Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer and the third leading cause of
cancer-associated mortality worldwide (1), and the incidence continues to increase
in numerous countries (2). In the
majority of cases, patients have a background of chronic liver
disease leading to liver cirrhosis, which is the main risk factor
for the development of HCC (3,4).
Currently, surgical resection and liver transplantation are the
only curative treatment options (5).
Transarterial chemoembolization (TACE) is a
minimally invasive treatment that is frequently used to reduce
tumor burden in inoperable situations or as bridging therapy prior
to transplantation. Although TACE may permit local tumor control
and increase survival time in patients with intermediate HCC
(Barcelona Clinic Liver Cancer stage B) (6), there is evidence that TACE enhances
angiogenesis in HCC (6,7). While hypoxia, which occurs during TACE
due to ischemia, is known to contribute to angiogenesis, little is
known about the undesirable effects of chemotherapeutic agents on
residual HCC cells subsequent to TACE (8).
The anthracycline doxorubicin is one of the most
commonly used drugs in TACE (9). Its
main mechanisms of action are intercalation into DNA, inhibition of
topoisomerase II and generation of reactive oxygen species (ROS),
inducing apoptotic pathways (10,11). While
a large proportion of doxorubicin is eliminated from the body
unchanged, the main pathway of doxorubicin metabolism is
two-electron reduction by cytosolic reductases, of which carbonyl
reductase 1 is the most important in the liver (10). However, doxorubicin resistance in HCC
cells is predominantly associated with the expression of adenosine
triphosphate-binding cassette (ABC) transporters such as ABCB1
(multi-drug resistance gene; MDR1) or ABCC1 (multidrug
resistance-associated protein 1; MRP1) (10,12–19).
Previous studies concerning the drug resistance of
HCC cells have used doxorubicin-resistant cell lines that were
generated through constant exposure to rising levels of doxorubicin
(13,20–22). By
contrast, the aim of the present study was to analyze the effects
of single-step doxorubicin treatment on surviving HCC cells in
vitro, mimicking the situation of HCC cells surviving TACE
treatment.
Materials and methods
Cells and cell culture
HCC HepG2 (cat. no. HB-8065) and Hep3B (cat. no.
HB-8064; American Type Culture Collection, Manassas, VA, USA) cell
lines were cultured as described previously (23). Briefly, cells were maintained in
high-glucose Dulbecco's modified Eagle's medium (DMEM)
(Sigma-Aldrich, Taufkirchen, Germany) supplemented with penicillin
(400 U/ml), streptomycin (50 µg/ml), L-glutamine (300 µg/ml) and
10% fetal calf serum (FCS; Sigma-Aldrich; Merck Millipore,
Deisenhofen, Germany) and were passaged at a 1:5 ratio every 3
days. To select cells that survive treatment with a defined
doxorubicin dose (1 µM), HepG2 and Hep3B cells were incubated with
doxorubicin for 48 h. Subsequently, medium was removed, the cell
culture dishes were carefully washed with PBS to remove dead cells,
and surviving HCC cells (HCCsurv) were further cultured
in normal, doxorubicin-free medium. Control cells
(HCCctr) were continuously cultured in normal medium
without doxorubicin. Subsequently, HCCsurv and
HCCctr cells were cultured in parallel and were split
when they became confluent. In the two HCCsurv cell
lines this occurred after 1 week. Subsequent to splitting,
HCCsurv cells were further cultured and regularly
passaged in parallel with HCCctr cells for another 2
weeks.
Microscope images were captured using an Olympus™
CKX41 microscope (Olympus Corporation, Tokyo Japan) with the ALTRA
20 Soft Imaging System™ and CellA software version 2.6
(Olympus Soft Imaging Solutions GmbH, Münster, Germany). Images
were processed using IrfanView™ software version 4.36 (Irfan
Skiljan, Jajce, Bosnia).
Analysis of cell viability and
proliferation
Cells were seeded in 6-well plates (200,000/well) or
96-well plates (30,000/well), respectively. After 24 h, analysis of
lactate dehydrogenase (LDH) secretion into the supernatant
(Cytotoxicity Detection Kit PLUS; Roche Diagnostics GmbH, Mannheim,
Germany) and a colorimetric XTT assay (Roche Diagnostics GmbH) were
used to analyze the viability of HCC cells subsequent to treatment
with doxorubicin as described (24).
Cell proliferation was assessed using the xCELLigence impedance
measurement system (Roche Diagnostics GmbH) according to the
manufacturer's protocol.
Analysis of cell migration
The migratory activity of HCC cells was quantified
using Cultrex 96-Well Cell Migration assay (Trevigen, Gaithersburg,
MD, USA) as described (25). Briefly,
HCC cells were seeded into the upper compartment of the provided
96-well micropore plate (10,000 cells/well) in DMEM. The lower
compartment was filled with DMEM to study spontaneous cell
migration. Subsequent to incubation at 37°C for 5 h, cell migration
was quantified by fluorometry with an EMax Microplate Reader (MWG
Biotech, Ebersberg, Germany).
Analysis of mRNA expression
Total cellular RNA was isolated from
doxorubicin-treated and control HepG2 and Hep3B cells using the
RNeasy Kit (Qiagen GmbH, Hilden, Germany) according to the
manufacturer's instructions. Reverse transcription was performed as
described previously (24).
Quantitative polymerase chain reaction was performed using a
LightCycler Real-Time PCR System (Roche Diagnostics) (24). In each well, 2 µl of cDNA template was
added to 8 µl master mix containing primers and SYBR Green (Bioline
GmbH, Luckenwalde, Germany). Melting, annealing and amplification
were performed at 95°C (5 sec), 58°C (10 sec) and 72°C (8 sec),
respectively and repeated for 45 cycles. ABCB1, ABCC1 and SNAIL
mRNA expression were analyzed using QuantiTect Primer assays
according to the manufacturer's protocol (Qiagen GmbH, Hilden,
Germany). Amplification of cDNA derived from 18S rRNA was used for
normalization (24), with the
following primer sequences: Forward, 5′-AAACGGCTACCACATCCAAG-3′,
and reverse, 5′-CCTCCAATGGATCCTCGTTA-3′. Results were evaluated
using the 2−ΔΔCq method (26). Analyses were performed in triplicates
and experiments were repeated three times.
Statistical analysis
Values are presented as the mean ± standard error of
the mean. Comparison between groups was made using the unpaired
Student's t-test or two-way analysis of variance. P<0.05
was considered to indicate a statistically significant difference.
All calculations were performed using the statistical computer
package GraphPad Prism version 6.01 for Windows (GraphPad Software,
Inc., La Jolla, CA, USA).
Results
Selection of HCC cells surviving
doxorubicin treatment
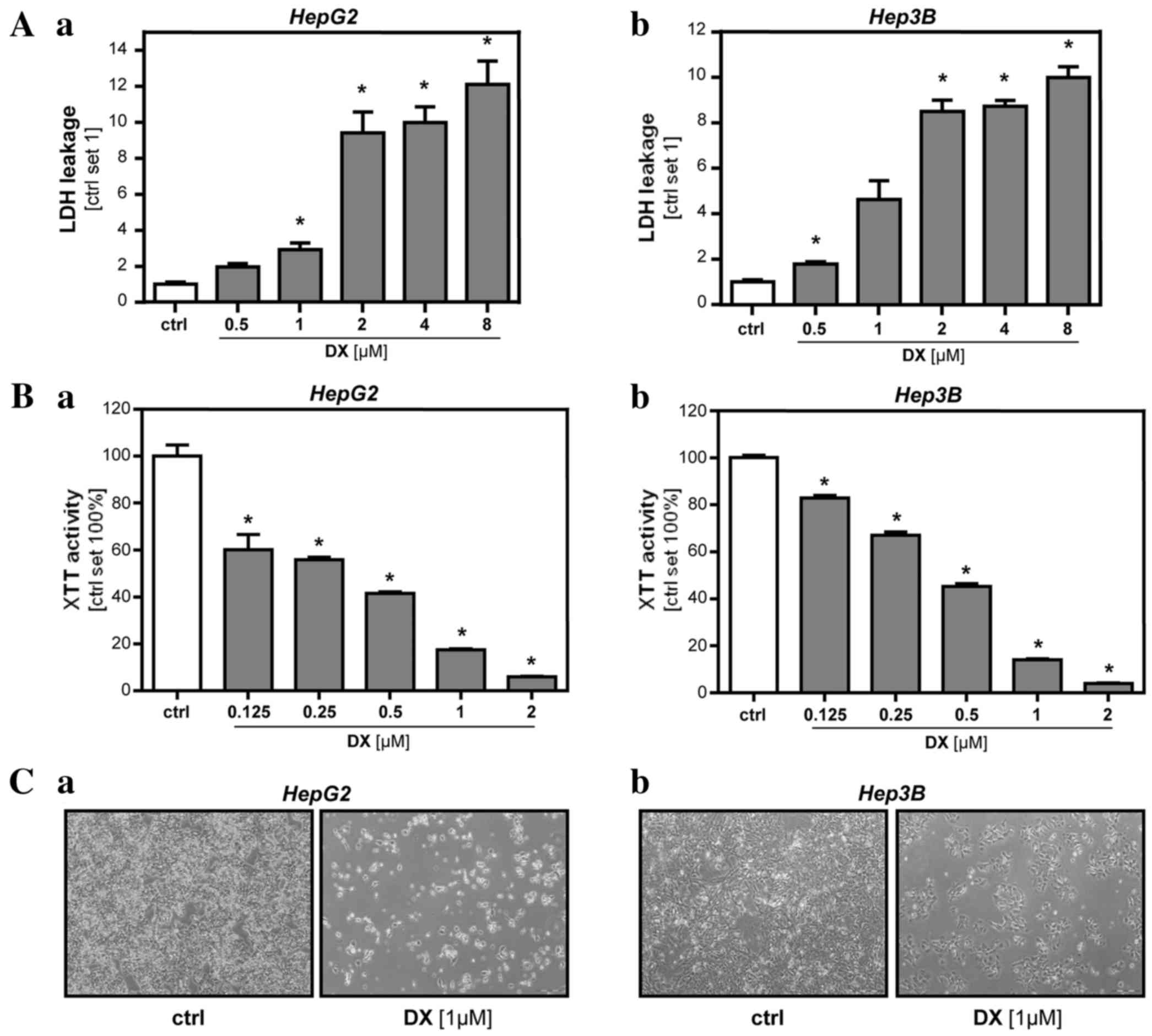
The present study analyzed the effective dose range
of doxorubicin on the HepG2 and Hep3B human HCC cell lines.
Analysis of LDH release into the supernatant (Fig. 1A) and XTT activity (Fig. 1B) showed that doxorubicin
dose-dependently reduced the viability of HCC cells during the 48 h
incubation time. Starting at a dose of 1 µM in HepG2 cells and 0.5
µM in Hep3B cells, doxorubicin caused a significant increase of LDH
levels in the supernatant (2.9-fold, P=0.0001 in HepG2; 1.8-fold,
P=0.004 in Hep3B). XTT activity was significantly reduced by
incubation with 0.125 µM doxorubicin in HepG2 cells (60%; P=0.0075)
and Hep3B cells (83%-fold; P=0.0003). The determined toxic dose
ranges were comparable to previous in vitro studies using
the same HCC cell lines (27–32). Phase-contrast microscopy confirmed
that, after 48 h incubation with a concentration of 1 µM
doxorubicin, 10–20% of HCC cells survived (Fig. 1C). For the next in vitro model
that was designed to mimic the circumstances of TACE, doxorubicin
was used at a concentration of 1 µM, which was in the range of
doxorubicin concentrations found in human HCC explants following
the administration of TACE (33,34). HCC
cells surviving incubation with this doxorubicin dose for 48 h
(HCCsurv) and control cells (HCCctr) were
generated as aforementioned.
Analysis of surviving HCC cells in the
early phase following doxorubicin treatment
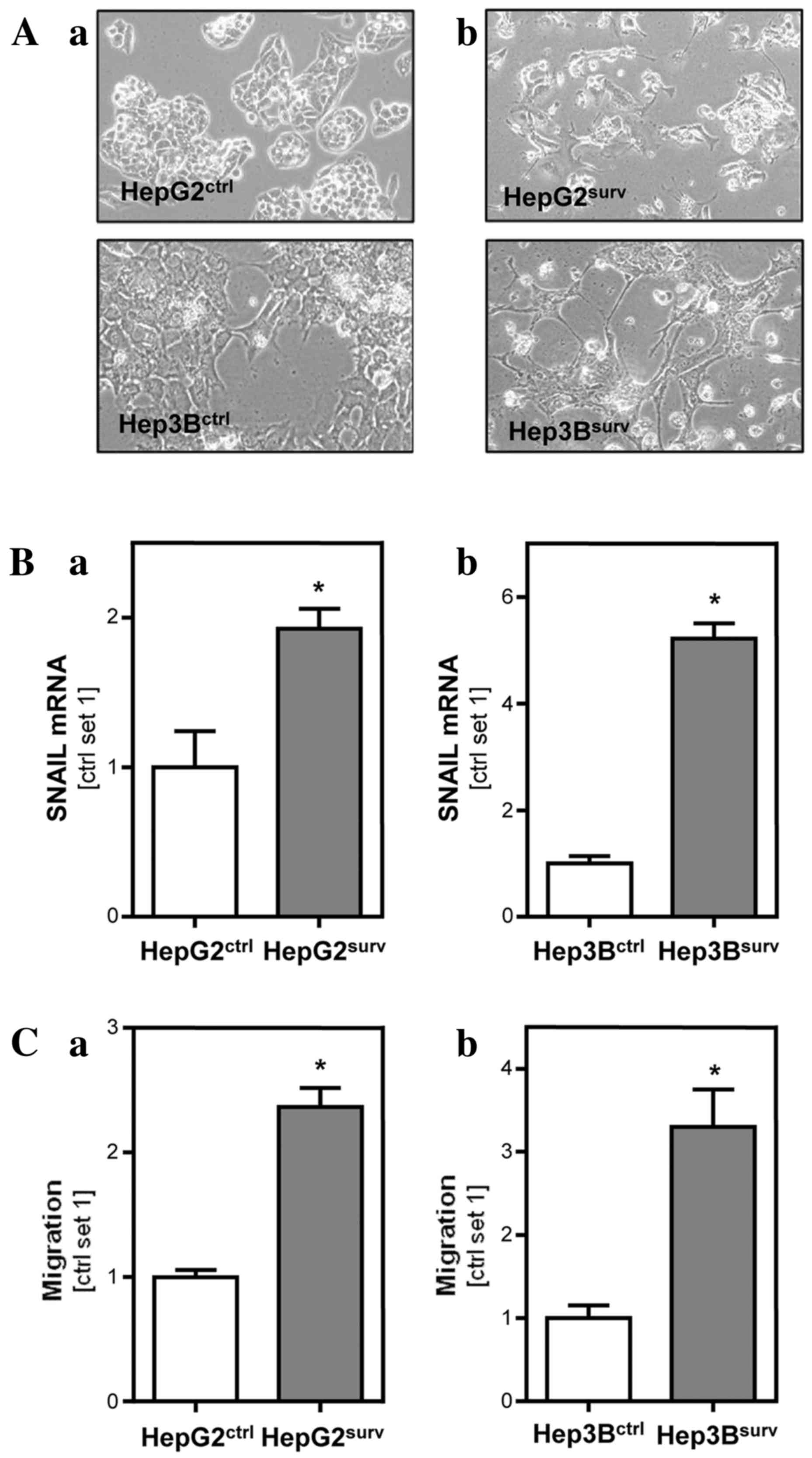
Monitoring of cell growth and morphology with
phase-contrast microscopy revealed that HCCsurv cells
developed a spindle-like, outstretched, mesenchymal shape within
the first 6 days after treatment with doxorubicin (Fig. 2A). By contrast, HepG2ctr
and Hep3Bctr did not change their characteristic, cubic
and compact cell form during the whole observation period.
Additionally, expression of the epithelial-mesenchymal transition
(EMT) marker SNAIL was 1.9-fold (P=0.03) increased in
HepG2surv compared to HepG2ctr cells
(Fig. 2B). Also in
Hep3Bsurv SNAIL expression was 5.2-fold (P=0.0002)
higher compared with Hep3Bctr cells (Fig. 2B). Functional analysis revealed
similar rates of proliferation of HCCsurv and
HCCctr cells (data not shown). However,
HCCsurv cells exhibited significantly increased
migration in Boyden chamber assays compared to HCCctr
cells (Fig. 2C). Migration ability in
HepG2surv was 2.4-fold increased (P=0.001) compared with
HepG2ctr. Hep3Bsurv exhibited a 3.3-fold
increase (P=0.009) in migratory potential compared with
Hep3Bctr.
Analysis of surviving HCC cells 3
weeks after doxorubicin treatment
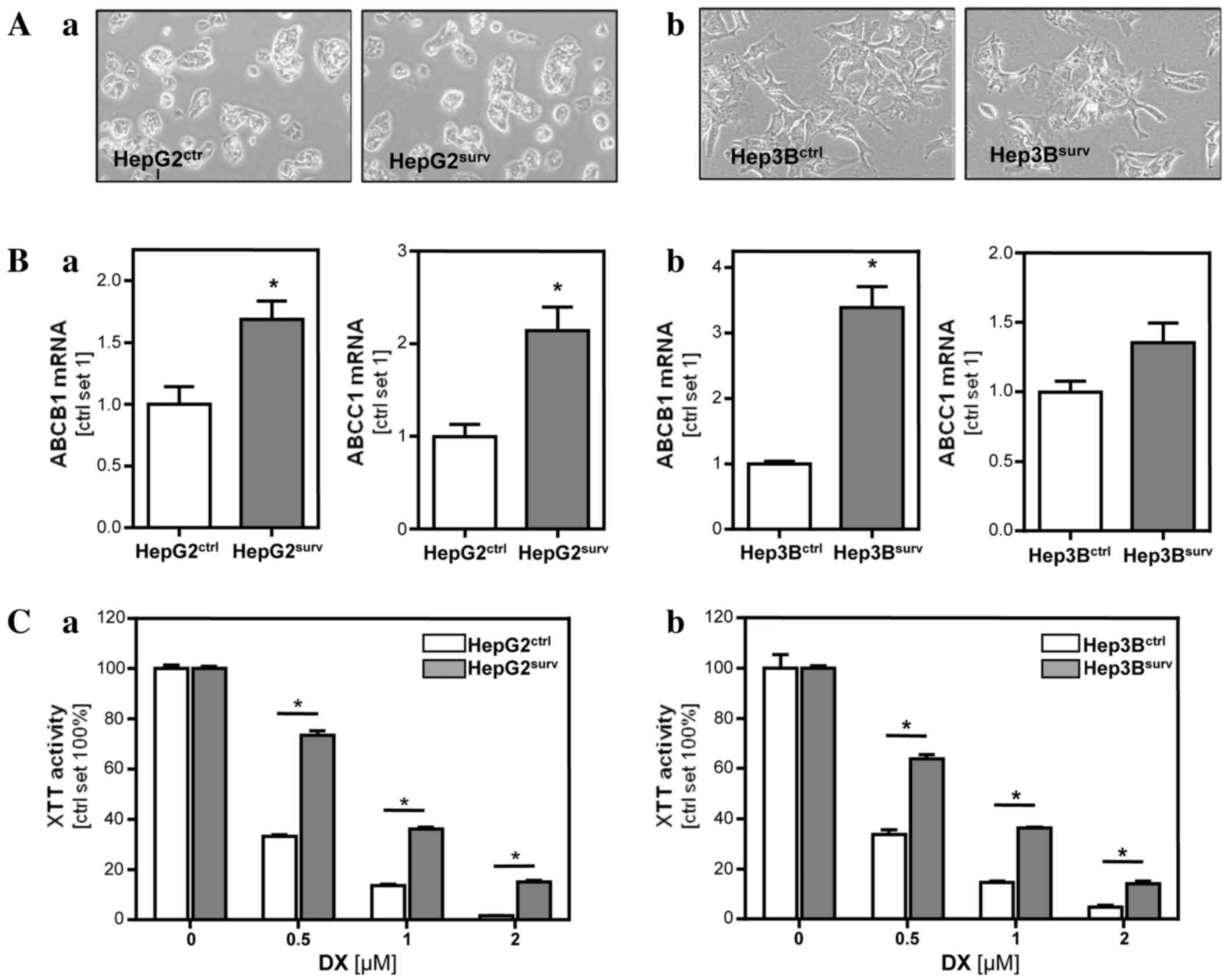
After ~1 week, HCCsurv cells became
confluent and required splitting. Subsequently, HCCsurv
cells were further cultured in parallel with HCCctr
cells for another 2 weeks. During that time, the HCCsurv
cells reverted to their original shape. The spindle-like,
outstretched cell form disappeared and the HepG2surv and
Hep3Bsurv no longer differed from their respective
control cells (Fig. 3A). SNAIL
expression and migratory potential were similar in
HCCsurv and HCCctr cells (data not shown).
However, 3 weeks following doxorubicin treatment,
HCCsurv cells exhibited significantly higher expression
levels of MDR1 (ABCB1) and MRP1 (ABCC1) compared to
HCCctr cells (Fig. 3B).
ABCB1 expression was 1.7-fold increased in HepG2surv
(P=0.029) and 3.4-fold in Hep3Bsurv (P=0.002) compared
with their respective control cells. ABCC1 expression was increased
2.1-fold in HepG2surv (P=0.016) and 1.4-fold in
Hep3Bsurv (P=0.09) cells compared with their respective
control cells. Consistently, HCCsurv cells tolerated
significantly increased doxorubicin concentrations compared with
HCCctr cells (Fig. 3C).
Although XTT-activity was reduced to 33% in HepG2ctr
treated with 0.5 µM doxorubicin, HepG2surv exhibited an
XTT-activity of 74% (P=0.0001) upon incubation with the same
doxorubicin dose. Similarly, impairment of XTT-activity in response
to 0.5 µM doxorubicin in Hep3Bsurv cells (64%) was
significantly lowered (P=0.0006) compared with the reduction of
XTT-activity (34%) in Hep3Bctr cells.
Discussion
The aim of the present study was to analyze human
HCC cells surviving doxorubicin treatment in vitro, in an
experimental setting resembling the circumstances of HCC cells
surviving doxorubicin application during TACE. For this, two
different human HCC cell lines were incubated with doxorubicin at a
concentration that killed >80% of the tumor cells within 48 h.
The applied concentration of doxorubicin was in the range of tissue
drug concentrations found in experimental TACE models in
vivo, as well as in HCC explants of patients after the
administration of TACE (33,34). After 2 days, cell culture of surviving
HCC cells was continued without doxorubicin exposure to mimic the
situation of a single doxorubicin dose application during TACE.
Applying these experimental conditions, the present
study observed an increased expression of the EMT marker SNAIL and
morphological changes to a mesenchymal cell shape in HCC cells
surviving doxorubicin exposure. Additionally, doxorubicin-surviving
HCC cells exhibited increased migratory activity. Expression of
SNAIL has been found to positively correlate with poor clinical
outcomes in different types of cancer, including HCC (35). Furthermore, several studies indicate
that EMT is a crucial event in HCC progression, being associated
with tumor cell invasion and metastasis (36). Accordingly, a previous study reported
that the incidences of poorly differentiated histology and
intrahepatic metastases are significantly increased in post-TACE
HCC tissues compared with in HCC tissues of patients who have not
undergone TACE treatment (37).
Furthermore, Zen et al (38)
found a combined hepato-cholangiocellular phenotype was more
frequently detected in HCC tissues after TACE compared to untreated
HCC. In the context of these previous studies and the present in
vitro data, one may hypothesize that doxorubicin application
during TACE promotes a more malignant phenotype in surviving HCC
cells. Currently, the present study can only speculate why the
alterations in cell morphology, SNAIL expression and migratory
activity in doxorubicin-surviving HCC cells regressed with
prolonged cell culture. It may indeed have been an intermediate
effect, or trypsinization and splitting of the cells may have
triggered this reversion.
However, for up to 3 weeks after a single
doxorubicin application, surviving HCC cells were significantly
less susceptible to retreatment with doxorubicin. As a potential
explanation for this increased chemotherapy resistance,
significantly increased expression levels of MDR1 (ABCB1) and MRP1
(ABCC1) were found; these genes are known to contribute to
multidrug resistance in HCC (12–15,17,18,20).
MRP1, which is overexpressed in HCC (39), performs an important role in the
intrinsic multidrug resistance of HCC and is also associated with
an aggressive tumor phenotype and has been suggested to indicate a
progenitor cell origin (18).
Hypoxia, which also occurs after TACE through
ischemia, is known to induce EMT and to enhance migration and
therapy resistance in HCC cells (40,41). The
findings of the present study suggest that the chemotherapeutic
agent doxorubicin may also cause unfavorable alterations in
surviving HCC cells. These findings are of importance for the
understanding of HCC recurrence observed subsequent to TACE. Future
studies are required to analyze whether maintaining doxorubicin
levels for a prolonged period, such as with doxorubicin-eluting
beads, or switching to other anticancer agents may omit certain
pathological alterations found in the present in vitro
model. Furthermore, it must be investigated whether such altered
therapeutic strategies may improve the outcome of HCC patients
following TACE treatment, and this in vitro model may be
used for preclinical analyses addressing these questions.
Acknowledgements
The authors would like to thank Mrs. Birgitta
Ott-Rötzer (University Hospital Regensburg, Germany) for excellent
technical assistance. This study was supported by grants from the
German Research Association (grant nos. FOR2127 and KFO262) and an
educational grant from the Medical Faculty of the University
Hospital Regensburg.
References
|
1
|
Lin S, Hoffmann K and Schemmer P:
Treatment of hepatocellular carcinoma: A systematic review. Liver
Cancer. 1:144–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mazzanti R, Arena U and Tassi R:
Hepatocellular carcinoma: Where are we? World J Exp Med. 6:21–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J, Boix L, Sala M and Llovet JM:
Focus on hepatocellular carcinoma. Cancer Cell. 5:215–219. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hernandez-Gea V, Toffanin S, Friedman SL
and Llovet JM: Role of the microenvironment in the pathogenesis and
treatment of hepatocellular carcinoma. Gastroenterology.
144:512–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belghiti J: Treatment of hepatocellular
carcinoma. Bull Acad Natl Med. 196:97–103. 2012.PubMed/NCBI
|
|
6
|
Biolato M, Marrone G, Racco S, Di Stasi C,
Miele L, Gasbarrini G, Landolfi R and Grieco A: Transarterial
chemoembolization (TACE) for unresectable HCC: A new life begins?
Eur Rev Med Pharmacol Sci. 14:356–362. 2010.PubMed/NCBI
|
|
7
|
Xiao EH, Guo D and Bian DJ: Effect of
preoperative transcatheter arterial chemoembolization on
angiogenesis of hepatocellular carcinoma cells. World J
Gastroenterol. 15:4582–4586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park W, Chung YH, Kim JA, Jin YJ, Lee D,
Shim JH, Lee D, Kim KM, Lim YS, Lee HC, et al: Recurrences of
hepatocellular carcinoma following complete remission by
transarterial chemoembolization or radiofrequency therapy: Focused
on the recurrence patterns. Hepatol Res. 43:1304–1312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niessen C, Wiggermann P, Velandia C,
Stroszczynski C and Pereira PL: Transarterial
chemoembolization-status quo in Germany. Rofo. 185:1089–1094. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thorn CF, Oshiro C, Marsh S,
Hernandez-Boussard T, McLeod H, Klein TE and Altman RB: Doxorubicin
pathways: Pharmacodynamics and adverse effects. Pharmacogenet
Genomics. 21:440–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tacar O, Sriamornsak P and Dass CR:
Doxorubicin: An update on anticancer molecular action, toxicity and
novel drug delivery systems. J Pharm Pharmacol. 65:157–170. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li G, Chen X, Wang Q, Xu Z, Zhang W and Ye
L: The roles of four multi-drug resistance proteins in
hepatocellular carcinoma multidrug resistance. J Huazhong Univ Sci
Technolog Med Sci. 27:173–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Chan JY, Fong CC, Tzang CH, Fung
KP and Yang M: Transcriptional analysis of doxorubicin-induced
cytotoxicity and resistance in human hepatocellular carcinoma cell
lines. Liver Int. 29:1338–1347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pang E, Hu Y, Chan KY, Lai PB, Squire JA,
Macgregor PF, Beheshti B, Albert M, Leung TW and Wong N: Karyotypic
imbalances and differential gene expressions in the acquired
doxorubicin resistance of hepatocellular carcinoma cells. Lab
Invest. 85:664–674. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE
and Gottesman MM: P-glycoprotein: From genomics to mechanism.
Oncogene. 22:7468–7485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye CG, Wu WK, Yeung JH, Li HT, Li ZJ, Wong
CC, Ren SX, Zhang L, Fung KP and Cho CH: Indomethacin and SC236
enhance the cytotoxicity of doxorubicin in human hepatocellular
carcinoma cells via inhibiting P-glycoprotein and MRP1 expression.
Cancer Lett. 304:90–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JG, Lee SK, Hong IG, Kim HS, Lim KH,
Choe KJ, Kim WH, Kim YI, Tsuruo T and Gottesman MM: MDR1 gene
expression: Its effect on drug resistance to doxorubicin in human
hepatocellular carcinoma cell lines. J Natl Cancer Inst.
86:700–705. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vander Borght S, Komuta M, Libbrecht L,
Katoonizadeh A, Aerts R, Dymarkowski S, Verslype C, Nevens F and
Roskams T: Expression of multidrug resistance-associated protein 1
in hepatocellular carcinoma is associated with a more aggressive
tumour phenotype and may reflect a progenitor cell origin. Liver
Int. 28:1370–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Itsubo M, Ishikawa T, Toda G and Tanaka M:
Immunohistochemical study of expression and cellular localization
of the multidrug resistance gene product P-glycoprotein in primary
liver carcinoma. Cancer. 73:298–303. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan JY, Chu AC and Fung KP: Inhibition of
P-glycoprotein expression and reversal of drug resistance of human
hepatoma HepG2 cells by multidrug resistance gene (mdr1) antisense
RNA. Life Sci. 67:2117–2124. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun BT, Zheng LH, Bao YL, Yu CL, Wu Y,
Meng XY and Li YX: Reversal effect of Dioscin on multidrug
resistance in human hepatoma HepG2/adriamycin cells. Eur J
Pharmacol. 654:129–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang L, Liu X, Lu Z, Yuet-Wa Chan J, Zhou
L, Fung KP, Wu P and Wu S: Ursolic acid induces
doxorubicin-resistant HepG2 cell death via the release of
apoptosis-inducing factor. Cancer Lett. 298:128–138. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bosserhoff AK, Moser M, Schölmerich J,
Buettner R and Hellerbrand C: Specific expression and regulation of
the new melanoma inhibitory activity-related gene MIA2 in
hepatocytes. J Biol Chem. 278:15225–311. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hellerbrand C, Mühlbauer M, Wallner S,
Schuierer M, Behrmann I, Bataille F, Weiss T, Schölmerich J and
Bosserhoff AK: Promoter-hypermethylation is causing functional
relevant downregulation of methylthioadenosine phosphorylase (MTAP)
expression in hepatocellular carcinoma. Carcinogenesis. 27:64–72.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dorn C, Weiss TS, Heilmann J and
Hellerbrand C: Xanthohumol, a prenylated chalcone derived from
hops, inhibits proliferation, migration and interleukin-8
expression of hepatocellular carcinoma cells. Int J Oncol.
36:435–441. 2010.PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Al-Qubaisi M, Rozita R, Yeap SK, Omar AR,
Ali AM and Alitheen NB: Selective cytotoxicity of goniothalamin
against hepatoblastoma HepG2 cells. Molecules. 16:2944–2959. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fan LL, Sun GP, Wei W, Wang ZG, Ge L, Fu
WZ and Wang H: Melatonin and doxorubicin synergistically induce
cell apoptosis in human hepatoma cell lines. World J Gastroenterol.
16:1473–1481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Al-Abd AM, Mahmoud AM, El-Sherbiny GA,
El-Moselhy MA, Nofal SM, El-Latif HA, El-Eraky WI and El-Shemy HA:
Resveratrol enhances the cytotoxic profile of docetaxel and
doxorubicin in solid tumour cell lines in vitro. Cell Prolif.
44:591–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu QD, Chen W, Yan TL, Ma T, Chen CL,
Liang C, Zhang Q, Xia XF, Liu H, Zhi X, et al: NSC 74859 enhances
doxorubicin cytotoxicity via inhibition of epithelial-mesenchymal
transition in hepatocellular carcinoma cells. Cancer Lett.
325:207–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gambari R, Hau DK, Wong WY and Chui CH:
Sensitization of Hep3B hepatoma cells to cisplatin and doxorubicin
by corilagin. Phytother Res. 28:781–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shiraga K, Sakaguchi K, Senoh T, Ohta T,
Ogawa S, Sawayama T, Mouri H, Fujiwara A and Tsuji T: Modulation of
doxorubicin sensitivity by cyclosporine A in hepatocellular
carcinoma cells and their doxorubicin-resistant sublines. J
Gastroenterol Hepatol. 16:460–466. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Namur J, Wassef M, Millot JM, Lewis AL,
Manfait M and Laurent A: Drug-eluting beads for liver embolization:
Concentration of doxorubicin in tissue and in beads in a pig model.
J Vasc Interv Radiol. 21:259–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Namur J, Citron SJ, Sellers MT, Dupuis MH,
Wassef M, Manfait M and Laurent A: Embolization of hepatocellular
carcinoma with drug-eluting beads: Doxorubicin tissue concentration
and distribution in patient liver explants. J Hepatol.
55:1332–1338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van Zijl F, Zulehner G, Petz M, Schneller
D, Kornauth C, Hau M, Machat G, Grubinger M, Huber H and Mikulits
W: Epithelial-mesenchymal transition in hepatocellular carcinoma.
Future Oncol. 5:1169–1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nishihara Y, Aishima S, Kuroda Y, Iguchi
T, Taguchi K, Asayama Y, Taketomi A, Kinukawa N, Honda H and
Tsuneyoshi M: Biliary phenotype of hepatocellular carcinoma after
preoperative transcatheter arterial chemoembolization. J
Gastroenterol Hepatol. 23:1860–1868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zen C, Zen Y, Mitry RR, Corbeil D,
Karbanová J, O'Grady J, Karani J, Kane P, Heaton N, Portmann BC and
Quaglia A: Mixed phenotype hepatocellular carcinoma after
transarterial chemoembolization and liver transplantation. Liver
Transpl. 17:943–954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hoffmann K, Shibo L, Xiao Z, Longerich T,
Büchler MW and Schemmer P: Correlation of gene expression of
ATP-binding cassette protein and tyrosine kinase signaling pathway
in patients with hepatocellular carcinoma. Anticancer Res.
31:3883–3890. 2011.PubMed/NCBI
|
|
40
|
Zhang L, Huang G, Li X, Zhang Y, Jiang Y,
Shen J, Liu J, Wang Q, Zhu J, Feng X, et al: Hypoxia induces
epithelial-mesenchymal transition via activation of SNAI1 by
hypoxia-inducible factor-1α in hepatocellular carcinoma. BMC
Cancer. 13:1082013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Luo D, Wang Z and Wu J, Jiang C and Wu J:
The role of hypoxia inducible factor-1 in hepatocellular carcinoma.
Biomed Res Int. 2014:4092722014. View Article : Google Scholar : PubMed/NCBI
|