AX expression between
cells treated with 4 Gy X-ray irradiation alone and cells treated
with poly(I:C)-HMW + 4 Gy X-ray irradiation at 1 and 3 h after the
irradiation. However, γ-H2AX expression was higher in cells treated
with poly(I:C)-HMW + 4 Gy X-ray irradiation than in cells treated
with 4 Gy X-ray irradiation alone at 24 h after the irradiation.
Similarly, γ-H2AX expression was higher in H1299 cells treated with
poly(I:C)-HMW + 4 Gy X-ray irradiation than in cells treated with 4
Gy X-ray irradiation alone at 48 h after the irradiation (Fig. 3C). These results suggest that
poly(I:C)-HMW inhibits DSB repair in irradiated cells, which leads
to their radiosensitization.
Effects of the RLR agonist and
ionizing radiation cotreatment on RLR and MAVS expression
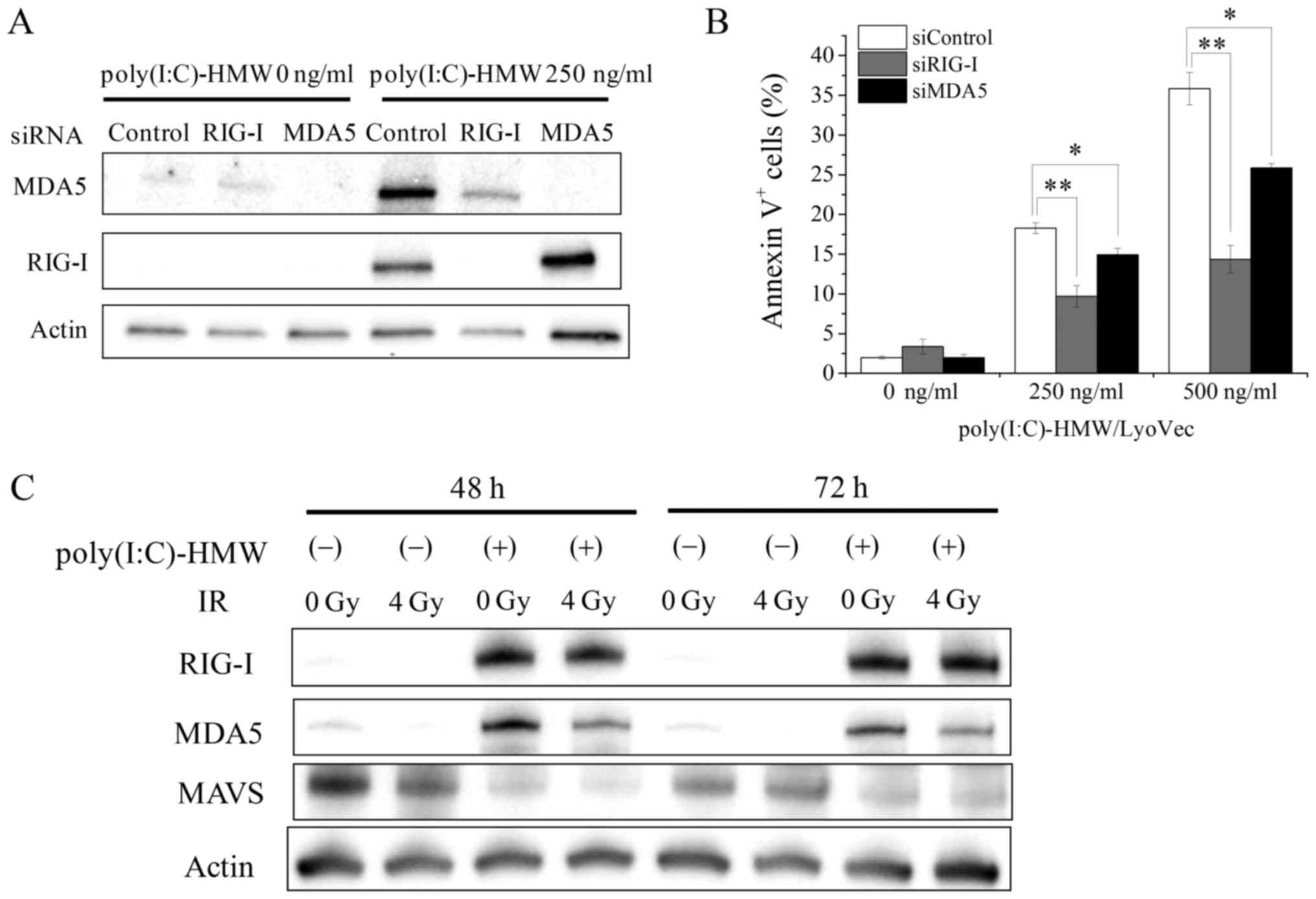
We next explored mechanisms underlying the effect of
poly(I:C)-HMW and ionizing radiation cotreatment on cell death
induction in A549 and H1299 cells. Knockdown of RIG-I and MDA5 in
A549 cells decreased poly(I:C)-HMW-induced RIG-I and MDA5
expression (Fig. 4A) and
significantly decreased poly(I:C)-HMW-induced Annexin V+
cells (Fig. 4B). Because these
results suggested that RLRs mediated poly(I:C)-HMW-induced cell
death, we hypothesized that the effects of poly(I:C)-HMW and
ionizing radiation cotreatment on cell death induction were induced
by the upregulation of RLR expression. However, ionizing radiation
did not increase poly(I:C)-HMW-induced increase in RIG-I and MDA5
expression (Fig. 4C). Next, we
analyzed the protein expression of MAVS, which functions as an
adaptor protein for RLR-mediated signaling pathways (15). It is reported that proteasome-mediated
MAVS degradation occurs after RLR activation, and this degradation
is required for downstream signaling leading to type I IFN
production (16). Consistently, we
observed that poly(I:C)-HMW treatment downregulated MAVS protein
expression in A549 cells. However, no significant difference in
MAVS protein expression was observed between cells treated with
poly(I:C)-HMW alone and cells treated with poly(I:C)-HMW + 4 Gy
X-ray irradiation (Fig. 4C).
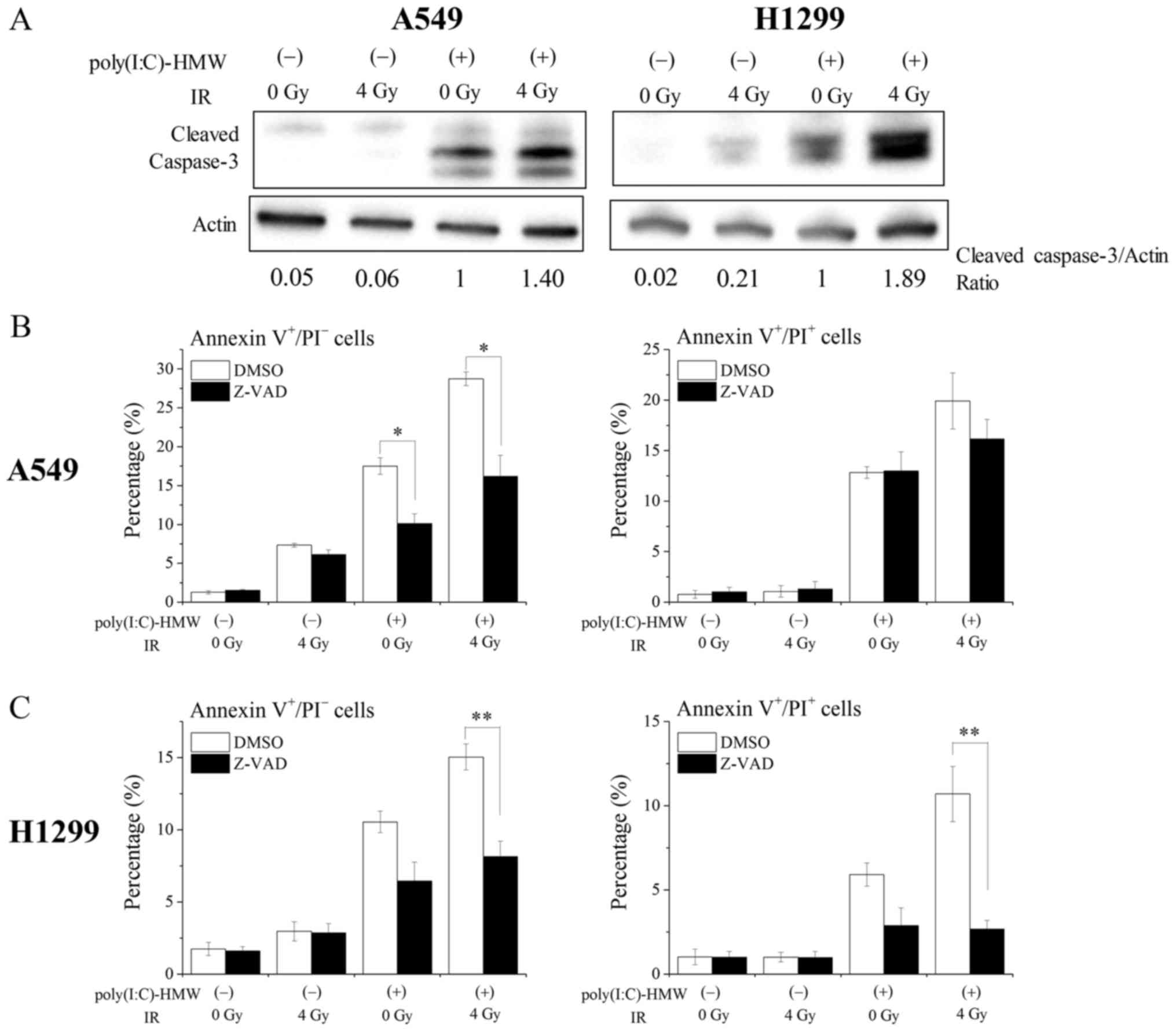
The RLR agonist and ionizing radiation
cotreatment effectively induce apoptosis by activating caspase
Caspases are involved in RLR-induced apoptosis
(7). As shown in Fig. 5A, poly(I:C)-HMW induced active
caspase-3 expression in A549 and H1299 cells (Fig. 5A). Furthermore, treatment with the
pan-caspase inhibitor Z-VAD-fmk significantly decreased
poly(I:C)-HMW-induced Annexin V+/PI− early
apoptotic cells in A549 cells (Fig.
5B). Similarly, Z-VAD-fmk tended to decrease Annexin
V+/PI− early apoptotic cells in H1299 cells
(P=0.052). These results indicate that RLR agonists induce
apoptosis in human NSCLC cells by activating caspases. Next, we
investigated the involvement of caspases in the effects of the RLR
agonist and ionizing radiation cotreatment. Cotreatment with
poly(I:C)-HMW and 4 Gy X-ray irradiation increased active caspase-3
expression compared with treatment with poly(I:C)-HMW or 4 Gy X-ray
irradiation alone (Fig. 5A).
Furthermore, the percentages of Annexin
V+/PI− early apoptotic cells in A549 and
H1299 cells were significantly decreased by treatment with
Z-VAD-fmk (Fig. 5B and C). The
percentages of Annexin V+/PI+ late apoptotic
cells/necrotic cells in H1299 cells, not A549 cells, were also
significantly decreased by treatment with Z-VAD-fmk (Fig. 5C). Together, these results suggest
that the effects of the RLR agonist and ionizing radiation
cotreatment on cell death induction including apoptosis are
mediated by caspase activation.
Discussion
We previously investigated the effect of ionizing
radiation on PRRs in human monocytic cells (9,10) and
showed that ionizing radiation negligibly affected RLR expression
and response to their agonists (10),
suggesting that RLR agonists could be used as effective
immunostimulants during radiation therapy. In the present study, we
investigated the effects of RLR agonists and ionizing radiation
cotreatment on cytotoxicity against NSCLC cells. We found that
cotreatment with poly(I:C)-HMW and ionizing radiation effectively
suppressed the growth of and induced cell death in NSCLC cells.
Furthermore, we found that poly(I:C)-HMW treatment exerted
radiosensitizing effects on NSCLC cells probably by affecting DSB
repair capacity. Although we did not determine mechanisms
underlying the attenuation of DSB repair by the activation of
mitochondria-mediated immune systems in the present study, our
results showed that cotreatment with the RLR agonist and ionizing
radiation is a promising strategy to enhance cytotoxicity against
NSCLC cells.
We found that poly(I:C)-HMW exerted a stronger
cytotoxic effect than poly(I:C)-LMW against NSCLC cells. The
difference between poly(I:C)-LMW and poly(I:C)-HMW is a molecular
weight. According to the manufacture's data sheet, the average size
of poly(I:C)-LMW and poly(I:C)-HMW is 0.2–1 and 1.5–8 kb,
respectively. It is thought that poly(I:C)-LMW and poly(I:C)-HMW
are mainly recognized by RIG-I and MDA5, respectively, because
RIG-I and MDA5 preferentially recognizes short (~0.3 kb) and long
(>4 kb) poly(I:C), respectively (4). These imply that MDA5 may be a better
target for inducing cytotoxicity than RIG-I. However, knockdown of
RIG-I effectively suppressed poly(I:C)-HMW-induced cell death
compared with knockdown of MDA5 (Fig.
4B), thus suggesting that not only MDA5 but also RIG-I are
involved in the poly(I:C)-HMW induced cell death. Interestingly, we
found that knockdown of RIG-I downregulated poly(I:C)-HMW-induced
MDA5 expression as well as RIG-I expression (Fig. 4A). Consistently, Imaizumi et al
reported that poly(I:C) transfection induced MDA5 expression
through RIG-I and IFN-β (17).
Therefore, it is possible that RIG-I mediates the
poly(I:C)-HMW-induced MDA5 expression, which contributes to the
poly(I:C)-HMW-induced cell death.
There are some researches to improve the
RLR-mediated antitumor activity. For example, some researcher
focused on the recognition of 5′-triphosphate single-stranded RNAs
by RIG-I, and designed the 5′-triphosphate-siRNA (8,18,19). Yuan et al designed a
5′-triphosphate-siRNA targeting vascular endothelial growth factor
(VEGF). The 5′-triphosphate-siRNA targeting VEGF showed multiple
antitumor effects against NSCLC through not only induction of
RIG-mediated apoptosis and antitumor immunity but also inhibition
of tumor angiogenesis by knockdown of VEGF (8). On the other hand, Duewell et al
reported a cotreatment with RLR agonists and an activating
monoclonal antibody for death receptor Fas on cytotoxicity. In
their report, it was demonstrated that RLR agonists increased the
cell surface Fas expression, which sensitized pancreatic cancer
cells towards Fas-mediated cell killing (20). In the present study, we focused on the
ionizing radiation to improve RLR agonist-induced cytotoxicity, and
showed that cotreatment with RLR agonist and ionizing radiation
effectively induced the cytotoxicity against cancer cells for the
first time.
Recently, Ranoa et al showed that depletion
of RIG-I protected mice from death following to total body
irradiation (21). They also showed
that ionizing radiation increased the RIG-I expression, not MDA5
expression, and that RIG-I recognize small endogenous noncoding RNA
induced by ionizing radiation, which resulted in the activation of
IFN signaling through MAVS. Considering that we did not observe the
enhancement of RIG-I and MDA5 expression by 4 Gy X-ray irradiation
in the presence or absence and poly(I:C)-HMW (Fig. 4C), there is a possibility that the
poly(I:C)-HMW-induced RIG-I recognizes small endogenous noncoding
RNA induced by ionizing radiation, which effectively increases the
poly(I:C)-HMW-induced cell death. However, we need to investigate
the RLR/MAVS-mediated signaling pathway in the cells cotreated with
RLR agonist and ionizing radiation in detail in a future study,
because the difference in the MAVS expression between poly(I:C)-HMW
and poly(I:C)-HMW + 4 Gy X-ray irradiation was not observed in the
present study (Fig. 4C).
RLR activation induces type I IFN. The type I IFN
such as IFN-β is known to improve the response of tumor to ionizing
radiation (22–24). For example, type I IFN affects the
efficacy of radiotherapy through activation of immune cells
(23). Furthermore, the
radiosensitizing effects of exogenous IFN-β was reported (24). Therefore, it is possible that the
radiosensitizing effect of poly(I:C)-HMW and the effects of
poly(I:C)-HMW and ionizing radiation cotreatment on cytotoxicity
are due to the induction of type I IFN. However, because it is
reported that RLR/MAVS signaling pathway induces apoptosis in a
IFN-independent manner (7,25,26), we
need a further study to clarify whether the effects of RLR agonist
and ionizing radiation cotreatment on cytotoxicity depends on the
type I IFN.
Results of the present study suggested the
involvement of caspase activation in the effects of the RLR agonist
and ionizing radiation cotreatment on cytotoxicity against NSCLC
cells. It is reported that RLR activation induces apoptosis via
intrinsic (caspase-9) and/or extrinsic (caspase-8) apoptotic
pathway. Besch et al reported that caspase-9 plays important
roles in RLR-induced apoptosis in human melanoma cells (7). On the other hand, the activation of MDA5
by Semliki Forest virus induces both caspase-9-mediated apoptosis
and caspase-8-mediated extrinsic apoptosis (27). Since ionizing radiation can modulate
the intrinsic and extrinsic apoptotic pathway (28–31),
further studies are needed to clarify the apoptotic pathway which
RLR agonist and ionizing radiation cooperatively activate.
In conclusion, results of the present study showed
that cotreatment with ionizing radiation and the RLR agonist
effectively induced cytotoxicity against human NSCLC cells,
suggesting that RLR activations effectively enhanced the apoptosis
and radiosensitivity of cancer cells and improved antitumor
immunity during radiation therapy. However, because treatment with
poly(I:C)-LMW (1 µg/ml) induces apoptosis in human hematopoietic
stem/progenitor cells (32), further
studies are needed to investigate the effects of cotreatment with
the RLR agonist and ionizing radiation on cytotoxicity against
normal cells and to determine a strategy for minimizing its side
effects.
Acknowledgements
This study was supported by JSPS KAKENHI (grant no.
JP25861053) and was partially supported by JSPS KAKENHI (grant no.
JP15K09985) and Takeda Science Foundation [HY].
References
|
1
|
Duchen MR: Mitochondria in health and
disease: Perspectives on a new mitochondrial biology. Mol Aspects
Med. 25:365–451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pourcelot M and Arnoult D: Mitochondrial
dynamics and the innate antiviral immune response. FEBS J.
281:3791–3802. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsumiya T and Stafforini DM: Function
and regulation of retinoic acid-inducible gene-I. Crit Rev Immunol.
30:489–513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kato H, Takeuchi O, Mikamo-Satoh E, Hirai
R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T and Akira
S: Length-dependent recognition of double-stranded ribonucleic
acids by retinoic acid-inducible gene-I and melanoma
differentiation-associated gene 5. J Exp Med. 205:1601–1610. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kato H, Takeuchi O, Sato S, Yoneyama M,
Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al:
Differential roles of MDA5 and RIG-I helicases in the recognition
of RNA viruses. Nature. 441:101–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li K, Qu S, Chen X, Wu Q and Shi M:
Promising targets for cancer immunotherapy: TLRs, RLRs, and
STING-mediated innate immune pathways. Int J Mol Sci. 18:pii:
E4042017. View Article : Google Scholar
|
|
7
|
Besch R, Poeck H, Hohenauer T, Senft D,
Häcker G, Berking C, Hornung V, Endres S, Ruzicka T, Rothenfusser S
and Hartmann G: Proapoptotic signaling induced by RIG-I and MDA-5
results in type I interferon-independent apoptosis in human
melanoma cells. J Clin Invest. 119:2399–2411. 2009.PubMed/NCBI
|
|
8
|
Yuan D, Xia M, Meng G, Xu C, Song Y and
Wei J: Anti-angiogenic efficacy of 5′-triphosphate siRNA combining
VEGF silencing and RIG-I activation in NSCLCs. Oncotarget.
6:29664–29674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshino H, Chiba K, Saitoh T and
Kashiwakura I: Ionizing radiation affects the expression of
Toll-like receptors 2 and 4 in human monocytic cells through c-Jun
N-terminal kinase activation. J Radiat Res. 55:876–884. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshino H, Saitoh T, Kozakai M and
Kashiwakura I: Effects of ionizing radiation on retinoic
acid-inducible gene-I-like receptors. Biomed Rep. 3:59–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsai MF, Wang CC and Chen JJ: Tumour
suppressor HLJ1: A potential diagnostic, preventive and therapeutic
target in non-small cell lung cancer. World J Clin Oncol.
5:865–873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshino H, Kumai Y and Kashiwakura I:
Effects of endoplasmic reticulum stress on apoptosis induction in
radioresistant macrophages. Mol Med Rep. 15:2867–2872. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukushi S, Yoshino H, Yoshizawa A and
Kashiwakura I: p53-independent structure-activity relationships of
3-ring mesogenic compounds' activity as cytotoxic effects against
human non-small cell lung cancer lines. BMC Cancer. 16:5212016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jacobs JL and Coyne CB: Mechanisms of MAVS
regulation at the mitochondrial membrane. J Mol Biol.
425:5009–5019. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Castanier C, Zemirli N, Portier A, Garcin
D, Bidère N, Vazquez A and Arnoult D: MAVS ubiquitination by the E3
ligase TRIM25 and degradation by the proteasome is involved in type
I interferon production after activation of the antiviral
RIG-I-like receptors. BMC Biol. 10:442012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Imaizumi T, Aizawa-Yashiro T, Tsuruga K,
Tanaka H, Matsumiya T, Yoshida H, Tatsuta T, Xing F, Hayakari R and
Satoh K: Melanoma differentiation-associated gene 5 regulates the
expression of a chemokine CXCL10 in human mesangial cells:
Implications for chronic inflammatory renal diseases. Tohoku J Exp
Med. 228:17–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poeck H, Besch R, Maihoefer C, Renn M,
Tormo D, Morskaya SS, Kirschnek S, Gaffal E, Landsberg J, Hellmuth
J, et al: 5′-Triphosphate-siRNA: Turning gene silencing and
Rig-Iactivation against melanoma. Nat Med. 14:1256–1263. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li D, Gale RP, Liu Y, Lei B, Wang Y, Diao
D and Zhang M: 5′-Triphosphate siRNA targeting MDR1 reverses
multi-drug resistance and activates RIG-I-induced
immune-stimulatory and apoptotic effects against human myeloid
leukaemia cells. Leuk Res. 58:23–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duewell P, Steger A, Lohr H, Bourhis H,
Hoelz H, Kirchleitner SV, Stieg MR, Grassmann S, Kobold S, Siveke
JT, et al: RIG-I-like helicases induce immunogenic cell death of
pancreatic cancer cells and sensitize tumors toward killing by
CD8(+) T cells. Cell Death Differ. 21:1825–1837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ranoa DR, Parekh AD, Pitroda SP, Huang X,
Darga T, Wong AC, Huang L, Andrade J, Staley JP, Satoh T, et al:
Cancer therapies activate RIG-I-like receptor pathway through
endogenous non-coding RNAs. Oncotarget. 7:26496–26515. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Widau RC, Parekh AD, Ranck MC, Golden DW,
Kumar KA, Sood RF, Pitroda SP, Liao Z, Huang X, Darga TE, et al:
RIG-I-like receptor LGP2 protects tumor cells from ionizing
radiation. Proc Natl Acad Sci USA. 111:pp. E484–E491. 2014;
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burnette BC, Liang H, Lee Y, Chlewicki L,
Khodarev NN, Weichselbaum RR, Fu YX and Auh SL: The efficacy of
radiotherapy relies upon induction of type i interferon-dependent
innate and adaptive immunity. Cancer Res. 71:2488–2496. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmidberger H, Rave-Fränk M, Lehmann J,
Schweinfurth S, Rehring E, Henckel K and Hess CF: The combined
effect of interferon beta and radiation on five human tumor cell
lines and embryonal lung fibroblasts. Int J Radiat Oncol Biol Phys.
43:405–412. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lei Y, Moore CB, Liesman RM, O'Connor BP,
Bergstralh DT, Chen ZJ, Pickles RJ and Ting JP: MAVS-mediated
apoptosis and its inhibition by viral proteins. PLoS One.
4:e54662009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu CY, Chiang RL, Chang TH, Liao CL and
Lin YL: The interferon stimulator mitochondrial antiviral signaling
protein facilitates cell death by disrupting the mitochondrial
membrane potential and by activating caspases. J Virol.
84:2421–2431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El Maadidi S, Faletti L, Berg B, Wenzl C,
Wieland K, Chen ZJ, Maurer U and Borner C: A novel mitochondrial
MAVS/Caspase-8 platform links RNA virus-induced innate antiviral
signaling to Bax/Bak-independent apoptosis. J Immunol.
192:1171–1183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verbrugge I, de Vries E, Tait SW, Wissink
EH, Walczak H, Verheij M and Borst J: Ionizing radiation modulates
the TRAIL death-inducing signaling complex, allowing bypass of the
mitochondrial apoptosis pathway. Oncogene. 27:574–584. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim MJ, Lee KH and Lee SJ: Ionizing
radiation utilizes c-Jun N-terminal kinase for amplification of
mitochondrial apoptotic cell death in human cervical cancer cells.
FEBS J. 275:2096–2108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maier P, Hartmann L, Wenz F and Herskind
C: Cellular pathways in response to ionizing radiation and their
targetability for tumor radiosensitization. Int J Mol Sci. 17:pii:
E1022016. View Article : Google Scholar
|
|
31
|
Kim JY, An YM, Choi WH, Kim JM, Cho S, Yoo
BR, Kang JW, Lee YS, Lee YJ and Cho J: Pro-apoptotic Noxa is
involved in ablative focal irradiation-induced lung injury. J Cell
Mol Med. 21:711–719. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Guo YM, Hirokawa M, Iwamoto K,
Ubukawa K, Michishita Y, Fujishima N, Tagawa H, Takahashi N, Xiao
W, et al: A synthetic double-stranded RNA, poly I:C, induces a
rapid apoptosis of human CD34(+) cells. Exp Hematol. 40:330–341.
2012. View Article : Google Scholar : PubMed/NCBI
|