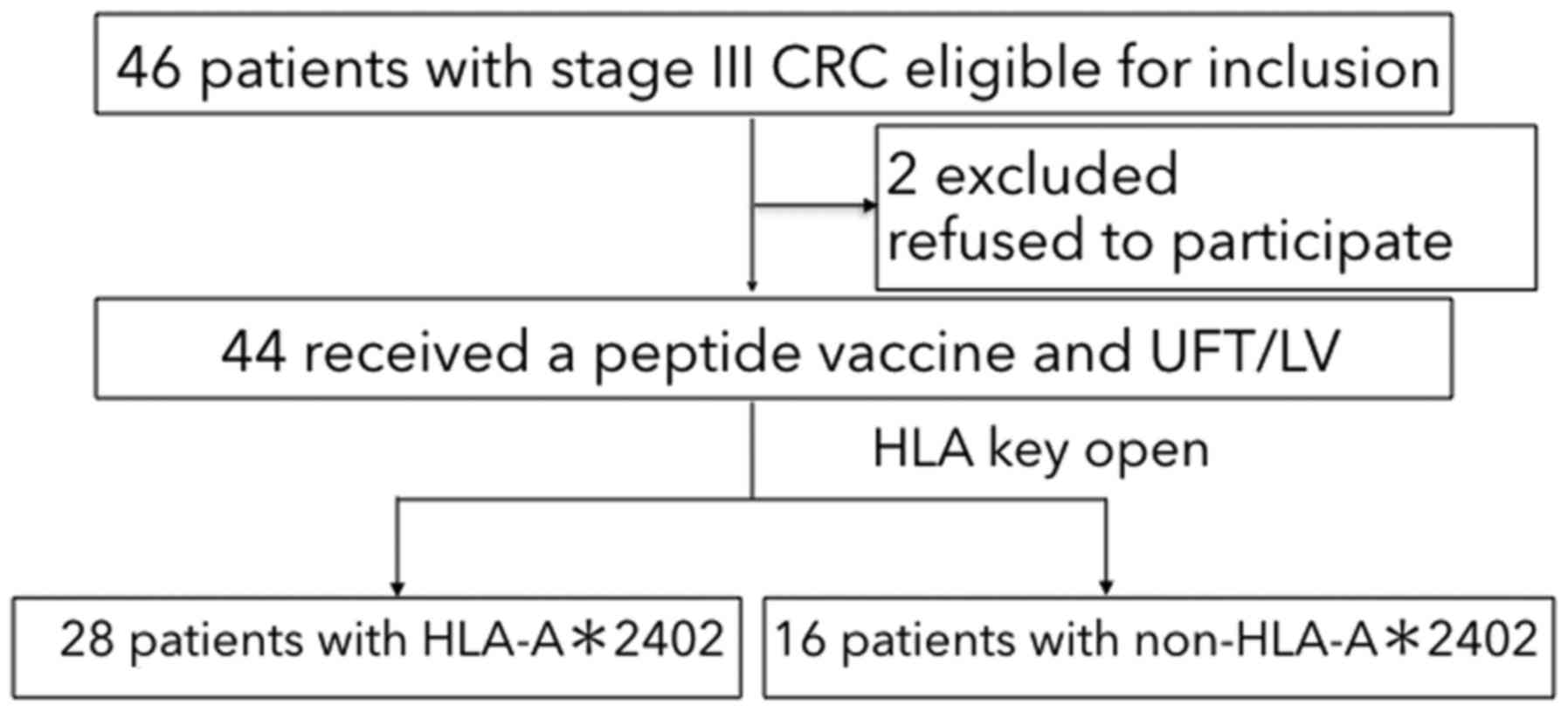
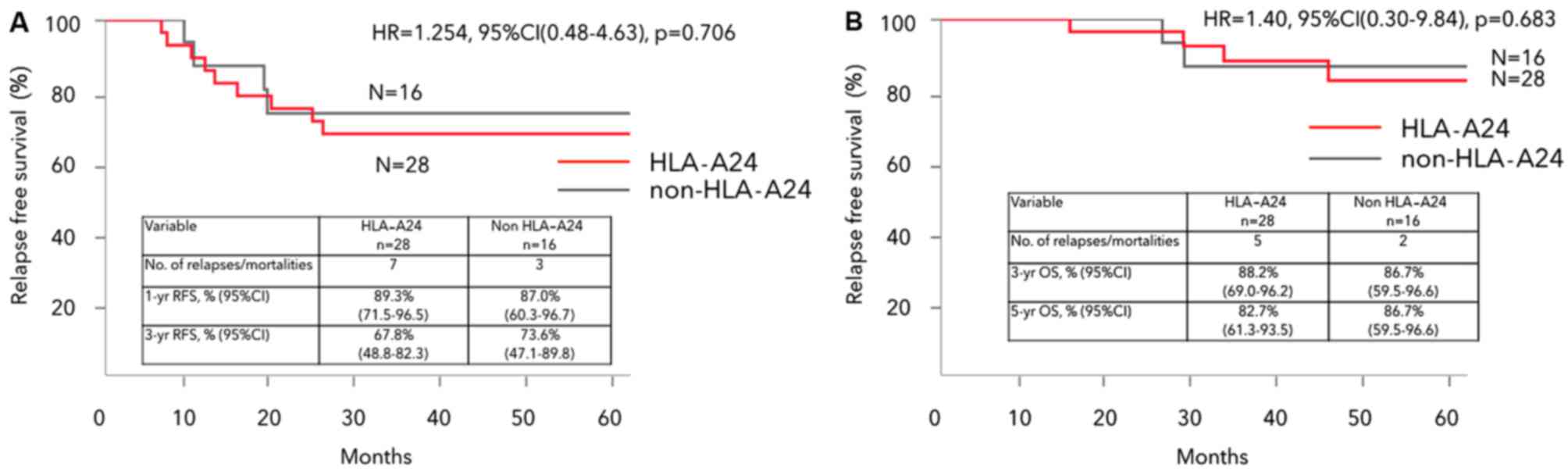
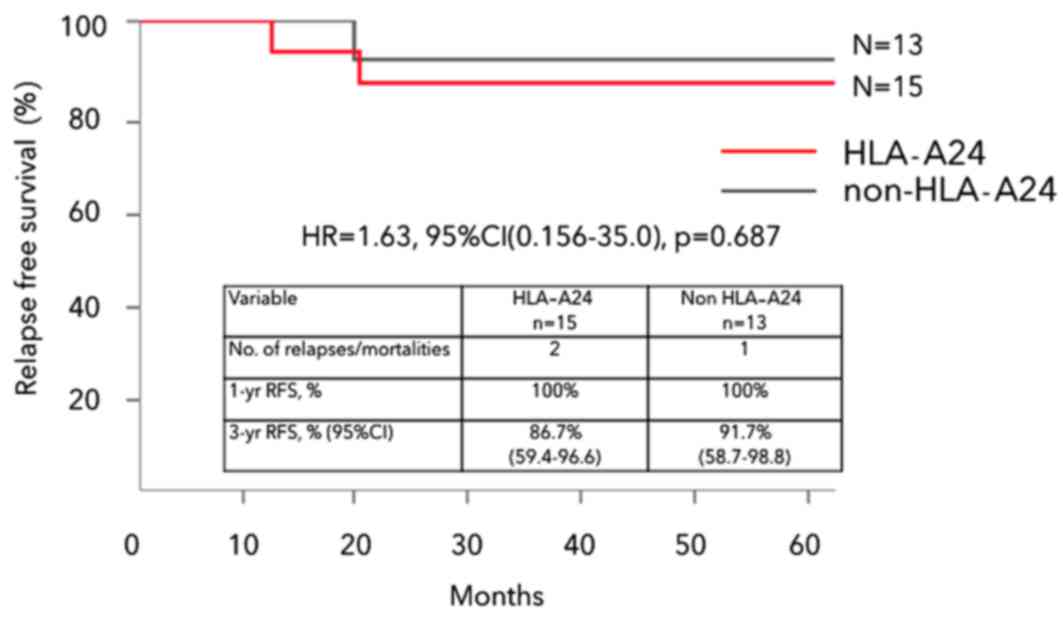
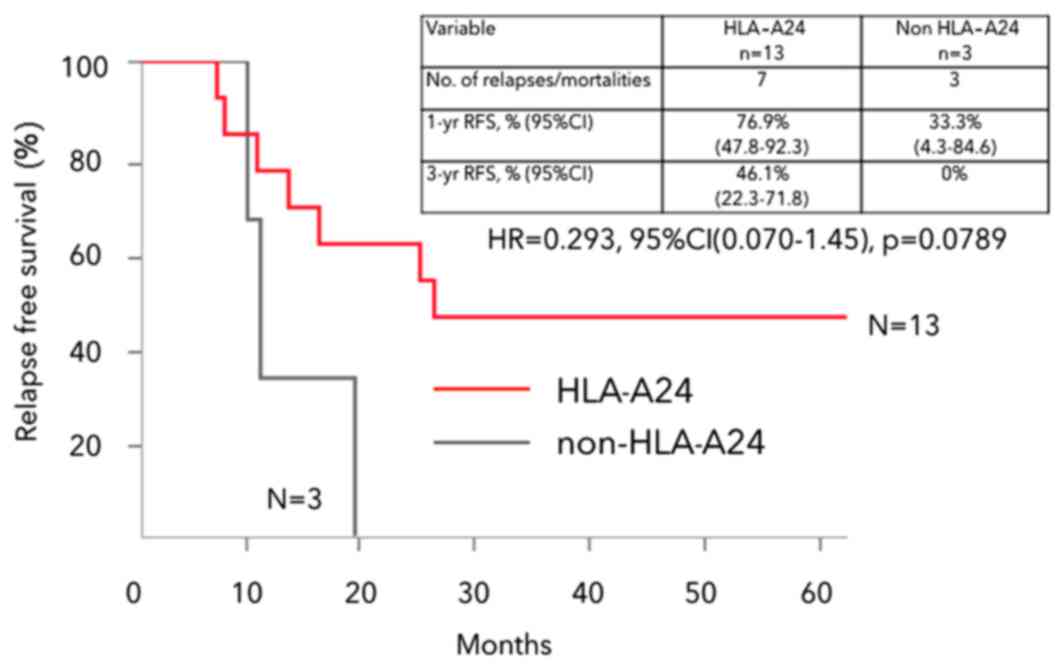
|
1
|
Data from Cancer Registry and Statistics.
Cancer Information Service, National Cancer Center, Japan.
https://ganjoho.jp/reg_stat/statistics/dl/index.html#incidenceOctober
24–2017(In Japanese).
|
|
2
|
Vital Statistics. Ministry of Health,
Labour and Welfare, Japan. http://www.mhlw.go.jp/toukei/list/81-1.htmlOctober
24–2017(In Japanese).
|
|
3
|
NIH consensus conference. Adjuvant therapy
for patients with colon and rectal cancer. JAMA. 264:1444–1450.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2014 for treatment of colorectal cancer. Int J Clin
Oncol. 20:207–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolmark N, Rockette H, Fisher B, Wickerham
DL, Redmond C, Fisher ER, Jones J, Mamounas EP, Ore L and Petrelli
NJ: The benefit of leucovorin-modulated fluorouracil as
postoperative adjuvant therapy for primary colon cancer: Results
from national surgical adjuvant breast and bowel project protocol
C-03. J Clin Oncol. 11:1879–1887. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimada Y, Hamaguchi T, Mizusawa J, Saito
N, Kanemitsu Y, Takiguchi N, Ohue M, Kato T, Takii Y, Sato T, et
al: Randomised phase III trial of adjuvant chemotherapy with oral
uracil and tegafur plus leucovorin versus intravenous fluorouracil
and levofolinate in patients with stage III colorectal cancer who
have undergone Japanese D2/D3 lymph node dissection: Final results
of JCOG0205. Eur J Cancer. 50:2231–2240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Twelves C, Wong A, Nowacki MP, Abt M,
Burris H III, Carrato A, Cassidy J, Cervantes A, Fagerberg J,
Georgoulias V, et al: Capecitabine as adjuvant treatment for stage
III colon cancer. N Engl J Med. 352:2696–2704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshida M, Ishiguro M, Ikejiri K,
Mochizuki I, Nakamoto Y, Kinugasa Y, Takagane A, Endo T, Shinozaki
H, Takii Y, et al: S-1 as adjuvant chemotherapy for stage III colon
cancer: A randomized phase III study (ACTS-CC trial). Ann Oncol.
25:1743–1749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuebler JP, Wieand HS, O'Connell MJ, Smith
RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE,
Atkins JN, et al: Oxaliplatin combined with weekly bolus
fluorouracil and leucovorin as surgical adjuvant chemotherapy for
stage II and III colon cancer: Results from NSABP C-07. J Clin
Oncol. 25:2198–2204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmoll HJ, Tabernero J, Maroun J, de
Braud F, Price T, Van Cutsem E, Hill M, Hoersch S, Rittweger K and
Haller DG: Capecitabine plus oxaliplatin compared with
fluorouracil/folinic acid as adjuvant therapy for stage III colon
cancer: Final results of the NO16968 randomized controlled phase
III trial. J Clin Oncol. 33:3733–3740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil, and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okuno K, Sugiura F, Hida JI, Tokoro T,
Ishimaru E, Sukegawa Y and Ueda K: Phase I clinical trial of a
novel peptide vaccine in combination with UFT/LV for metastatic
colorectal cancer. Exp Ther Med. 2:73–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanaka F: UFT (tegafur and uracil) as
postoperative adjuvant chemotherapy for solid tumors (carcinoma of
the lung, stomach, colon/rectum and breast): Clinical evidence,
mechanism of action, and future direction. Surg Today. 37:923–943.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carmichael J, Popiela T, Radstone D, Falk
S, Borner M, Oza A, Skovsgaard T, Munier S and Martin C: Randomized
comparative study of tegafur/uracil and oral leucovorin versus
parenteral fluorouracil and leucovorin in patients with previously
untreated metastatic colorectal cancer. J Clin Oncol. 20:3617–3627.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Douillard JY, Hoff PM, Skillings JR,
Eisenberg P, Davidson N, Harper P, Vincent MD, Lembersky BC,
Thompson S, Maniero A and Benner SE: Multicenter phase III study of
uracil/tegafur and oral leucovorin versus fluorouracil and
leucovorin in patients with previously untreated metastatic
colorectal cancer. J Clin Oncol. 20:3605–3616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shirao K, Hoff PM, Ohtsu A, Loehrer PJ,
Hyodo I, Wadler S, Wadleigh RG, O'Dwyer PJ, Muro K, Yamada Y, et
al: Comparison of the efficacy, toxicity, and pharmacokinetics of a
uracil/tegafur (UFT) plus oral leucovorin (LV) regimen between
Japanese and American patients with advanced colorectal cancer:
Joint United States and Japan study of UFT/LV. J Clin Oncol.
22:3466–3474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hattori T, Mine T, Komatsu N, Yamada A,
Itoh K, Shiozaki H and Okuno K: Immunological evaluation of
personalized peptide vaccination in combination with UFT and UZEL
for metastatic colorectal carcinoma patients. Cancer Immunol
Immunother. 58:1843–1852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
U.S. Department of Health and Human
Services, National Institutes of Health and National Cancer
Institute, . Common Terminology Criteria for Adverse Events
(CTCAE). Version4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdfMay.
2009
|
|
20
|
Okuno K, Sugiura F, Inoue K and Sukegawa
Y: Clinical trial of a 7-peptide cocktail vaccine with oral
chemotherapy for patients with metastatic colorectal cancer.
Anticancer Res. 34:3045–3052. 2014.PubMed/NCBI
|
|
21
|
Kono K, Iinuma H, Akutsu Y, Tanaka H,
Hayashi N, Uchikado Y, Noguchi T, Fujii H, Okinaka K, Fukushima R,
et al: Multicenter, phase II clinical trial of cancer vaccination
for advanced esophageal cancer with three peptides derived from
novel cancer-testis antigens. J Transl Med. 10:1412012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Japanese Society for Cancer of the Colon
anc Rectum: Japanese Classification of Colorectal Carcinoma. 8th.
Kanehara & Co., Ltd.; Tokyo: 2013
|