Introduction
Hepatocellular carcinoma (HCC) is the most common
type of primary liver malignancy (1).
Currently, HCC ranks as the third leading cause of
cancer-associated mortality worldwide and the sixth leading cause
of human cancer (2–4). During the past few decades, a number of
HCC treatments have been developed. However, the therapeutic
effects are not optimal due to tumor recurrence, metastasis and, in
particular, a low survival rate (5).
Therefore, novel treatment options based on an improved
understanding of the molecular mechanisms of HCC need to be
investigated.
MicroRNAs (miRNAs) are a class of small non-coding
RNA molecules (20–24 nucleotides), processed as miRNA precursors
with a hairpin structure (6–8). The specific involvement of miRNAs in
different tumor types and associations with prognosis are of
interest. miRNAs are highly conserved and tissue-specific. Previous
studies have shown that miRNA expression varies in different types
of cancer, indicating that miRNAs may be involved not only in
tissue function and cell specificity, but also in complex gene
regulation (9–11).
In recent years, a plethora of studies have
suggested that dysregulation of miRNA-32 (miR-32) is associated
with tumor progression and poor prognosis in different types of
tumors. miR-32 has been shown to be upregulated in colorectal
cancer (CRC) (12), renal cell
carcinoma (13) and prostate cancer
(14), and downregulated in non-small
cell lung cancer (NSCLC) (15),
osteosarcoma (16), gastric cancer
(17) and oral squamous cell
carcinoma (18). Yan et al
(19) reported that miR-32 levels
were upregulated in HCC tissue, and cell proliferation, invasion
and migration were upregulated by targeting phosphatase and tensin
homolog (PTEN). These results indicate that miR-32 may be a
potential biomarker for HCC diagnosis. However, the clinical
relevance and function of miR-32 in HCC remains elusive.
The present study aimed to investigate the
expression levels of miR-32 in HCC tissues by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). In
addition, the association of miR-32 and patient clinicopathological
characteristics was analyzed.
Materials and methods
Ethics statement
The present study was approved by the Ethics
Committee of Jilin University (approval no. 20151101; Jilin,
China). Each patient provided written informed consent prior to
participation in the present study.
miRNA expression in HCC from Gene
Expression Omnibus (GEO) datasets
miRNA expression levels were investigated in HCC
tissues and normal tissue samples in the GEO datasets using the
NCBI Platform (http://www.ncbi.nlm.nih.gov/). A total of three
original datasets were downloaded (GEO accession no. GSE31383,
GSE21362 and GSE22058), and differentially expressed miRNAs in HCC
samples and adjacent non-tumor tissue were analyzed. Fold-change
(FC) ≥2 or ≤0.5 and P<0.01 were used as basic screening
parameters for cluster analysis. Hierarchical clustering was
performed using the Multiple Experiment Viewer (MeV) 4.7.1 software
program (http://www.tm4.org/).
Clinical specimens
A total of 154 HCC primary tumor tissues obtained
from patients with HCC (123 male, 30 female and 1 unknown) with a
median age of 65.4 years (range, 45–81 years), who underwent
surgical resection between July 2004 and October 2013 at
China-Japan Union Hospital, Jilin University (Changchun, China),
were analyzed. In total, 33 HCC samples were paired with adjacent
non-tumor tissues. No patient received chemotherapy or radiotherapy
prior to surgery. Follow-up data and statistics were recorded for
all patients until September 2014.
RNA extraction and RT-qPCR for
miRNA
Total RNA was isolated from HCC and normal tissue
specimens using TRIzol reagent (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol. RNA
concentration was determined using a Nanodrop 1000
spectrophotometer (Thermo Fisher Scientific, Inc.), and purity was
identified in 1.5% denaturing agarose gels. TaqMan probe-based qPCR
was carried out using TaqMan MicroRNA Reverse Transcription kit
(no. 4366597) and Universal Master Mix II (no. 4440048) (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
protocol of the manufacturer. The specific primers are as follows:
for hsa-miR-32, forward, 5′-GCACATTCATCATACACGCCG-3′ and reverse,
5′-GACCACTGAGGTTAGAGCCA-3′; for U6, forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′. Thermocycling conditions for RT
reaction were as follows: Initial reaction at 16°C for 30 min,
followed by 42°C for 30 min, 85°C for 5 min, with a final reaction
at 4°C. Thermocycling conditions for PCR were as follows: Initial
denaturation at 94°C for 10 min, followed by 35 cycles of 94°C for
30 sec, 60°C for 30 sec and 72°C for 30 sec, with a final extension
at 72°C for 10 min. Each reaction was independently tested in
duplicate a minimum of three times. U6 small nuclear RNA was used
as an internal control, and the 2−ΔΔCq method (20) was used to analyze miR-32 expression
levels.
Statistical analysis
All statistical analyses were performed using the
IBM SPSS statistical software (version 22.0; IBM SPSS, Armonk, NY,
USA). χ2 test and t-test were used to examine the
associations between clinical characteristics and miR-32
expression. Overall survival (OS) curves were constructed using
Kaplan-Meier survival analysis, and results were compared using the
log-rank test. In order to estimate independent prognostic factors
associated with survival, univariate and multivariate survival
analyses were performed using the Cox regression model. P<0.05
was considered to indicate a statistically significant
difference.
Results
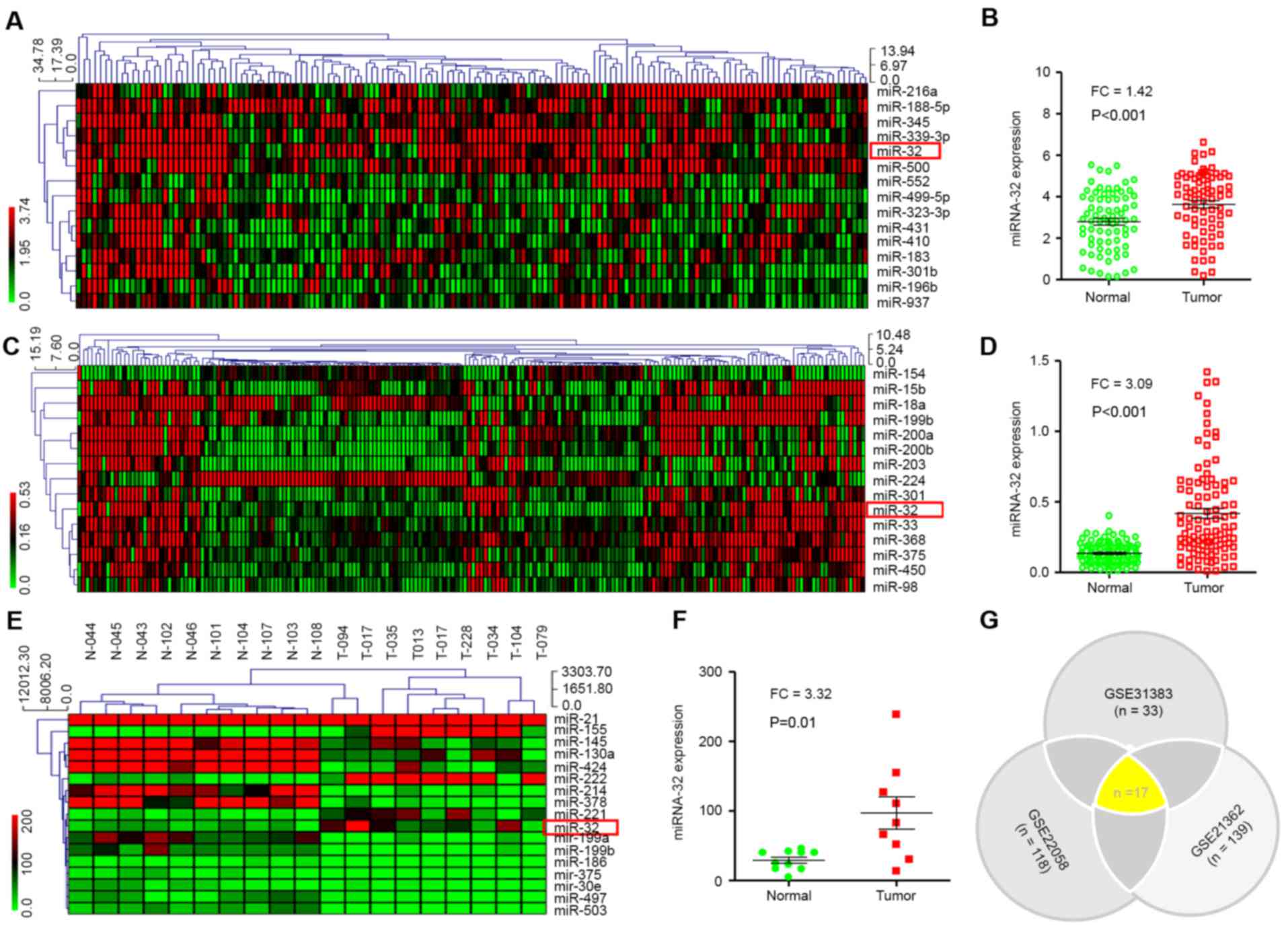
Cluster analysis of miRNA expression
in HCC using MEV4.7.1 software
miRNA expression was investigated in HCC and normal
tissues (n=73). Raw data were retrieved using the search terms
‘GSE#21362’ in the GEO dataset. A total of 15 different miRNAs with
P<0.01 and FC ≥1.4 were identified. The MEV4.7.1 clustering
software was used to analyze 15 different miRNAs (Fig. 1A). The analysis indicated that miR-32
expression in HCC tissues was significantly higher compared with
non-tumor tissues (P<0.001; Fig.
1B).
Next, the raw data were downloaded from the GEO
database (GEO accession no. GSE22058). In the present study, the
genome-wide expression profiles of miRNAs from paired tumor and
adjacent non-tumor tissues from a cohort of 96 patients with HCC in
Hong Kong were evaluated. A total of 15 dysregulated miRNAs were
screened out using FC ≥3 or ≤0.3 and P<0.01 (Fig. 1C). It was revealed that miR-32
expression was also significantly higher in HCC compared with
non-tumor tissues (P<0.001; Fig.
1D).
The same method was then used to analyze the
‘GSE31383’ GEO dataset (Fig. 1E).
Approximately 57 miRNAs were tested in HCC samples (n=9) and normal
tissue (n=10). A total of 17 dysregulated miRNAs were screened with
P<0.01. miR-32 expression in HCC tissue was significantly higher
(P=0.01) compared with non-tumor tissue (Fig. 1F).
Altogether, differentially expressed miRNAs were
screened using three different raw datasets (GEO accession no.
GSE31383, GSE22058 and GSE21362). Notably, 17 miRNAs were
identified, including miR-32, which were common in all datasets
(P<0.01; Fig. 1G). These results
indicated that miR-32 is increased in HCC compared with non-tumor
tissues, and therefore miR-32 may be a potential biomarker for
diagnosis of HCC.
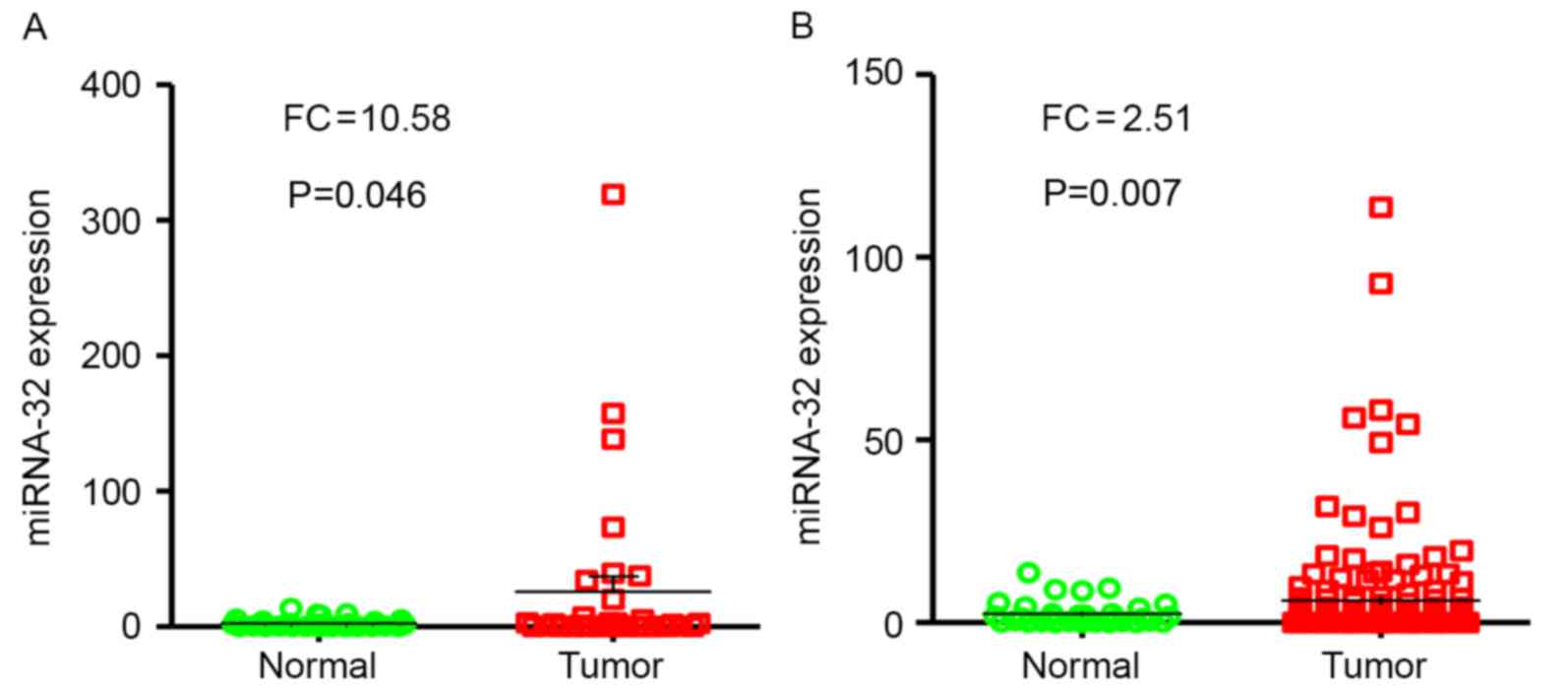
Increased expression of miR-32 in
HCC
miR-32 expression was evaluated in tumor samples
(n=33) compared with adjacent non-tumor tissues (n=33) by RT-qPCR.
Results of the present study revealed that miR-32 expression levels
were significantly higher in HCC tumor specimens compared with
adjacent non-neoplastic tissues (P<0.05; FC, 10.58; Fig. 2A).
miR-32 expression levels were examined in tumor
samples (n=154), and levels of expression were compared with paired
adjacent non-tumor tissues (n=33) from patients with HCC using
RT-qPCR. Notably, results demonstrated that the levels of miR-32
expression were significantly higher in HCC tumor specimens
compared with normal tissues (P=0.007; FC, 2.51; Fig. 2B).
Association between miR-32 expression
and clinicopathological characteristics of patients with HCC
The association between miR-32 expression levels and
the individual clinicopathological characteristics were evaluated
in patients with HCC. As shown in Table
I, miR-32 expression was positively associated with the number
and diameter of foci (P<0.05). However, no significant
association was observed between miR-32 expression and other
clinical characteristics, including age, sex and tumor
differentiation (P>0.05).
 | Table I.miR-32 expression and
clinicopathological characteristics of patients with hepatocellular
carcinoma. |
Table I.
miR-32 expression and
clinicopathological characteristics of patients with hepatocellular
carcinoma.
|
|
| miR-32
expression |
| Overall survival,
months |
|
|---|
|
|
|
|
|
|
|
|---|
| Factor | n | Low | High | P-value | Mean | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
≥60 | 84 | 42 | 42 | 0.996 | 45.98 | 39.11–52.84 | 0.472 |
|
<60 | 70 | 35 | 35 |
| 37.97 | 32.42–43.52 |
|
| Sex |
|
|
|
|
|
|
|
|
Male | 123 | 61 | 62 | 0.957 | 41.33 | 37.14–47.54 | 0.101 |
|
Female | 30 | 15 | 15 |
| 42.46 | 31.48–51.44 |
|
|
Unknown |
1 | 1 | 0 |
|
|
|
|
| Tumor
differentiation |
|
|
|
|
|
|
|
|
Poor | 11 | 4 | 7 | 0.541 | 20.91 | 5.77–36.04 | 0.015 |
|
Good | 125 | 65 | 60 |
| 44.42 | 39.30–49.55 |
|
|
Unknown | 18 | 8 | 10 |
|
|
|
|
| Tumor diameter,
cm |
|
|
|
|
|
|
|
| ≥5 | 58 | 21 | 37 | 0.012 | 36.51 | 30.79–42.22 | 0.009 |
|
<5 | 96 | 56 | 40 |
| 46.01 | 39.63–52.39 |
|
| Number of foci |
|
|
|
|
|
|
|
|
Multiple | 81 | 32 | 49 | 0.022 | 34.41 | 27.86–40.96 | 0.014 |
|
Single | 67 | 39 | 28 |
| 51.96 | 46.57–57.34 |
|
|
Unknown |
6 | 6 | 0 |
| – | – |
|
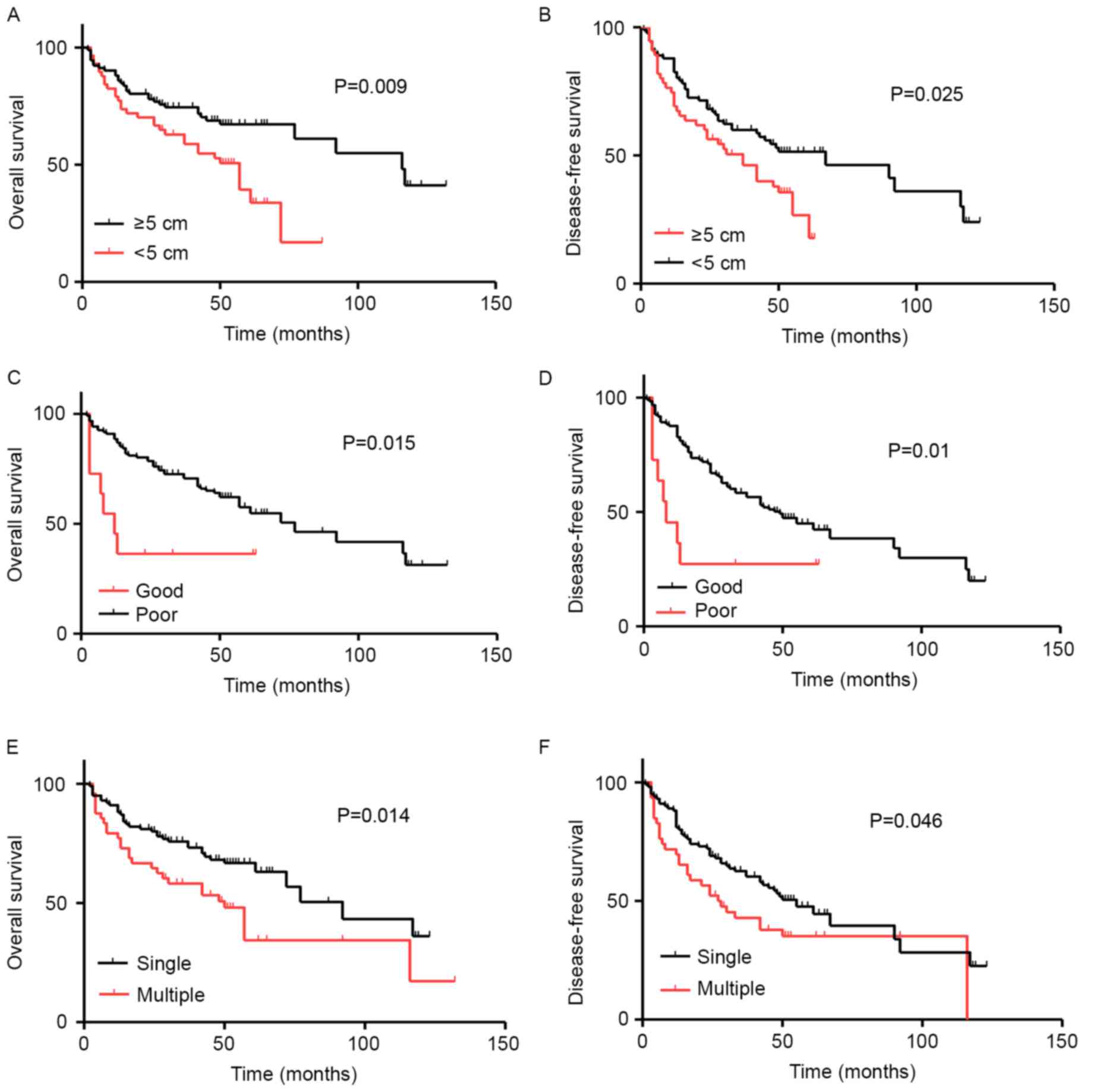
Association between clinical
characteristics and HCC prognosis
In order to analyze whether clinical factors,
including sex, age, diameter, tumor differentiation and number of
foci affect HCC prognosis, Kaplan-Meier survival curves were
plotted and compared using a log-rank test (Table I). It was observed that tumor diameter
was significantly associated with decreased duration of OS
(P=0.009; Fig. 3A) and disease-free
survival (DFS) (P=0.025; Fig. 3B) in
patients with HCC. In addition, tumor differentiation was
significantly associated with decreased duration of OS (P=0.015;
Fig. 3C) and DFS (P=0.01; Fig. 3D) in patients with HCC. Similar
results were obtained regarding the number of foci and duration of
OS (P=0.014; Fig. 3E) and DFS
(P=0.046; Fig. 3F).
As shown in Table II,
univariate analysis using the Cox regression model revealed that
miR-32 expression levels [hazard ratio (HR)=2.51; confidence
interval (CI): 1.48–4.27; P=0.001], number of foci (HR=36.78; CI:
8.98–150.68; P<0.001) and tumor diameter (HR=1.95; CI:
1.17–3.24; P=0.011) were positively associated with poor prognosis
(P<0.05). However, clinicopathological characteristics,
including age, sex and tumor differentiation exhibited no
association with HCC prognosis (P>0.05).
 | Table II.Cox regression model analysis for OS
based on the clinicopathological characteristics of patients with
hepatocellular carcinoma. |
Table II.
Cox regression model analysis for OS
based on the clinicopathological characteristics of patients with
hepatocellular carcinoma.
| A, Univariate
analysis |
|---|
|
|---|
| Factor | HR | 95% CI | P-value |
|---|
| Sex (male vs.
female) | 0.526 | 0.24–1.16 | 0.111 |
| Age, years (≥60 vs.
<60) | 1.21 | 0.42–1.08 | 0.481 |
| Tumor
differentiation (poorly vs. moderately/well) | 0.9 | 0.60–1.36 | 0.632 |
| Tumor diameter, cm
(≥5 vs. <5) | 1.95 | 1.17–3.24 | 0.011 |
| Number of foci
(multiple vs. single) | 36.78 |
8.98–150.68 | <0.001 |
| miR-32 expression
(high vs. low) | 2.51 | 1.48–4.27 | 0.001 |
|
| B, Multivariate
analysis |
|
| Factor | HR | 95% CI | P-value |
|
| Sex |
|
|
|
| Age, years |
|
|
|
| Tumor
differentiation |
|
|
|
| Tumor diameter,
cm | 4.47 | 2.13–9.35 | <0.001 |
| Number of foci | 4.63 | 2.20–9.76 | <0.001 |
| miR-32
expression |
|
|
|
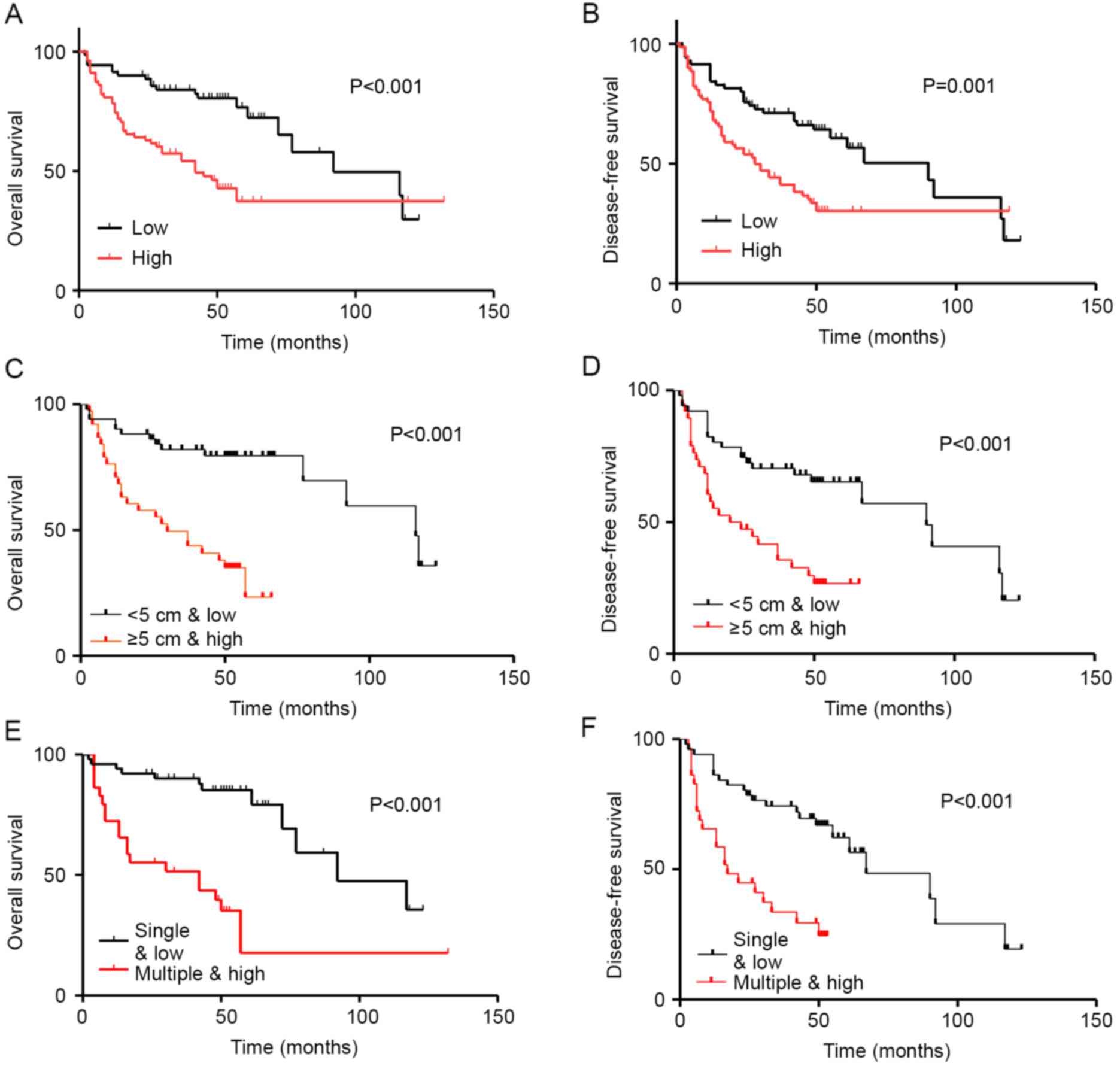
miR-32 upregulation is a prognostic
marker for survival in patients with HCC
To determine the prognostic value of miR-32
expression in HCC, Kaplan-Meier survival analysis was used to
evaluate the associations between miR-32 expression and OS and DFS.
The results revealed that high miR-32 expression was associated
with poorer OS, whereas low miR-32 mRNA levels were associated with
increased OS. Therefore, miR-32 expression was significantly
associated with decreased duration of OS (P<0.001; Fig. 4A) and DFS (P=0.001; Fig. 4B) in patients with HCC.
Kaplan-Meier survival analysis indicated that
patients with HCC with high miR-32 expression and large tumor size
(≥5 cm) had significantly decreased duration of Kaplan-Meier
survival analysis indicated that patients with HCC with high miR-32
expression and large tumor size (≥5 cm) had significantly decreased
duration of OS (P<0.001; Fig. 4C)
and DFS (P=0.001; Fig. 4D). In
addition, low miR-32 expression and multiple tumor foci were
associated with poorer OS and DFS (Fig.
4E and F).
Discussion
HCC ranks as the sixth most common type of cancer
worldwide. HCC treatment includes radical surgery or chemotherapy,
but the postoperative recurrence rate is high (21). Therefore, the prognosis of HCC
currently remains unsatisfactory. The toxicity of chemotherapeutic
drugs and cancer-associated tumor resistance are matters of
concern, and improvements in therapy are required. In the past few
years, the development of new molecular biology techniques have
enabled targeted HCC therapy (22).
miRNAs are regarded as ideal biomarkers, since the diagnostic and
therapeutic sensitivity of miRNAs is associated with clinical
outcome compared with other biomarkers, including metabolites,
antibodies and nucleic acids (23–25).
Recently, a study confirmed that the differences in
miRNA expression levels in HCC tissues compared with normal tissues
are not only statistically significant, but also associated with
HCC diagnosis, prognosis and treatment (26). Li et al (27) reported that six different miRNAs were
significantly upregulated in HCC samples compared with non-tumor
tissues. Notably, it was revealed that miR-375 alone exhibited
diagnostic value for HCC (27).
Sukata et al (28)
demonstrated that miR-98, let-7f and let-7a are potential early
diagnostic markers for liver cancer. Pineau et al (29) revealed that downregulation of miR-221
in HCC performs an important role in tumorigenesis and drug
resistance by inducing apoptosis.
miR-32 is able to function as a tumor suppressor and
as a tumor promoter in different types of cancer (30). Due to the tissue specificity of
miR-32, its biological function has been thoroughly studied. miR-32
functions as a tumor suppressor in osteosarcoma and NSCLC (16,31). In
another study, Wu et al (12,32)
reported that miR-32 is also involved in development of CRC, in
part, due to suppression of PTEN. Furthermore, upregulation of
miR-32 was associated with specific clinicopathological
characteristics of patients with CRC. Therefore, miR-32 is
considered to be a putative molecular marker of poor prognosis in
patients with CRC.
Next, raw datasets from the GEO database were
analyzed. In the raw datasets, it was revealed that miR-32
expression levels were significantly higher in HCC tumor specimens
compared with non-neoplastic tissues (33–35).
Indeed, using FC ≥2 or ≤0.5 and P<0.01 for screening in cluster
analysis revealed that miR-32 was a common denominator of the three
datasets. In a series of in vitro experiments performed by
Yan et al (19), it was
reported that miR-32 induces cell migration, proliferation and
invasion in HCC by targeting PTEN. However, the clinical relevance
between miR-32 and HCC currently remains unknown.
In the present study, it was revealed that miR-32
expression in HCC samples was significantly higher compared with
normal tissues. The association between miR-32 and the clinical
parameters was also examined in patients with HCC. High miR-32
expression was associated with tumor diameter and the number of
foci, indicating that the upregulation of miR-32 has a crucial role
in the progression of liver cancer. In order to investigate the
potential prognostic value of miR-32, the association between the
expression of miR-32 and OS in patients with HCC was analyzed.
Kaplan-Meier survival analysis revealed that patients with high
miR-32 expression levels had decreased OS time. Furthermore, Cox
analysis demonstrated that miR-32 was an independent prognostic
indicator of HCC. Therefore, levels of miR-32 expression may be an
optimal indicator and risk factor for reduced OS and DFS in
patients with HCC.
In summary, the present study demonstrated the
clinical and prognostic significance of miR-32 in HCC. High miR-32
expression may be an optimal indicator of poor HCC prognosis.
Altogether, the present analysis indicated that high miR-32
expression may be a potential biomarker for patients with HCC.
Acknowledgements
This study was supported partly by grants from the
National Natural Science Foundation of China (grant nos. 81201535,
81302065, 81472202, 81772932, 81472209 and 81702243), Jilin
Provincial Science and Technology Department (grant no.
20140414061GH), Shanghai Natural Science Foundation (grant nos.
12ZR1436000 and 16ZR1428900) and Shanghai Municipal Commission of
Health and Family Planning (grant nos. 201440398 and 201540228).
The funding agencies had no role in study design, data collection
and analysis, decision to publish or preparation of the
manuscript.
References
|
1
|
Wang Y, Ma Y, Fang Y, Wu S, Liu L, Fu D
and Shen X: Regulatory T cell: A protection for tumor cells. J Cell
Mol Med. 16:425–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. Ca Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fang Y, Fu D, Tang WQ, Cai Y, Ma D, Wang
HJ, Xue RY, Liu TT, Huang XW, Dong L, et al: Ubiquitin C-terminal
hydrolase 37, a novel predictor for hepatocellular carcinoma
recurrence, promotes cell migration and invasion via interacting
and deubiquitinating PRP19. Biochim Biophys Acta. 1833:559–572.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen YJ, Ma YS, Fang Y, Wang Y, Fu D and
Shen XZ: Power and promise of ubiquitin carboxyl-terminal hydrolase
37 as a target of cancer therapy. Asian Pac J Cancer Prev.
14:2173–2179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu LL, Fu D, Ma Y and Shen XZ: The power
and the promise of liver cancer stem cell markers. Stem Cells Dev.
20:2023–2030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou LK, Ma YS, Han Y, Lu GX, Luo P, Chang
ZY, Xie RT, Yang HQ, Chai L, Cai MX, et al: Association of
microRNA-33a molecular signature with non-small cell lung cancer
diagnosis and prognosis after chemotherapy. PLoS One.
12:e01704312017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho WC: OncomiRs: The discovery and
progress of microRNAs in cancers. Mol Cancer. 6:602007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jay C, Nemunaitis J, Chen P, Fulgham P and
Tong AW: miRNA profiling for diagnosis and prognosis of human
cancer. DNA Cell Biol. 26:293–300. 1007. View Article : Google Scholar
|
|
12
|
Wu W, Yang J, Feng X, Wang H, Ye S, Yang
P, Tan W, Wei G and Zhou Y: MicroRNA-32 (miR-32) regulates
phosphatase and tensin homologue (PTEN) expression and promotes
growth, migration, and invasion in colorectal carcinoma cells. Mol
Cancer. 12:302013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang Y, Fu D and Shen XZ: The potential
role of ubiquitin C-terminal hydrolases in oncogenesis. Biochim
Biophys Acta. 1806:1–6. 2010.PubMed/NCBI
|
|
14
|
Jalava SE, Urbanucci A, Latonen L,
Waltering KK, Sahu B, Jänne OA, Seppälä J, Lähdesmäki H, Tammela TL
and Visakorpi T: Androgen-regulated miR-32 targets BTG2 and is
overexpressed in castration-resistant prostate cancer. Oncogene.
31:4460–4471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bai Y, Wang YL, Yao WJ, Guo L, Xi HF, Li
SY and Zhao BS: Expression of miR-32 in human non-small cell lung
cancer and its correlation with tumor progression and patient
survival. Int J Clin Exp Pathol. 8:824–829. 2015.PubMed/NCBI
|
|
16
|
Xu JQ, Zhang WB, Wan R and Yang YQ:
MicroRNA-32 inhibits osteosarcoma cell proliferation and invasion
by targeting Sox9. Tumor Biol. 35:9847–953. 2014. View Article : Google Scholar
|
|
17
|
Zhang J, Kuai X, Song M, Chen X, Yu Z,
Zhang H and Mao Z: microRNA32 inhibits the proliferation and
invasion of the SGC-7901 gastric cancer cell line in vitro. Oncol
Lett. 7:270–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang D, Ni Z and Xu X: Decreased
microRNA-32 in oral squamous cell carcinoma. Med Sci Monit.
20:2527–2535. 2014.PubMed/NCBI
|
|
19
|
Yan SY, Chen MM, Li GM, Wang YQ and Fan
JG: miR-32 induces cell proliferation, migration, and invasion in
hepatocellular carcinoma by targeting PTEN. Tumor Biol.
36:4747–4755. 2015. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang Y, Mu J, Ma Y, Ma D, Fu D and Shen X:
The interaction between ubiquitin C-terminal hydrolase 37 and
glucose-regulated protein 78 in hepatocellular carcinoma. Mol Cell
Biochem. 359:59–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma YS, Wu TM, Lv ZW, Lu GX, Cong XL, Xie
RT, Yang HQ, Chang ZY, Sun R, Chai L, et al: High expression of
miR-105-1 positively correlates with clinical prognosis of
hepatocellular carcinoma by targeting oncogene NCOA1. Oncotarget.
8:11896–11905. 2017.PubMed/NCBI
|
|
23
|
Wu SD, Ma YS, Fang Y, Liu LL, Fu D and
Shen XZ: Role of the microenvironment in hepatocellular carcinoma
development and progression. Cancer Treat Rev. 38:218–225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ge S and Huang D: Systemic therapies for
hepatocellular carcinoma. Drug Discov Ther. 9:352–362. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bartels CL and Tsongalis GJ: MicroRNAs:
Novel biomarkers for human cancer. Ann Biol Clin (Paris).
68:263–272. 2010.(In French). PubMed/NCBI
|
|
26
|
Ma Y, Hou L, Yu F, Lu G, Qin S, Xie R,
Yang H, Wu T, Luo P, Chai L, et al: Incidence and physiological
mechanism of carboplatin-induced electrolyte abnormality among
patients with non-small cell lung cancer. Oncotarget.
8:18417–18423. 2017.PubMed/NCBI
|
|
27
|
Li LM, Hu ZB, Zhou ZX, Chen X, Liu FY,
Zhang JF, Shen HB, Zhang CY and Zen K: Serum microRNA profiles
serve as novel biomarkers for HBV infection and diagnosis of
HBV-positive hepatocarcinoma. Cancer Res. 70:9798–9807. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sukata T, Sumida K, Kushida M, Ogata K,
Miyata K, Yabushita S and Uwagawa S: Circulating microRNAs,
possible indicators of progress of rat hepatocarcinogenesis from
early stages. Toxicol Lett. 200:46–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pineau P, Volinia S, McJunkin K, Marchio
A, Battiston C, Terris B, Mazzaferro V, Lowe SW, Croce CM and
Dejean A: miR-221 overexpression contributes to liver
tumorigebesis. Proc Natl Acad Sci USA. 107:pp. 264–269. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Zhu Y, Ma Y, Wang J, Zhang F, Xia
Q and Fu D: The role of cancer stem cells in cancer metastasis: New
perspective and progress. Cancer Epidemiol. 37:60–63. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu D, Chen H, Yang X, Chen W, Wang L, Xu
J and Yu L: miR-32 functions as a tumor suppressor and directly
targets SOX9 in human non-small cell lung cancer. Onco Targets
Ther. 8:1773–1783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu W, Yang P, Feng X, Wang H, Qiu Y, Tian
T, He Y, Yu C, Yang J, Ye S and Zhou Y: The relationship between
and clinical significance of MicroRNA-32 and phosphatase and tensin
homologue expression in colorectal cancer. Genes Chromosomes
Cancer. 52:1133–1140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Xu T, Jiang Y, Xu H, Yan Y, Fu D
and Chen J: The challenges and the promise of molecular targeted
therapy in malignant gliomas. Neoplasia. 17:239–255. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sato F, Hatano E, Kitamura K, Myomoto A,
Fujiwara T, Takizawa S, Tsuchiya S, Tsujimoto G, Uemoto S and
Shimizu K: MicroRNA profile predicts recurrence after resection in
patients with hepatocellular carcinoma within the Milan Criteria.
PLoS One. 6:e164352011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burchard J, Zhang C, Liu AM, Poon RT, Lee
NP, Wong KF, Sham PC, Lam BY, Ferguson MD, Tokiwa G, et al:
microRNA-122 as a regulator of mitochondrial metabolic gene network
in hepatocellular carcinoma. Mol Syst Biol. 6:4022010. View Article : Google Scholar : PubMed/NCBI
|