Introduction
In the 1800s, Halsted first practiced and
standardized radical mastectomy (RM) (1). Subsequently, surgery for breast cancer
has significantly improved with regards to oncological safety and
cosmetic outcome. In 1965, Madden (2)
first introduced modified RM (MRM), which has since been frequently
performed clinically. RM and MRM are grouped with conventional
mastectomy (CM), and the local recurrence (LR) rate following CM
was ~10% 10 years after surgery (3–5).
In 1991, Toth and Lappert (6) first described skin-sparing mastectomy
(SSM), which involves removing the mammary glands, including the
nipple-areola complex (NAC), while preserving the native skin
envelope and the inframammary fold. The oncological safety (local
disease control) of SSM has been demonstrated to be equivalent to
that of MRM (7,8). Nipple-sparing mastectomy (NSM) is
similar to SSM, but does not involve removal of the NAC (9). In 1984, Hinton et al (10) first reported that the LR and survival
rates following NSM was equivalent to those following MRM; however,
this conclusion was not accepted by prominent surgeons at the time
due to the fact that the NAC may harbor occult tumor cells, thereby
increasing the risk of LR (11,12).
Previous studies have reported NAC involvement in 5–12% of cases
(13,14). Over time, an increasing number of
studies have reported that NSM may provide oncological safety
compared with CM for carefully selected patients (15–19).
The young age of certain patients has been revealed
to be associated with a poor prognosis and an increased risk of LR
in breast cancer (20–22), an effect which may be explained, in
part, by diagnosis at a later stage and a higher proportion of
unfavorable tumor characteristics (23–25).
Although young age was previously a contraindication for
breast-conserving surgery (BCS), later studies have reported that
young patients who had undergone BCS may exhibit oncological safety
compared with those who had undergone CM (26). Bantema-Joppe et al (27) reported that the 10-year overall
survival (OS) rate of patients following BCS was not impaired,
compared with traditional mastectomy in young patients with
early-stage breast cancer. Furthermore, the oncological safety of
NSM in young patients with breast cancer has rarely been reported
and remains poorly understood.
In the present study, LR, disease-free survival
(DFS) and OS rates were investigated in young patients with
early-stage breast cancer who had undergone NSM or CM.
Materials and methods
Data collection
The information of young patients with stage 0–IIB
breast cancer who had undergone NSM (163 cases) or CM (194 cases)
at Guangxi Medical University Affiliated Tumor Hospital (Nanning,
China; 103 NSM cases and 126 CM cases) and Liuzhou People's
Hospital (Liuzhou, China; 60 NSM cases and 68 CM cases), between
January 2007 and June 2016, was collected. Patient
clinicopathological data were collected, including age at
diagnosis, tumor size, nodal status, Tumor-Node-Metastasis (TNM)
stage (28), histological grade,
estrogen receptor (ER) and progesterone receptor (PR) status, human
epidermal growth factor receptor 2 (HER-2) expression, surgical
procedures, complications and adjuvant treatment regimens.
Follow-up data included LR time, systemic recurrence time and
patient mortality.
Inclusion and exclusion criteria
The inclusion criteria for the present study were as
follows: i) Patients who had undergone NSM or CM; ii) patients aged
<35 years; iii) female patients; and iv) patients with TNM
stages 0-IIB at initial diagnosis. The exclusion criteria for the
present study were as follows: i) Patients who had received
preoperative treatment; ii) patients who had not received adjuvant
treatment; iii) patients without available pathological data; iv)
patients with synchronous bilateral invasive breast cancer or
metachronous contralateral breast cancer; and v) patients without
follow-up records. A total of 357 patients were included in the
present study.
Surgery
Patients who had been found to be contraindicated
with BCS by preoperative imaging, including magnetic resonance
imaging, ultrasonography and mammography, and those who had
explicitly rejected BCS, were eligible for NSM. Patients with
possible tumor involvement in the NAC or surrounding skin,
according to preoperative imaging, were not eligible for NSM. Based
on the results of fine needle biopsies and clinical staging, CM was
performed with or without a sentinel lymph node biopsy. Axillary
lymph node dissection was routinely performed when metastasis to
the sentinel lymph node had occurred. NSM removed the entire
mammary gland parenchyma, including the skin overlying superficial
tumors, when possible. Patients with a tumor-to-NAC distance <2
cm, nipple discharge or Paget's disease were not eligible for NSM.
When performing NSM, in order to confirm that there was no tumor
invasion to the NAC borders, frozen sections of the retroareolar
tissue were routinely acquired for intraoperative histological
diagnosis. The puncture point of the core biopsy was as far from
the NAC and as close to the lump as possible. NSM was followed by
immediate breast reconstruction with a permanent implant,
latissimus dorsi myocutaneous flap (LD), extended LD (ELD),
transverse rectus abdominis myocutaneous flap (TRAM) and deep
inferior epigastric artery perforator flap (DIEP), which was
performed by the same team in a single operative procedure. The
reconstructive procedure was selected depending on the anatomical
conditions and personal preferences of patients.
Adjuvant therapy
Adjuvant systemic treatment and radiation therapy
were administered according to the National Comprehensive Cancer
Network guidelines (29) and based on
the postoperative pathological examinations. Adjuvant therapy
included chemotherapy, endocrine therapy, radiotherapy and targeted
therapy. Patients were normatively treated according to the most
recent guidelines at the time of treatment.
Statistical analysis
The patient clinicopathological features were
compared between the NSM and CM groups using the χ2
(when the theoretical frequency was >1) or Fisher's exact tests
(when the theoretical frequency was <1). The present study was
not a randomized controlled trial and statistical differences in
certain clinicopathological features, including the clinical TNM
stage, of a number of patients were observed between the 2 groups.
Therefore, Kaplan-Meier survival analysis, followed by the log-rank
test, was used to compare the LR, DFS and OS rates between the two
groups; and the clinical TNM stage-stratified log-rank test was
also used to compare the LR and DFS rates. The Cox proportional
hazards model was used to evaluate the prognostic factors of DFS.
All statistical analyses were performed using SPSS version 22.0
(IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate
a statistically significant difference.
Cosmetic assessment
The postoperative cosmetic outcome of the patients
who had undergone NSM, followed by immediate breast reconstruction,
was evaluated by a panel comprising three surgeons and patients,
and was stratified into 5 grades (excellent, good, acceptable, poor
or very poor). Evaluation was based on 4 criteria, including
symmetry of size and shape, symmetry of NAC and inframammary fold,
the visibility of scarring and skin color match. Each criterion was
evaluated with 0 to 5 points. The total score 17 to 20 was
categorized as excellent, 13 to 16 as good, 9 to 12 as acceptable,
5 to 8 as poor and 0 to 4 as very poor.
Ethical approval and informed
consent
All procedures in the present study involving human
participants were performed in accordance with the ethical
standards of Guangxi Medical University Affiliated Tumor Hospital
research committee (approval no. 14–06) and with the 1964
Declaration of Helsinki and its later amendments (30). The present study was approved by the
Review Board of Guangxi Medical University Affiliated Tumor
Hospital and Liuzhou People's Hospital. The study used only
unidentifiable patient information and therefore, no written
informed consent was required.
Results
Patient characteristics
A total of 357 patients were selected for the
present study; of which, 163 patients (45.7%) had undergone NSM and
194 (54.3%) had undergone CM. The patient clinicopathological
features are summarized in Table I.
Patients who had undergone NSM exhibited a smaller tumor size, a
lower proportion of lymph node metastasis and a lower proportion of
stage II disease, compared with the CM group. There were no
statistical differences in age, histological grade, ER and PR
status and HER-2 status between the NSM and CM groups.
 | Table I.Comparison of clinical
characteristics between the NSM group (n=163) and the CM group
(n=194). |
Table I.
Comparison of clinical
characteristics between the NSM group (n=163) and the CM group
(n=194).
|
| NSM group | CM group |
|
|---|
|
|
|
|
|
|---|
| Characteristic | n | % | n | % | P-value |
|---|
| Age, years |
|
|
|
|
|
|
<30 | 29 | 17.8 | 23 | 11.9 | 0.113 |
|
30–34 | 134 | 82.2 | 171 | 88.1 |
|
| T stage |
|
|
|
|
|
| T0 | 3 | 1.8 | 5 | 2.6 | 0.047a |
| T1 | 70 | 42.9 | 58 | 29.9 |
|
| T2 | 90 | 55.2 | 129 | 66.5 |
|
| T3 | 0 | 0 | 2 | 1.0 |
|
| Nodal status |
|
|
|
|
|
| + | 54 | 33.1 | 89 | 45.9 | 0.014a |
| − | 109 | 66.9 | 105 | 54.1 |
|
| Clinical TNM
stage |
|
|
|
|
|
| 0 | 3 | 1.8 | 5 | 2.6 | 0.015a |
| I | 59 | 36.2 | 44 | 22.7 |
|
|
IIA | 58 | 35.6 | 68 | 35.1 |
|
|
IIB | 43 | 26.4 | 77 | 39.7 |
|
| Histological
grade |
|
|
|
|
|
| I | 35 | 21.5 | 31 | 16.0 | 0.357 |
| II | 70 | 42.9 | 84 | 43.3 |
|
|
III | 58 | 35.6 | 79 | 40.7 |
|
| ER status |
|
|
|
|
|
| + | 111 | 68.1 | 116 | 59.8 | 0.104 |
| − | 52 | 31.9 | 78 | 40.2 |
|
| PR status |
|
|
|
|
|
| + | 101 | 62.0 | 110 | 56.7 | 0.314 |
| − | 62 | 38.0 | 84 | 43.3 |
|
| HER-2 status |
|
|
|
|
|
| + | 69 | 42.3 | 97 | 50.0 | 0.148 |
| − | 78 | 47.9 | 73 | 37.6 |
|
|
Unknown | 16 | 9.8 | 24 | 12.4 |
|
Surgery
All patients from the NSM group had undergone breast
reconstruction, of which 148 patients (90.8%) had undergone
immediate breast reconstruction and 15 patients (9.2%) had
undergone delayed breast reconstruction. The reconstruction method
included permanent implant (32 cases, 19.6%), LD (51 cases, 31.2%),
ELD (59 cases, 36.2%), TRAM (18 cases, 11.0%) and DIEP (3 cases,
1.8%). Only 4 patients in the CM group had undergone delayed breast
reconstruction with ELD or a tissue expander followed by a
permanent implant. None of the patients in the CM group had
undergone immediate breast reconstruction.
Complications
Nipple necrosis occurred in 4 patients (2.5%) who
had undergone breast reconstruction with permanent implants, ELD
and TRAM. Partial necrosis of breast skin or the myocutaneous flap
was observed in 18 patients (11.0%) who had undergone breast
reconstruction with permanent implants and TRAM. Complete necrosis
of the myocutaneous flap was observed in 1 patient (0.6%) who had
undergone breast reconstruction with DIEP. No flap necrosis was
observed in the patients that had undergone breast reconstruction
with LD or ELD. There were no patients with a hematoma requiring
surgical intervention or an infection requiring removal of the
implant.
Oncological outcomes
All patients were followed up for a period of 4–118
months, with a median follow-up time of 49 months. The median
follow-up time in the NSM and CM groups were 39 months (range,
4–112 months) and 53 months (range, 4–118 months), respectively. In
the NSM group, 22 patients (13.5%) experienced recurrence, of which
6 (27.3%) exhibited LR only, 15 (68.2%) exhibited systemic
recurrence only and 1 (4.5%) exhibited LR and systemic recurrence.
In the CM group, 33 patients (17.0%) experienced recurrence, of
which 3 (9.1%) exhibited LR only, 27 (81.8%) exhibited systemic
recurrence only and 3 (9.1%) exhibited LR and systemic
recurrence.
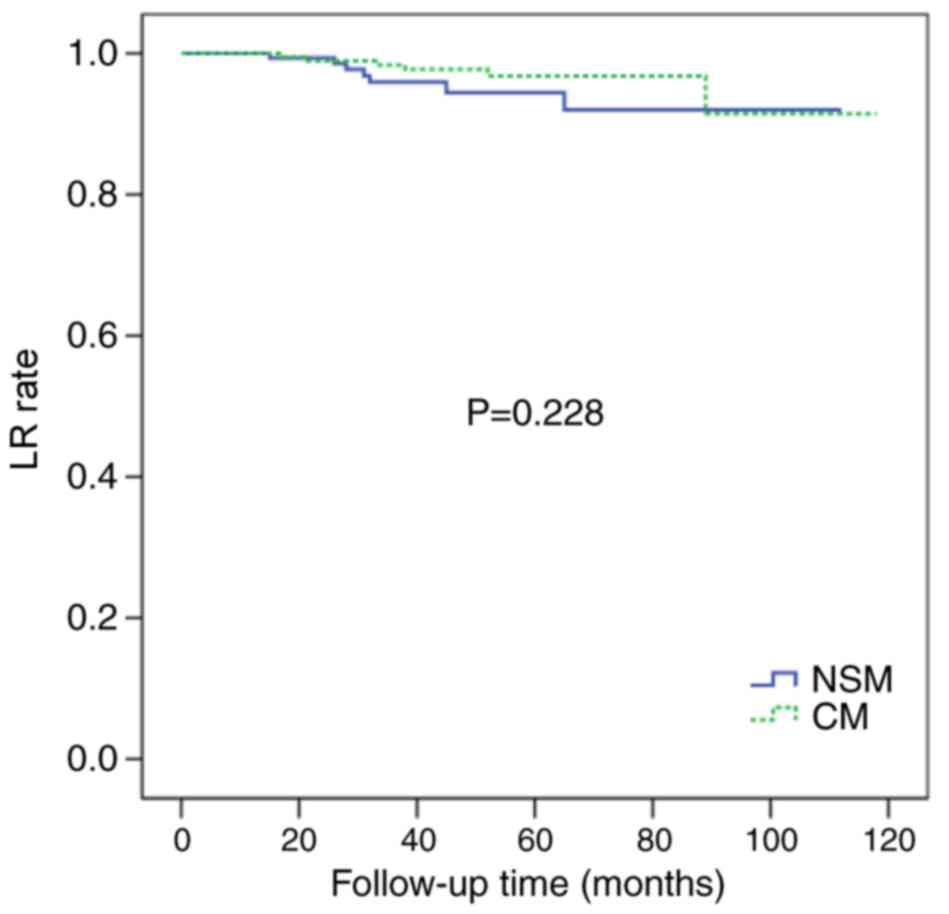
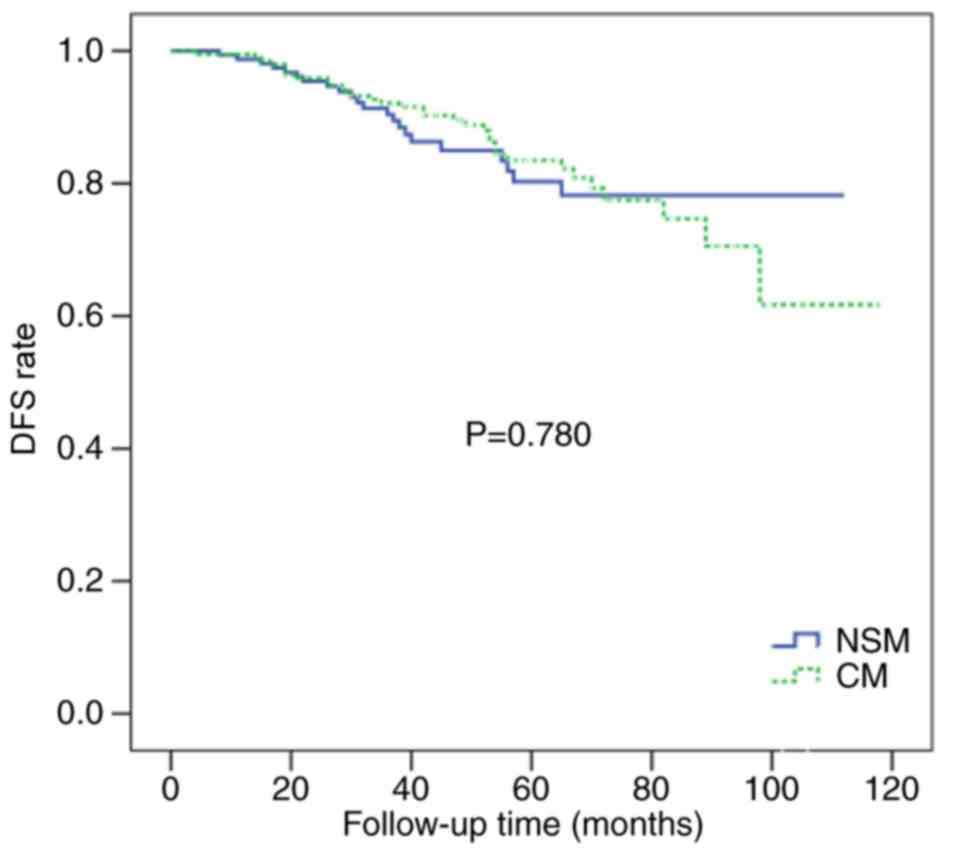
There were no significant differences in the 5-year
LR rate of the NSM and CM groups (4.3 vs. 3.1%; P=0.228; Fig. 1) or in the 5-year DFS rate of the two
groups (86.5 vs. 83.0%; P=0.780; Fig.
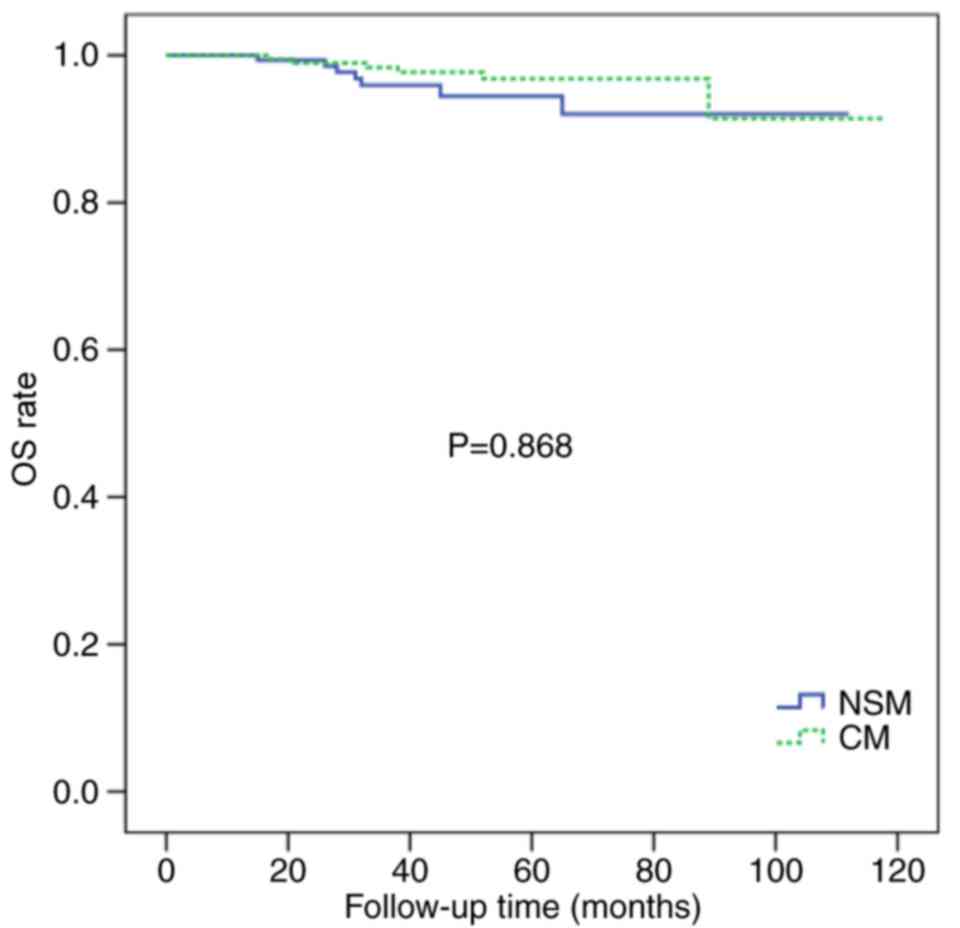
2). The 5-year OS rates were similar for the two groups (94.5
vs. 92.3%; P=0.868; Fig. 3).
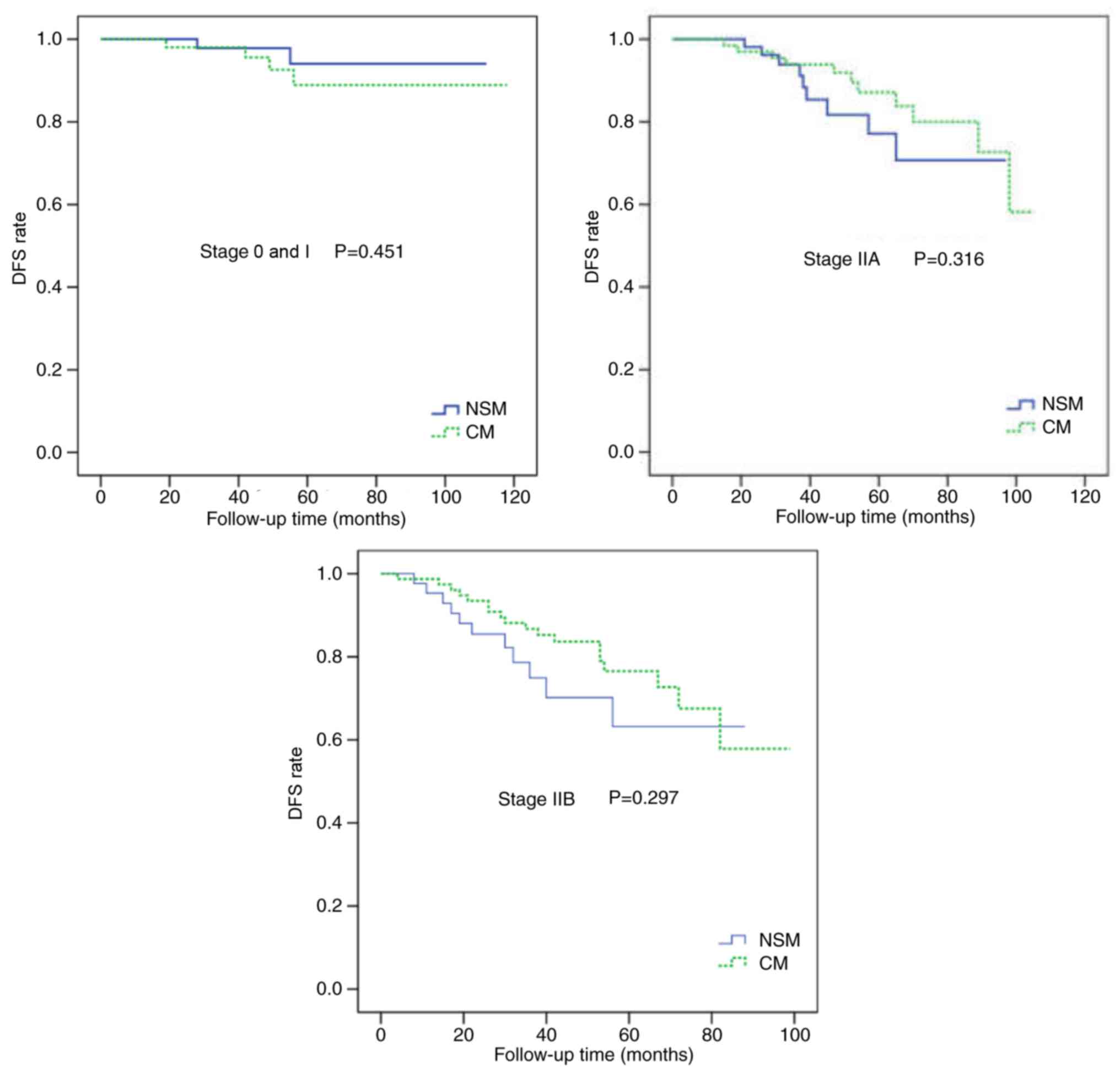
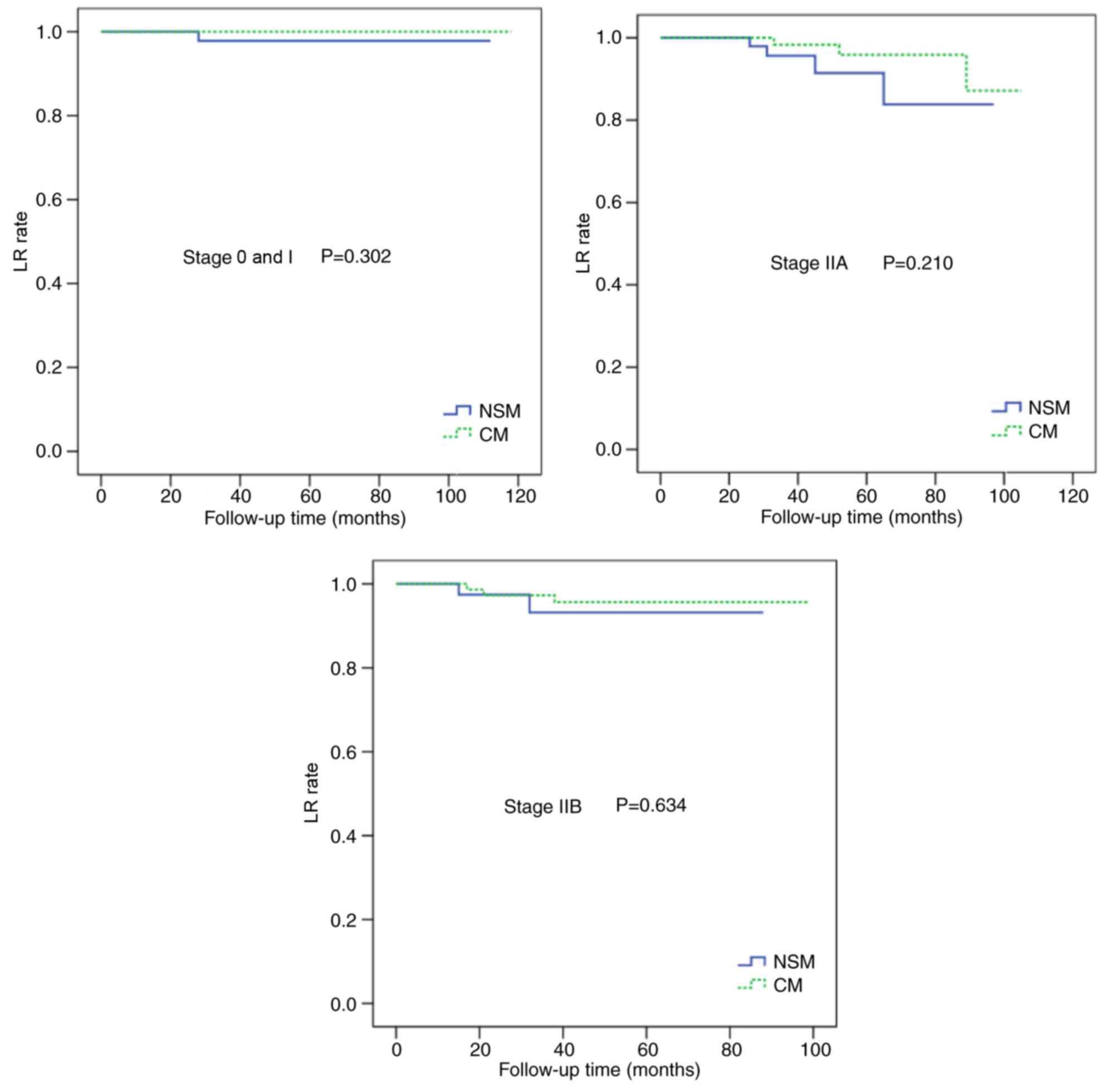
Following adjustment for the clinical TNM stage, there were no
statistically significant differences in the LR and DFS rates
between the two groups (Figs. 4 and
5). In multivariate analysis
(Table II), the significant risk
factors for DFS following NSM or CM were positive lymph nodes,
higher clinical TNM stage, negative ER/PR expression and positive
HER-2 expression. The surgical procedure was not a significant risk
factor for DFS (HR=1.041; 95% confidence interval=0.585–1.851;
P=0.892).
 | Table II.Multivariate analysis of clinical
characteristics and prognosis in young females treated with NSM or
CM. |
Table II.
Multivariate analysis of clinical
characteristics and prognosis in young females treated with NSM or
CM.
|
|
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Clinical
characteristic | B | SE | Wald | d.f. | P-value | Exp(B) | Lower limit | Upper limit |
|---|
| Surgical
procedure | 0.040 | 0.294 | 0.018 | 1 | 0.892 | 1.041 | 0.585 | 1.851 |
| Age | −0.031 | 0.057 | 0.301 | 1 | 0.583 | 0.969 | 0.867 | 1.084 |
| Tumor size | −0.301 | 0.334 | 0.812 | 1 | 0.368 | 0.740 | 0.384 | 1.425 |
| Nodal status | 1.229 | 0.541 | 5.172 | 1 | 0.023a | 3.419 | 1.185 | 9.865 |
| Clinical TNM
stage | 0.984 | 0.395 | 6.212 | 1 | 0.013a | 2.676 | 1.234 | 5.803 |
| Histological
grade | −0.083 | 0.195 | 0.179 | 1 | 0.672 | 0.921 | 0.628 | 1.349 |
| ER status | −0.944 | 0.290 | 10.592 | 1 | 0.001a | 0.389 | 0.220 | 0.687 |
| PR status | −1.234 | 0.306 | 16.302 | 1 | 0.006a | 0.291 | 0.160 | 0.530 |
| HER-2 status | −0.562 | 0.241 | 5.415 | 1 | 0.020a | 0.570 | 0.355 | 0.915 |
Treatment of LR with NSM
In the NSM group, 7 patients presented with LR. Of
these cases, NAC, skin and incision scar recurrences were observed
in 2 (1.2%), 4 (2.5%) and 1 case(s) (0.6%), respectively. The local
recurrent lesions of the 6 patients with LR only were excised
completely. All margins were negative and none of the patients
required total mastectomy. All these patients underwent
chemotherapy and radiation therapy following surgery. No novel
recurrences were observed during follow-up. The patient with LR and
systemic recurrence underwent chemotherapy and radiation therapy,
but liver failure caused by liver metastasis resulted in mortality
18 months later.
Cosmetic outcome
The postoperative cosmetic outcome evaluated by
patients was ‘excellent’ in 45/163 patients (27.6%), ‘good’ in
85/163 patients (52.1%), ‘acceptable’ in 24/163 patients (14.7%),
‘poor’ in 8/163 patients (4.9%) and ‘very poor’ in 1/163 patients
(0.6%). The postoperative cosmetic outcome evaluated by the panel
was ‘excellent’ in 38/163 patients (23.3%), ‘good’ in 77/163
patients (47.2%), ‘acceptable’ in 36/163 patients (22.1%), ‘poor’
in 11/163 patients (6.7%) and ‘very poor’ in 1/163 patients (0.6%).
There was only one patient who was graded as ‘very poor’ due to
complete necrosis of the DIEP flap. The overall percentage of
patients with ‘excellent’ or ‘good’ rating was 75.2%.
Discussion
The typical SSM procedure involves removal of the
mammary glands and NAC while sparing the skin envelope and the
native inframammary fold (6). It has
been demonstrated to be a safe procedure that provides good
cosmetic results with excellent local tumor control rate (7,8,31). NSM is similar to SSM but it also
preserves the NAC. Due to its preservation of the NAC, NSM has been
considered to have potentially higher LR risks (11). NSM has been used for breast tumors
with peripheral locations and even advanced disease (32).
Numerous previous prospective and retrospective
studies, comprising patients with varying sample sizes, inclusion
criteria and follow-up durations, have reported an LR rate of 0–24%
following NSM (12,16–18,32–34).
Although Benediktsson and Perbeck (33) reported a highest LR rate of 24% among
these studies, the NAC recurrence rate was 4%. While a study
undertaken by Adam et al (16)
reported no LR in a group comprising 67 patients after 36 months.
Shimo et al (34) determined
an LR rate of 5.8% after 46.8 months (range, 6–158 months) for 425
patients who had undergone NSM and the cumulative LR rate at the
NAC was 2.3%. Orzalesi et al (35) evaluated the oncological safety of NSM
in a large sample size (1,006 cases), and reported an LR rate of
2.9% and a NAC recurrence rate of 0.7%. A meta-analysis of 73
studies (12,358 cases) between 1970 and 2015 assessed the incidence
of LR following NSM (19). The mean
follow-up time was 38 months (range, 7.4–156 months) and the
overall LR rate was 2.38%. In recent years, studies have indicated
that NSM was safe for oncology and led to similar LR and OS rates
compared with those of patients treated with CM (18,34). These
results were similar to those of the present study. In the present
study, LR was observed in 7 patients (4.3%) in the NSM group,
including 2 NAC recurrences (1.2%), and no significant difference
was observed in the 5-year LR rate between the NSM and CM groups
(4.3 vs. 3.1%; P=0.228).
A number of previous studies have specialized in the
oncological safety of young patients who have undergone NSM. In
previous studies, the tumors observed in young patients have
frequently exhibited a high histological grade, ER/PR negative
status, HER-2 positive status, multifocal or multicenter
presentation, high proliferation and lymphovascular invasion
(23–25,36–39). The
presence of these characteristics in young patients with breast
cancer has generally been associated with a poorer prognosis
(20,21). Fredholm et al (22) conducted a population-based study
comprising 22,017 female patients with breast cancer and revealed
that young age was a risk factor for loco-regional recurrence. In
the present study, it was determined that young age did not
increase the oncological risk for the female patients with
early-stage breast cancer who had undergone NSM. There were no
significant statistical differences in the 5-year LR, DFS and OS
rates between the NSM and CM groups (Figs. 1–3).
In the present study, patients who had undergone CM
exhibited a larger tumor size, a higher proportion of positive
lymph nodes and a higher proportion of stage II disease, compared
with those who had undergone NSM. There may have been a selection
bias towards patients and the patients with early-stage cancer may
have been offered NSM more frequently. Patients with a larger tumor
size and positive lymph nodes exhibited a higher clinical TNM stage
more frequently. In order to reduce the selection bias, analysis
was adjusted for clinical TNM stage. It was determined that there
were no significant statistical differences in the LR and DFS rates
between the two groups when adjusted for clinical TNM stage
(Figs. 4 and 5).
Benediktsson and Perbeck reported that LR was
dependent on the lymph node status and clinical TNM stage (33). Petit et al (40) determined that the number of positive
lymph nodes, histological type and Ki-67 index were significant
predictive factors of LR based on multivariate analysis. Due to the
limited number of LR cases, the prognostic factors of LR based on
multivariate analysis could not be evaluated. However, it was
revealed that the significant risk factors for DFS following NSM or
CM were positive lymph nodes, negative ER/PR expression and
positive HER-2 expression.
In addition to oncological safety, complication
rates and cosmetic outcome are two other concerns associated with
NSM. Due to the removal of the tissue beneath the NAC, NSM may
cause an increased incidence of NAC necrosis. Headon et al
(19) analyzed 73 studies in their
meta-analysis and determined a nipple necrosis rate of 5.8% for
12,358 NSM procedures. In the present study, nipple necrosis
occurred in 4 patients (2.5%), less than the result reported by
Headon et al (19).
Arteriosclerosis rarely occurs in young female patients and the
majority of patients in the present study were non-smokers, which
may be a reason for the low nipple necrosis rate observed.
Furthermore, avoiding the use of high frequency electrotomes when
removing the tissue beneath the NAC (41) and maintaining the thickness of the
areola flap under the premise of oncological safety are also
important for reducing nipple areola necrosis (42). One advantage of the NSM procedure, the
cosmetic outcome, has been recognized by the majority of experts
(43,44). Yueh et al (44) reported that the NSM procedure provided
patients with a superior cosmetic outcome and psychological
satisfaction. The majority of the patients that had undergone NSM
followed by breast reconstruction in the present study obtained a
satisfactory cosmetic appearance. The overall rates of ‘excellent’
and ‘good’ grades averaged 75.2%. A positive body image is
particularly important for these young female patients with breast
cancer. Physical defects in young women can lead to poorer mental
health, lower self-esteem and sexual dysfunction, thereby
significantly affecting their quality of life (45).
There were certain limitations associated with the
present study. To begin with, the present study was retrospective
and only comprised a small number of patients (357 cases), thereby
resulting in unavoidable bias. In particular, the tendency to
perform NSM on patients with early-stage cancer led to a selection
bias. Secondly, the median follow-up time of the present study was
49 months (39 months for the NSM group and 53 months for the CM
group), which was relatively short, compared with the long natural
course of breast cancer. The present study only included patients
<35 years of age. A longer follow-up is required to demonstrate
the natural course of breast cancer in these young patients.
Furthermore, the clinical TNM stages of patients in the present
study were between stage 0 and IIB, but these patients exhibited a
relatively high proportion of recurrence and mortality. A
considerable proportion of HER-2 positive patients may not have
received Herceptin® therapy due to financial reasons,
which posed a notable social problem.
In summary, compared with CM, NSM did not increase
the risk of local and systemic recurrence in young patients with
early-stage breast cancer in the present study. The NSM procedure
achieved oncological safety and superior cosmetic outcomes for the
young females with breast cancer. Therefore, NSM may become one of
the standard procedures for the young patients with early-stage
breast cancer when breast reconstruction is performed.
Acknowledgements
The authors would like to thank the colleagues at
the Medical Records Room of Guangxi Medical University Affiliated
Tumor Hospital and Liuzhou People's Hospital for extracting the
necessary information from the database and the technicians at the
Department of Pathology at Guangxi Medical University Affiliated
Tumor Hospital and Liuzhou People's Hospital for providing
assistance in dealing with archived samples. The authors also would
like to thank the Statistics Teaching and Research Department of
Guangxi Medical University for providing advice regarding
statistical methods. The present study was supported by the
National Natural Science Foundation of China (grant no. 81360396)
and the Science and Technology Research Fund of Guangxi Zhuang
Autonomous Region Science and Technology Department (grant no.
1355005-3-12).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Halsted WS: I. The results of radical
operations for the cure of carcinoma of the breast. Ann Surg.
46:1–19. 1907. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Madden JL: Modified radical mastectomy.
Surg Gynecol Obstet. 121:1221–1230. 1965.PubMed/NCBI
|
|
3
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fisher B, Anderson S, Redmond CK, Wolmark
N, Wickerham DL and Cronin WM: Reanalysis and results after 12
years of follow-up in a randomized clinical trial comparing total
mastectomy with lumpectomy with or without irradiation in the
treatment of breast cancer. N Engl J Med. 333:1456–1461. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Voogd AC, Nielsen M, Peterse JL,
Blichert-Toft M, Bartelink H, Overgaard M, van Tienhoven G,
Andersen KW, Sylvester RJ and van Dongen JA; Danish Breast Cancer
Cooperative Group, ; Breast Cancer Cooperative Group of the
European Organization for Research and Treatment of Cancer, :
Differences in risk factors for local and distant recurrence after
breast-conserving therapy or mastectomy for stage I and II breast
cancer: Pooled results of two large European randomized trials. J
Clin Oncol. 19:1688–1697. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toth BA and Lappert P: Modified skin
incisions for mastectomy: The need for plastic surgical input in
preoperative planning. Plast Reconstr Surg. 87:1048–1053. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lanitis S, Tekkis PP, Sgourakis G,
Dimopoulos N, Al Mufti R and Hadjiminas DJ: Comparison of
skin-sparing mastectomy versus non-skin-sparing mastectomy for
breast cancer: A meta-analysis of observational studies. Ann Surg.
251:632–639. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yi M, Kronowitz SJ, Meric-Bernstam F, Feig
BW, Symmans WF, Lucci A, Ross MI, Babiera GV, Kuerer HM and Hunt
KK: Local, regional, and systemic recurrence rates in patients
undergoing skin-sparing mastectomy compared with conventional
mastectomy. Cancer. 117:916–924. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petit JY, Veronesi U, Orecchia R, Rey P,
Didier F, Giraldo A, Luini A, De Lorenzi F, Rietjens M, Garusi C,
et al: The nipple-sparing mastectomy: Early results of a
feasibility study of a new application of perioperative
radiotherapy (ELIOT) in the treatment of breast cancer when
mastectomy is indicated. Tumori. 89:288–291. 2003.PubMed/NCBI
|
|
10
|
Hinton CP, Doyle PJ, Blamey RW, Davies CJ,
Holliday HW and Elston CW: Subcutaneous mastectomy for primary
operable breast cancer. Br J Surg. 71:469–472. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cense HA, Rutgers EJ, Lopes Cardozo M and
Van Lanschot JJ: Nipple-sparing mastectomy in breast cancer: A
viable option? Eur J Surg Oncol. 27:1–526. 2001. View Article : Google Scholar
|
|
12
|
Simmons RM, Brennan M, Christos P, King V
and Osborne M: Analysis of nipple/areolar involvement with
mastectomy: Can the areola be preserved? Ann Surg Oncol. 9:165–168.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Laronga C, Kemp B, Johnston D, Robb GL and
Singletary SE: The incidence of occult nipple-areola complex
involvement in breast cancer patients receiving a skin-sparing
mastectomy. Ann Surg Oncol. 6:609–613. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brachtel EF, Rusby JE, Michaelson JS, Chen
LL, Muzikansky A, Smith BL and Koerner FC: Occult nipple
involvement in breast cancer: Clinicopathologic findings in 316
consecutive mastectomy specimens. J Clin Oncol. 27:4948–4954. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gerber B, Krause A, Dieterich M, Kundt G
and Reimer T: The oncological safety of skin sparing mastectomy
with conservation of the nipple-areola complex and autologous
reconstruction: An extended follow-up study. Ann Surg. 249:461–468.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adam H, Bygdeson M and de Boniface J: The
oncological safety of nipple-sparing mastectomy-a Swedish matched
cohort study. Eur J Surg Oncol. 40:1209–1215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ou KW, Yu JC, Ho MH, Chiu WK, Ou KL, Chen
TM and Chen SG: Oncological safety and outcomes of nipple-sparing
mastectomy with breast reconstruction: A single-centered experience
in Taiwan. Ann Plast Surg. 74 Suppl 2:S127–S131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seki T, Jinno H, Okabayashi K, Murata T,
Matsumoto A, Takahashi M, Hayashida T and Kitagawa Y: Comparison of
oncological safety between nipple sparing mastectomy and total
mastectomy using propensity score matching. Ann R Coll Surg Engl.
97:291–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Headon HL, Kasem A and Mokbel K: The
oncological safety of Nipple-sparing mastectomy: A systematic
review of the literature with a pooled analysis of 12,358
procedures. Arch Plast Surg. 43:328–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung WB, Yi JE, Jin JY, Choi YS, Park CS,
Park WC, Song BJ and Youn HJ: Early cardiac function monitoring for
detection of subclinical Doxorubicin cardiotoxicity in young adult
patients with breast cancer. J Breast Cancer. 16:178–183. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anders CK, Hsu DS, Broadwater G, Acharya
CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, et
al: Young age at diagnosis correlates with worse prognosis and
defines a subset of breast cancers with shared patterns of gene
expression. J Clin Oncol. 26:3324–3330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fredholm H, Magnusson K, Lindstrom LS,
Garmo H, Fält SE, Lindman H, Bergh J, Holmberg L, Pontén F, Frisell
J and Fredriksson I: Long-term outcome in young women with breast
cancer: A population-based study. Breast Cancer Res Treat.
160:131–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bharat A, Aft RL, Gao F and Margenthaler
JA: Patient and tumor characteristics associated with increased
mortality in young women (< or =40 years) with breast cancer. J
Surg Oncol. 100:248–251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fredholm H, Eaker S, Frisell J, Holmberg
L, Fredriksson I and Lindman H: Breast cancer in young women: Poor
survival despite intensive treatment. PLoS One. 4:e76952009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gnerlich JL, Deshpande AD, Jeffe DB, Sweet
A, White N and Margenthaler JA: Elevated breast cancer mortality in
women younger than age 40 years compared with older women is
attributed to poorer survival in early-stage disease. J Am Coll
Surg. 208:341–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kroman N, Holtveg H, Wohlfahrt J, Jensen
MB, Mouridsen HT, Blichert-Toft M and Melbye M: Effect of
breast-conserving therapy versus radical mastectomy on prognosis
for young women with breast carcinoma. Cancer. 100:688–693. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bantema-Joppe EJ, de Munck L, Visser O,
Willemse PH, Langendijk JA, Siesling S and Maduro JH: Early-stage
young breast cancer patients: Impact of local treatment on
survival. Int J Radiat Oncol Biol Phys. 81:e553–e559. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amin MB, Edge SB, Greene FL, et al:
American Joint committee on cancer (AJCC)AJCC cancer staging
manual. 8th. New York: Springer; 2017, View Article : Google Scholar
|
|
29
|
National Comprehensive Cancer Network
(NCCN), . Clinical practice guidelines in oncology. Breast cancer,
version, 2007–2016. https://www.nccn.org/professionals/physician_gls/default.aspx#breast
|
|
30
|
Rits IA: Declaration of helsinki.
Recommendations guidings doctors in clinical research. World Med J.
11:2811964.PubMed/NCBI
|
|
31
|
Kinoshita S, Nojima K, Takeishi M, Imawari
Y, Kyoda S, Hirano A, Akiba T, Kobayashi S, Takeyama H, Uchida K
and Morikawa T: Retrospective comparison of non-skin-sparing
mastectomy and skin-sparing mastectomy with immediate breast
reconstruction. Int J Surg Oncol. 2011:8765202011.PubMed/NCBI
|
|
32
|
Burdge EC, Yuen J, Hardee M, Gadgil PV,
Das C, Henry-Tillman R, Ochoa D, Korourian S and Suzanne Klimberg
V: Nipple skin-sparing mastectomy is feasible for advanced disease.
Ann Surg Oncol. 20:3294–3302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Benediktsson KP and Perbeck L: Survival in
breast cancer after nipple-sparing subcutaneous mastectomy and
immediate reconstruction with implants: A prospective trial with 13
years median follow-up in 216 patients. Eur J Surg Oncol.
34:143–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shimo A, Tsugawa K, Tsuchiya S, Yoshie R,
Tsuchiya K, Uejima T, Kojima Y, Shimo A, Hayami R, Nishikawa T, et
al: Oncologic outcomes and technical considerations of
nipple-sparing mastectomies in breast cancer: Experience of 425
cases from a single institution. Breast Cancer. 23:851–860. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Orzalesi L, Casella D, Santi C, Cecconi L,
Murgo R, Rinaldi S, Regolo L, Amanti C, Roncella M, Serra M, et al:
Nipple sparing mastectomy: Surgical and oncological outcomes from a
national multicentric registry with 913 patients (1006 cases) over
a six year period. Breast. 25:75–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morrison DH, Rahardja D, King E, Peng Y
and Sarode VR: Tumour biomarker expression relative to age and
molecular subtypes of invasive breast cancer. Br J Cancer.
107:382–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tang LC, Jin X, Yang HY, He M, Chang H,
Shao ZM and Di GH: Luminal B subtype: A key factor for the worse
prognosis of young breast cancer patients in China. BMC Cancer.
15:2012015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lund MJ, Butler EN, Hair BY, Ward KC,
Andrews JH, Oprea-Ilies G, Bayakly AR, O'Regan RM, Vertino PM and
Eley JW: Age/race differences in HER2 testing and in incidence
rates for breast cancer triple subtypes: A population-based study
and first report. Cancer. 116:2549–2559. 2010.PubMed/NCBI
|
|
39
|
Colleoni M, Rotmensz N, Robertson C,
Orlando L, Viale G, Renne G, Luini A, Veronesi P, Intra M, Orecchia
R, et al: Very young women (<35 years) with operable breast
cancer: Features of disease at presentation. Ann Oncol. 13:273–279.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Petit JY, Veronesi U, Orecchia R,
Curigliano G, Rey PC, Botteri E, Rotmensz N, Lohsiriwat V, Cassilha
Kneubil M and Rietjens M: Risk factors associated with recurrence
after nipple-sparing mastectomy for invasive and intraepithelial
neoplasia. Ann Oncol. 23:2053–2058. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Donovan CA, Harit AP, Chung A, Bao J,
Giuliano AE and Amersi F: Oncological and surgical outcomes after
Nipple-sparing mastectomy: Do incisions matter? Ann Surg Oncol.
23:3226–3231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Laporta R, Longo B, Sorotos M, Farcomeni
A, Patti C, Mastrangeli MR, Rubino C and Santanelli di Pompeo F:
Breast reconstruction following nipple-sparing mastectomy: Clinical
outcomes and risk factors related complications. J Plast Surg Hand
Surg. 51:427–435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Salgarello M, Visconti G and Barone-Adesi
L: Nipple-sparing mastectomy with immediate implant reconstruction:
Cosmetic outcomes and technical refinements. Plast Reconstr Surg.
126:1460–1471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yueh JH, Houlihan MJ, Slavin SA, Lee BT,
Pories SE and Morris DJ: Nipple-sparing mastectomy: Evaluation of
patient satisfaction, aesthetic results, and sensation. Ann Plast
Surg. 62:586–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fobair P, Stewart SL, Chang S, D'Onofrio
C, Banks PJ and Bloom JR: Body image and sexual problems in young
women with breast cancer. Psycho Oncol. 15:579–594. 2006.
View Article : Google Scholar
|